化工进展 ›› 2023, Vol. 42 ›› Issue (3): 1572-1582.DOI: 10.16085/j.issn.1000-6613.2022-0813
碱性工业固废矿化封存二氧化碳研究进展
- 华东交通大学土木建筑学院,江西 南昌 330013
-
收稿日期:2022-05-05修回日期:2022-07-11出版日期:2023-03-15发布日期:2023-04-10 -
通讯作者:张卫风 -
作者简介:王秋华(1977—),女,博士,讲师,研究方向为给水管网水质监测、温室气体CO2减排。E-mail:58773299@qq.com。 -
基金资助:江西省研究生创新专项(YC2021-S444)
Research progress of alkaline industrial solid wastes mineralization for carbon dioxide sequestration
WANG Qiuhua( ), WU Jiashuai, ZHANG Weifeng(
), WU Jiashuai, ZHANG Weifeng( )
)
- School of Civil Engineering and Architecture, East China Jiaotong University, Nanchang 330013, Jiangxi, China
-
Received:2022-05-05Revised:2022-07-11Online:2023-03-15Published:2023-04-10 -
Contact:ZHANG Weifeng
摘要:
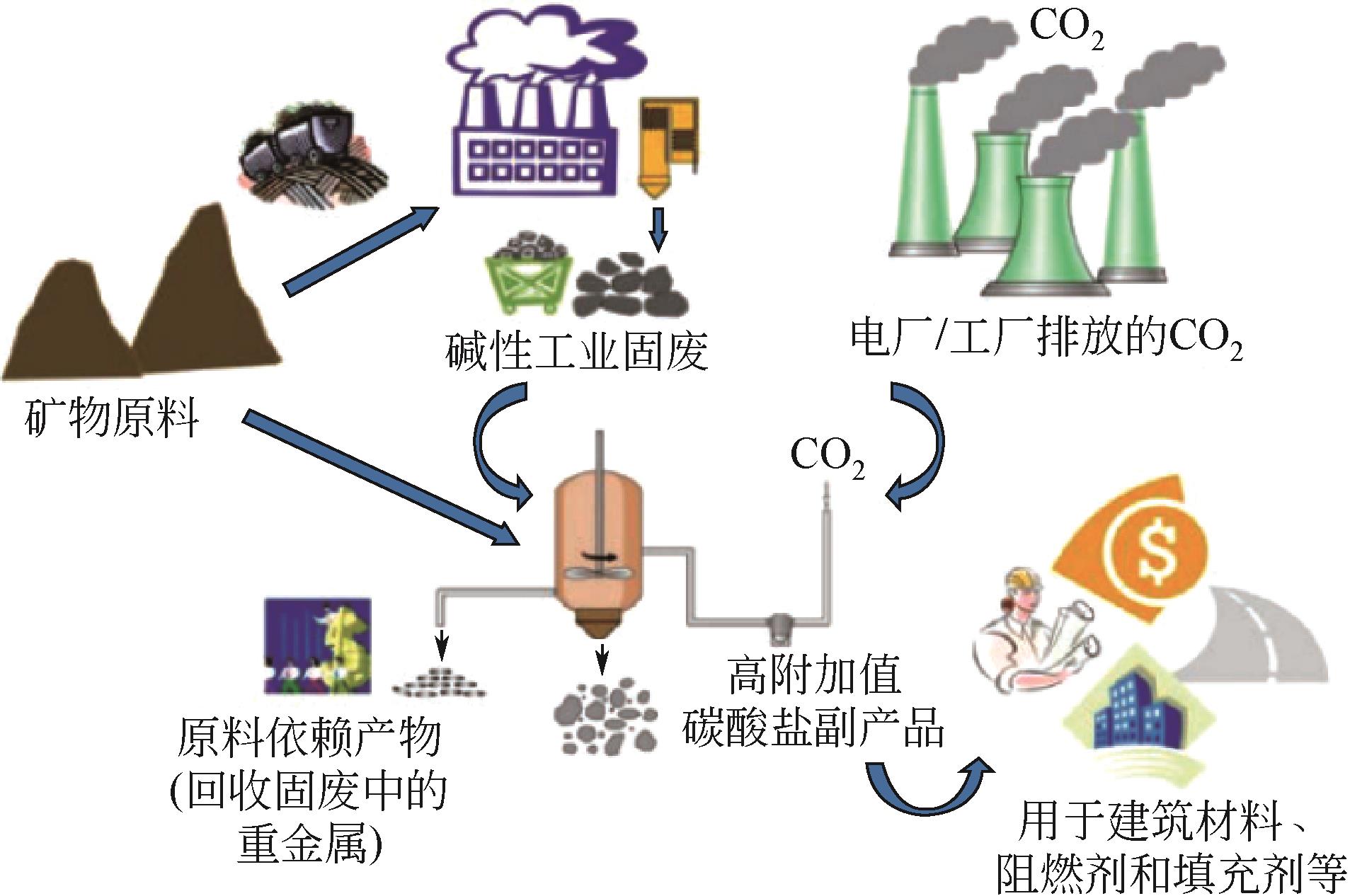
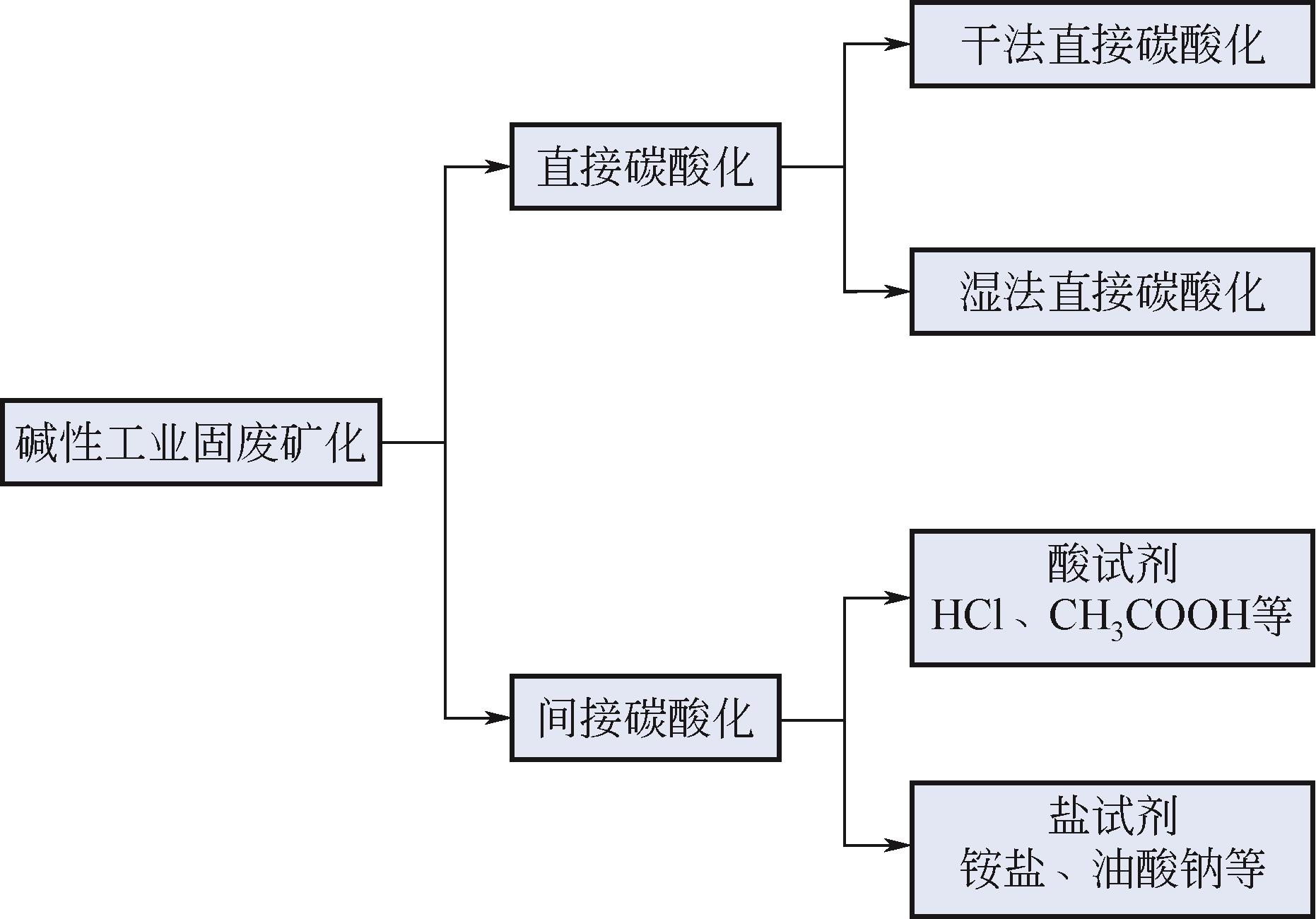
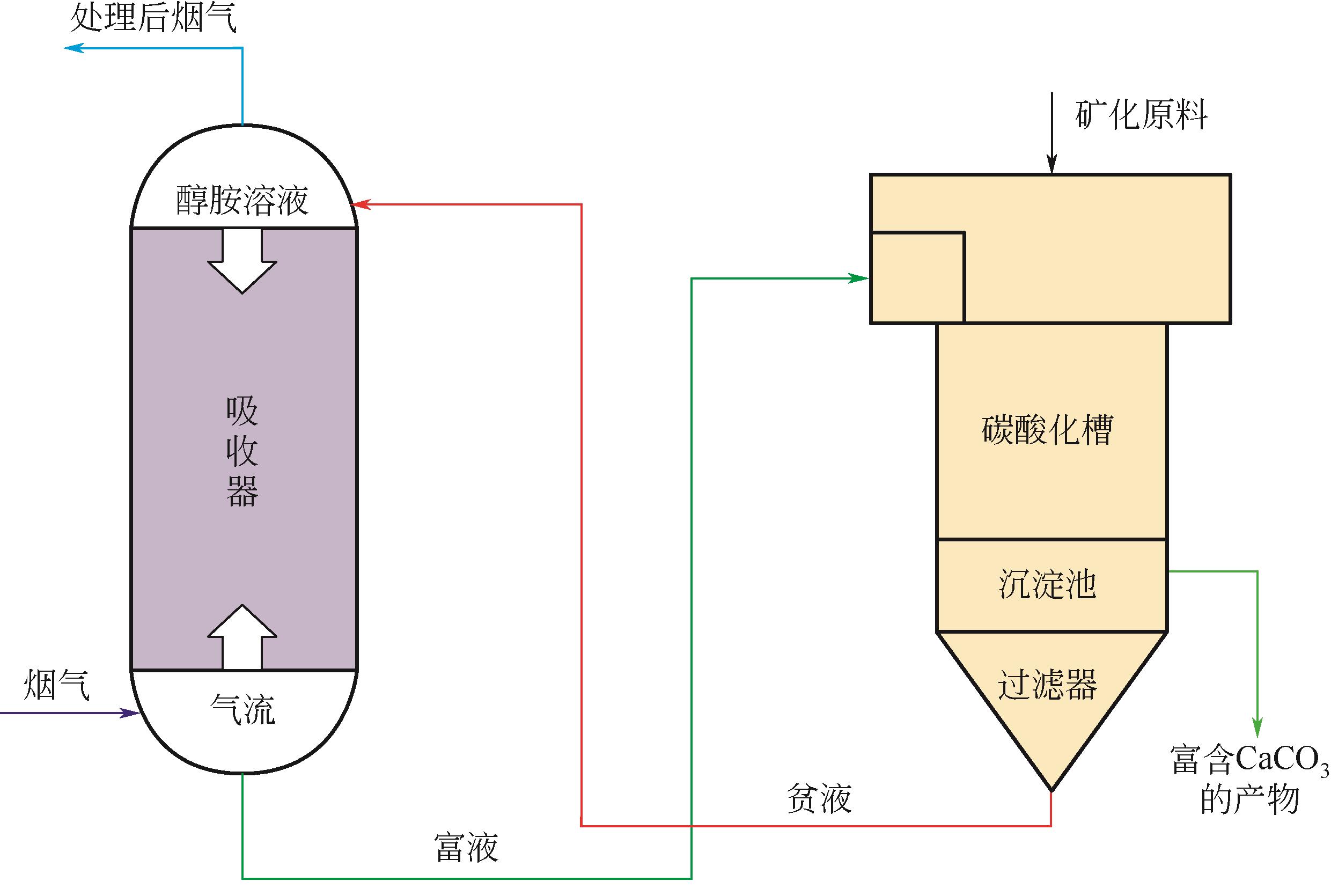
温室效应引起的全球变暖已经影响到人类的生存和发展,CO2减排刻不容缓。CO2矿物碳酸化作为一种CO2减排技术,受到越来越多的关注。相对于传统天然矿化原料,碱性工业固废具有反应速率快、碳酸化效率更高、能耗低等特点,并且利用碱性工业固废进行CO2矿化还可以产出高附加值产物用于化工、建筑等领域。本文主要综述了碱性工业固废的矿化机理,利用碱性工业固废(粉煤灰、钢渣、电石渣)进行CO2碳酸化的研究进展及吸收-矿化一体化(IAM)技术。对于以碱性工业固废为原料的碳酸化技术,未来应进一步加强机理和生命周期影响评价的研究并优化工艺流程;针对IAM工艺今后应开发出高效、经济的吸收剂和封存能力更好的矿化原料,并加强对IAM工艺反应机理的研究。
中图分类号:
引用本文
王秋华, 吴嘉帅, 张卫风. 碱性工业固废矿化封存二氧化碳研究进展[J]. 化工进展, 2023, 42(3): 1572-1582.
WANG Qiuhua, WU Jiashuai, ZHANG Weifeng. Research progress of alkaline industrial solid wastes mineralization for carbon dioxide sequestration[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1572-1582.
| 矿化原料 | 年产量 /Mt·a-1 | 主要反应 成分 | 主要化学反应 |
|---|---|---|---|
| 粉煤灰 | 750~1000 | CaO | CaO + CO2 CaO + H2O Ca(OH)2(aq) + CO2 |
| 钢渣 | 240 | CaO、MgO | CaO + CO2 MgO + CO2 |
| 电石渣 | 2.8 | Ca(OH)2 | Ca(OH)2(aq) + CO2 |
表1 矿化原料、主要成分及矿化主要化学反应[13-14]
| 矿化原料 | 年产量 /Mt·a-1 | 主要反应 成分 | 主要化学反应 |
|---|---|---|---|
| 粉煤灰 | 750~1000 | CaO | CaO + CO2 CaO + H2O Ca(OH)2(aq) + CO2 |
| 钢渣 | 240 | CaO、MgO | CaO + CO2 MgO + CO2 |
| 电石渣 | 2.8 | Ca(OH)2 | Ca(OH)2(aq) + CO2 |
| 矿化原料 | Ca质量分数/% | 温度/℃ | 压力/MPa | 反应时间/h | CO2封存能力/kg·t-1 | 碳酸化效率η/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| 粉煤灰 | 35 | 300~500 | 0.1 | 0.16 | 250 | 65 | Baciocchi等[ |
| 粉煤灰 | 31.95 | 45 | 1.5 | 2.4 | 180 | 74 | Mazzella等[ |
| 电石渣 | 90.9 | 580 | 0.1 | 1.7 | 382.21 | — | 张亚朋等[ |
表2 碱性工业固废干法直接碳酸化条件及CO2封存能力
| 矿化原料 | Ca质量分数/% | 温度/℃ | 压力/MPa | 反应时间/h | CO2封存能力/kg·t-1 | 碳酸化效率η/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| 粉煤灰 | 35 | 300~500 | 0.1 | 0.16 | 250 | 65 | Baciocchi等[ |
| 粉煤灰 | 31.95 | 45 | 1.5 | 2.4 | 180 | 74 | Mazzella等[ |
| 电石渣 | 90.9 | 580 | 0.1 | 1.7 | 382.21 | — | 张亚朋等[ |
| 矿化原料 | Ca质量分数 /% | 添加剂 | 温度 /℃ | 压力 /MPa | 液固比 /mL·g-1 | 反应时间 /min | CO2封存能力 /kg·t-1 | 碳酸化效率η /% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 粉煤灰 | 26.6 | — | 70 | 0.01 | 20 | 120~270 | 230 | 75 | Back等[ |
