化工进展 ›› 2023, Vol. 42 ›› Issue (7): 3652-3663.DOI: 10.16085/j.issn.1000-6613.2022-1588
炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响
李海东1( ), 杨远坤1,2, 郭姝姝1, 汪本金1, 岳婷婷1, 傅开彬1,2, 王哲1,2, 何守琴3, 姚俊4, 谌书1,2(
), 杨远坤1,2, 郭姝姝1, 汪本金1, 岳婷婷1, 傅开彬1,2, 王哲1,2, 何守琴3, 姚俊4, 谌书1,2( )
)
- 1.西南科技大学环境与资源学院,四川 绵阳 621000
2.固体废物处理与资源化教育部重点实验室,四川 绵阳 621000
3.西南科技大学经济管理学院,四川 绵阳 621000
4.中国地质大学(北京)水资源与 环境学院,北京 100083
-
收稿日期:2022-08-29修回日期:2022-11-02出版日期:2023-07-15发布日期:2023-08-14 -
通讯作者:谌书 -
作者简介:李海东(1997—),男,硕士研究生,研究方向为水污染治理。E-mail:3064651627@qq.com。 -
基金资助:国家重点研发计划(2019YFC1803500);国家自然科学基金青年基金(42107481);川省科技计划-重点研发项目(2021YFS0289);西南科技大学科研基金(20zx7150);四川循环经济研究中心科技项目(XHJJ-1417)
Effect of carbonization and calcination temperature on As(Ⅲ) removal performance of plant-based Fe-C microelectrolytic materials
