化工进展 ›› 2025, Vol. 44 ›› Issue (5): 2938-2954.DOI: 10.16085/j.issn.1000-6613.2024-2067
轻烃分离材料和机理的研究进展
罗伊雯( ), 赵亮(
), 赵亮( ), 张宇豪, 刘东阳, 高金森, 徐春明
), 张宇豪, 刘东阳, 高金森, 徐春明
- 中国石油大学(北京)重质油全国重点实验室,北京 102249
-
收稿日期:2024-12-19修回日期:2025-02-21出版日期:2025-05-25发布日期:2025-05-20 -
通讯作者:赵亮 -
作者简介:罗伊雯(1999—),女,博士研究生,研究方向为膜材料分离。E-mail:2021310248@student.cup.edu.cn。 -
基金资助:国家重点研发计划青年科学家项目(2024YFB3815600);创新研究群体项目(2202100004);国家杰出青年科学基金(22325808);国家自然科学基金委员会联合基金(U22B20140)
Progress on separation materials and mechanisms of light hydrocarbons
LUO Yiwen( ), ZHAO Liang(
), ZHAO Liang( ), ZHANG Yuhao, LIU Dongyang, GAO Jinsen, XU Chunming
), ZHANG Yuhao, LIU Dongyang, GAO Jinsen, XU Chunming
- State Key Laboratory of Heavy Oil Progressing, China University of Petroleum, Beijing 102249, China
-
Received:2024-12-19Revised:2025-02-21Online:2025-05-25Published:2025-05-20 -
Contact:ZHAO Liang
摘要:
随着我国新能源的发展和石油需求峰值的临近,石油行业面临炼油产能过剩的问题。将石油通过轻烃分离后转向生产化工原料是缓解炼油产能过剩、弥补当前化工原料短缺和实现石油高值化利用的有效途径。本文首先介绍了轻烃在化工行业的重要性和轻烃分离的意义,讨论了轻烃分离机理,具体介绍了分子筛效应、动力学效应、热力学平衡效应和协同效应的分离原理和适用材料。然后按照不同烃类的分离进行分类,详细讨论了各种轻烃分离的研究现状,并对不同材料的分离效果进行对比,总结了不同分离材料的适用范围。最后,对未来轻烃分离的研究方向进行了展望,为今后开发分离效果更好、成本更低的轻烃分离技术和分离材料提供了借鉴。
中图分类号:
引用本文
罗伊雯, 赵亮, 张宇豪, 刘东阳, 高金森, 徐春明. 轻烃分离材料和机理的研究进展[J]. 化工进展, 2025, 44(5): 2938-2954.
LUO Yiwen, ZHAO Liang, ZHANG Yuhao, LIU Dongyang, GAO Jinsen, XU Chunming. Progress on separation materials and mechanisms of light hydrocarbons[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2938-2954.
| 名称 | 化学式 | 沸点/K | 动力学直径/Å | 偶极矩/D | 极化率/10-25cm3 |
|---|---|---|---|---|---|
| 甲烷 | CH4 | 111.65 | 4.1 | 0 | 25.93 |
| 乙烷 | C2H6 | 184.55 | 4.44 | 0 | 44.3~44.7 |
| 丙烷 | C3H8 | 231.05 | 4.3~5.11 | 0.084 | 62.9~63.7 |
| 正丁烷 | C4H10 | 272.65 | 4.4 | 0.3 | 82.4 |
| 异丁烷 | C4H10 | 262.65 | 4.8 | 0.253 | 81.5 |
| 乙烯 | C2H4 | 169.45 | 4.16 | 0 | 42.52 |
| 丙烯 | C3H6 | 225.45 | 4.68 | 0.366 | 62.6 |
| 乙炔 | C2H2 | 189.15 | 3.3 | 2.6 | 33.3~39.3 |
表1 轻烃分子的性质
| 名称 | 化学式 | 沸点/K | 动力学直径/Å | 偶极矩/D | 极化率/10-25cm3 |
|---|---|---|---|---|---|
| 甲烷 | CH4 | 111.65 | 4.1 | 0 | 25.93 |
| 乙烷 | C2H6 | 184.55 | 4.44 | 0 | 44.3~44.7 |
| 丙烷 | C3H8 | 231.05 | 4.3~5.11 | 0.084 | 62.9~63.7 |
| 正丁烷 | C4H10 | 272.65 | 4.4 | 0.3 | 82.4 |
| 异丁烷 | C4H10 | 262.65 | 4.8 | 0.253 | 81.5 |
| 乙烯 | C2H4 | 169.45 | 4.16 | 0 | 42.52 |
| 丙烯 | C3H6 | 225.45 | 4.68 | 0.366 | 62.6 |
| 乙炔 | C2H2 | 189.15 | 3.3 | 2.6 | 33.3~39.3 |
| 原料 | 材料类型 | 原料状态 | 温度/℃ | 压力/MPa | 分离效果 | 参考文献 |
|---|---|---|---|---|---|---|
| CH4/C2H4/C2H6/C3H6 | 改性4A分子筛 | 气相 | 50 | 常压 | 乙烯和丙烯的穿透时间为15min和33min,甲烷和乙烷的穿透时间为12min和30min | [ |
| C2H4/C2H6 | AgA分子筛 | 气相 | 30 | 0.1 | 对乙烷不吸附,对乙烯的吸附量为2.3mmol/g | [ |
| C2H4/C2H6 | AgX分子筛 | 气相 | 30 | 0.1 | 对乙烷的吸附量为1.4mmol/g,对乙烯的吸附量为2.4mmol/g | [ |
| C3H6/C3H8 | 13X | 气相 | 25 | 0.1 | 选择性为22 | [ |
| C3H6/C3H8 | Na-ETS-10 | 气相 | 25 | 0.1 | 选择性为7.4 | [ |
| C3H6/C3H8 | CMS | 气相 | 90 | 10 | 分离因子为27 | [ |
| C2H4/C2H6 | CMS | 气相 | 25 | 10 | 吸附选择性为3.0~20.6 | [ |
| C2H4/C2H6 | CuCl2改性CMS | 气相 | 30 | 0.1 | 吸附选择性为70 | [ |
| C3H6/C3H8 | Na+、K+和Rb+改性CMS | 气相 | — | — | 吸附量比值比5A分子筛和ZSM-5分子筛高78% | [ |
| C2H4/C2H6 | Cu-BTC | 气相 | 22 | 0.08 | C2H6吸附量为4.7mmol/g,C2H4吸附量为5.8mmol/g | [ |
| C2H4/C2H6 | M-gallate | 气相 | 25 | 0.1 | 选择性为52 | [ |
| C2H4/C2H6 | NOTT-300 | 气相 | 20 | 0.1 | 选择性为48.7 | [ |
| C2H4/C2H6 | 碳分子筛膜 | 气相 | — | — | 选择性为17.5 | [ |
| C2H4/C2H6和C3H6/C3H8 | 碳分子筛膜 | 气相 | 35 | — | C2H4/C2H6的选择性接近11 | [ |
| C2H4/C2H6 | 碳分子筛膜 | 气相 | 35 | 0.4 | 800℃热解的CMSM对C2H4/C2H6理想气体选择性为24.1 | [ |
| C3H6/C3H8 | 混合基质膜 | 气相 | — | — | C3H6渗透率为174 Barrer,C3H6/C3H8分离选择性为14.4 | [ |
| C3H6/C3H8 | 混合基质膜 | 气相 | 35 | 0.28 | 选择性约为34 | [ |
表2 烷烃/烯烃分离的研究成果总结
| 原料 | 材料类型 | 原料状态 | 温度/℃ | 压力/MPa | 分离效果 | 参考文献 |
|---|---|---|---|---|---|---|
| CH4/C2H4/C2H6/C3H6 | 改性4A分子筛 | 气相 | 50 | 常压 | 乙烯和丙烯的穿透时间为15min和33min,甲烷和乙烷的穿透时间为12min和30min | [ |
| C2H4/C2H6 | AgA分子筛 | 气相 | 30 | 0.1 | 对乙烷不吸附,对乙烯的吸附量为2.3mmol/g | [ |
| C2H4/C2H6 | AgX分子筛 | 气相 | 30 | 0.1 | 对乙烷的吸附量为1.4mmol/g,对乙烯的吸附量为2.4mmol/g | [ |
| C3H6/C3H8 | 13X | 气相 | 25 | 0.1 | 选择性为22 | [ |
| C3H6/C3H8 | Na-ETS-10 | 气相 | 25 | 0.1 | 选择性为7.4 | [ |
| C3H6/C3H8 | CMS | 气相 | 90 | 10 | 分离因子为27 | [ |
| C2H4/C2H6 | CMS | 气相 | 25 | 10 | 吸附选择性为3.0~20.6 | [ |
| C2H4/C2H6 | CuCl2改性CMS | 气相 | 30 | 0.1 | 吸附选择性为70 | [ |
| C3H6/C3H8 | Na+、K+和Rb+改性CMS | 气相 | — | — | 吸附量比值比5A分子筛和ZSM-5分子筛高78% | [ |
| C2H4/C2H6 | Cu-BTC | 气相 | 22 | 0.08 | C2H6吸附量为4.7mmol/g,C2H4吸附量为5.8mmol/g | [ |