| 粉煤灰 | — | — | 40 | 6 | 5 | 600 | 7.66 | 13.6 | Ukwattage等[ |
| 粉煤灰 | 4.1 | — | 25~60 | 1~4 | 6.67~20 | 120 | 26 | 82 | Montes-Hernandez等[ |
| 粉煤灰 | 15.72 | — | 35 | 0.015 | 0.67~1.67 | 1440 | 55 | 19 | Bocheńczyk等[ |
| 钢渣 | 52.82 | — | 40-160 | 4.83 | 10 | 720 | 283 | 68 | Chang等[ |
| 钢渣 | 42.43 | — | 65 | 0.1 | 20 | 30 | 290 | 93.5 | Chang等[ |
| 电石渣 | — | — | 25 | 5 | 4 | — | 470 | — | Yang等[ |
| 电石渣 | 82.1 | — | 常温 | 常压 | 8.26 | 10 | — | 93.58 | 郑鹏等[ |
| 粉煤灰 | 24.9 | NaCl | 75 | 0.1 | 60 | 39 | 196 | 70.8 | Mayoral等[ |
| 粉煤灰 | 16.41 | Na2CO3 | 275 | 2 | 10 | 120 | 102 | 79 | Ji等[ |
| 电石渣 | 98.5 | 油酸钠 | 80 | 0.167 | 0.67 | 39 | — | 94.65 | Altiner[ |
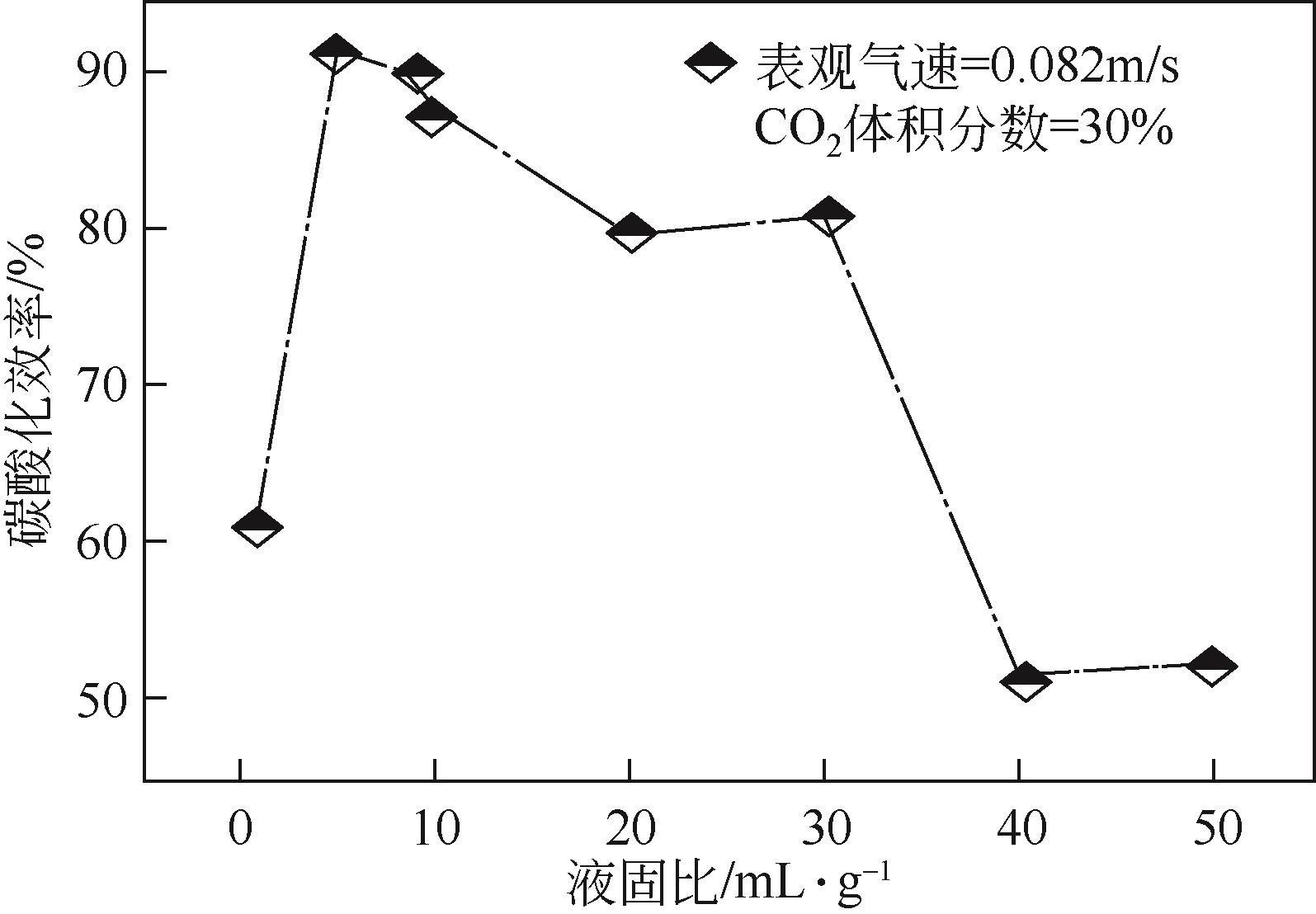
表3 碱性工业固废湿法直接碳酸化的条件及最大碳酸化效率
| 矿化原料 | Ca质量分数 /% | 添加剂 | 温度 /℃ | 压力 /MPa | 液固比 /mL·g-1 | 反应时间 /min | CO2封存能力 /kg·t-1 | 碳酸化效率η /% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 粉煤灰 | 26.6 | — | 70 | 0.01 | 20 | 120~270 | 230 | 75 | Back等[ |
| 粉煤灰 | — | — | 40 | 6 | 5 | 600 | 7.66 | 13.6 | Ukwattage等[ |
| 粉煤灰 | 4.1 | — | 25~60 | 1~4 | 6.67~20 | 120 | 26 | 82 | Montes-Hernandez等[ |
| 粉煤灰 | 15.72 | — | 35 | 0.015 | 0.67~1.67 | 1440 | 55 | 19 | Bocheńczyk等[ |
| 钢渣 | 52.82 | — | 40-160 | 4.83 | 10 | 720 | 283 | 68 | Chang等[ |
| 钢渣 | 42.43 | — | 65 | 0.1 | 20 | 30 | 290 | 93.5 | Chang等[ |
| 电石渣 | — | — | 25 | 5 | 4 | — | 470 | — | Yang等[ |
| 电石渣 | 82.1 | — | 常温 | 常压 | 8.26 | 10 | — | 93.58 | 郑鹏等[ |
| 粉煤灰 | 24.9 | NaCl | 75 | 0.1 | 60 | 39 | 196 | 70.8 | Mayoral等[ |
| 粉煤灰 | 16.41 | Na2CO3 | 275 | 2 | 10 | 120 | 102 | 79 | Ji等[ |
| 电石渣 | 98.5 | 油酸钠 | 80 | 0.167 | 0.67 | 39 | — | 94.65 | Altiner[ |
| 矿化原料 | 工艺 | 添加剂 | T/℃ | 液固比(L/S) | 反应时间/h | Ca2+浸出率η或 浸出浓度 | CO2封存能力或 碳酸化效率 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 粉煤灰 | 间接碳酸化 | CH3COOH | 60 | — | 1 | 14g/L | 264kg/t | Sun等[ |
| 粉煤灰 | 间接碳酸化 | 甘氨酸 | 25 | 5mL/g | 3 | 42% | 86kg/t | Zheng等[ |
| 钢渣 | 间接碳酸化 | CH3COOH | 50 | 71.4mL/g | 2 | 90% | — | Teir等[ |
| 钢渣 | 间接碳酸化 | HCl | 25 | 60mL/g | 1 | 93.4% | 56.5% | 唐海燕等[ |
| 钢渣 | 间接碳酸化 | NH4Cl | 60 | 20mL/g | 1 | 15g/L | 211kg/t | Sun等[ |
| 粉煤灰 | 间接碳酸化 | CH3COONH4 | 25 | 20mL/g | 1 | 40% | 111kg/t | He等[ |
| NH4Cl | ||||||||
| NH4NO3 | ||||||||
| H粉煤灰 | 间接碳酸化 | NH4Cl | 80 | 60mL/g | 1 | 32% | 90% | Hosseini等[ |
| Y粉煤灰 | 间接碳酸化 | NH4Cl | 80 | 60mL/g | 1 | 37% | 40% | Hosseini等[ |
| 钢渣 | 间接碳酸化 | NH4Cl | 80 | — | 1 | 60% | — | Kodama等[ |
| 电石渣 | 间接碳酸化 | NH4Cl | 40 | — | 0.83 | — | 763.33kg/t | Zhang等[ |
| 电石渣 | 间接碳酸化 | (NH4)2SO4 | 40 | 9.52mL/g | 0.5 | — | 500kg/t | Li等[ |
| 钢渣 | 间接碳酸化(超声波) | NH4Cl | 23 | 50mL/g | 1 | 96% | — | Said等[ |