LI Haidong1( ), YANG Yuankun1,2, GUO Shushu1, WANG Benjin1, YUE Tingting1, FU Kaibin1,2, WANG Zhe1,2, HE Shouqin3, YAO Jun4, CHEN Shu1,2(
), YANG Yuankun1,2, GUO Shushu1, WANG Benjin1, YUE Tingting1, FU Kaibin1,2, WANG Zhe1,2, HE Shouqin3, YAO Jun4, CHEN Shu1,2( )
)
- 1.School of Environment and Resource, Southwest University of Science and Technology, Mianyang 621010, Sichuan, China
2.Key Laboratory of Solid Waste Treatment and Resource Recycle, Ministry of Education, Mianyang 621010, Sichuan, China
3.School of Economics and Management, Southwest University of Science and Technology, Mianyang 621010, Sichuan, China
4.China University of Geosciences in Beijing, Beijing 100083, China
-
Received:2022-08-29Revised:2022-11-02Online:2023-07-15Published:2023-08-14 -
Contact:CHEN Shu
摘要:
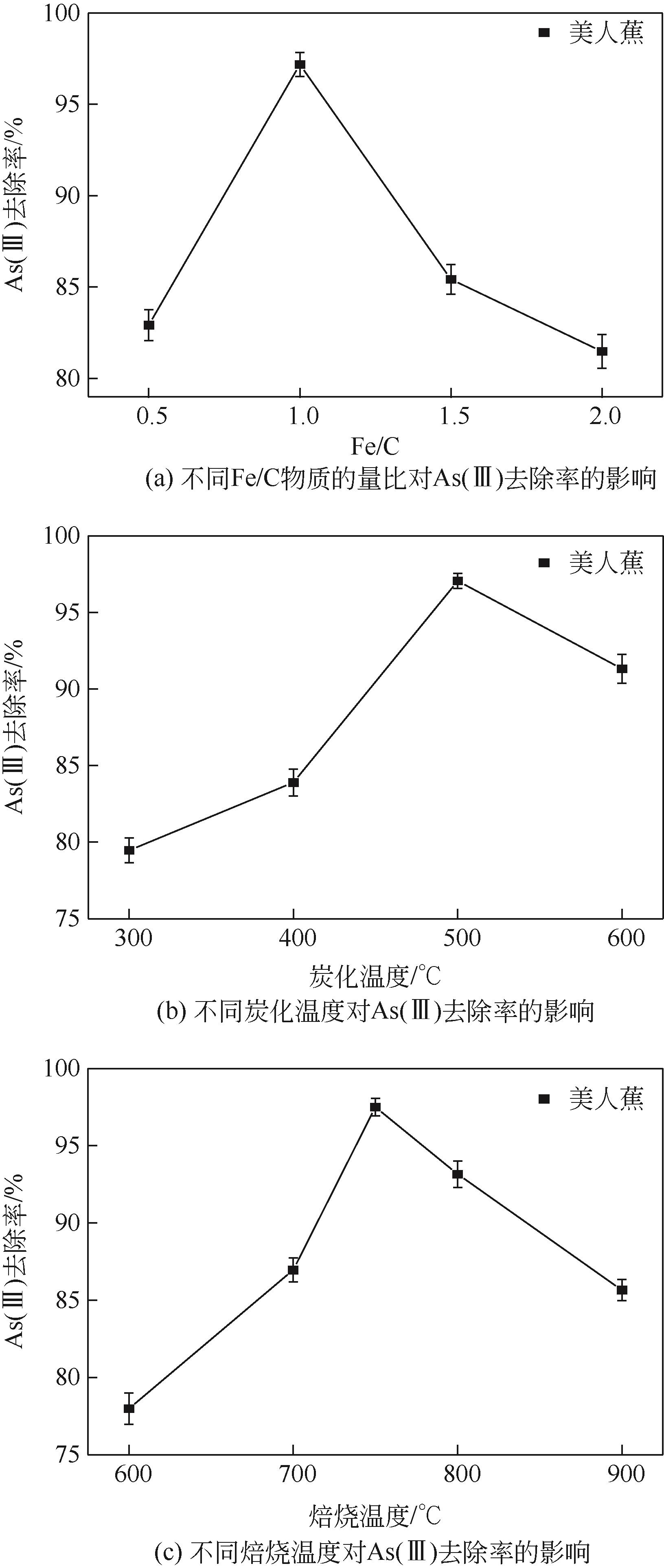
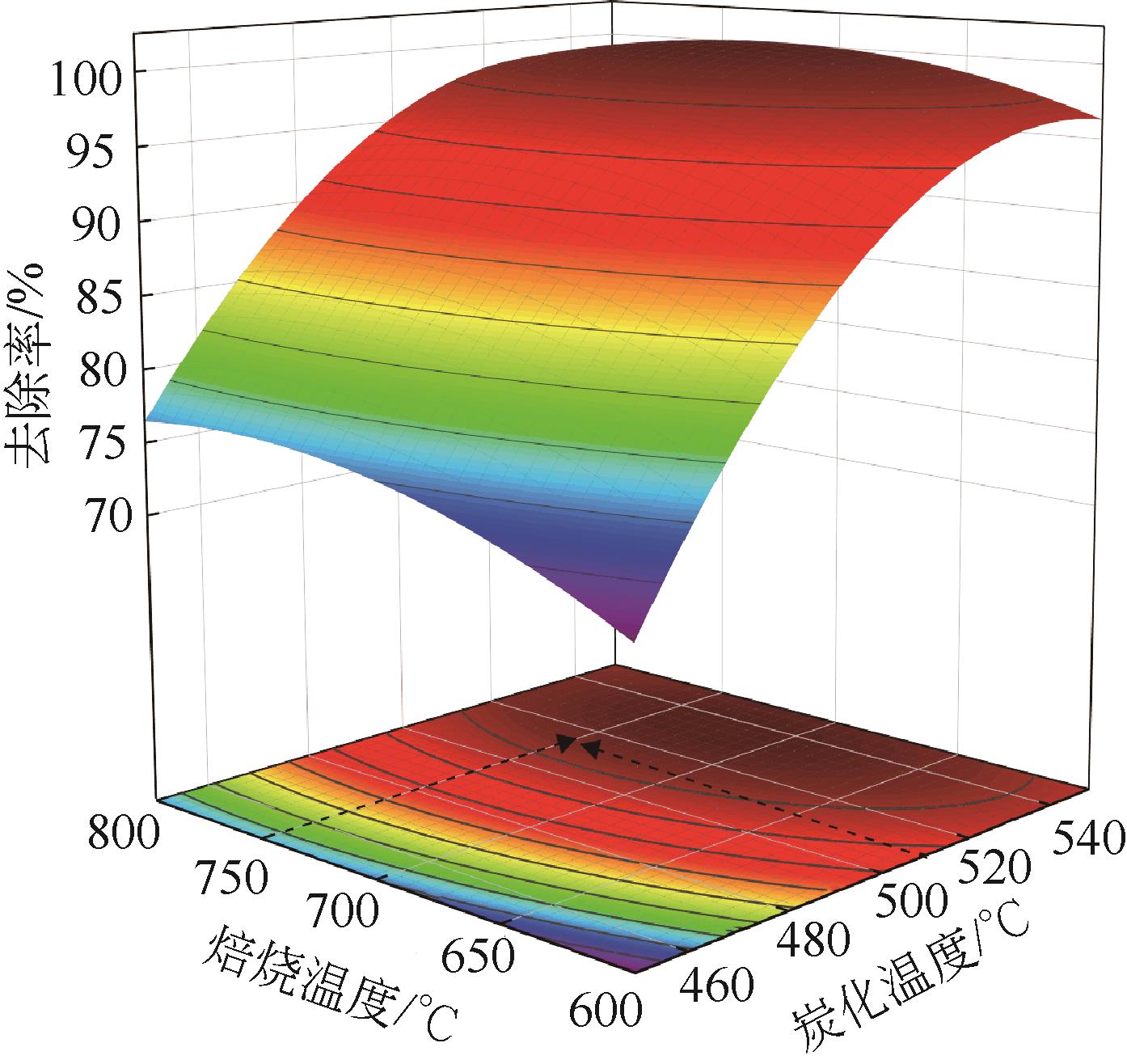
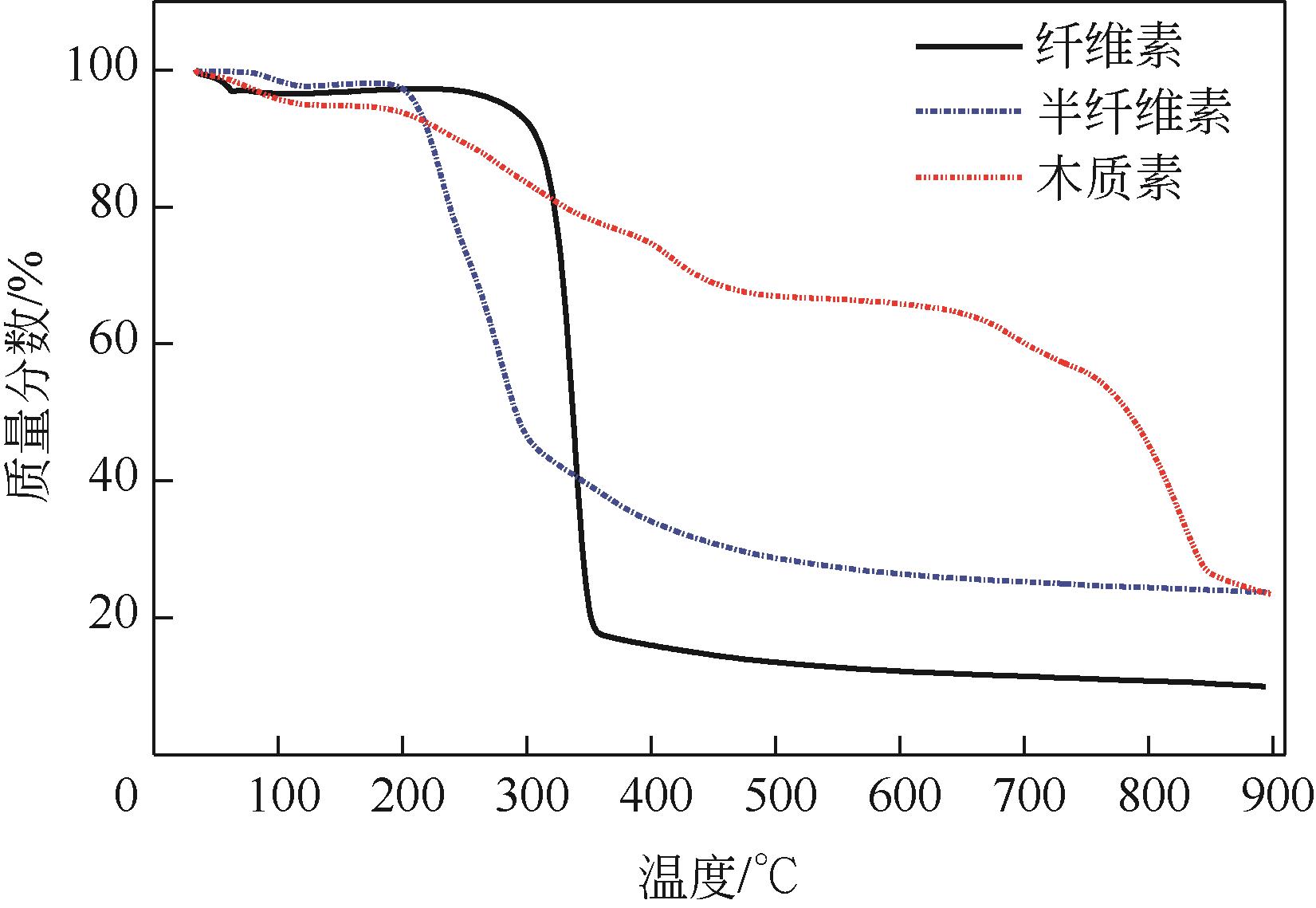
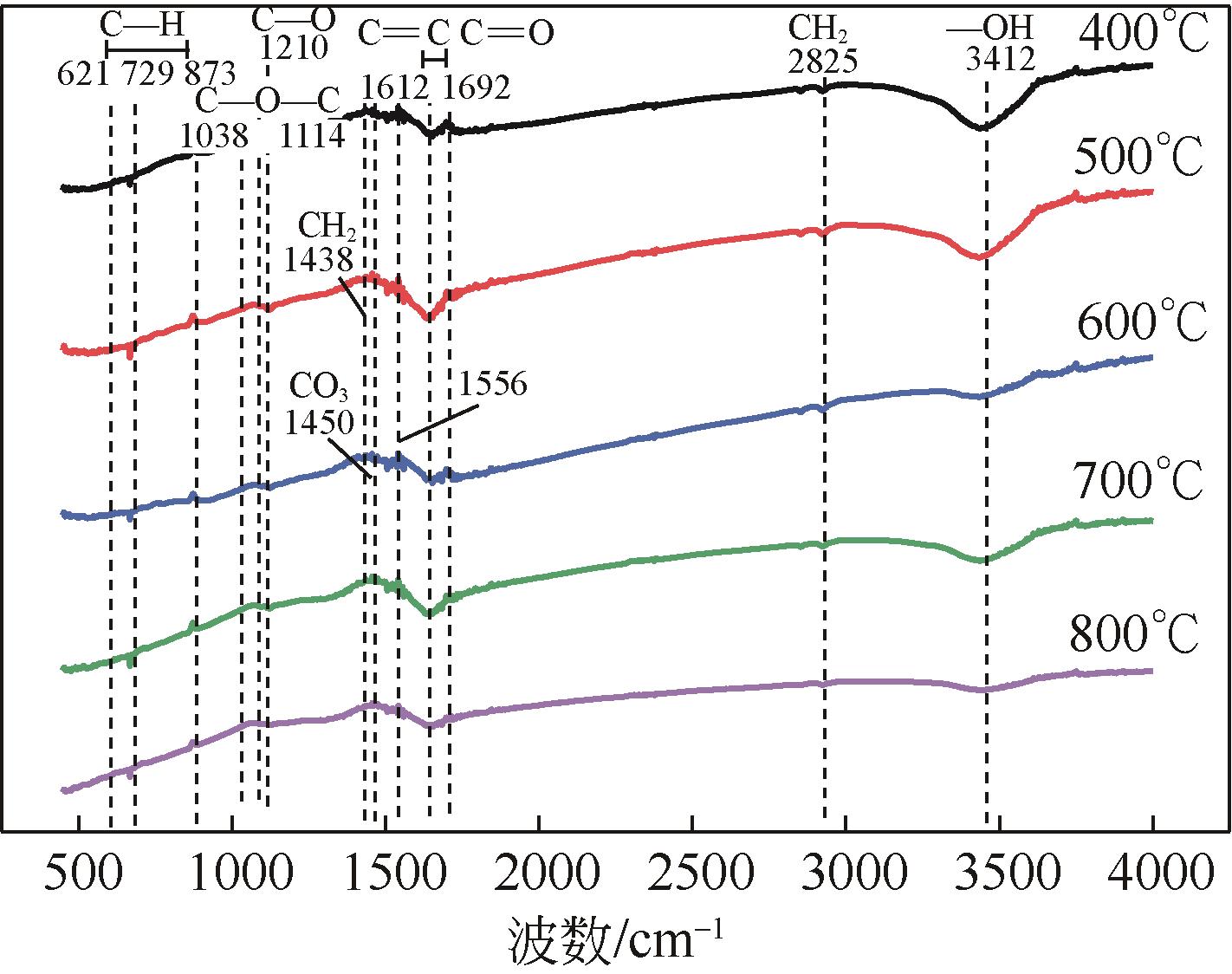
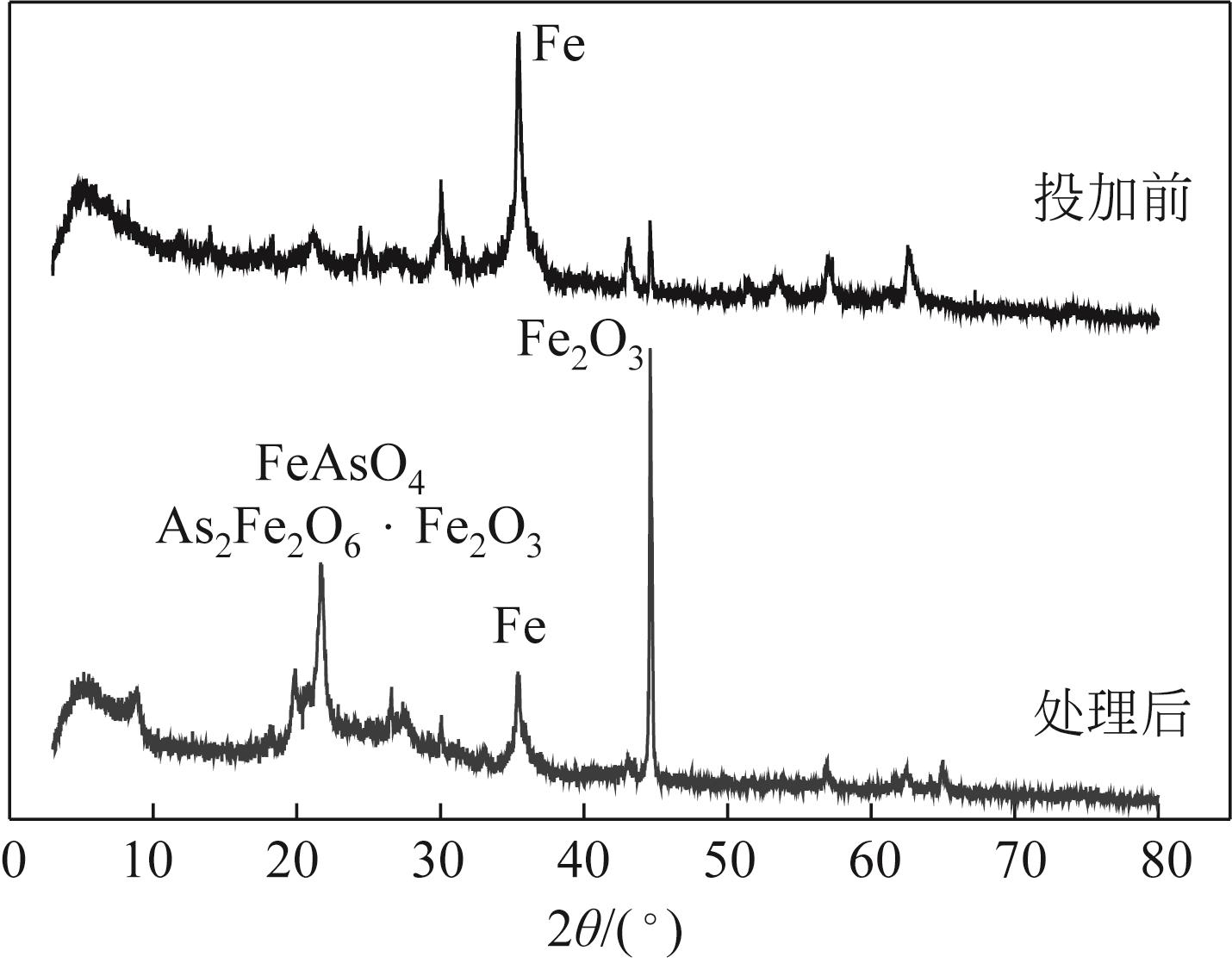
以美人蕉、还原铁粉和膨润土为原材料制备植物基铁碳微电解材料,先通过单因素实验确定Fe/C物质的量比、炭化温度和焙烧温度3个影响因素,后采用Box-Behnken响应面法对“均质化-炭化-焙烧”制备工艺进行优化,以确定最优制备条件。并结合X射线衍射(XRD)、电子顺磁共振波谱仪(EPR)、傅里叶红外光谱仪(FTIR)等表征方法,探究烧制温度对植物基铁碳微电解材料固有性质及其去除As(Ⅲ)性能的影响。结果表明,最优制备条件为Fe/C=1.05、炭化温度502.87℃、焙烧温度760.92℃。烧制温度的升高,有利于增强碳基组分得电子能力,加速As(Ⅲ)氧化为As(Ⅴ),降低水体生物毒性的同时提高对As(Ⅲ)的去除率;当焙烧温度高于700℃时,膨润土晶体结构层瓦解,渗透性提高的同时加速Ca2+、Mg2+离子释放,并促进Fe3+的水解沉积物互斥作用减弱,提高对As(Ⅲ)的吸附容量;还原铁粉过量5%,在保证反应时微原电池数量的同时,表面氧化产生的Fe3O4、Fe2O3在酸性条件下易反应形成Fe2+、Fe3+,对As(Ⅲ)的吸附去除作用加强。
中图分类号:
引用本文
李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663.
