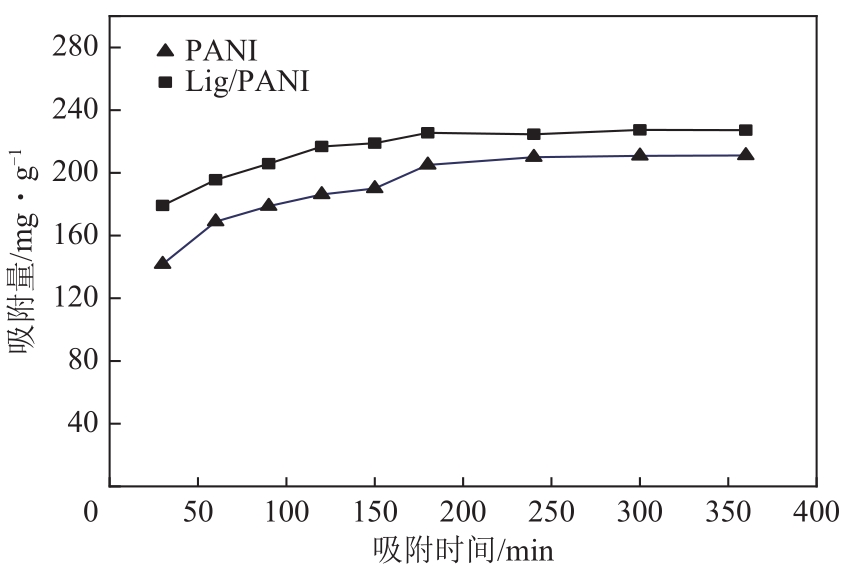
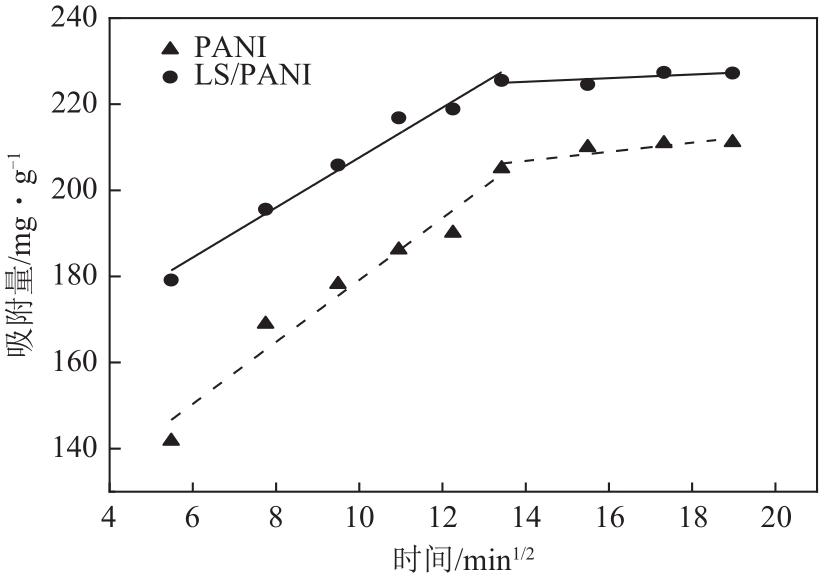
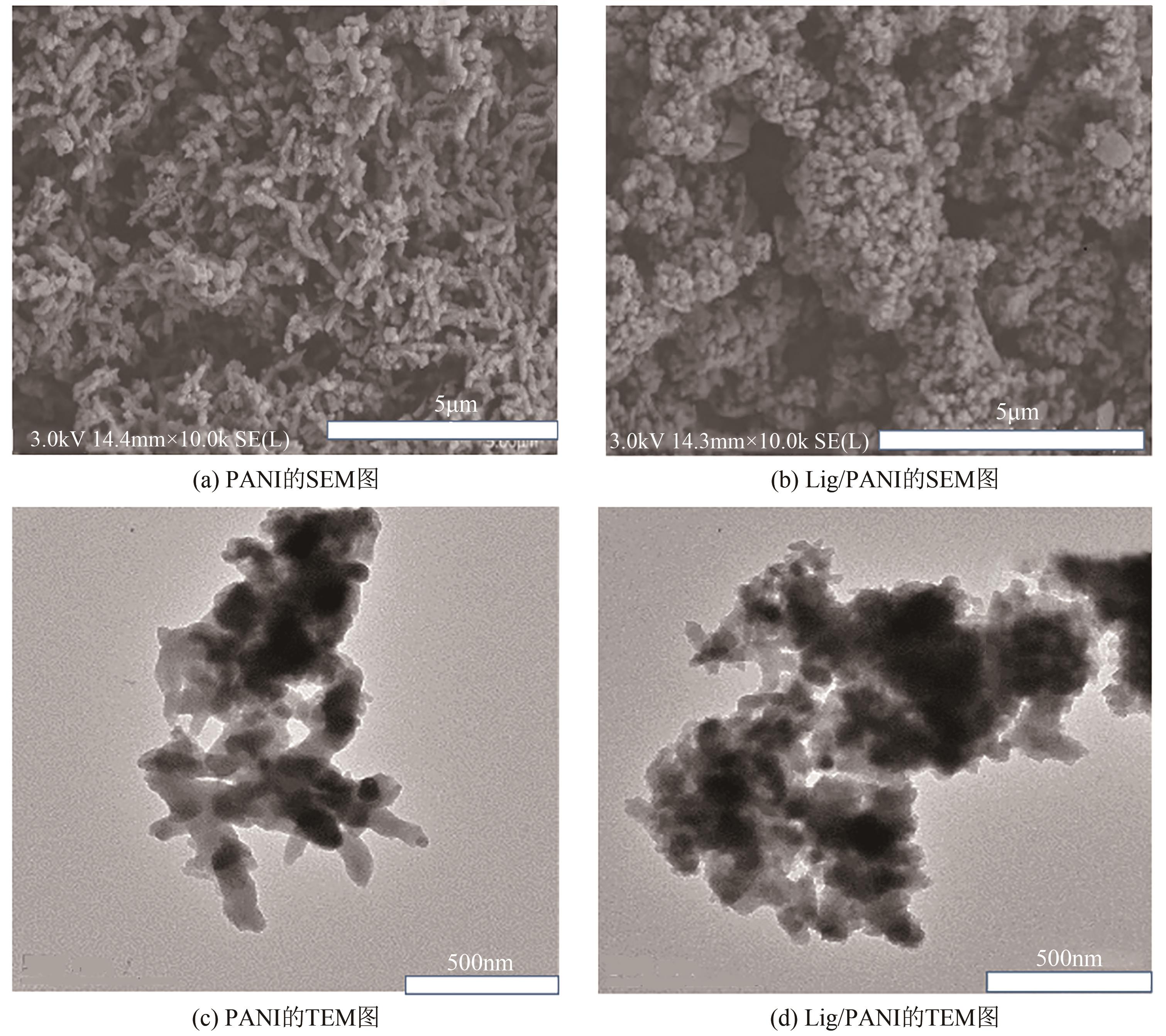
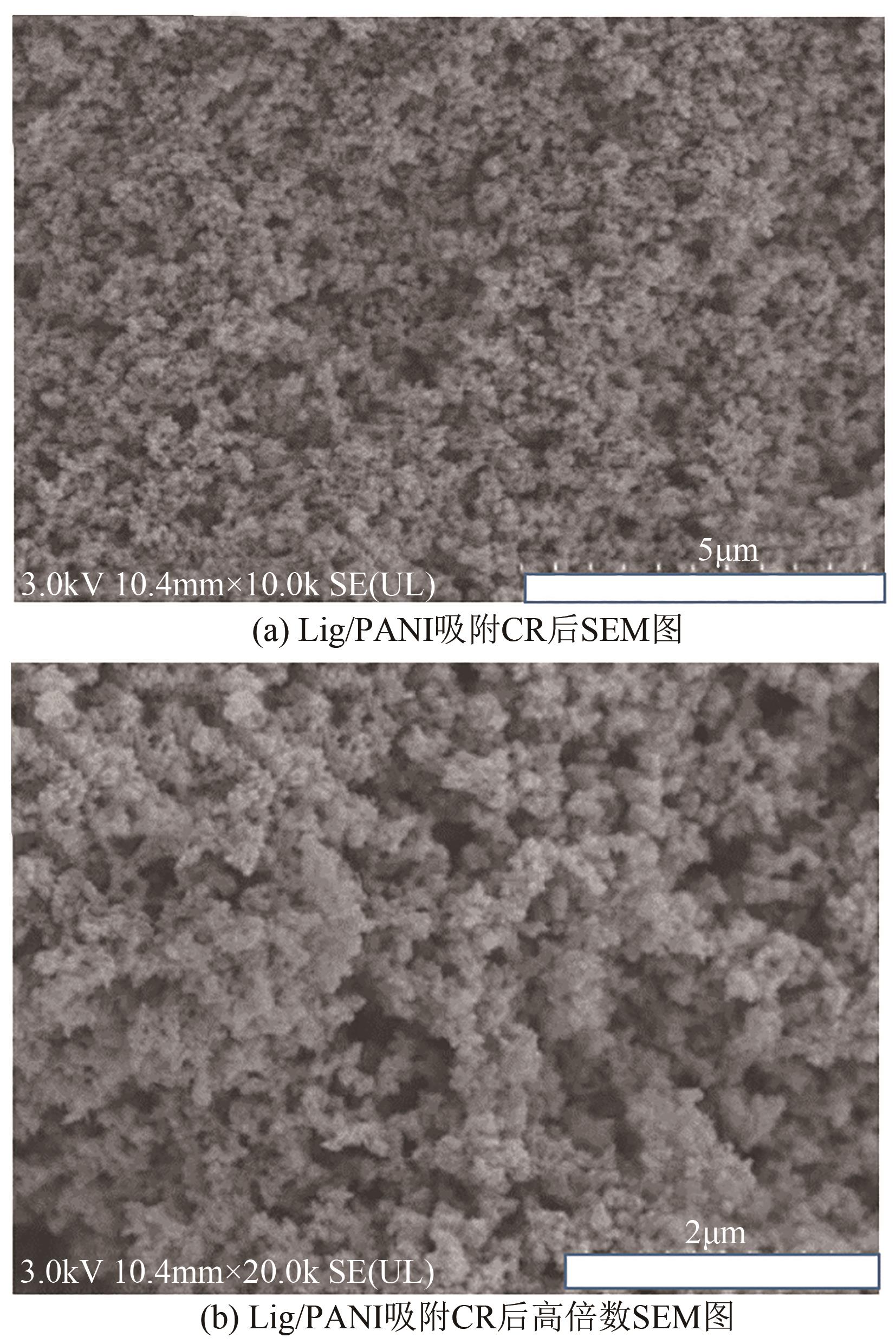
| 1 |
MINISY I M, SALAHUDDIN N A, AYAD M M. Chitosan/polyaniline hybrid for the removal of cationic and anionic dyes from aqueous solutions[J]. Journal of Applied Polymer Science, 2019, 136(6): 47056.
|
| 2 |
SUN Chencheng, XIONG Bowen, PAN Yang, et al. Adsorption removal of tannic acid from aqueous solution by polyaniline: Analysis of operating parameters and mechanism[J]. Journal of Colloid and Interface Science, 2017, 487: 175-181.
|
| 3 |
YAN Bo, CHEN Zhonghui, CAI Lu, et al. Fabrication of polyaniline hydrogel: Synthesis, characterization and adsorption of Methylene blue[J]. Applied Surface Science, 2015, 356: 39-47.