| C2H4/C2H6 | M-gallate | 气相 | 25 | 0.1 | 选择性为52 | [ |
| C2H4/C2H6 | NOTT-300 | 气相 | 20 | 0.1 | 选择性为48.7 | [ |
| C2H4/C2H6 | 碳分子筛膜 | 气相 | — | — | 选择性为17.5 | [ |
| C2H4/C2H6和C3H6/C3H8 | 碳分子筛膜 | 气相 | 35 | — | C2H4/C2H6的选择性接近11 | [ |
| C2H4/C2H6 | 碳分子筛膜 | 气相 | 35 | 0.4 | 800℃热解的CMSM对C2H4/C2H6理想气体选择性为24.1 | [ |
| C3H6/C3H8 | 混合基质膜 | 气相 | — | — | C3H6渗透率为174 Barrer,C3H6/C3H8分离选择性为14.4 | [ |
| C3H6/C3H8 | 混合基质膜 | 气相 | 35 | 0.28 | 选择性约为34 | [ |
| 原料 | 材料类型 | 原料状态 | 温度/℃ | 压力/MPa | 分离效果 | 参考文献 |
|---|---|---|---|---|---|---|
| C2H2/C2H4 | M'MOF | 气相 | 室温 | — | 分离选择性为5.23 | [ |
| C2H2/C2H4 | NCU-100 | 气相 | 25 | 0.001 | C2H2/C2H4混合气穿过填充床,乙烯纯度能超过99.99% | [ |
| C2H2/C2H4 | JCM-1 | 气相 | 25 | 0.1 | 选择性为3.1 | [ |
| C3H4/C3H6 | ELM-12 | 气相 | 25 | 0.001 | C3H4吸附量为1.83mmol/g,C3H6的吸附量为0.67mmol/g | [ |
表3 烯烃/炔烃分离的研究成果总结
| 原料 | 材料类型 | 原料状态 | 温度/℃ | 压力/MPa | 分离效果 | 参考文献 |
|---|---|---|---|---|---|---|
| C2H2/C2H4 | M'MOF | 气相 | 室温 | — | 分离选择性为5.23 | [ |
| C2H2/C2H4 | NCU-100 | 气相 | 25 | 0.001 | C2H2/C2H4混合气穿过填充床,乙烯纯度能超过99.99% | [ |
| C2H2/C2H4 | JCM-1 | 气相 | 25 | 0.1 | 选择性为3.1 | [ |
| C3H4/C3H6 | ELM-12 | 气相 | 25 | 0.001 | C3H4吸附量为1.83mmol/g,C3H6的吸附量为0.67mmol/g | [ |
| 原料 | 材料类型 | 原料状态 | 温度/℃ | 压力/MPa | 分离效果 | 参考文献 |
|---|---|---|---|---|---|---|
| C11~16正构烷烃 | 5A分子筛 | 液相 | 120~200 | 1.2~2.5 | 在180℃时,5A分子筛对正构烷烃的分离度:C11(1.31)>C12(1.14)>C13(1.01)>C14(0.98)>C15(0.95)>C16(0.94) | [ |
| 正戊烷、正己烷、正庚烷 | 多级孔道5A分子筛 | 液相 | — | — | 正戊烷的液相扩散系数增大81.2%,正己烷增大89.9%,正庚烷增大98.3% | [ |
| 正丁烷/甲烷 | 聚二甲基硅氧烷膜 | 液相 | 25 | — | 当正丁烷进料体积分数从1%增加到8%时,正丁烷/甲烷选择性从10增加到12 | [ |
| 正丁烷/甲烷 | 聚乙烯-烯丙基-二甲基硅烷膜 | 液相 | — | — | 选择性为14.1 | [ |
表4 不同碳数烷烃分离的研究成果总结
| 原料 | 材料类型 | 原料状态 | 温度/℃ | 压力/MPa | 分离效果 | 参考文献 |
|---|---|---|---|---|---|---|
| C11~16正构烷烃 | 5A分子筛 | 液相 | 120~200 | 1.2~2.5 | 在180℃时,5A分子筛对正构烷烃的分离度:C11(1.31)>C12(1.14)>C13(1.01)>C14(0.98)>C15(0.95)>C16(0.94) | [ |
| 正戊烷、正己烷、正庚烷 | 多级孔道5A分子筛 | 液相 | — | — | 正戊烷的液相扩散系数增大81.2%,正己烷增大89.9%,正庚烷增大98.3% | [ |
| 正丁烷/甲烷 | 聚二甲基硅氧烷膜 | 液相 | 25 | — | 当正丁烷进料体积分数从1%增加到8%时,正丁烷/甲烷选择性从10增加到12 | [ |
| 正丁烷/甲烷 | 聚乙烯-烯丙基-二甲基硅烷膜 | 液相 | — | — | 选择性为14.1 | [ |
| 原料 | 材料类型 | 原料状态 | 温度/℃ | 压力/MPa | 分离效果 | 参考文献 |
|---|---|---|---|---|---|---|
| 1-丁烯、顺式-2-丁烯、反式-2-丁烯 | SAPO-17分子筛 | 气体 | 60 | — | 选择性为75 | [ |
| n-C4H10/i-C4H10 | MFI分子筛 | 气相 | 100 | 0.1 | 选择性为2.14 | [ |
| n-C4H10/i-C4H10 | Zr-fum-fcu-MOF | 气相 | 20 | 0.08 | 无缺陷的Zr-fum-fcu-MOF的选择性为20 | [ |
| n-C4H8/i-C4H8 | MnINA | 气相 | — | 0~0.01 | 选择性为327.7 | [ |
| n-C4H10/i-C4H10 | MFI膜 | 气相 | — | — | 选择性为5.4 | [ |
| n-C4H10/i-C4H10 | silicalite-1膜 | 气相 | 60 | 0.103 | 混合物的分离系数为45 | [ |
| n-C4H10、i-C4H10 | B-ZSM-5沸石分子膜 | 气相 | 200 | 0.125 | 理想分离系数达104.73 | [ |
| n-C4H10/i-C4H10 | CMS | 气相 | 25 | 0.1 | 异丁烷直接穿过色谱柱而正丁烷在色谱柱中保留35min/g | [ |
| n-C4H10/i-C4H10 | 碳分子筛膜 | 气相 | 25 | 0.1 | 分离因子为74 | [ |
表5 C4异构烷烃分离的研究成果总结
| 原料 | 材料类型 | 原料状态 | 温度/℃ | 压力/MPa | 分离效果 | 参考文献 |
|---|---|---|---|---|---|---|
| 1-丁烯、顺式-2-丁烯、反式-2-丁烯 | SAPO-17分子筛 | 气体 | 60 | — | 选择性为75 | [ |
| n-C4H10/i-C4H10 | MFI分子筛 | 气相 | 100 | 0.1 | 选择性为2.14 | [ |
| n-C4H10/i-C4H10 | Zr-fum-fcu-MOF | 气相 | 20 | 0.08 | 无缺陷的Zr-fum-fcu-MOF的选择性为20 | [ |
| n-C4H8/i-C4H8 | MnINA | 气相 | — | 0~0.01 | 选择性为327.7 | [ |
| n-C4H10/i-C4H10 | MFI膜 | 气相 | — | — | 选择性为5.4 | [ |
| n-C4H10/i-C4H10 | silicalite-1膜 | 气相 | 60 | 0.103 | 混合物的分离系数为45 | [ |
| n-C4H10、i-C4H10 | B-ZSM-5沸石分子膜 | 气相 | 200 | 0.125 | 理想分离系数达104.73 | [ |
| n-C4H10/i-C4H10 | CMS | 气相 | 25 | 0.1 | 异丁烷直接穿过色谱柱而正丁烷在色谱柱中保留35min/g | [ |
| n-C4H10/i-C4H10 | 碳分子筛膜 | 气相 | 25 | 0.1 | 分离因子为74 | [ |
| 原料 | 材料类型 | 原料状态 | 温度/℃ | 压力/MPa | 分离效果 | 参考文献 |
|---|---|---|---|---|---|---|
| 石脑油 | 5A分子筛 | 液相 | 220 | — | 正构烷烃的分离纯度>99.9% | [ |
| 石脑油 | 5A分子筛 | 液相 | 300 | 0.5 | 正构烷烃回收率达到95% | [ |
| 2-甲基戊烷/正己烷 | 5A和ZSM-5分子筛 | 气相 | 30、80、150 | 0~1 | 在30℃时,5A分子筛对正己烷的吸附量为1.77mmol/g,ZSM-5分子筛对正己烷的吸附量为1.34mmol/g | [ |
石脑油(5A) 2-甲基己烷/3-甲基己烷(ZSM-5) | 5A和ZSM-5分子筛 | 气相 | 270、290、310(5A) 25(ZSM-5) | — | 正构烷烃脱除率达到99%(5A) 3-甲基己烷的分离率为66.2%(ZSM-5) | [ |
| PX/OX | 分子筛膜 | 液相 | 100 | — | 选择性为150 | [ |
| PX/OX | HZSM-5/氧化铝复合膜 | 液相 | 500 | — | 分离因子大于400 | [ |
| PX/OX | 碳分子筛膜 | 液相 | 25 | 5~12 | 分离因子大于4 | [ |
| PX/OX | 碳分子筛膜 | 气相 | 35 | 7~11 | 选择性为39.88 | [ |
表6 C5以上异构体分离的研究成果总结
| 原料 | 材料类型 | 原料状态 | 温度/℃ | 压力/MPa | 分离效果 | 参考文献 |
|---|---|---|---|---|---|---|
| 石脑油 | 5A分子筛 | 液相 | 220 | — | 正构烷烃的分离纯度>99.9% | [ |
| 石脑油 | 5A分子筛 | 液相 | 300 | 0.5 | 正构烷烃回收率达到95% | [ |