| 钢渣 | 间接碳酸化(微波照射) | NH4Cl | 60 | 1~10mL/g | — | 55% | ≈100% | Tong等[ |
表4 碱性工业固废间接碳酸化的碳酸化条件及Ca2+浸出情况
| 矿化原料 | 工艺 | 添加剂 | T/℃ | 液固比(L/S) | 反应时间/h | Ca2+浸出率η或 浸出浓度 | CO2封存能力或 碳酸化效率 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 粉煤灰 | 间接碳酸化 | CH3COOH | 60 | — | 1 | 14g/L | 264kg/t | Sun等[ |
| 粉煤灰 | 间接碳酸化 | 甘氨酸 | 25 | 5mL/g | 3 | 42% | 86kg/t | Zheng等[ |
| 钢渣 | 间接碳酸化 | CH3COOH | 50 | 71.4mL/g | 2 | 90% | — | Teir等[ |
| 钢渣 | 间接碳酸化 | HCl | 25 | 60mL/g | 1 | 93.4% | 56.5% | 唐海燕等[ |
| 钢渣 | 间接碳酸化 | NH4Cl | 60 | 20mL/g | 1 | 15g/L | 211kg/t | Sun等[ |
| 粉煤灰 | 间接碳酸化 | CH3COONH4 | 25 | 20mL/g | 1 | 40% | 111kg/t | He等[ |
| NH4Cl | ||||||||
| NH4NO3 | ||||||||
| H粉煤灰 | 间接碳酸化 | NH4Cl | 80 | 60mL/g | 1 | 32% | 90% | Hosseini等[ |
| Y粉煤灰 | 间接碳酸化 | NH4Cl | 80 | 60mL/g | 1 | 37% | 40% | Hosseini等[ |
| 钢渣 | 间接碳酸化 | NH4Cl | 80 | — | 1 | 60% | — | Kodama等[ |
| 电石渣 | 间接碳酸化 | NH4Cl | 40 | — | 0.83 | — | 763.33kg/t | Zhang等[ |
| 电石渣 | 间接碳酸化 | (NH4)2SO4 | 40 | 9.52mL/g | 0.5 | — | 500kg/t | Li等[ |
| 钢渣 | 间接碳酸化(超声波) | NH4Cl | 23 | 50mL/g | 1 | 96% | — | Said等[ |
| 钢渣 | 间接碳酸化(微波照射) | NH4Cl | 60 | 1~10mL/g | — | 55% | ≈100% | Tong等[ |
| 1 | ALLEN M R, FRAME D J, HUNTINGFORD C, et al. Warming caused by cumulative carbon emissions towards the trillionth tonne[J]. Nature, 2009, 458(7242): 1163-1166. |
| 2 | 项目综合报告编写组. 《中国长期低碳发展战略与转型路径研究》综合报告[J]. 中国人口·资源与环境, 2020, 30(11): 1-25. |
| Project Synthesis Report Writing Team. Synthesis report on China’s long-term low carbon development strategy and transition pathway study[J]. China Population,Resources and Environment, 2020, 30(11): 1-25. | |
| 3 | 赵宏图, 韩立群, 孙立鹏, 等. 国际碳中和发展态势及前景[J]. 现代国际关系, 2022(2): 20-28. |
| ZHAO Hongtu, HAN Liqun, SUN Lipeng, et al. International carbon neutral development trends and prospects[J]. Modern International Relations, 2022(2): 20-28. | |
| 4 | 米剑锋, 马晓芳. 中国CCUS技术发展趋势分析[J]. 中国电机工程学报, 2019, 39(9): 2537-2544. |
| Mi Jianfeng, MA Xiaofang. Development trend analysis of carbon capture, utilization and storage technology in China[J]. Proceedings of the CSEE, 2019, 39(9): 2537-2544. | |
| 5 | ORR F M. Carbon capture, utilization, and storage: an update[J]. SPE Journal, 2018, 23(6): 2444-2455. |
| 6 | 孙玉景, 周立发, 李越. CO2海洋封存的发展现状[J]. 地质科技情报, 2018, 37(4): 212-218. |
| SUN Yujing, ZHOU Lifa, LI Yue. Development status of CO2 marine sequestration[J]. Geological Science and Technology Information, 2018, 37(4): 212-218. | |
| 7 | 李琦, 蔡博峰, 陈帆, 等. 二氧化碳地质封存的环境风险评价方法研究综述[J]. 环境工程, 2019, 37(2): 13-21. |
| LI Qi, CAI Bofeng, CHEN Fan, et al. Review of environmental risk assessment methods for carbon dioxide geological storage[J]. Environmental Engineering, 2019, 37(2): 13-21. | |