LI Haidong, YANG Yuankun, GUO Shushu, WANG Benjin, YUE Tingting, FU Kaibin, WANG Zhe, HE Shouqin, YAO Jun, CHEN Shu. Effect of carbonization and calcination temperature on As(Ⅲ) removal performance of plant-based Fe-C microelectrolytic materials[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3652-3663.
样品 名称 | 元素组成(质量分数)/% | 原子比 | 灰分 /% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | H/C | O/C | (O+N/C) | |||
| 美人蕉 | 36.35 | 3.72 | 1.68 | 50.09 | 0.29 | 1.23 | 1.03 | 1.07 | 11.23 | |
表1 美人蕉植物材料元素组成及原子比
样品 名称 | 元素组成(质量分数)/% | 原子比 | 灰分 /% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | H/C | O/C | (O+N/C) | |||
| 美人蕉 | 36.35 | 3.72 | 1.68 | 50.09 | 0.29 | 1.23 | 1.03 | 1.07 | 11.23 | |
| 自变量 | 因数 | 范围和水平 | ||
|---|---|---|---|---|
| 低(-1) | 中(0) | 高(+1) | ||
| Fe/C | A | 0.8 | 1.0 | 1.2 |
| 炭化温度/℃ | B | 450 | 500 | 550 |
| 焙烧温度/℃ | C | 600 | 700 | 800 |
表2 Box-Behnken设计实验因数与水平
| 自变量 | 因数 | 范围和水平 | ||
|---|---|---|---|---|
| 低(-1) | 中(0) | 高(+1) | ||
| Fe/C | A | 0.8 | 1.0 | 1.2 |
| 炭化温度/℃ | B | 450 | 500 | 550 |
| 焙烧温度/℃ | C | 600 | 700 | 800 |
实验 编号 | 因素 | As(Ⅲ)去除率(美人蕉) /% | ||
|---|---|---|---|---|
| A | B | C | ||
| 1 | 0.8 | 450 | 700 | 85.47 |
| 2 | 1.2 | 450 | 700 | 91.86 |
| 3 | 0.8 | 550 | 700 | 88.83 |
| 4 | 1.2 | 550 | 700 | 93.36 |
| 5 | 0.8 | 500 | 600 | 68.36 |
| 6 | 1.2 | 500 | 600 | 77.39 |
| 7 | 0.8 | 500 | 800 | 95.49 |
| 8 | 1.2 | 500 | 800 | 98.56 |
| 9 | 1 | 450 | 600 | 68.18 |
| 10 | 1 | 550 | 600 | 74.68 |
| 11 | 1 | 450 | 800 | 96.04 |
| 12 | 1 | 550 | 800 | 96.48 |
| 13 | 1 | 500 | 700 | 95.53 |
| 14 | 1 | 500 | 700 | 95.05 |
| 15 | 1 | 500 | 700 | 94.81 |
| 16 | 1 | 500 | 700 | 95.42 |
| 17 | 1 | 500 | 700 | 95.63 |
表3 Box-Bennken响应面实验设计及结果
实验 编号 | 因素 | As(Ⅲ)去除率(美人蕉) /% | ||
|---|---|---|---|---|
| A | B | C | ||
| 1 | 0.8 | 450 | 700 | 85.47 |
| 2 | 1.2 | 450 | 700 | 91.86 |
| 3 | 0.8 | 550 | 700 | 88.83 |
| 4 | 1.2 | 550 | 700 | 93.36 |
| 5 | 0.8 | 500 | 600 | 68.36 |
| 6 | 1.2 | 500 | 600 | 77.39 |
| 7 | 0.8 | 500 | 800 | 95.49 |
| 8 | 1.2 | 500 | 800 | 98.56 |
| 9 | 1 | 450 | 600 | 68.18 |
| 10 | 1 | 550 | 600 | 74.68 |
| 11 | 1 | 450 | 800 | 96.04 |
| 12 | 1 | 550 | 800 | 96.48 |
| 13 | 1 | 500 | 700 | 95.53 |
| 14 | 1 | 500 | 700 | 95.05 |
| 15 | 1 | 500 | 700 | 94.81 |
| 16 | 1 | 500 | 700 | 95.42 |
| 17 | 1 | 500 | 700 | 95.63 |
| 模型项 | 平方和 | 自由度 | 均方 | F值 | P值 |
|---|---|---|---|---|---|
| 模型 | 1674.59 | 9 | 186.07 | 914.56 | <0.0001 |
| Fe/C(A) | 66.24 | 1 | 66.24 | 325.59 | <0.0001 |
| 炭化温度(B) | 17.41 | 1 | 17.41 | 85.55 | <0.0001 |
| 焙烧温度(C) | 1199.52 | 1 | 1199.52 | 5895.98 | <0.0001 |
| AB | 0.8649 | 1 | 0.8649 | 4.25 | 0.0482 |
| AC | 8.88 | 1 | 8.88 | 43.65 | 0.0003 |
| BC | 9.18 | 1 | 9.18 | 45.13 | 0.0003 |
| A² | 19.49 | 1 | 19.49 | 95.80 | <0.0001 |
| B² | 44.65 | 1 | 44.65 | 219.48 | <0.0001 |
| C² | 282.18 | 1 | 282.18 | 1387.02 | <0.0001 |
| 残差 | 1.42 | 7 | 0.2034 | ||
| 失拟项 | 0.9460 | 3 | 0.3153 | 2.64 | 0.1859 |
| 误差值 | 0.4781 | 4 | 0.1195 | ||
| 总和 | 1676.01 | 16 | |||
| R2adj | 0.9981 | R2 | 0.9992 | ||
| 精密度 | 87.4283 | R2Pred | 0.9905 | ||
表4 二次回归模型检验及方差分析
| 模型项 | 平方和 | 自由度 | 均方 | F值 | P值 |
|---|---|---|---|---|---|
| 模型 | 1674.59 | 9 | 186.07 | 914.56 | <0.0001 |
| Fe/C(A) | 66.24 | 1 | 66.24 | 325.59 | <0.0001 |
| 炭化温度(B) | 17.41 | 1 | 17.41 | 85.55 | <0.0001 |