| 2-甲基戊烷/正己烷 | 5A和ZSM-5分子筛 | 气相 | 30、80、150 | 0~1 | 在30℃时,5A分子筛对正己烷的吸附量为1.77mmol/g,ZSM-5分子筛对正己烷的吸附量为1.34mmol/g | [ |
石脑油(5A) 2-甲基己烷/3-甲基己烷(ZSM-5) | 5A和ZSM-5分子筛 | 气相 | 270、290、310(5A) 25(ZSM-5) | — | 正构烷烃脱除率达到99%(5A) 3-甲基己烷的分离率为66.2%(ZSM-5) | [ |
| PX/OX | 分子筛膜 | 液相 | 100 | — | 选择性为150 | [ |
| PX/OX | HZSM-5/氧化铝复合膜 | 液相 | 500 | — | 分离因子大于400 | [ |
| PX/OX | 碳分子筛膜 | 液相 | 25 | 5~12 | 分离因子大于4 | [ |
| PX/OX | 碳分子筛膜 | 气相 | 35 | 7~11 | 选择性为39.88 | [ |
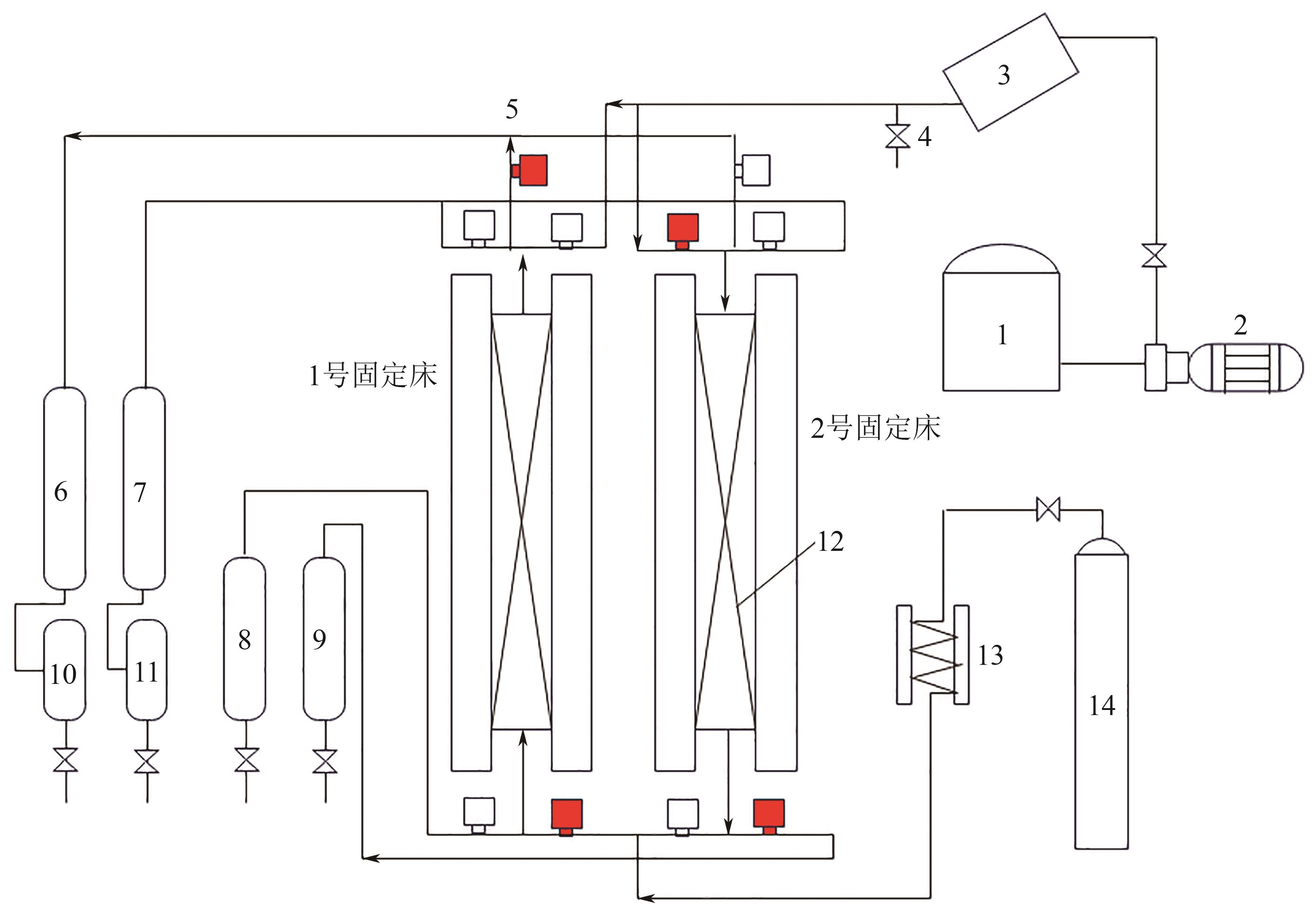
图4 双塔并联吸附分离实验装置工艺流程[104]1—原料油储罐;2—原料油进料泵;3—原料油汽化炉;4—手动阀;5—电磁阀(红色表示打开状态,该状态表示1号床脱附、2号床吸附);6—中间油冷凝器;7—脱附油冷凝器;8—1号床层吸余油冷凝器;9—2号床层吸余油冷凝器;10—中间油气液分离器;11—脱附油气液分离器;12—分子筛床层;13—脱附油预热器;14—氮气储罐
| 参数 | 分子筛 | 碳分子筛 | 金属有机框架材料 | 碳分子筛膜 | 分子筛膜 | 聚合物膜 | 混合基质膜 |
|---|---|---|---|---|---|---|---|
| 孔径大小/nm | 0.3~1 | 0.3~1 | 0.5~2 | 0.3~0.5 | 0.3~1 | 0.5~10(取决于聚合物类型) | 0.3~2(取决于填料和基质) |
| 吸附量或渗透率① | 乙烷0~1.4、乙烯2.3~2.4、丙烯1.75~3.1、正丁烷1.37、异丁烷0.64、反式-2-丁烯0.75、正己烷1.37~1.77 | 乙烷7.12、乙烯2.57~6.25 | 乙烯3.37~6、丙烯0.67、丙炔1.83、正丁烷3.5~4、正丁烯1.79 | 乙烯10.4~244.6、正丁烷3.84×106、PX1.0~32×10-3mol/(m2·s) | 正丁烷1.3~3.5×10-7mol/(m2·s·Pa)、异丁烷5.71×0-10mol/(m2·s·Pa)、PX3.5×10-6mol/(m2·s·Pa) | 甲烷32.1、正丁烷451.2 | 丙烯19 |
| 选择性 | 丙烷/丙烯7.4~22、正/异丁烷2.14、丁烯异构体75 | 乙烷/乙烯3~70、丙烷/丙烯27 | 乙烷/乙烯48.7~52、乙烯/乙炔3.1~5.23、正/异丁烷20~327.7 | 乙烷/乙烯4.8~24.1、正/异丁烷74、PX/OX39.88 | 正/异丁烷5.4~104.73、PX/OX150 | 正丁烷/甲烷10~14.1 | 丙烷/丙烯14.4~34 |
| 稳定性 | 高热稳定性(>500℃)和化学稳定性 | 高化学稳定性,中等热稳定性(<300℃) | 中等化学稳定性,中等热稳定性(<400℃) | 高化学稳定性,中等热稳定性(<300℃) | 高热稳定性(>500℃)和化学稳定性 | 中等化学稳定性,中等热稳定性(<200℃) | 中等至高(取决于填料和基质) |
| 分离原料状态 | 气相、液相 | 气相 | 气相 | 气相、液相 | 液相 | 液相 | 气相 |
| 分离轻烃范围 | 烷烃/烯烃、不同碳数的烷烃、C4及以上异构体 | 烷烃/烯烃、C4异构体 | 烷烃/烯烃、烯烃/炔烃、C4异构体 | 烷烃/烯烃、C4及以上异构体 | C4及以上异构体 | 不同碳数的烷烃 | 烷烃/烯烃 |
| 优点 | 应用范围广、稳定性好 | 选择性较高 | 选择性高、孔径可微调、易功能化 | 选择性较高、孔径分布均匀 | 稳定性好、选择性高 | 渗透率高 | 制备成本较低、易加工 |
| 缺点 | 吸附量小、孔径难精准控制 | 热稳定性一般、孔径难精准控制 | 制备成本高、难规模化 | 热稳定性一般、易污染 | 渗透率低、易污染 | 选择性低、稳定性一般、易污染 | 选择性一般、易污染 |
表7 不同轻烃分离材料的分离性能
| 参数 | 分子筛 | 碳分子筛 | 金属有机框架材料 | 碳分子筛膜 | 分子筛膜 | 聚合物膜 | 混合基质膜 |
|---|---|---|---|---|---|---|---|
| 孔径大小/nm | 0.3~1 | 0.3~1 | 0.5~2 | 0.3~0.5 | 0.3~1 | 0.5~10(取决于聚合物类型) | 0.3~2(取决于填料和基质) |
| 吸附量或渗透率① | 乙烷0~1.4、乙烯2.3~2.4、丙烯1.75~3.1、正丁烷1.37、异丁烷0.64、反式-2-丁烯0.75、正己烷1.37~1.77 | 乙烷7.12、乙烯2.57~6.25 | 乙烯3.37~6、丙烯0.67、丙炔1.83、正丁烷3.5~4、正丁烯1.79 | 乙烯10.4~244.6、正丁烷3.84×106、PX1.0~32×10-3mol/(m2·s) | 正丁烷1.3~3.5×10-7mol/(m2·s·Pa)、异丁烷5.71×0-10mol/(m2·s·Pa)、PX3.5×10-6mol/(m2·s·Pa) | 甲烷32.1、正丁烷451.2 | 丙烯19 |
| 选择性 | 丙烷/丙烯7.4~22、正/异丁烷2.14、丁烯异构体75 | 乙烷/乙烯3~70、丙烷/丙烯27 | 乙烷/乙烯48.7~52、乙烯/乙炔3.1~5.23、正/异丁烷20~327.7 | 乙烷/乙烯4.8~24.1、正/异丁烷74、PX/OX39.88 | 正/异丁烷5.4~104.73、PX/OX150 | 正丁烷/甲烷10~14.1 | 丙烷/丙烯14.4~34 |
| 稳定性 | 高热稳定性(>500℃)和化学稳定性 | 高化学稳定性,中等热稳定性(<300℃) | 中等化学稳定性,中等热稳定性(<400℃) | 高化学稳定性,中等热稳定性(<300℃) | 高热稳定性(>500℃)和化学稳定性 | 中等化学稳定性,中等热稳定性(<200℃) | 中等至高(取决于填料和基质) |
| 分离原料状态 | 气相、液相 | 气相 | 气相 | 气相、液相 | 液相 | 液相 | 气相 |
| 分离轻烃范围 | 烷烃/烯烃、不同碳数的烷烃、C4及以上异构体 | 烷烃/烯烃、C4异构体 | 烷烃/烯烃、烯烃/炔烃、C4异构体 | 烷烃/烯烃、C4及以上异构体 | C4及以上异构体 | 不同碳数的烷烃 | 烷烃/烯烃 |
| 优点 | 应用范围广、稳定性好 | 选择性较高 | 选择性高、孔径可微调、易功能化 | 选择性较高、孔径分布均匀 | 稳定性好、选择性高 | 渗透率高 | 制备成本较低、易加工 |
| 缺点 | 吸附量小、孔径难精准控制 | 热稳定性一般、孔径难精准控制 | 制备成本高、难规模化 | 热稳定性一般、易污染 | 渗透率低、易污染 | 选择性低、稳定性一般、易污染 | 选择性一般、易污染 |
| 1 | 孙仁金, 董秀成, 王文澜. 中国石油流通行业发展蓝皮书(2019—2020)[M]. 北京: 中国经济出版社, 2020. |
| SUN Renjin, DONG Xiucheng, WANG Wenlan. Blue book of China oil circulation industry development (2019—2020) [M]. Beijing: Economic Press China, 2020. | |
| 2 | 涂是. 基于筛分效应的超微孔MOFs材料设计合成及其对轻烃的吸附分离性能[D]. 广州: 华南理工大学, 2023. |
| TU Shi. Design and synthesis of ultramicroporous MOFs based on sieving effect and its adsorption and separation performance for light hydrocarbons[D]. Guangzhou: South China University of Technology, 2023. | |
| 3 | ZHOU Yousheng, LI Peicheng, WANG Yifan, et al. Progress in the separation and purification of carbon hydrocarbon compounds using MOFs and molecular sieves[J]. Separations, 2023, 10(10): 543. |
| 4 | 吴厚晓. 几种微孔MOFs的合成及其对C2H4/C2H6和C3H6/C3H8的吸附分离[D]. 广州: 华南理工大学, 2021. |
| WU Houxiao. The synthesis of several micro-porous metal-organic frameworks and their separation performances toward C2H4/C2H 6 and C3H6/C3H8 mixtures[D]. Guangzhou: South China University of Technology, 2021. | |
| 5 | 翟万军. 5A分子筛吸附法分离石脑油中正构烷烃的工艺研究[J]. 化工时刊, 2009, 23(5): 60-63. |
| ZHAI Wanjun. Study on the adsorption process of separating normal paraffin from naphtha by 5A sieves[J]. Chemical Industry Times, 2009, 23(5): 60-63. | |