| 8 | SEIFRITZ W. CO2 disposal by means of silicates[J]. Nature, 1990, 345(6275): 486. |
| 9 | BOBICKI E R, LIU Q X, XU Z H, et al. Carbon capture and storage using alkaline industrial wastes[J]. Progress in Energy and Combustion Science, 2012, 38(2): 302-320. |
| 10 | OLAJIRE A A. A review of mineral carbonation technology in sequestration of CO2 [J]. Journal of Petroleum Science and Engineering, 2013, 109: 364-392. |
| 11 | LIU W, TENG L, ROHANI S, et al. CO2 mineral carbonation using industrial solid wastes: a review of recent developments[J]. Chemical Engineering Journal, 2021, 416: 129093. |
| 12 | 张兵兵, 王慧敏, 曾尚红, 等. 二氧化碳矿物封存技术现状及展望[J]. 化工进展, 2012, 31(9): 2075-2083. |
| ZHANG Bingbing, WANG Huimin, ZENG Shanghong, et al. Current status and outlook of carbon dioxide mineral carbonation technologies[J]. Chemical Industry and Engineering Progress, 2012, 31(9): 2075-2083. | |
| 13 | DINDI A, QUANG D V, VEGA L F, et al. Applications of fly ash for CO2 capture, utilization, and storage[J]. Journal of CO2 Utilization, 2019, 29: 82-102. |
| 14 | 王中辉, 苏胜, 尹子骏, 等. CO2矿化及吸收-矿化一体化(IAM)方法研究进展[J]. 化工进展, 2021, 40(4): 2318-2327. |
| WANG Zhonghui, SU Sheng, YIN Zijun, et al. Research progress of CO2 mineralization and integrated absorption-mineralization (IAM) method[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2318-2327. | |
| 15 | WANG Tao, HUANG Hao, HU Xutao, et al. Accelerated mineral carbonation curing of cement paste for CO2 sequestration and enhanced properties of blended calcium silicate[J]. Chemical Engineering Journal, 2017, 323: 320-329. |
| 16 | HUANG Hao, GUO Ruonan, WANG Tao, et al. Carbonation curing for wollastonite-Portland cementitious materials: CO2 sequestration potential and feasibility assessment[J]. Journal of Cleaner Production,2019, 211: 830-841. |
| 17 | YI Zhenwei, WANG Tao, GUO Ruonan, et al. Sustainable building material from CO2 mineralization slag: aggregate for concretes and effect of CO2 curing[J]. Journal of CO2 Utilization, 2020, 40: 101196. |
| 18 | WANG Tao, YI Zhenwei, SONG Jiayi, et al. An industrial demonstration study on CO2 mineralization curing for concrete[J]. iScience, 2022, 25(5): 104261. |
| 19 | GUO Ruonan, CHEN Qiyang, HUANG Hao, et al. Carbonation curing of industrial solid waste-based aerated concretes[J]. Greenhouse Gases Science and Technology, 2019, 9: 433-443. |
| 20 | 黄浩, 王涛, 方梦祥. 二氧化碳矿化养护混凝土技术及新型材料研究进展[J]. 化工进展, 2019, 38(10): 4363-4373. |
| HUANG Hao, WANG Tao, FANG Mengxiang. Review on carbon dioxide mineral carbonation curing technology of concrete and novel material development[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4363-4373. | |
