化工进展 ›› 2022, Vol. 41 ›› Issue (10): 5200-5213.DOI: 10.16085/j.issn.1000-6613.2021-2512
费托合成水相副产物混合醇分离: 馏分切割工艺设计及控制
黄洋1( ), 张稼骏1, 李家腾1, 夏铭2(
), 张稼骏1, 李家腾1, 夏铭2( ), 许春建1(
), 许春建1( )
)
- 1.天津大学化工学院,化学工程研究所,化学工程联合国家重点实验室,天津 300350
2.中国科学院山西煤炭 化学研究所煤转化国家重点实验室,山西 太原 030001
-
收稿日期:2021-12-09修回日期:2021-12-21出版日期:2022-10-20发布日期:2022-10-21 -
通讯作者:夏铭,许春建 -
作者简介:黄洋(1997—),男,硕士研究生,研究方向为精馏分离。E-mail:yh_1@tju.edu.cn。
Separation of mixed alcohols from Fischer-Tropsch aqueous by-product: design, optimization and control of fraction cutting
HUANG Yang1( ), ZHANG Jiajun1, LI Jiateng1, XIA Ming2(
), ZHANG Jiajun1, LI Jiateng1, XIA Ming2( ), XU Chunjian1(
), XU Chunjian1( )
)
- 1.State Key Laboratory of Chemical Engineering, Chemical Engineering Research Center, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China
2.State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, Shanxi, China
-
Received:2021-12-09Revised:2021-12-21Online:2022-10-20Published:2022-10-21 -
Contact:XIA Ming, XU Chunjian
摘要:
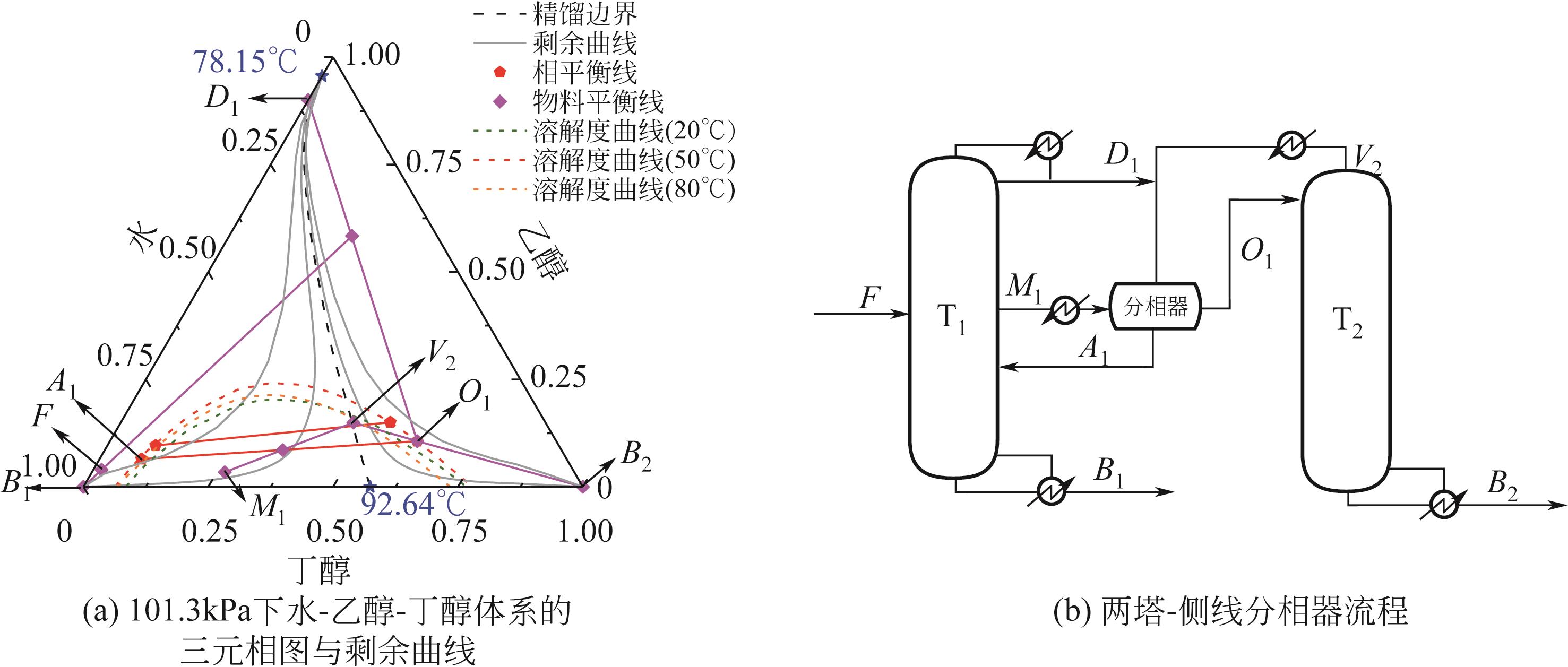
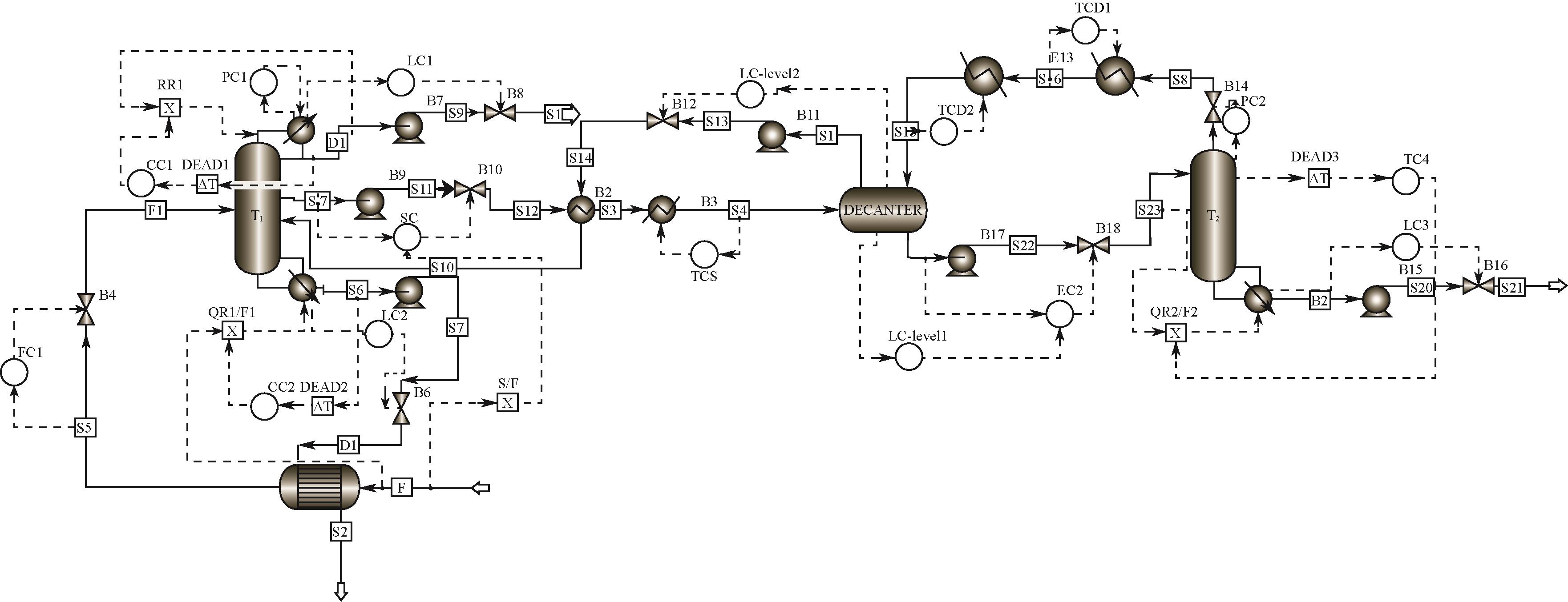
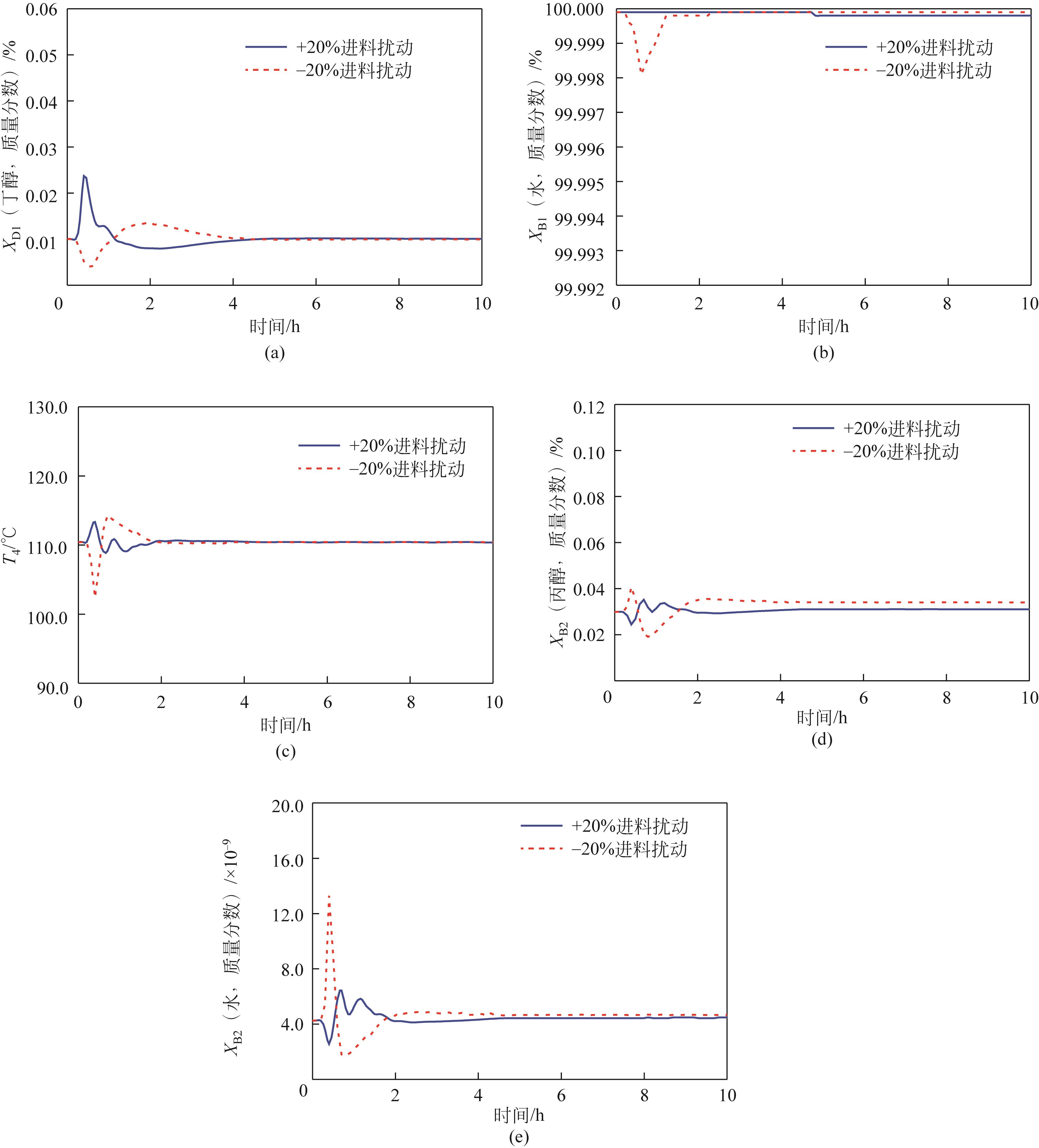
费托合成水相副产物主要为C1~C8醇(甲醇、乙醇、丙醇、丁醇、戊醇、己醇、庚醇和辛醇)与水的混合物,其中水的质量分数高达95%,且C2~C8醇与水均形成最低共沸物。此类醇水混合物的完全分离虽具重要价值,但难度大、能耗高,一直是学界和工业界的关注热点。