| 6 | WANG Yongsheng, ZHANG Xuejie, BA Yaqi, et al. Recent advances in carbon-based adsorbents for adsorptive separation of light hydrocarbons[J]. Research, 2022, 2022: 9780864. |
| 7 | AGUADO Sonia, BERGERET Gérard, DANIEL Cecile, et al. Absolute molecular sieve separation of ethylene/ethane mixtures with silver zeolite A[J]. Journal of the American Chemical Society, 2012, 134(36): 14635-14637. |
| 8 | LIN Ruibiao, LI Libo, WU Hui, et al. Optimized separation of acetylene from carbon dioxide and ethylene in a microporous material[J]. Journal of the American Chemical Society, 2017, 139(23): 8022-8028. |
| 9 | LIN Ruibiao, LI Libo, ZHOU Haolong, et al. Molecular sieving of ethylene from ethane using a rigid metal-organic framework[J]. Nature Materials, 2018, 17(12): 1128-1133. |
| 10 | Federico JIMÉNEZ-CRUZ, HERNÁNDEZ J A, LAREDO Georgina C, et al. Adsorption of n-heptane and 2-methylheptane in the gas phase on polyvinylidene chloride-based microporous activated carbon[J]. Energy & Fuels, 2007, 21(5): 2929-2934. |
| 11 | WU Xinmin, CHANG Bor Kae, HSIEH Chieh-Ming. Computational study on the effect of steric hindrance in functionalised Zr-based metal-organic frameworks on hydrocarbon storage and separation[J]. Molecular Simulation, 2021, 47(7): 565-574. |
| 12 | SRIDHAR S, KHAN A A. Simulation studies for the separation of propylene and propane by ethylcellulose membrane[J]. Journal of Membrane Science, 1999, 159(1/2): 209-219. |
| 13 | CHANG Zhiduo, LIN Ruibiao, YE Yingxiang, et al. Construction of a thiourea-based metal-organic framework with open Ag+ sites for the separation of propene/propane mixtures[J]. Journal of Materials Chemistry A, 2019, 7(44): 25567-25572. |
| 14 | WANG Yu, HUANG Ningyu, ZHANG Xuewen, et al. Selective aerobic oxidation of a metal-organic framework boosts thermodynamic and kinetic propylene/propane selectivity[J]. Angewandte Chemie International Edition, 2019, 58(23): 7692-7696. |
| 15 | 赵丽, 何畅, 舒逸聃, 等. 丙烯/丙烷在分子筛上吸附热力学的Monte Carlo模拟[J]. 清华大学学报(自然科学版), 2023, 63(5): 714-722. |
| ZHAO Li, HE Chang, SHU Yidan, et al. Monte Carlo simulation of propylene/propane adsorption thermodynamics on molecular sieves[J]. Journal of Tsinghua University (Science and Technology), 2023, 63(5): 714-722. | |
| 16 | COUCK Sarah, Tom RÉMY, BARON Gino V, et al. A pulse chromatographic study of the adsorption properties of the amino-MIL-53 (Al) metal-organic framework[J]. Physical Chemistry Chemical Physics, 2010, 12(32): 9413-9418. |
| 17 | XU Yinxiang, XU Junbo, YANG Chao. Separation of diverse alkenes from C2—C4 alkanes through nanoporous graphene membranes via local size sieving[J]. Journal of Membrane Science, 2019, 584: 227-235. |
| 18 | LIANG Wanwen, WU Ying, XIAO Huiyu, et al. Ethane-selective carbon composites CPDA@A-ACs with high uptake and its enhanced ethane/ethylene adsorption selectivity[J]. AIChE Journal, 2018, 64(9): 3390-3399. |
| 19 | 邬娇娇, 徐向亚, 刘红梅, 等. 金属有机骨架材料在轻烃分离领域的研究进展[J]. 石油化工, 2022, 51(8): 970-975. |
| WU Jiaojiao, XU Xiangya, LIU Hongmei, et al. Research progress of metal-organic framework materials for separation of light hydrocarbons[J]. Petrochemical Technology, 2022, 51(8): 970-975. | |
| 20 | WANG Yuxiang, Shing Bo PEH, ZHAO Dan. Alternatives to cryogenic distillation: Advanced porous materials in adsorptive light olefin/paraffin separations[J]. Small, 2019, 15(25): 1900058. |
| 21 | YANG Lifeng, QIAN Siheng, WANG Xiaobing, et al. Energy-efficient separation alternatives: Metal-organic frameworks and membranes for hydrocarbon separation[J]. Chemical Society Reviews, 2020, 49(15): 5359-5406. |
| 22 | 朱炜, 李庆, 豆琳琳, 等. 正己烷+1-丁基-3-甲基咪唑硫氰酸盐的分子动力学[J]. 纺织高校基础科学学报, 2020, 33(2): 93-99. |
| ZHU Wei, LI Qing, DOU Linlin, et al. Molecular dynamics of n-hexane+1-butyl-3-methylimidazolium thiocyanate binary system[J]. Basic Sciences Journal of Textile Universities, 2020, 33(2): 93-99. | |
| 23 | SCHOELLNER R, MUELLER U. Influence of mono-and bivalent cations in 4A-zeolites on the adsorptive separation of ethene and propene from crack-gases[J]. Adsorption Science & Technology, 1986, 3(3): 167-171. |
| 24 | DIVEKAR Swapnil, NANOTI Anshu, DASGUPTA Soumen, et al. Adsorption equilibria of propylene and propane on zeolites and prediction of their binary adsorption with the ideal adsorbed solution theory[J]. Journal of Chemical & Engineering Data, 2016, 61(7): 2629-2637. |