| 焙烧温度(C) | 1199.52 | 1 | 1199.52 | 5895.98 | <0.0001 |
| AB | 0.8649 | 1 | 0.8649 | 4.25 | 0.0482 |
| AC | 8.88 | 1 | 8.88 | 43.65 | 0.0003 |
| BC | 9.18 | 1 | 9.18 | 45.13 | 0.0003 |
| A² | 19.49 | 1 | 19.49 | 95.80 | <0.0001 |
| B² | 44.65 | 1 | 44.65 | 219.48 | <0.0001 |
| C² | 282.18 | 1 | 282.18 | 1387.02 | <0.0001 |
| 残差 | 1.42 | 7 | 0.2034 | ||
| 失拟项 | 0.9460 | 3 | 0.3153 | 2.64 | 0.1859 |
| 误差值 | 0.4781 | 4 | 0.1195 | ||
| 总和 | 1676.01 | 16 | |||
| R2adj | 0.9981 | R2 | 0.9992 | ||
| 精密度 | 87.4283 | R2Pred | 0.9905 | ||
| 样品 | g值 | 线宽/Gs |
|---|---|---|
| 400 | 2.00452±0.00006 | 6.27 |
| 500 | 2.00386±0.00002 | 5.29 |
| 600 | 2.00377±0.00003 | 4.75 |
| 700 | 2.00342±0.00001 | 4.38 |
| 800 | 2.00337±0.00002 | 3.65 |
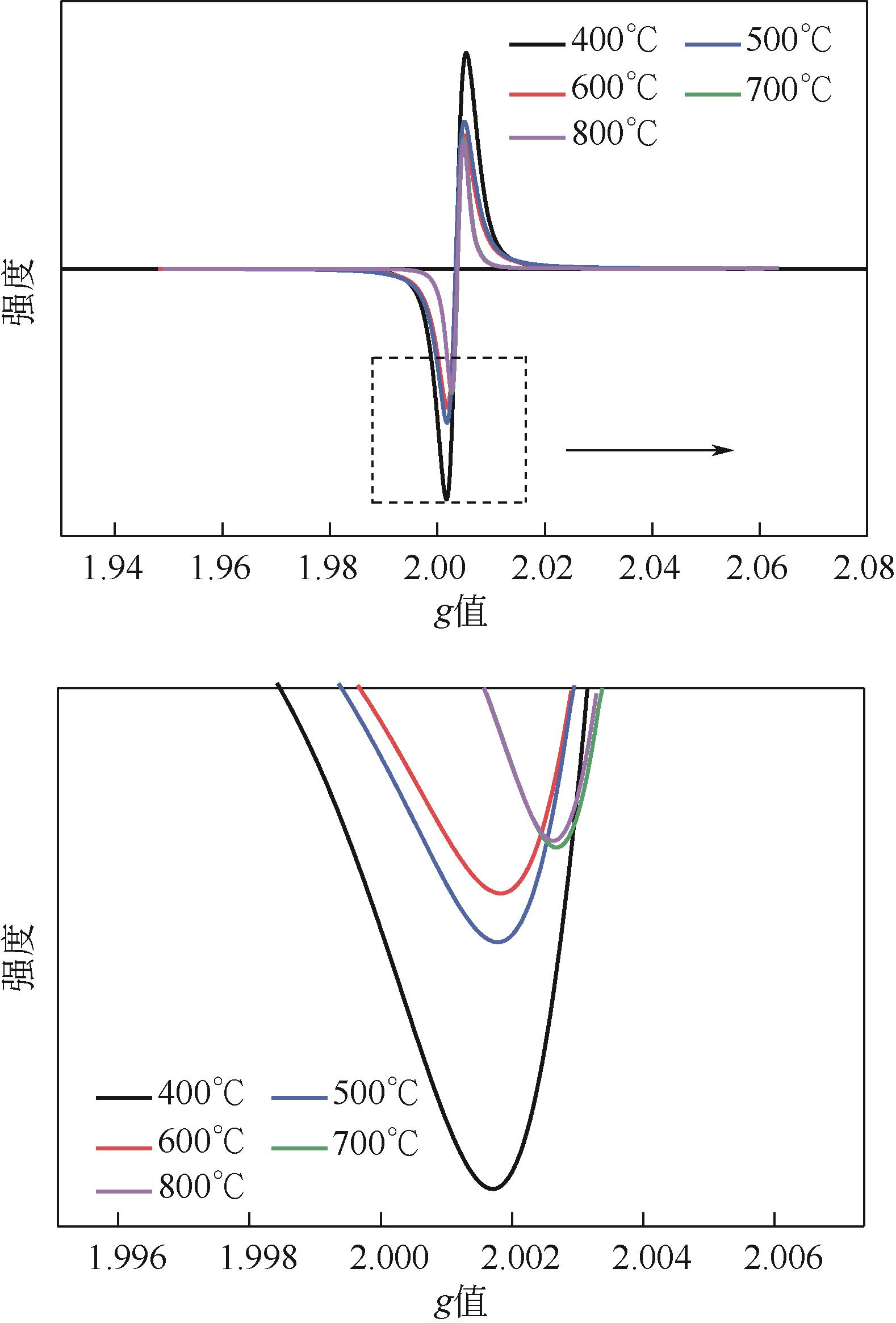
表5 生物炭PFRs参数
| 样品 | g值 | 线宽/Gs |
|---|---|---|
| 400 | 2.00452±0.00006 | 6.27 |
| 500 | 2.00386±0.00002 | 5.29 |
| 600 | 2.00377±0.00003 | 4.75 |
| 700 | 2.00342±0.00001 | 4.38 |
| 800 | 2.00337±0.00002 | 3.65 |
| 编号 | 反应式 | 标准摩尔吉布斯自由能变化表达式 |
|---|---|---|
| Y1 | 4Fe3O4+O2 | |
| Y2 | 3/2Fe+O2 | |
| Y3 | 6Fe+O2 | |
| Y4 | 6Fe+O2 |
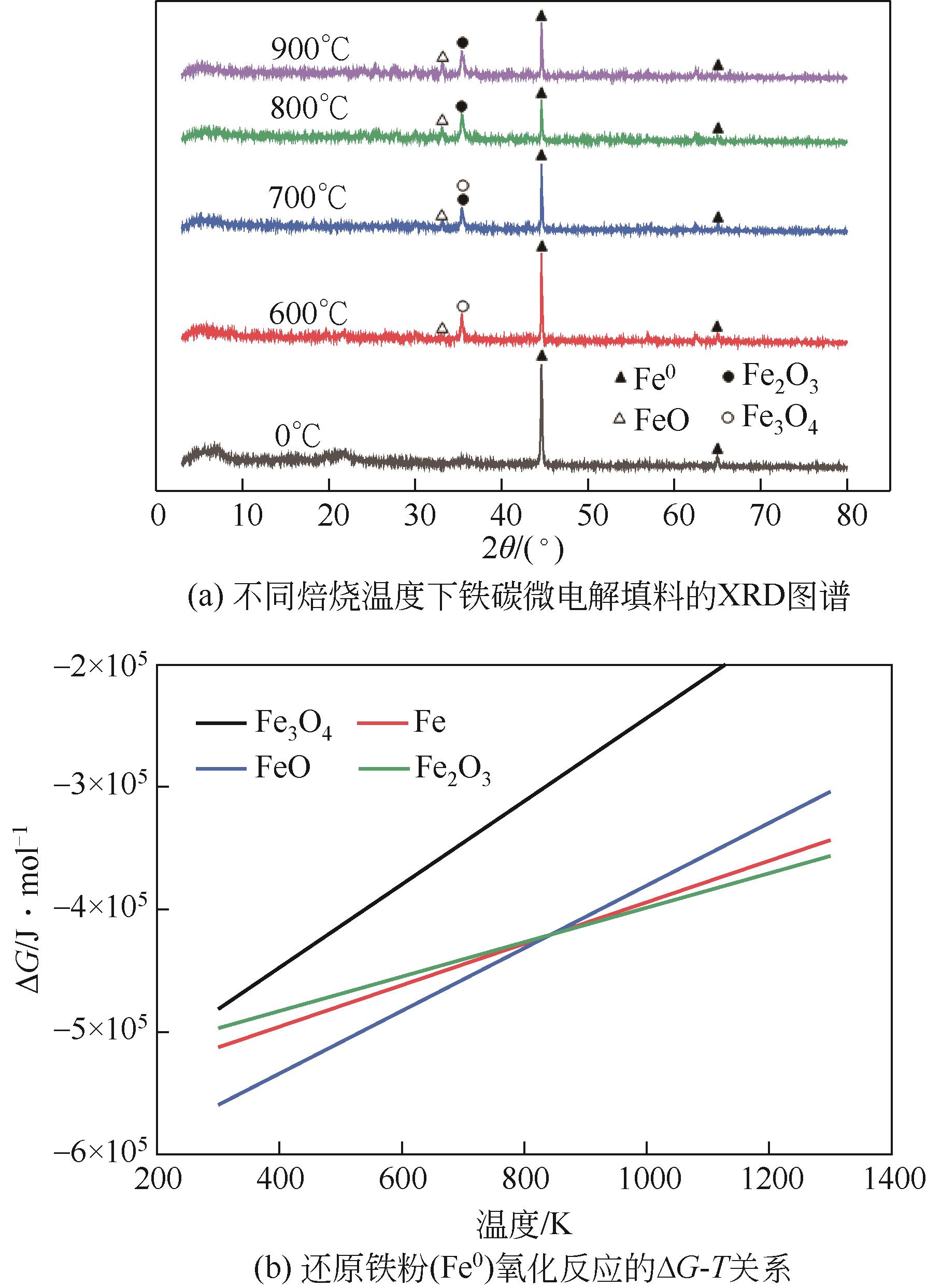
表6 铁氧化反应的标准摩尔吉布斯自由能变化表达式
| 编号 | 反应式 | 标准摩尔吉布斯自由能变化表达式 |
|---|---|---|
| Y1 | 4Fe3O4+O2 | |
| Y2 | 3/2Fe+O2 | |
| Y3 | 6Fe+O2 | |
| Y4 | 6Fe+O2 |
碳基 材料 | Fe/C 摩尔比 | 炭化温度 /℃ | 焙烧温度 /℃ | As(Ⅲ)去除率/% | 相对偏差 /% | |
|---|---|---|---|---|---|---|
| 预测值 | 实验值 | |||||
| 美人蕉 | 1.05 | 502.87 | 760.92 | 98.69 | 97.32 | 1.39 |
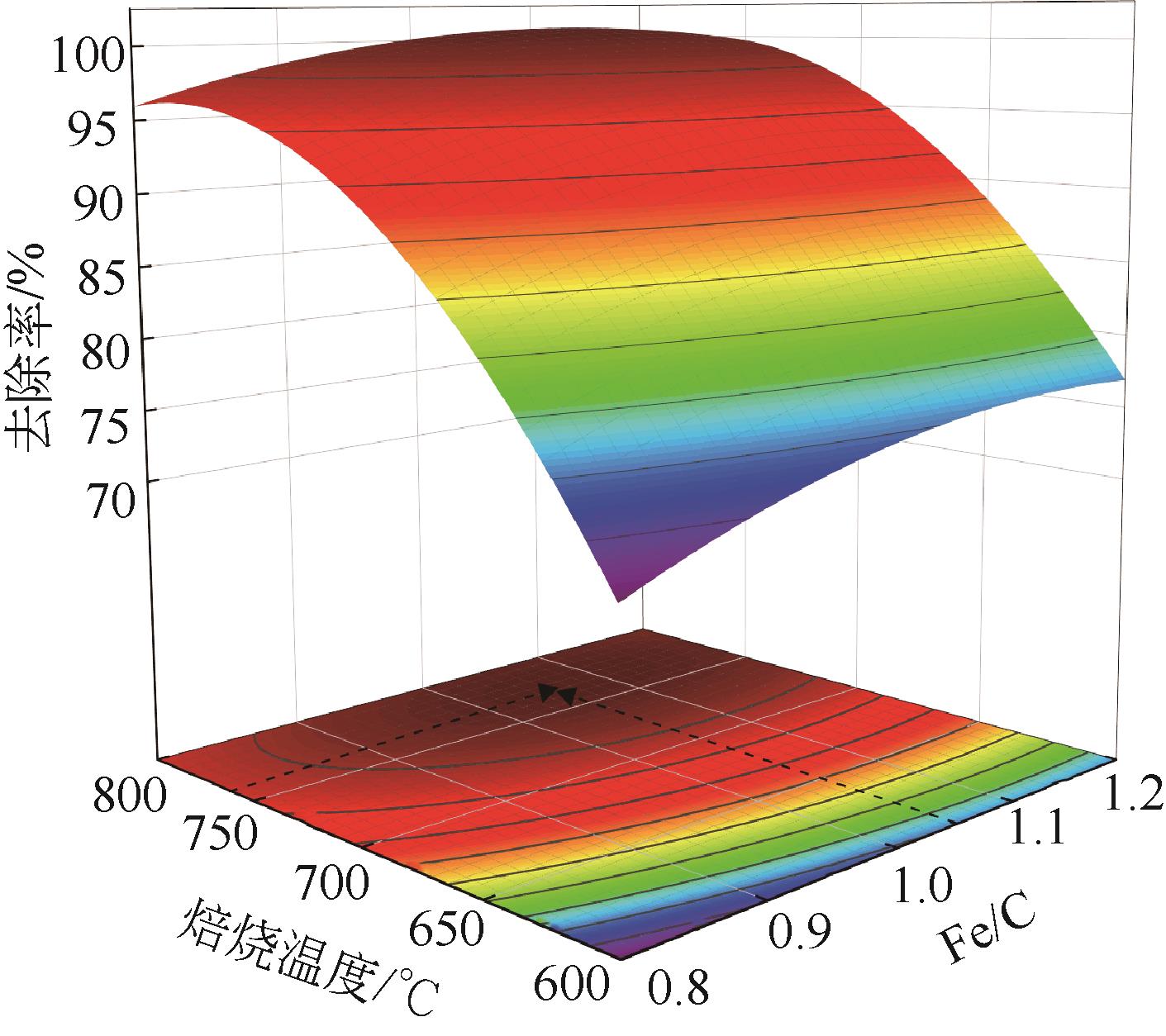
表7 美人蕉植物基铁碳微电解材料最优制备条件
碳基 材料 | Fe/C 摩尔比 | 炭化温度 /℃ | 焙烧温度 /℃ | As(Ⅲ)去除率/% | 相对偏差 /% | |
|---|---|---|---|---|---|---|
| 预测值 | 实验值 | |||||
| 美人蕉 | 1.05 | 502.87 | 760.92 | 98.69 | 97.32 | 1.39 |
| 1 | 肖细元, 陈同斌, 廖晓勇, 等. 中国主要含砷矿产资源的区域分布与砷污染问题[J]. 地理研究, 2008, 27(1): 201-212. |
| XIAO Xiyuan, CHEN Tongbin, LIAO Xiaoyong, et al. Regional distribution of arsenic contained minerals and arsenic pollution in China[J]. Geographical Research, 2008, 27(1): 201-212. | |
| 2 | HE Zongliang, TIAN Senlin, NING Ping. Adsorption of arsenate and arsenite from aqueous solutions by cerium-loaded cation exchange resin[J]. Journal of Rare Earths, 2012, 30(6): 563-572. |
| 3 | 包稚群, 丘克强. 关于我国砷污染现状与治理砷建议[J]. 云南冶金, 2019, 48(3): 60-64. |
| BAO Zhiqun, QIU Keqiang. The current status and treatment suggestions of arsenic pollution in China[J]. Yunnan Metallurgy, 2019, 48(3): 60-64. | |
| 4 | ZENG L, WANG Y P. 200 Million people in the world drink arsenic exceeding the standard[J]. Ecological Economy, 2018, 34(11): 6-9. |
| 5 | SARWAR T, KHAN S, MUHAMMAD S, et al. Arsenic speciation, mechanisms, and factors affecting rice uptake and potential human health risk: A systematic review[J]. Environmental Technology & Innovation, 2021, 22: 101392. |
| 6 | DAS A, JOARDAR M, CHOWDHURY N R, et al. Arsenic toxicity in livestock growing in arsenic endemic and control sites of West Bengal: Risk for human and environment[J]. Environmental Geochemistry and Health, 2021, 43(8): 3005-3025. |
| 7 | 国家市场监督管理总局, 国家标准化管理委员会. 关于批准发布《生活饮用水卫生标准》等5项强制性国家标准的公告[J]. 中国标准化, 2022(7): 272. |
| State Administration for Market Regulation, Administration Standardization. Notice on the approval and promulgation of five mandatory national standards, including the sanitary standards for drinking water[J]. China Standardization, 2022(7): 272. | |