本研究充分利用C2~C3醇水混合物的均相共沸物特性和C4~C8醇水混合物的高度非均相共沸物特性,提出两塔-侧线分相器工艺:通过侧线精馏塔实现C1~C3醇水、富C4~C8醇水和水的精准馏分切割;富C4~C8醇水混合物通入分相器以打破精馏边界,其中富水相返回侧线精馏塔,富醇相进入汽提塔,得到无水C4~C8醇混合物。基于年度总成本(total annual cost,TAC)的稳态优化表明,与常规三塔粗分流程相比,两塔-侧线分相器工艺能够降低TAC 14.79%,节约能耗15.96%。进一步,建立了两塔-侧线分相器工艺的控制结构,动态模拟表明,结合浓度控制器与前馈比例的控制结构表现出良好的控制性能。
中图分类号:
引用本文
黄洋, 张稼骏, 李家腾, 夏铭, 许春建. 费托合成水相副产物混合醇分离: 馏分切割工艺设计及控制[J]. 化工进展, 2022, 41(10): 5200-5213.
HUANG Yang, ZHANG Jiajun, LI Jiateng, XIA Ming, XU Chunjian. Separation of mixed alcohols from Fischer-Tropsch aqueous by-product: design, optimization and control of fraction cutting[J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5200-5213.