| 25 | LIU Junqiang, LIU Yujun, KAYRAK TALAY Defne, et al. A new carbon molecular sieve for propylene/propane separations[J]. Carbon, 2015, 85: 201-211. |
| 26 | GAO Fei, WANG Yaquan, WANG Xiao, et al. Ethylene/ethane separation by CuCl/AC adsorbent prepared using CuCl2 as a precursor[J]. Adsorption, 2016, 22(7): 1013-1022. |
| 27 | 杜胜君. 新型超微孔碳分子筛的制备及其对小分子气体的吸附分离性能[D]. 广州: 华南理工大学, 2023. |
| DU Shengjun. Preparation of novel ultramicroporous carbon molecular sieves and their adsorptive separation performance for small molecule gases[D]. Guangzhou: South China University of Technology, 2023. | |
| 28 | WANG Qingmin, SHEN Dongmin, Martin BÜLOW, et al. Metallo-organic molecular sieve for gas separation and purification[J]. Microporous and Mesoporous Materials, 2002, 55(2): 217-230. |
| 29 | BAO Zongbi, WANG Jiawei, ZHANG Zhiguo, et al. Molecular sieving of ethane from ethylene through the molecular cross-section size differentiation in gallate-based metal-organic frameworks[J]. Angewandte Chemie International Edition, 2018, 57(49): 16020-16025. |
| 30 | YANG Sihai, RAMIREZ-CUESTA Anibal J, NEWBY Ruth, et al. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal-organic framework[J]. Nature Chemistry, 2014, 7(2): 121-129. |
| 31 | SALINAS Octavio, MA Xiaohua, LITWILLER Eric, et al. High-performance carbon molecular sieve membranes for ethylene/ethane separation derived from an intrinsically microporous polyimide[J]. Journal of Membrane Science, 2016, 500: 115-123. |
| 32 | CHU Yu-Han, YANCEY David, XU Liren, et al. Iron-containing carbon molecular sieve membranes for advanced olefin/paraffin separations[J]. Journal of Membrane Science, 2018, 548: 609-620. |
| 33 | WANG Qixiang, HUANG Fei, CORNELIUS Chris J, et al. Carbon molecular sieve membranes derived from crosslinkable polyimides for CO2/CH4 and C2H4/C2H6 separations[J]. Journal of Membrane Science, 2021, 621: 118785. |
| 34 | 徐笑峰, 徐瑞松, 王月, 等. 用于C3H6/C3H8分离的ZIF-8混合基质炭膜[J]. 膜科学与技术, 2023, 43(4): 84-89, 98. |
| XU Xiaofeng, XU Ruisong, WANG Yue, et al. ZIF-8-based mixed matrix carbon membranes for C3H6/C3H8 separation[J]. Membrane Science and Technology, 2023, 43(4): 84-89, 98. | |
| 35 | LIU Yang, CHEN Zhijie, LIU Gongping, et al. Conformation-controlled molecular sieving effects for membrane-based propylene/propane separation[J]. Advanced Materials, 2019, 31(14): 1807513. |
| 36 | YANG Ruihan, GAO Ruomei, QIAN Zhen, et al. Batch and fixed bed column selective adsorption of C6, C8 and C10 linear α-olefins from binary liquid olefin/paraffin mixtures onto 5A and 13X microporous molecular sieves[J]. Separation and Purification Technology, 2020, 230: 115884. |
| 37 | ROMANOVSKY B V, ONISHCHENKO M I, TYABLIKOV I A, et al. Catalytic properties of Pd-containing systems based on SBA-15 molecular sieve modified with imidazolium ionic liquid[J]. Kinetics and Catalysis, 2013, 54(3): 353-357. |
| 38 | 吴庆玲. 基于分子管理的石脑油中不同族烃类分子定向分离研究[D]. 上海: 华东理工大学, 2018. |
| WU Qingling. Studies on the directional separation of hydrocarbon molecules in naphtha based on molecular scale management[D]. Shanghai: East China University of Science and Technology, 2018. | |
| 39 | AGBAJE Taofeeqah A, VEGA Lourdes F, KHALEEL Maryam, et al. Membranes and adsorbents in separation of C4 hydrocarbons: A review and the definition of the current upper bounds[J]. Separation and Purification Technology, 2021, 278: 119530. |
| 40 | 马宝岐, 张秋民. 半焦的利用[M]. 北京: 冶金工业出版社, 2014. |
| MA Baoqi, ZHANG Qiumin. Utilization of semi-coke[M]. Beijing: Metallurgical Industry Press, 2014. | |
| 41 | MA Mingjie, YING Huijuan, CAO Fangfang, et al. Adsorption of congo red on mesoporous activated carbon prepared by CO2 physical activation[J]. Chinese Journal of Chemical Engineering, 2020, 28(4): 1069-1076. |
| 42 | ZHOU Jiazhen, LUO Anran, ZHAO Youcai. Preparation and characterisation of activated carbon from waste tea by physical activation using steam[J]. Journal of the Air & Waste Management Association, 2018, 68(12): 1269-1277. |
| 43 | 梁力友, 鲁德华, 袁英, 等. 基于碳分子筛吸附剂的CH4/N2变压吸附工艺研究[J]. 低碳化学与化工, 2024, 49(6): 115-121, 128. |
| LIANG Liyou, LU Dehua, YUAN Ying, et al. Process research on CH4/N2 pressure swing adsorption based on carbon molecular sieve adsorbents[J]. Low-Carbon Chemistry and Chemical Engineering, 2024, 49(6): 115-121, 128. | |