| 21 | BACIOCCHI R, COSTA G, POLETTINI A, et al. Comparison of different reaction routes for carbonation of APC residues[J]. Energy Procedia, 2009, 1(1): 4851-4858. |
| 22 | MAZZELLA A, ERRICO M, SPIGA D. CO2 uptake capacity of coal fly ash: influence of pressure and temperature on direct gas-solid carbonation[J]. Journal of Environmental Chemical Engineering, 2016, 4(4): 4120-4128. |
| 23 | 张亚朋, 崔龙鹏, 刘艳芳, 等. 3种典型工业固废的CO2矿化封存性能[J]. 环境工程学报, 2021, 15(7): 2344-2355. |
| ZHANG Yapeng, CUI Longpeng, LIU Yanfang, et al. Comparison of three typical industrial solid wastes on the performance of CO2 mineralization and sequestration[J]. Chinese Journal of Environmental Engineering, 2021, 15(7): 2344-2355. | |
| 24 | 胡绍洋, 戴晓天, 那贤昭. 钢渣的处理工艺及综合利用[J]. 铸造技术, 2019, 40(2): 220-224. |
| HU Shaoyang, DAI Xiaotian, NA Xianzhao. Treatment process and comprehensive utilization of steel slag[j]. Foundry Technology, 2019, 40(2): 220-224. | |
| 25 | BACK M, KUEHN M, STANJEK H, et al. Reactivity of alkaline lignite fly ashes towards CO2 in water[J]. Environmental Science & Technology, 2008, 42(12): 4520-4526. |
| 26 | UKWATTAGE N L, RANJITH P G, WANG S H. Investigation of the potential of coal combustion fly ash for mineral sequestration of CO2 by accelerated carbonation[J]. Energy, 2013, 52: 230-236. |
| 27 | MONTES-HERNANDEZ G, PÉREZ-LÓPEZ R, RENARD F, et al. Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash[J]. Journal of Hazardous Materials, 2009, 161(2/3): 1347-1354. |
| 28 | ULIASZ-BOCHEŃCZYK A, MOKRZYCKI E, PIOTROWSKI Z, et al. Estimation of CO2 sequestration potential via mineral carbonation in fly ash from lignite combustion in Poland[J]. Energy Procedia, 2009, 1(1): 4873-4879. |
| 29 | CHANG E E, PAN S Y, CHEN Y H, et al. CO2 sequestration by carbonation of steelmaking slags in an autoclave reactor[J]. Journal of Hazardous Materials, 2011, 195: 107-114. |
| 30 | CHANG E E, PAN S Y, CHEN Y H, et al. Accelerated carbonation of steelmaking slags in a high-gravity rotating packed bed[J]. Journal of Hazardous Materials, 2012, 227/228: 97-106. |
| 31 | YANG Yuan, LIU Wei, CHENG Weimin, et al. Method for rapid mineralization of CO2 with carbide slag in the constant-pressure and continuous-feed way and its reaction heat[J]. Powder Technology, 2022, 398:117148. |
| 32 | 郑鹏, 李蔚玲, 郭亚飞, 等. 鼓泡床中电石渣加速碳酸化分析与响应面优化[J]. 化工进展, 2022, 41(3): 1528-1538. |
| ZHENG Peng, LI Weiling, GUO Yafei, et al. Analysis of carbide slag accelerated carbonation in bubble column and response surface optimization[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1528-1538. | |