| 8 | 国家环境保护总局、国家质量监督检验检疫总局发布地表水环境质量标准[J]. 中国环保产业, 2002(6): 8-9. |
| The State Environmental Protection Administration and the General Administration of Quality Supervision, Inspection and Quarantine issue standards for surface water environmental quality[J]. China Environmental Protection Industry, 2002(6): 8-9. | |
| 9 | SHUKLA R, SARIM K M, SINGH D P. Microbe-mediated management of arsenic contamination: Current status and future prospects[J]. Environmental Sustainability, 2020, 3(1): 83-90. |
| 10 | BO Y, YAN L. Preparation and application of modified biochar for arsenic pollution remediation[J]. International Core Journal of Engineering, 2021, 7(11): 46-50. |
| 11 | 贾艳萍, 张真, 毕朕豪, 等. 铁碳微电解处理印染废水的效能及生物毒性变化[J]. 化工进展, 2020, 39(2): 790-797. |
| JIA Yanping, ZHANG Zhen, BI Zhenhao, et al. Efficiency and biological toxicity of iron-carbon microelectrolysis in treatment of the dye wastewater[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 790-797. | |
| 12 | MOHINDRU J, GARG U, GUPTA R. Coagulation-flocculation technologies for arsenic removal—A review[J]. Asian Journal of Research in Chemistry, 2017, 10(3): 405-413. |
| 13 | BHATTACHARYA S, SHARMA P, MITRA S, et al. Arsenic uptake and bioaccumulation in plants: A review on remediation and socio-economic perspective in Southeast Asia[J]. Environmental Nanotechnology, Monitoring & Management, 2021, 15: 100430. |
| 14 | ARIF S, SAQIB H, MUBASHIR M, et al. Comparison of Nigella sativa and Trachyspermum ammi via experimental investigation and biotechnological potential[J]. Chemical Engineering and Processing: Process Intensification, 2021, 161: 108313. |
| 15 | KANG Yan, SUN Huiling, GAO Balai, et al. Enhanced reduction of Cr( Ⅵ ) in iron-carbon micro-electrolysis constructed wetlands: Mechanisms of iron cycle and microbial interactions[J]. Chemical Engineering Journal, 2022, 439: 135742. |
| 16 | PENG Changsheng, CHEN Linheng, WU Xiange, et al. Identification of adsorption or degradation mechanism for the removal of different ionic dyes with iron-carbon micro-electrolysis process[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105690. |
| 17 | ZHU Xinyan, CHEN Xinjian, YANG Zemeng, et al. Investigating the influences of electrode material property on degradation behavior of organic wastewaters by iron-carbon micro-electrolysis[J]. Chemical Engineering Journal, 2018, 338: 46-54. |
| 18 | ZHENG Xiaoying, JIN Mengqi, ZHOU Xiang, et al. Enhanced removal mechanism of iron carbon micro-electrolysis constructed wetland on C, N, and P in salty permitted effluent of wastewater treatment plant[J]. Science of the Total Environment, 2019, 649: 21-30. |
| 19 | 张啸跃, 闻帅, 张海艳, 等. 铁碳微电解技术预处理抗生素制药废水的研究[J]. 环境科学与技术, 2021, 44(10): 144-151. |
| ZHANG Xiaoyue, WEN Shuai, ZHANG Haiyan, et al. Study on the application of iron-carbon micro-electrolysis in antibiotic wastewater treatment[J]. Environmental Science & Technology, 2021, 44(10): 144-151. | |
| 20 | HAN Yanhe, WU Chuantao, FU Xiaolu, et al. Sulfate removal mechanism by internal circulation iron-carbon micro-electrolysis[J]. Separation and Purification Technology, 2021, 279: 119762. |
| 21 | 中华人民共和国水利部. 铅、镉、钒、磷等34种元素的测定: [S]. |
| Ministry of Water Resources of the People’s Republic of China. Determination of 34 elements (Pb, Cd, V, P,etc.) : [S]. | |
| 22 | 陶厚永, 曹伟. 多项式回归与响应面分析的原理及应用[J]. 统计与决策, 2020, 36(8): 36-40. |