| 组分 | 分子式 | 组成(质量分数)/% | 沸点/℃ | 共沸物中的醇质量分数/% | 共沸物沸点/℃ | 溶解度(水) |
|---|---|---|---|---|---|---|
| 甲醇 | CH4O | 0.96 | 65.40 | — | — | 混溶 |
| 乙醇 | C2H6O | 2.00 | 78.01 | 95.6 | 78.16 | 混溶 |
| 正丙醇 | C3H8O | 1.00 | 97.20 | 69.0 | 87.63 | 混溶 |
| 正丁醇 | C4H10O | 0.83 | 118.66 | 56.5 | 91.78 | 微溶 |
| 正戊醇 | C5H12O | 0.53 | 137.80 | 45.5 | 94.30 | 微溶 |
| 正己醇 | C6H14O | 0.25 | 157.40 | 25.0 | 97.80 | 微溶 |
| 正庚醇 | C7H16O | 0.04 | 176.30 | 17.0 | 98.70 | 微溶 |
| 正辛醇 | C8H18O | 0.01 | 195.20 | 10.0 | 99.40 | 不溶 |
| 水 | H2O | 94.38 | 100.00 | — | — | — |
表1 混合醇组成及物性数据
| 组分 | 分子式 | 组成(质量分数)/% | 沸点/℃ | 共沸物中的醇质量分数/% | 共沸物沸点/℃ | 溶解度(水) |
|---|---|---|---|---|---|---|
| 甲醇 | CH4O | 0.96 | 65.40 | — | — | 混溶 |
| 乙醇 | C2H6O | 2.00 | 78.01 | 95.6 | 78.16 | 混溶 |
| 正丙醇 | C3H8O | 1.00 | 97.20 | 69.0 | 87.63 | 混溶 |
| 正丁醇 | C4H10O | 0.83 | 118.66 | 56.5 | 91.78 | 微溶 |
| 正戊醇 | C5H12O | 0.53 | 137.80 | 45.5 | 94.30 | 微溶 |
| 正己醇 | C6H14O | 0.25 | 157.40 | 25.0 | 97.80 | 微溶 |
| 正庚醇 | C7H16O | 0.04 | 176.30 | 17.0 | 98.70 | 微溶 |
| 正辛醇 | C8H18O | 0.01 | 195.20 | 10.0 | 99.40 | 不溶 |
| 水 | H2O | 94.38 | 100.00 | — | — | — |
| 共沸物 | 模拟值/(kg/kg,℃) | 实验值/(kg/kg,℃) |
|---|---|---|
| 乙醇/水 | 0.9581/0.0419,78.17 | 0.956/0.044,78.16 |
| 丙醇/水 | 0.6914/0.3086,87.73 | 0.690/0.310,87.63 |
| 丁醇/水 | 0.5742/0.4258,92.64 | 0.565/0.435,91.78 |
| 戊醇/水 | 0.4492/0.5508,95.87 | 0.455/0.545,94.30 |
| 己醇/水 | 0.2793/0.7207,98.21 | 0.250/0.750,97.80 |
| 庚醇/水 | 0.1614/0.8386,99.21 | 0.170/0.830,98.70 |
| 辛醇/水 | 0.1159/0.8841,99.52 | 0.100/0.900,99.40 |
表2 混合醇体系存在的共沸点的预测值与实验值比较(101.3kPa)
| 共沸物 | 模拟值/(kg/kg,℃) | 实验值/(kg/kg,℃) |
|---|---|---|
| 乙醇/水 | 0.9581/0.0419,78.17 | 0.956/0.044,78.16 |
| 丙醇/水 | 0.6914/0.3086,87.73 | 0.690/0.310,87.63 |
| 丁醇/水 | 0.5742/0.4258,92.64 | 0.565/0.435,91.78 |
| 戊醇/水 | 0.4492/0.5508,95.87 | 0.455/0.545,94.30 |
| 己醇/水 | 0.2793/0.7207,98.21 | 0.250/0.750,97.80 |
| 庚醇/水 | 0.1614/0.8386,99.21 | 0.170/0.830,98.70 |
| 辛醇/水 | 0.1159/0.8841,99.52 | 0.100/0.900,99.40 |
| 参数 | 公式 |
|---|---|
| 塔壳费用/USD | (M&S/280)×596.115d1.066H0.802 |
| 塔板费用/USD | (M&S/280)×12.69d1.55H |
| 换热器费用①/USD | (M&S/280)×101.3A0.65(2.29+3.75Fd) |
| 塔高(H)/m | 1.2h (NT-2) |
| 换热面积(A)②/m2 | Q/(UΔT) |
低压蒸汽(500kPa,160℃) /USD·GJ-1 | 13.28 |
| 冷却水(30~35℃)/USD·GJ-1 | 0.354 |
| M&S | 1638.2(2018)[ |
表3 TAC计算公式和参数
| 参数 | 公式 |
|---|---|
| 塔壳费用/USD | (M&S/280)×596.115d1.066H0.802 |
| 塔板费用/USD | (M&S/280)×12.69d1.55H |
| 换热器费用①/USD | (M&S/280)×101.3A0.65(2.29+3.75Fd) |
| 塔高(H)/m | 1.2h (NT-2) |
| 换热面积(A)②/m2 | Q/(UΔT) |
低压蒸汽(500kPa,160℃) /USD·GJ-1 | 13.28 |
| 冷却水(30~35℃)/USD·GJ-1 | 0.354 |
| M&S | 1638.2(2018)[ |
| 参数 | S1 | S2 |
|---|---|---|
| 进料板 | 19 | 19 |
| 侧线出料位置 | 18 | 49 |
| NT1 | 60 | 60 |
| NT2 | 15 | 15 |
| 塔径/m | ||
| d1 | 1.1 | 1.4 |
| d2 | 0.5 | 0.25 |
| 塔板间距/m | ||
| h1 | 0.6 | 0.8 |