| 33 | 武鸽, 刘艳芳, 崔龙鹏, 等. 典型工业固体废物碳酸化反应性能的比较[J]. 石油学报(石油加工), 2020, 36(1): 169-178. |
| WU Ge, LIU Yanfang, CUI Longpeng, et al. Comparison of the carbonation reaction properties of typical industrial solid wastes[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2020, 36(1): 169-178. | |
| 34 | JI Long, YU Hai, WANG Xiaolong, et al. CO2 sequestration by direct mineralisation using fly ash from Chinese Shenfu coal[J]. Fuel Processing Technology, 2017, 156: 429-437. |
| 35 | YUAN Qixin, ZHANG Yongsheng, WANG Tao, et al. Mineralization characteristics of coal fly ash in the transition from non-supercritical CO2 to supercritical CO2 [J]. Fuel, 2022, 318: 123636. |
| 36 | MAYORAL M C, ANDRÉS J M, GIMENO M P. Optimization of mineral carbonation process for CO2 sequestration by lime-rich coal ashes[J]. Fuel, 2013, 106: 448-454. |
| 37 | ALTINER M. Use of Taguchi approach for synthesis of calcite particles from calcium carbide slag for CO2 fixation by accelerated mineral carbonation[J]. Arabian Journal of Chemistry, 2019, 12(4): 531-540. |
| 38 | SUN Y, PARIKH V, ZHANG L. Sequestration of carbon dioxide by indirect mineralization using Victorian brown coal fly ash[J]. Journal of Hazardous Materials, 2012, 209/210: 458-466. |
| 39 | ZHENG Xuan, LIU Jiayao, WEI Yibin, et al. Glycine-mediated leaching-mineralization cycle for CO2 sequestration and CaCO3 production from coal fly ash: dual functions of glycine as a proton donor and receptor[J]. Chemical Engineering Journal, 2022, 440: 135900. |
| 40 | TEIR S, ELONEVA S, FOGELHOLM C J, et al. Dissolution of steelmaking slags in acetic acid for precipitated calcium carbonate production[J]. Energy, 2007, 32(4): 528-539. |
| 41 | 唐海燕, 孟文佳, 孙绍恒, 等. 炼钢炉渣的浸出和碳酸化[J]. 北京科技大学学报, 2014, 36(S1): 27-31. |
| TANG Haiyan, MENG Wenjia, SUN Shaoheng, et al. Leaching and carbonation of steelmaking slag[J]. Journal of University of Science and Technology Beijing, 2014, 36(S1): 27-31. | |
| 42 | HE Lanlan, YU Dunxi, Weizhi LYU, et al. A novel method for CO2 sequestration via indirect carbonation of coal fly ash[J]. Industrial & Engineering Chemistry Research, 2013, 52(43): 15138-15145. |
| 43 | HOSSEINI T, SELOMULYA C, HAQUE N, et al. Indirect carbonation of Victorian brown coal fly ash for CO2 sequestration: multiple-cycle leaching-carbonation and magnesium leaching kinetic modeling[J]. Energy & Fuels, 2014, 28(10): 6481-6493. |
| 44 | KODAMA S, NISHIMOTO T, YAMAMOTO N, et al. Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution[J]. Energy, 2008, 33(5): 776-784. |
| 45 | SUN Y, YAO M S, ZHANG J P, et al. Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution[J]. Chemical Engineering Journal, 2011, 173(2): 437-445. |