|
| 4 |
DUHAN Monika, KAUR Raminder. Nano-structured polyaniline as a potential adsorbent for Methylene blue dye removal from effluent[J]. Journal of Composites Science, 2020, 5(1): 7.
|
| 5 |
BHAUMIK Madhumita, MCCRINDLE Rob I, MAITY Arjun, et al. Polyaniline nanofibers as highly effective re-usable adsorbent for removal of Reactive black 5 from aqueous solutions[J]. Journal of Colloid and Interface Science, 2016, 466: 442-451.
|
| 6 |
LI Changzhi, ZHAO Xiaochen, WANG Aiqin, et al. Catalytic transformation of lignin for the production of chemicals and fuels[J]. Chemical Reviews, 2015, 115(21): 11559-11624.
|
| 7 |
XU Wenjing, CHEN Yizhen, KANG Jianxun, et al. Synthesis of polyaniline/lignosulfonate for highly efficient removal of Acid red 94 from aqueous solution[J]. Polymer Bulletin, 2019, 76(8): 4103-4116.
|
| 8 |
DEBNATH Sushanta, BALLAV Niladri, MAITY Arjun, et al. Development of a polyaniline-lignocellulose composite for optimal adsorption of Congo red[J]. International Journal of Biological Macromolecules, 2015, 75: 199-209.
|
| 9 |
贾羽洁, 孙康, 陈超. 聚苯胺-改性木质素磺酸的制备及电化学性能[J]. 电源技术, 2018, 42(8): 1204-1208.
|
|
JIA Yujie, SUN Kang, CHEN Chao. Synthesis and electrochemical performance of polyaniline doped with sulfonation modified lignosulfonate[J]. Chinese Journal of Power Sources, 2018, 42(8): 1204-1208.
|
| 10 |
Qiufeng LYU, HE Zhiwei, ZHANG Jiayin, et al. Preparation and properties of nitrogen-containing hollow carbon nanospheres by pyrolysis of polyaniline-lignosulfonate composites[J]. Journal of Analytical and Applied Pyrolysis, 2011, 92(1): 152-157.
|
| 11 |
Zümriye AKSU. Biosorption of reactive dyes by dried activated sludge: Equilibrium and kinetic modelling[J]. Biochemical Engineering Journal, 2001, 7(1): 79-84.
|
| 12 |
ZAIR Zyad R, ALISMAEEL Ziad T, EYSSA Mohammed Y, et al. Optimization, equilibrium, kinetics and thermodynamic study of Congo red dye adsorption from aqueous solutions using Iraqi porcelanite rocks[J]. Heat and Mass Transfer, 2022, 58(8): 1393-1410.
|
| 13 |
ALSENANI Ghadah. Removal of Congo red dye from aqueous solution by date palm leaf base[J]. American Journal of Applied Sciences, 2014, 11(9): 1553-1557.
|
| 14 |
MEHRIZAD Ali, BEHNAJADY Mohammad A, GHARBANI Parvin, et al. Sonocatalytic degradation of Acid Red 1 by sonochemically synthesized zinc sulfide-titanium dioxide nanotubes: Optimization, kinetics and thermodynamics studies[J]. Journal of Cleaner Production, 2019, 215: 1341-1350.
|
| 15 |
HU Lishuang, GUANG Chunyu, LIU Yang, et al. Adsorption behavior of dyes from an aqueous solution onto composite magnetic lignin adsorbent[J]. Chemosphere, 2020, 246: 1-12.
|
| 16 |
ASEFA Misikir Tamiru, FEYISA Gebisa Bekele. Comparative investigation on two synthesizing methods of zeolites for removal of Methylene blue from aqueous solution[J]. International Journal of Chemical Engineering, 2022, 2022: 9378712.
|
| 17 |
WU Chung Hsin. Adsorption of reactive dye onto carbon nanotubes: Equilibrium, kinetics and thermodynamics[J]. Journal of Hazardous Materials, 2007, 144(1/2): 93-100.