| h2 | 0.4 | 0.2 |
| 再沸器热负荷/kW | ||
| QR1 | 2882.54(差异31.48%①) | 4206.67 |
| QR2 | 278.65 | 99.27 |
| 总再沸器热负荷QR/kW | 3161.19(差异26.59%②) | 4305.94 |
| 冷凝器热负荷QC/kW | -2502.16 | -3828.01 |
| FCI/kUSD·a-1 | 1032.86 | 1275.04 |
| OC/kUSD·a-1 | 1237.21 | 1686.71 |
| TAC/kUSD·a-1 | 1547.07(差异25.23%③) | 2069.22 |
表4 S1和S2两种分离序列比较
| 参数 | S1 | S2 |
|---|---|---|
| 进料板 | 19 | 19 |
| 侧线出料位置 | 18 | 49 |
| NT1 | 60 | 60 |
| NT2 | 15 | 15 |
| 塔径/m | ||
| d1 | 1.1 | 1.4 |
| d2 | 0.5 | 0.25 |
| 塔板间距/m | ||
| h1 | 0.6 | 0.8 |
| h2 | 0.4 | 0.2 |
| 再沸器热负荷/kW | ||
| QR1 | 2882.54(差异31.48%①) | 4206.67 |
| QR2 | 278.65 | 99.27 |
| 总再沸器热负荷QR/kW | 3161.19(差异26.59%②) | 4305.94 |
| 冷凝器热负荷QC/kW | -2502.16 | -3828.01 |
| FCI/kUSD·a-1 | 1032.86 | 1275.04 |
| OC/kUSD·a-1 | 1237.21 | 1686.71 |
| TAC/kUSD·a-1 | 1547.07(差异25.23%③) | 2069.22 |
| 参数 | 两塔-侧线分相器流程 | 常规三塔粗分流程 | ||||
|---|---|---|---|---|---|---|
| 侧线精馏塔 | 重醇塔 | 脱水塔 | 醇分塔 | 重醇塔 | ||
| p/kPa | 101.3 | 121.3 | 101.3 | 121.3 | ||
| NT | 62 | 14 | 47 | 51 | 8 | |
| NF | 25 | 1 | 10 | 29 | 1 | |
| RR | 6.13 | — | 1.61 | 1.69 | — | |
| d/m | 1.1 | 0.5 | 1.0 | 0.7 | 0.25 | |
| h/m | 0.6 | 0.4 | 0.6 | 0.4 | 0.2 | |
| QC/kW | -2224.74 | -272.31 | -2111.65 | -840.84 | -80.20 | |
| QR/kW | 2571.21 | 291.39 | 2446.14 | 858.11 | 101.95 | |
| 合计QR/kW | 2862.60(差异15.96%①) | 3406.20 | ||||
| FCI/kUSD·a-1 | 1016.67 | 1136.08 | ||||
| OC/kUSD·a-1 | 1120.30 | 1333.67 | ||||
| TAC/kUSD·a-1 | 1456.40(差异14.79%②) | 1709.25 | ||||
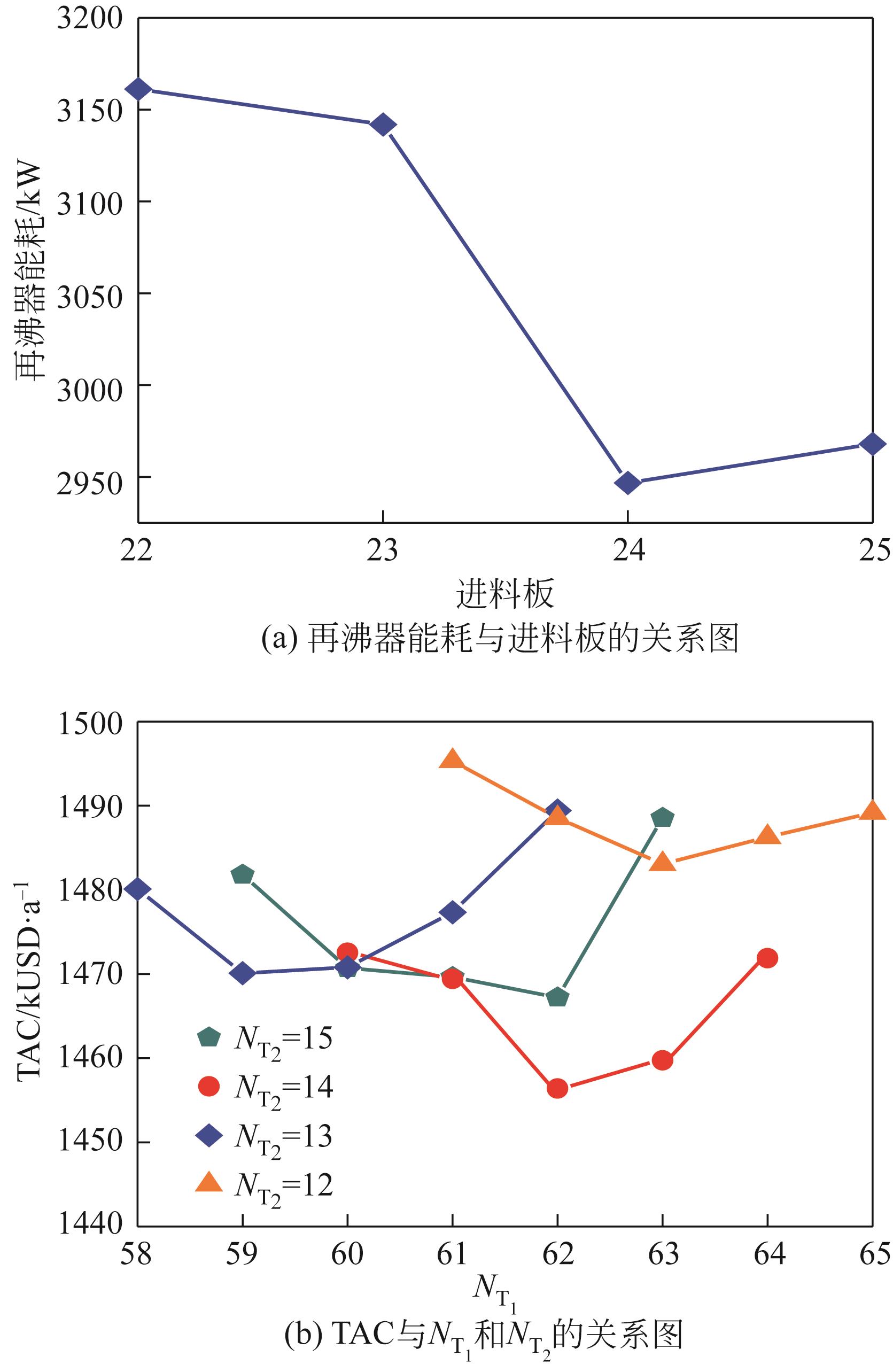
表5 两塔-侧线分相器和常规三塔粗分流程比较
| 参数 | 两塔-侧线分相器流程 | 常规三塔粗分流程 | ||||
|---|---|---|---|---|---|---|
| 侧线精馏塔 | 重醇塔 | 脱水塔 | 醇分塔 | 重醇塔 | ||
| p/kPa | 101.3 | 121.3 | 101.3 | 121.3 | ||
| NT | 62 | 14 | 47 | 51 | 8 | |
| NF | 25 | 1 | 10 | 29 | 1 | |
| RR | 6.13 | — | 1.61 | 1.69 | — | |
| d/m | 1.1 | 0.5 | 1.0 | 0.7 | 0.25 | |
| h/m | 0.6 | 0.4 | 0.6 | 0.4 | 0.2 | |
| QC/kW | -2224.74 | -272.31 | -2111.65 | -840.84 | -80.20 | |
| QR/kW | 2571.21 | 291.39 | 2446.14 | 858.11 | 101.95 | |
| 合计QR/kW | 2862.60(差异15.96%①) | 3406.20 | ||||
| FCI/kUSD·a-1 | 1016.67 | 1136.08 | ||||
| OC/kUSD·a-1 | 1120.30 | 1333.67 | ||||
| TAC/kUSD·a-1 | 1456.40(差异14.79%②) | 1709.25 | ||||
| 项目 | 控制变量 | 操纵变量 | 增益/Kc | 积分时间τI/min |
|---|---|---|---|---|
| CC1 | xD1(丁醇) | 回流比(侧线精馏塔) | 0.10 | 40.92 |
| CC2 | xB1(水) | 再沸器热负荷(侧线精馏塔) | 0.20 | 77.88 |
| T4 | xB1(丙醇) | 再沸器热负荷(重醇塔) | 0.35 | 10.56 |
表6 控制器调谐参数
| 项目 | 控制变量 | 操纵变量 | 增益/Kc | 积分时间τI/min |
|---|---|---|---|---|
| CC1 | xD1(丁醇) | 回流比(侧线精馏塔) | 0.10 | 40.92 |
| CC2 | xB1(水) | 再沸器热负荷(侧线精馏塔) | 0.20 | 77.88 |
| T4 | xB1(丙醇) | 再沸器热负荷(重醇塔) | 0.35 | 10.56 |
| 1 | LARSON E D, REN T J. Synthetic fuel production by indirect coal liquefaction[J]. Energy for Sustainable Development, 2003, 7(4): 79-102. |