| 46 | ZHANG T Y, CHU G R, LYU J L, et al. CO2 mineralization of carbide slag for the production of light calcium carbonates[J]. Chinese Journal of Chemical Engineering, 2022, 43: 86-98. |
| 47 | LI Wenxiu, HUANG Yan, WANG Tao, et al. Preparation of calcium carbonate nanoparticles from waste carbide slag based on CO2 mineralization[J]. Journal of Cleaner Production, 2022, 363: 132463. |
| 48 | SAID A, MATTILA O, ELONEVA S, et al. Enhancement of calcium dissolution from steel slag by ultrasound[J]. Chemical Engineering and Processing: Process Intensification, 2015, 89: 1-8. |
| 49 | TONG Z B, MA G J, ZHOU D, et al. The indirect mineral carbonation of electric arc furnace slag under microwave irradiation[J]. Scientific Reports, 2019, 9(1): 7676. |
| 50 | 林海周, 裴爱国, 方梦祥. 燃煤电厂烟气二氧化碳胺法捕集工艺改进研究进展[J]. 化工进展, 2018, 37(12): 4874-4886. |
| LIN Haizhou, PEI Aiguo, FANG Mengxiang. Progress of research on process modifications for amine solvent-based post combustion CO2 capture from coal-fired power plant[J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4874-4886. | |
| 51 | YU C H, HUANG C H, TAN C S. A review of CO2 capture by absorption and adsorption[J]. Aerosol and Air Quality Research, 2012, 12(5): 745-769. |
| 52 | LI K K, LEIGH W, FERON P, et al. Systematic study of aqueous monoethanolamine (MEA)-based CO2 capture process: techno economic assessment of the MEA process and its improvements[J]. Applied Energy, 2016, 165: 648-659. |
| 53 | SHAKERIAN F, KIM K H, SZULEJKO J E, et al. A comparative review between amines and ammonia as sorptive media for post-combustion CO2 capture[J]. Applied Energy, 2015, 148: 10-22. |
| 54 | HANG D, BIELICKI J M, OPPENHEIMER M, et al. Leakage risks of geologic CO2 storage and the impacts on the global energy system and climate change mitigation[J]. Climatic Change, 2017, 144:151-163. |
| 55 | LIU M S, GADIKOTA G. Integrated CO2 capture, conversion, and storage to produce calcium carbonate using an amine looping strategy[J]. Energy & Fuels, 2019, 33(3): 1722-1733. |
| 56 | 纪龙. 利用粉煤灰矿化封存二氧化碳的研究[D]. 北京: 中国矿业大学(北京), 2018. |
| JI Long. Carbon dioxide sequestration by mineralisation of coal fly ash[D]. Beijing: China University of Mining & Technology, Beijing, 2018. | |
| 57 | PARK S, MIN J, LEE M G, et al. Characteristics of CO2 fixation by chemical conversion to carbonate salts[J]. Chemical Engineering Journal, 2013, 231: 287-293. |
| 58 | KANG J M, MURNANDARI A, YOUN M H, et al. Energy-efficient chemical regeneration of AMP using calcium hydroxide for operating carbon dioxide capture process[J]. Chemical Engineering Journal,2018, 335: 338-344. |