| 44 | FOLEY Henry C. Carbogenic molecular sieves: Synthesis, properties and applications[J]. Microporous Materials, 1995, 4(6): 407-433. |
| 45 | 高明, 朱元璐, 闫江毅, 等. 金属有机骨架材料在气体分离膜中的应用[J]. 化学通报, 2022, 85(4): 425-431. |
| GAO Ming, ZHU Yuanlu, YAN Jiangyi, et al. Application of metal organic frameworks materials in gas separation membrane[J]. Chemistry, 2022, 85(4): 425-431. | |
| 46 | LEE Jaechul, CHUAH Chong Yang, KIM Jaheon, et al. Separation of acetylene from carbon dioxide and ethylene by a water-stable microporous metal-organic framework with aligned imidazolium groups inside the channels[J]. Angewandte Chemie International Edition, 2018, 57(26): 7869-7873. |
| 47 | SUMIDA Kenji, ROGOW David L, MASON Jarad A, et al. Carbon dioxide capture in metal-organic frameworks[J]. Chemical Reviews, 2012, 112(2): 724-781. |
| 48 | YAGHI Omar M. Reticular chemistry-construction, properties, and precision reactions of frameworks[J]. Journal of the American Chemical Society, 2016, 138(48): 15507-15509. |
| 49 | GUILLERM Vincent, KIM Dongwook, EUBANK Jarrod F, et al. A supermolecular building approach for the design and construction of metal-organic frameworks[J]. Chemical Society Reviews, 2014, 43(16): 6141-6172. |
| 50 | LU Weigang, WEI Zhangwen, GU Zhiyuan, et al. Tuning the structure and function of metal-organic frameworks via linker design[J]. Chemical Society Reviews, 2014, 43(16): 5561-5593. |
| 51 | DENG Hexiang, GRUNDER Sergio, CORDOVA Kyle E, et al. Large-pore apertures in a series of metal-organic frameworks[J]. Science, 2012, 336(6084): 1018-1023. |
| 52 | WEN Huimin, LI Bin, LI Libo, et al. A metal-organic framework with optimized porosity and functional sites for high gravimetric and volumetric methane storage working capacities[J]. Advanced Materials, 2018, 30(16): 1704792. |
| 53 | YAN Yong, YANG Sihai, BLAKE Alexander J, et al. Studies on metal-organic frameworks of Cu(Ⅱ) with isophthalate linkers for hydrogen storage[J]. Accounts of Chemical Research, 2014, 47(2): 296-307. |
| 54 | STOCK Norbert, BISWAS Shyam. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites[J]. Chemical reviews, 2012, 112(3): 933-969. |
| 55 | LI Baiyan, CHRZANOWSKI Matthew, ZHANG Yiming, et al. Applications of metal-organic frameworks featuring multi-functional sites[J]. Coordination Chemistry Reviews, 2016, 307: 106-129. |
| 56 | WEN Huimin, LIAO Caijun, LI Libo, et al. A metal-organic framework with suitable pore size and dual functionalities for highly efficient post-combustion CO2 capture[J]. Journal of Materials Chemistry A, 2019, 7(7): 3128-3134. |
| 57 | WANG Cheng, LIU Demin, LIN Wenbin. Metal-organic frameworks as a tunable platform for designing functional molecular materials[J]. Journal of the American Chemical Society, 2013, 135(36): 13222-13234. |
| 58 | CUI Yuanjing, LI Bin, HE Huajun, et al. Metal-organic frameworks as platforms for functional materials[J]. Accounts of Chemical Research, 2016, 49(3): 483-493. |
| 59 | ZHANG Zhangjing, YAO Zizhu, XIANG Shengchang, et al. Perspective of microporous metal-organic frameworks for CO2 capture and separation[J]. Energy & Environmental Science, 2014, 7(9): 2868-2899. |
| 60 | FURUKAWA Shuhei, REBOUL Julien, DIRING Stéphane, et al. Structuring of metal-organic frameworks at the mesoscopic/macroscopic scale[J]. Chemical Society Reviews, 2014, 43(16): 5700-5734. |
| 61 | EDDAOUDI Mohamed, KIM Jaheon, ROSI Nathaniel, et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage[J]. Science, 2002, 295(5554): 469-472. |
| 62 | BAI Yan, DOU Yibo, XIE Linhua, et al. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications[J]. Chemical Society reviews, 2016, 45(8): 2327-2367. |
| 63 | 李旭飞, 闫保有, 黄维秋, 等. 金属有机骨架及其复合材料基于筛分复合效应的C2分离的研究进展[J]. 化学学报, 2021, 79(4): 459-471. |
| LI Xufei, YAN Baoyou, HUANG Weiqiu, et al. Research progress in metal-organic framework and its composites for separation of C2 based on sieving multiple effects[J]. Acta Chimica Sinica, 2021, 79(4): 459-471. | |
| 64 | 王启祥. 碳分子筛膜的设计制备及气体分离性能评价[D]. 北京: 中国石油大学(北京), 2020. |
| WANG Qixiang. Design and preparation of carbon molecular sieve membranes and gas separation performance evaluation[D]. Beijing: China University of Petroleum (Beijing), 2020. | |