| 2 | LIU Z Y, SHI S D, LI Y W. Coal liquefaction technologies—Development in China and challenges in chemical reaction engineering[J]. Chemical Engineering Science, 2010, 65(1): 12-17. |
| 3 | KHODAKOV A Y, CHU W, FONGARLAND P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels[J]. Chemical Reviews, 2007, 107(5): 1692-1744. |
| 4 | DRY M E. The fischer-tropsch process: 1950—2000[J]. Catalysis Today, 2002, 71(3/4): 227-241. |
| 5 | DÜRRE P. Biobutanol: an attractive biofuel[J]. Biotechnology Journal: Healthcare Nutrition Technology, 2007, 2(12): 1525-1534. |
| 6 | MOSER W R, CONNOLLY K E. Synthesis and characterization of copper-modified zinc chromites by the high temperature aerosol decomposition process for higher alcohol synthesis[J]. The Chemical Engineering Journal and the Biochemical Engineering Journal, 1996, 64(2). |
| 7 | MAHDAVI V, PEYROVI M H. Synthesis of C1-C6 alcohols over copper/cobalt catalysts: investigation of the influence of preparative procedures on the activity and selectivity of Cu-Co2O3/ZnO, Al2O3 catalyst[J]. Catalysis Communications, 2006, 7(8): 542-549. |
| 8 | 马爱华, 云志. 费托合成水相副产物中具有共沸组成的低碳混合醇-水体系分离方法的研究进展[J]. 石油学报(石油加工), 2013, 29(4): 738-743. |
| MA A H, YUN Z. Research progress of separation technologies for azeotropic mixture of lower alcohols-water system of the by-product in water of fischer-Tropsch synthesis[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2013, 29(4): 738-743. | |
| 9 | SIE S T. Process development and scale up: Ⅳ. Case history of the development of a Fischer-Tropsch synthesis process[J]. Reviews in Chemical Engineering, 1998, 14(2): 109-157. |
| 10 | 李云华. 费托合成水相副产物馏分切割工艺的技术开发[D]. 天津: 天津大学, 2003. |
| LI Y H. Technology exploitation of cutting the by-product in water of Fischer-Tropsch synthesis[D]. Tianjin: Tianjin University, 2003. | |
| 11 | 汪俊锋, 王红星, 杨金杯, 等. 费托合成水相副产物混合醇分离脱水工艺模拟及优化[J]. 计算机与应用化学, 2015, 32(5): 567-571. |
| WANG J F, WANG H X, YANG J B, et al. F-T of mixed alcohol aqueous by-product separation dehydration technology simulation and optimization[J]. Computers and Applied Chemistry, 2015, 32(5): 567-571. | |
| 12 | 李玲, 柴士阳, 刘来春, 等. 费托合成水相副产物混合醇渗透蒸发分离工艺[J]. 化工进展, 2017, 36(6): 2086-2093. |
| LI L, CHAI S Y, LIU L C, et al. Study on separation of mixed alcohol from water phase by-product in the F-T synthesis by pervaporation technology[J]. Chemical Industry and Engineering Progress, 2017, 36(6): 2086-2093. | |
| 13 | GARCIA M, SANZ M T, BELTRAN S. Separation by pervaporation of ethanol from aqueous solutions and effect of other components present in fermentation broths[J]. Journal of Chemical Technology & Biotechnology, 2009, 84(12): 1873-1882. |
| 14 | ARIFIN S, CHIEN I L. Design and control of an isopropyl alcohol dehydration process via extractive distillation using dimethyl sulfoxide as an entrainer[J]. Industrial & Engineering Chemistry Research, 2008, 47(3): 790-803. |