| TAO Houyong, CAO Wei. Principle and application of polynomial regression and response surface analysis[J]. Statistics & Decision, 2020, 36(8): 36-40. | |
| 23 | HAMMOUDI A, MOUSSACEB K, BELEBCHOUCHE C, et al. Comparison of artificial neural network (ANN) and response surface methodology (RSM) prediction in compressive strength of recycled concrete aggregates[J]. Construction and Building Materials, 2019, 209: 425-436. |
| 24 | KUMAR S, MAZUMDER R. Development and optimization of venlafaxine hydrochloride floating microspheres using response surface plots[J]. Marmara Pharmaceutical Journal, 2018, 22(2): 277-285. |
| 25 | ZHAO Ling, CAO Xinde, MAŠEK O, et al. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures[J]. Journal of Hazardous Materials, 2013, 256/257: 1-9. |
| 26 | LIBRA J A, RO K S, KAMMANN C, et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis[J]. Biofuels, 2011, 2(1): 71-106. |
| 27 | PATWARDHAN P R, BROWN R C, SHANKS B H. Product distribution from the fast pyrolysis of hemicellulose[J]. ChemSusChem, 2011, 4(5): 636-643. |
| 28 | 程辉, 余剑, 姚梅琴, 等. 木质素慢速热解机理[J]. 化工学报, 2013, 64(5): 1757-1765. |
| CHENG Hui, YU Jian, YAO Meiqin, et al. Mechanism analysis of lignin slow pyrolysis[J]. CIESC Journal, 2013, 64(5): 1757-1765. | |
| 29 | KLÜPFEL L, KEILUWEIT M, KLEBER M, et al. Redox properties of plant biomass-derived black carbon (biochar)[J]. Environmental Science & Technology, 2014, 48(10): 5601-5611. |
| 30 | PFAFFENEDER-KMEN M, CASAS I F, NAGHILOU A, et al. A multivariate curve resolution evaluation of an in situ ATR-FTIR spectroscopy investigation of the electrochemical reduction of graphene oxide[J]. Electrochimica Acta, 2017, 255: 160-167. |
| 31 | KEILUWEIT M, NICO P, JOHNSON Mark G, et al. Dynamic molecular structure of plant biomass-derived black carbon (biochar) [J]. Environmental Science & Technology, 2010, 44(4): 1247-1253. |
| 32 | 唐正, 赵松, 钱雅洁, 等. 生物炭持久性自由基形成机制及环境应用研究进展[J]. 化工进展, 2020, 39(4): 1521-1527. |
| TANG Zheng, ZHAO Song, QIAN Yajie, et al. Formation mechanisms and environmental applications of persistent free radicals in biochar: A review[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1521-1527. | |
| 33 | ARANGIO A M, TONG Haijie, SOCORRO J, et al. Quantification of environmentally persistent free radicals and reactive oxygen species in atmospheric aerosol particles[J]. Atmospheric Chemistry and Physics, 2016, 16(20): 13105-13119. |
| 34 | DELA CRUZ A L N, COOK R L, LOMNICKI S M, et al. Effect of low temperature thermal treatment on soils contaminated with pentachlorophenol and environmentally persistent free radicals[J]. Environmental Science & Technology, 2012, 46(11): 5971-5978. |
| 35 | RABIA A, HAMNA B, IRSHAD B, et al. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions[J]. Chemical Engineering Journal, 2020, 396(C): 125195. |
| 36 | DONG Xiaoling, MA L Q, GRESS J, et al. Enhanced Cr(Ⅵ) reduction and As(Ⅲ) oxidation in ice phase: Important role of dissolved organic matter from biochar[J]. Journal of Hazardous Materials, 2014, 267: 62-70. |
| 37 | RINKLEBE J, SHAHEEN S, FROHNE T. Amendment of biochar reduces the release of toxic elements under dynamic redox conditions in a contaminated floodplain soil[J]. Chemosphere, 2016,142 (7):41-47. |
| 38 | 张伟彬. Fe2O3和Fe3O4相变机制研究[D]. 沈阳: 东北大学, 2017. |