|
| 18 |
TANZIFI Marjan, TAVAKKOLI YARAKI Mohammad, KARAMI Mojtaba, et al. Modelling of dye adsorption from aqueous solution on polyaniline/carboxymethyl cellulose/TiO2 nanocomposites[J]. Journal of Colloid and Interface Science, 2018, 519: 154-173.
|
| 19 |
陈凤贵, 李娇, 黄露, 等. 木质素改性及其对染料的吸附性能[J]. 中山大学学报(自然科学版), 2019, 58(2): 103-109.
|
|
CHEN Fenggui, LI Jiao, HUANG Lu, et al. The application of freeze-dried and cross-linked lignin in water treatment[J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2019, 58(2): 103-109.
|
| 20 |
LI Penghui, WEI Yumeng, WU Caiwen, et al. Lignin-based composites for high-performance supercapacitor electrode materials[J]. RSC Advances, 2022, 12(30): 19485-19494.
|
| 21 |
BHAUMIK Madhumita, MCCRINDLE Rob, MAITY Arjun. Efficient removal of Congo red from aqueous solutions by adsorption onto interconnected polypyrrole-polyaniline nanofibres[J]. Chemical Engineering Journal, 2013, 228: 506-515.
|
| 22 |
Alica BARTOŠOVÁ, Lenka BLINOVÁ, SIROTIAK Maroš, et al. Usage of FTIR-ATR as non-destructive analysis of selected toxic dyes[J]. Research Papers Faculty of Materials Science and Technology Slovak University of Technology, 2017, 25(40): 103-111.
|
| 23 |
宋文琦, 霍文娟, 杨金腾, 等. 氨丙基咪唑离子液体修饰纤维素气凝胶吸附剂对刚果红的清除研究:高性能与吸附机理[J]. 材料导报, 2022, 36(12): 202-208.
|
|
SONG Wenqi, HUO Wenjuan, YANG Jinteng, et al. Aminopropyl imidazolium ionic liquid modified cellulosic aerogel adsorbent for Congo red removal: High-performance and adsorption mechanism[J]. Materials Reports, 2022, 36(12): 202-208.
|
| 24 |
RAZZAQ Saba, AKHTAR Mehwish, ZULFIQAR Sonia, et al. Adsorption removal of Congo red onto L-cysteine/rGO/PANI nanocomposite: Equilibrium, kinetics and thermodynamic studies[J]. Journal of Taibah University for Science, 2021, 15(1): 50-62.
|
| 25 |
SINGH Somendra, PERWEEN Shama, RANJAN Amit. Dramatic enhancement in adsorption of Congo red dye in polymer-nanoparticle composite of polyaniline-zinc titanate[J]. Journal of Environmental Chemical Engineering, 2021, 9(3): 105149.
|
| 26 |
HEGDE Vinayak, UTHAPPA U T, ARVIND SWAMI O R, et al. Sustainable green functional nano aluminium fumarate-MOF decorated on 3D low-cost natural diatoms for the removal of Congo red dye and fabric whitening agent from wastewater: Batch & continuous adsorption process[J]. Materials Today Communications, 2022, 32: 103887.
|
| 27 |
HAN Mingyu, SHEN Xiaoyi, SHAO Hongmei, et al. Adsorption of Congo red by fibrous xonotlite prepared from waste silicon residue[J]. Water Science and Technology, 2022, 85(11): 3159-3168.
|
| 28 |
MOSAVI S H, ZARE‐DORABEI R, BEREYHI M. Rapid and effective ultrasonic-assisted adsorptive removal of Congo red onto MOF‐5 modified by CuCl2 in ambient conditions: Adsorption isotherms and kinetics studies[J]. ChemistrySelect, 2021, 6(18):4432-4439.
|
 ), WU Caiwen, LIU Huijun, WU Wenjuan(
), WU Caiwen, LIU Huijun, WU Wenjuan( )
)