| 15 | LUYBEN W L. Economic optimum design of the heterogeneous azeotropic dehydration of ethanol[J]. Industrial & Engineering Chemistry Research, 2012, 51(50): 16427-16432. |
| 16 | MILESTONE N B, BIBBY D M. Concentration of alcohols by adsorption on silicalite[J]. Journal of Chemical Technology & Biotechnology, 2010, 31(1): 732-736. |
| 17 | 胡子益, 李洪波, 谭宇鑫, 等. 分子筛膜-精馏耦合用于费托合成水相副产物混合醇回收的工艺流程模拟[J]. 化工进展, 2016, 35(S2): 56-60. |
| HU Z Y, LI H B, TANG Y X, et al. Zeolite membrane dehydration and distillation coupling process simulation of F-T water by-product recovery[J]. Chemical Industry and Engineering Progress, 2016, 35(S2): 56-60. | |
| 18 | CUI C T, ZHANG X D, SUN J S. Design and optimization of energy-efficient liquid-only side-stream distillation configurations using a stochastic algorithm[J]. Chemical Engineering Research and Design, 2019, 145: 48-52. |
| 19 | SMITH R. Chemical process: design and integration[M]. State of New Jersey: John Wiley & Sons, 2005. |
| 20 | ROOKS R E, MALONE M F, DOHERTY M F. A geometric design method for side-stream distillation columns[J]. Industrial & Engineering Chemistry Research, 1996, 35(10): 3653-3664. |
| 21 | TEDDER D W, RUDD D F. Parametric studies in industrial distillation: Part Ⅰ. Design comparisons[J]. AIChE Journal, 1978, 24(2): 303-315. |
| 22 | GLINOS K N, MALONE M F. Design of sidestream distillation columns[J]. Industrial & Engineering Chemistry Process Design and Development, 1985, 24(3): 822-828. |
| 23 | FERREIRA M C, MEIRELLES A J A, BATISTA E A C. Study of the fusel oil distillation process[J]. Industrial & Engineering Chemistry Research, 2013, 52(6): 2336-2351. |
| 24 | PUENTES C, JOULIA X, ATHÈS V, et al. Review and thermodynamic modeling with NRTL model of vapor-liquid equilibria (VLE) of aroma compounds highly diluted in ethanol-water mixtures at 101.3kPa[J]. Industrial & Engineering Chemistry Research, 2018, 57(10): 3443-3470. |
| 25 | IWAKABE K, KOSUGE H. Isobaric vapor-liquid-liquid equilibria with a newly developed still[J]. Fluid Phase Equilibria, 2001, 192(1/2): 171-186. |
| 26 | 任冉冉. 费托合成水中醇分离过程的热力学模型评价[D]. 北京: 北京石油化工学院, 2016. |
| REN R R. Evaluation of thermodynamic model for alcohol separation process of Fischer-Tropsch synthesis water[D]. Beijing: Beijing Institute of Petrochemical Technology, 2016. | |
| 27 | ORCHILLÉS A V, VERCHER E, MARTÍNEZ-ANDREU A, et al. Isobaric vapor-liquid equilibria for 1-propanol + water + 1-ethyl-3-methylimidazolium trifluoromethanesulfonate at 100kPa[J]. Journal of Chemical & Engineering Data, 2008, 53(10): 2426-2431. |