| ZHANG Weibin. Investigation on phase transition mechanism of iron(Ⅲ) oxide and ferroferric oxide[D]. Shenyang: Northeastern University, 2017. | |
| 39 | 王玉霞. 铁盐混凝去除As(Ⅲ)和As(Ⅴ)及钛盐光催化氧化混凝去除As(Ⅲ)的机理研究[D]. 西安: 西安建筑科技大学, 2015. |
WANG Yuxia. Mechanism of As( Ⅲ ) and As( ) removal by coagulation of ferric salts and As( Ⅲ ) removal by simultaneous photocatalytic oxidation-coagulation of titanium salts[D]. Xi’an: Xi’an University of Architecture and Technology, 2015. ) removal by coagulation of ferric salts and As( Ⅲ ) removal by simultaneous photocatalytic oxidation-coagulation of titanium salts[D]. Xi’an: Xi’an University of Architecture and Technology, 2015.
|
|
| 40 | WOLTERS F, EMMERICH K. Thermal reactions of smectites—Relation of dehydroxylation temperature to octahedral structure[J]. Thermochimica Acta, 2007, 462(1/2): 80-88. |
| 41 | JELÍNEK P, DOBOSZ St M, BEŇO J, et al. The behavior of bentonite binders for the elevated and high temperatures[J]. Archives of Metallurgy and Materials, 2014, 59(3): 1041-1044. |
| 42 | LIU Ruiping, LI Xing, XIA Shengji, et al. Calcium-enhanced ferric hydroxide co-precipitation of arsenic in the presence of silicate[J]. Water Environment Research: a Research Publication of the Water Environment Federation, 2007, 79(11): 2260-2264. |
| 43 | GUAN Xiaohong, MA Jun, DONG Haoran, et al. Removal of arsenic from water: Effect of calcium ions on As(Ⅲ) removal in the KMnO4-Fe(Ⅱ) process[J]. Water Research, 2009, 43(20): 5119-5128. |
| 44 | WEI Z, SOMASUNDARAN P. Cyclic voltammetric study of arsenic reduction and oxidation in hydrochloric acid using a Pt RDE[J]. Journal of Applied Electrochemistry, 2004, 34(2): 241-244. |
| 45 | OTTAKAM THOTIYL M M, BASIT H, SÁNCHEZ J A, et al. Multilayer assemblies of polyelectrolyte-gold nanoparticles for the electrocatalytic oxidation and detection of arsenic(Ⅲ)[J]. Journal of Colloid and Interface Science, 2012, 383(1): 130-139. |
| 46 | WU Chuan, AN Wenhui, LIU Ziyu, et al. The effects of biochar as the electron shuttle on the ferrihydrite reduction and related arsenic (As) fate[J]. Journal of Hazardous Materials, 2020, 390: 121391. |
| [1] | 孙继鹏, 韩靖, 唐杨超, 闫汉博, 张杰瑶, 肖苹, 吴峰. 硫黄湿法成型过程数值模拟与操作参数优化[J]. 化工进展, 2023, 42(S1): 189-196. |
| [2] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [3] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [4] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [5] | 雷伟, 姜维佳, 王玉高, 和明豪, 申峻. N、S共掺杂煤基碳量子点的电化学氧化法制备及用于Fe3+检测[J]. 化工进展, 2023, 42(9): 4799-4807. |
| [6] | 邵志国, 任雯, 许世佩, 聂凡, 许毓, 刘龙杰, 谢水祥, 李兴春, 王庆吉, 谢加才. 终温对油基钻屑热解产物分布和特性影响[J]. 化工进展, 2023, 42(9): 4929-4938. |
| [7] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [8] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [9] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [10] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [11] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [12] | 姚丽铭, 王亚琢, 范洪刚, 顾菁, 袁浩然, 陈勇. 餐厨垃圾处理现状及其热解技术研究进展[J]. 化工进展, 2023, 42(7): 3791-3801. |
| [13] | 张杉, 仲兆平, 杨宇轩, 杜浩然, 李骞. 磷酸盐改性高岭土对生活垃圾热解过程中重金属的富集[J]. 化工进展, 2023, 42(7): 3893-3903. |
| [14] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [15] | 李若琳, 何少林, 苑宏英, 刘伯约, 纪冬丽, 宋阳, 刘博, 余绩庆, 徐英俊. 原位热解对油页岩物性及地下水水质影响探索[J]. 化工进展, 2023, 42(6): 3309-3318. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||