| 65 | FU Shilu, SANDERS Edgar S, KULKARNI Sudhir S, et al. Carbon molecular sieve membrane structure-property relationships for four novel 6FDA based polyimide precursors[J]. Journal of Membrane Science, 2015, 487: 60-73. |
| 66 | RUNGTA Meha, WENZ Graham B, ZHANG Chen, et al. Carbon molecular sieve structure development and membrane performance relationships[J]. Carbon, 2017, 115: 237-248. |
| 67 | HU Chun-Po, POLINTAN Clarisse K, TAYO Lemmuel L, et al. The gas separation performance adjustment of carbon molecular sieve membrane depending on the chain rigidity and free volume characteristic of the polymeric precursor[J]. Carbon, 2019, 143: 343-351. |
| 68 | KIYONO Mayumi, WILLIAMS Paul J, KOROS William J. Effect of pyrolysis atmosphere on separation performance of carbon molecular sieve membranes[J]. Journal of Membrane Science, 2010, 359(1/2): 2-10. |
| 69 | RUNGTA Meha, XU Liren, KOROS William J. Structure-performance characterization for carbon molecular sieve membranes using molecular scale gas probes[J]. Carbon, 2015, 85: 429-442. |
| 70 | XIANG Shengchang, ZHANG Zhangjing, ZHAO Conggui, et al. Rationally tuned micropores within enantiopure metal-organic frameworks for highly selective separation of acetylene and ethylene[J]. Nature Communications, 2011, 2: 204. |
| 71 | WANG Jun, ZHANG Yan, ZHANG Peixin, et al. Optimizing pore space for flexible-robust metal-organic framework to boost trace acetylene removal[J]. Journal of the American Chemical Society, 2020, 142(21): 9744-9751. |
| 72 | LI Libo, LIN Ruibiao, KRISHNA Rajamani, et al. Flexible-robust metal-organic framework for efficient removal of propyne from propylene[J]. Journal of the American Chemical Society, 2017, 139(23):7733-7736. |
| 73 | 刘宇斯, 王红超, 乔晓菲, 等. 5A分子筛吸附剂吸附分离正构烷烃的研究[J]. 石油化工, 2021, 50(6): 534-540. |
| LIU Yusi, WANG Hongchao, QIAO Xiaofei, et al. Study on adsorption and separation of n-alkanes by 5A molecular sieve adsorbent[J]. Petrochemical Technology, 2021, 50(6): 534-540. | |
| 74 | 陈翔, 赵世敏, 刘纪昌, 等. 多级孔道5A分子筛的合成及其对正构烷烃的吸附性能研究[J]. 现代化工, 2016, 36(6): 87-91. |
| CHEN Xiang, ZHAO Shimin, LIU Jichang, et al. Synthesis and adsorption performance of hierarchical 5A zeolite for normal paraffin adsorption[J]. Modern Chemical Industry, 2016, 36(6): 87-91. | |
| 75 | PINNAU Ingo, HE Zhenjie. Pure-and mixed-gas permeation properties of polydimethylsiloxane for hydrocarbon/methane and hydrocarbon/hydrogen separation[J]. Journal of Membrane Science, 2004, 244(1/2): 227-233. |
| 76 | KIM Taek-Joong, BRYANTSEVA I S, BORISEVICH O B, et al. Synthesis and permeability properties of crosslinkable elastomeric poly(vinyl allyl dimethylsilane)s[J]. Journal of Applied Polymer Science, 2005, 96(3): 927-935. |
| 77 | VAVLITIS A P, RUTHVEN D M, LOUGHLIN K F. Sorption of n-pentane, N-octane, and N-decane in 5A zeolite crystals[J]. Journal of Colloid and Interface Science, 1981, 84(2): 526-531. |
| 78 | SLATER Anna G, COOPER Andrew I. Function-led design of new porous materials[J]. Science, 2015, 348(6238): aaa8075. |
| 79 | MINHAS Bhupender S, STAUBS David W. Membrane process for LPG recovery: US7799964[P]. 2010-09-21. |
| 80 | LEE Guo-Shuh J, MCCAIN James H, BHASIN Madan M. Synthetic organic chemicals[M]//Kent and Riegel’s Handbook of Industrial Chemistry and Biotechnology. Boston, MA: Springer US, 2007: 345-403. |
| 81 | RICHTER Manfred, ROOST Udo, LOHSE Ursula. Molecular sieving of n-butenes by microporous silicoaluminophosphates[J]. Journal of the Chemical Society, Chemical Communications, 1993, (21): 1616. |
| 82 | FERREIRA Alexandre F P, MITTELMEIJER-HAZELEGER Marjo C, BLIEK Alfred. Adsorption and differential heats of adsorption of normal and iso-butane on zeolite MFI[J]. Microporous and Mesoporous Materials, 2006, 91(1/2/3): 47-52. |
| 83 | CHEN Zhijie, FENG Liang, LIU Lingmei, et al. Enhanced separation of butane isomers via defect control in a fumarate/zirconium-based metal organic framework[J]. Langmuir, 2018, 34(48): 14546-14551. |
| 84 | CUI Jiyu, ZHANG Zhaoqiang, TAN Bin, et al. Efficient separation of n-butene and iso-butene by flexible ultramicroporous metal-organic frameworks with pocket-like cavities[J]. Chemistry—An Asian Journal, 2019, 14(20): 3572-3576. |