| 28 | KAMIHAMA N, MATSUDA H, KURIHARA K, et al. Isobaric vapor-liquid equilibria for ethanol + water + ethylene glycol and its constituent three binary systems[J]. Journal of Chemical & Engineering Data, 2012, 57(2): 339-344. |
| 29 | IWAKABE K, KOSUGE H. Isobaric vapor-liquid-liquid equilibria with a newly developed still[J]. Fluid Phase Equilibria, 2001, 192(1/2): 171-186. |
| 30 | 程能林. 溶剂手册[M]. 4版. 北京: 化学工业出版社, 2007: 405-439. |
| CHENG N L. Solvents Handbook[M]. 4th ed. Beijing: Chemical Industry Press, 2007: 405-439. | |
| 31 | STEPHENSON R, STUART J, TABAK M. Mutual solubility of water and aliphatic alcohols[J]. Journal of Chemical and Engineering Data, 1984, 29(3): 287-290. |
| 32 | GMEHLING J, MENKE J, KRAFCZYK J, et al. Azeotropic data[M]. Weinheim: Wiley-VCH, 2004. |
| 33 | DOUGLAS J M. Conceptual design of chemical processes[M]. New York: McGraw-Hill, 1988. |
| 34 | WEI F, DIAO B T, GAO J, et al. Process design, evaluation and control for separation of 2, 2, 3, 3‐tetrafluoro‐1‐propanol and water by extractive distillation using ionic liquid 1‐ethyl‐3‐methylimidazolium acetate[J]. Journal of Chemical Technology & Biotechnology, 2021, 96(11): 3175-3184. |
| 35 | FIEN G J A F, LIU Y A. Heuristic synthesis and shortcut design of separation processes using residue curve maps: a review[J]. Industrial & Engineering Chemistry Research, 1994, 33(11): 2505-2522. |
| 36 | BARBOSA D, DOHERTY M F. The simple distillation of homogeneous reactive mixtures[J]. Chemical Engineering Science, 1988, 43(3): 541-550. |
| 37 | WALPOT H E. Theoretical modeling of residue curve maps for a reactive distillation concept for the production of n-propyl propionate[D]. Vancouver: the University of British Columbia, 2011. |
| 38 | LUYBEN W L. Distillation design and control using Aspen simulation[M]. New York: John Wiley & Sons Inc., 2013. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [3] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [4] | 徐若思, 谭蔚. C形管池沸腾两相流流场模拟与流固耦合分析[J]. 化工进展, 2023, 42(S1): 47-55. |
| [5] | 张凤岐, 崔成东, 鲍学伟, 朱炜玄, 董宏光. 胺液吸收-分步解吸脱硫工艺的设计与评价[J]. 化工进展, 2023, 42(S1): 518-528. |
| [6] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [7] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [8] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [9] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [10] | 张瑞杰, 刘志林, 王俊文, 张玮, 韩德求, 李婷, 邹雄. 水冷式复叠制冷系统的在线动态模拟与优化[J]. 化工进展, 2023, 42(S1): 124-132. |
| [11] | 王太, 苏硕, 李晟瑞, 马小龙, 刘春涛. 交流电场中贴壁气泡的动力学行为[J]. 化工进展, 2023, 42(S1): 133-141. |
| [12] | 孙继鹏, 韩靖, 唐杨超, 闫汉博, 张杰瑶, 肖苹, 吴峰. 硫黄湿法成型过程数值模拟与操作参数优化[J]. 化工进展, 2023, 42(S1): 189-196. |
| [13] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [14] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [15] | 贺美晋. 分子管理在炼油领域分离技术中的应用和发展趋势[J]. 化工进展, 2023, 42(S1): 260-266. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||