| 59 | ARTI M, YOUN M H, PARK K T, et al. Single process for CO2 capture and mineralization in various alkanolamines using calcium chloride[J].Energy & Fuels, 2017, 31(1): 763-769. |
| 60 | ZHANG Weifeng, DENG Zhaoxiong, WANG Qiuhua, et al. Experimental research on chemical desorption based on CO2-rich absorption solutions[J]. International Journal of Greenhouse Gas Control, 2021, 109: 103356. |
| 61 | ZHANG Weifeng, XU Yuanlong, WANG Qiuhua. Coupled CO2 absorption and mineralization with low-concentration monoethanolamine[J]. Energy, 2022, 241: 122524. |
| 62 | YU Bing, LI Kangkang, JI Long, et al. Coupling a sterically hindered amine-based absorption and coal fly ash triggered amine regeneration: a high energy-saving process for CO2 absorption and sequestration[J]. International Journal of Greenhouse Gas Control, 2019, 87: 58-65. |
| 63 | YU Bing, YU Hai, LI Kangkang, et al. Integration of a diamine solvent based absorption and coal fly ash based mineralisation for CO2 sequestration[J]. Fuel Processing Technology, 2019, 192:220-226. |
| 64 | JI Long, YU Hai, YU Bing, et al. Integrated absorption-mineralisation for energy-efficient CO2 sequestration: reaction mechanism and feasibility of using fly ash as a feedstock[J]. Chemical Engineering Journal, 2018, 352: 151-162. |
| 65 | JI Long, YU Hai, LI Kangkang, et al. Integrated absorption-mineralisation for low-energy CO2 capture and sequestration[J]. Applied Energy, 2018, 225: 356-366. |
| 66 | 何民宇, 刘维燥, 刘清才, 等. CO2矿物封存技术研究进展[J]. 化工进展, 2022, 41(4): 1825-1833. |
| HE Minyu, LIU Weizao, LIU Qingcai, et al. Research progress in CO2 mineral sequestration technology[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1825-1833. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [3] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [4] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [5] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [6] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [7] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [8] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [9] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [10] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [11] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [12] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [13] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [14] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| [15] | 马源, 肖晴月, 岳君容, 崔彦斌, 刘姣, 许光文. CeO2-Al2O3复合载体负载Ni基催化剂催化CO x 共甲烷化性能[J]. 化工进展, 2023, 42(5): 2421-2428. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||