| 85 | ZHANG Han, XIAO Qiang, GUO Xianghai, et al. Open-pore two-dimensional MFI zeolite nanosheets for the fabrication of hydrocarbon-isomer-selective membranes on porous polymer supports[J]. Angewandte Chemie International Edition, 2016, 55(25): 7184-7187. |
| 86 | SUN Kuo, LIU Bo, ZHONG Shenglai, et al. Fast preparation of oriented silicalite-1 membranes by microwave heating for butane isomer separation[J]. Separation and Purification Technology, 2019, 219: 90-99. |
| 87 | 李良清, 李佳佳, 赵孟宇, 等. B-ZSM-5沸石分子筛膜的制备及正/异丁烷分离性能[J]. 化学世界, 2021, 62(1): 59-64. |
| LI Liangqing, LI Jiajia, ZHAO Mengyu, et al. Preparation of B-ZSM-5 membrane for butane isomer separation[J]. Chemical World, 2021, 62(1): 59-64. | |
| 88 | LI Baiyan, BELMABKHOUT Youssef, ZHANG Yiming, et al. From an equilibrium based MOF adsorbent to a kinetic selective carbon molecular sieve for paraffin/iso-paraffin separation[J]. Chemical Communications, 2016, 52(96): 13897-13900. |
| 89 | ZHOU Yingwu, WANG Yuecheng, BAN Yujie, et al. Carbon molecular sieving membranes for butane isomer separation[J]. AIChE Journal, 2019, 65(11): e16749. |
| 90 | LAN Jiancheng, WU Haowen, SAULAT Hammad, et al. Synthesis of ethanol perm-selective MFI zeolite membranes by binary structure directing agents[J]. Journal of Membrane Science, 2020, 598: 117647. |
| 91 | LIU Jichang, SHEN Benxian, SUN Hui. Adsorption behaviour of normal paraffins in a fixed bed adsorber containing 5Å molecular sieves[J]. Adsorption Science & Technology, 2006, 24(4): 311-320. |
| 92 | 田鹏, 王焕, 莫周胜, 等. 甲基戊烷和正己烷在ZSM-5上的吸附与扩散行为研究[J]. 石油化工高等学校学报, 2018, 31(3): 16-21. |
| TIAN Peng, WANG Huan, MO Zhousheng, et al. Adsorption/desorption behavior of methylpentane and n-hexane on ZSM-5 zeolites[J]. Journal of Petrochemical Universities, 2018, 31(3): 16-21. | |
| 93 | 江蕾, 吴庆玲, 程栖桐, 等. 5A和ZSM-5分子筛吸附分离石脑油中烷烃组分的研究[J]. 石油炼制与化工, 2020, 51(3): 6-12. |
| JIANG Lei, WU Qingling, CHENG Qitong, et al. Separation of alkanes from naphtha by coupled adsorption process of 5A and ZSM-5 zeolites[J]. Petroleum Processing and Petrochemicals, 2020, 51(3): 6-12. | |
| 94 | XOMERITAKIS George, LAI Zhiping, TSAPATSIS Michael. Separation of xylene isomer vapors with oriented MFI membranes made by seeded growth[J]. Industrial & Engineering Chemistry Research, 2001, 40(2): 544-552. |
| 95 | DARAMOLA M O, BURGER A J, PERA-TITUS M, et al. Xylene vapor mixture separation in nanocomposite MFI-alumina tubular membranes: Influence of operating variables[J]. Separation Science and Technology, 2009, 45(1): 21-27. |
| 96 | Dong-Yeun KOH, MCCOOL Benjamin A, DECKMAN Harry W, et al. Reverse osmosis molecular differentiation of organic liquids using carbon molecular sieve membranes[J]. Science, 2016, 353(6301): 804-807. |
| 97 | JANG Min-Jun, SEO Hyeokjun, Dong-Yeun KOH. Separation of liquid xylene isomers using thin-film composite carbon molecular sieve hollow fiber membranes[J]. Industrial & Engineering Chemistry Research, 2024, 63(27): 12166-12176. |
| 98 | 姚志龙, 赵毓璋. 正构烷烃吸附分离技术进展[J]. 化工进展, 2003, 22(6):589-592. |
| YAO Zhilong, ZHAO Yuzhang. Recent development of normal paraffin adsorption-separation technology[J]. Chemical Industry and Engineering Progress, 2003, 22(6):589-592. | |
| 99 | 刘纪昌, 沈本贤. 正构烷烃含量对裂解烯烃收率的影响及乙烯裂解的原料调配[J]. 华东理工大学学报(自然科学版), 2006, 32(5): 535-539. |
| LIU Jichang, SHEN Benxian. Effect of normal paraffin content on ethylene yield and feedstock allocation of ethylene pyrolysis process[J]. Journal of East China University of Science and Technology(Natural Science Edition), 2006, 32(5): 535-539. | |
| 100 | 刘纪昌, 沈本贤. 吸附富集裂解原料中的正构烷烃蒸汽裂解制乙烯效果研究[J]. 石油炼制与化工, 2010, 41(11): 57-60. |
| LIU Jichang, SHEN Benxian. Steam cracking performance of feedstocks rich in normal paraffins gathered by adsorption process[J]. Petroleum Processing and Petrochemicals, 2010, 41(11): 57-60. | |
| 101 | 徐向荣. 1.2Mt/a石脑油吸附分离装置运行状况分析[J]. 石油炼制与化工, 2019, 50(5): 33-38. |
| XU Xiangrong. Operation analysis of 1.2Mt/a maxene unit[J]. Petroleum Processing and Petrochemicals, 2019, 50(5): 33-38. | |
| 102 | KOCAL Joseph A, VORA Bipin V, IMAI Tamotsu. Production of linear alkylbenzenes[J]. Applied Catalysis A: General, 2001, 221(1/2): 295-301. |
| 103 | SHOKRI Aref, KARIMI Safoora. A review in linear alkylbenzene (LAB) production processes in the petrochemical industry[J]. Russian Journal of Applied Chemistry, 2021, 94(11): 1546-1559. |
| 104 | 曹昕, 刘纪昌, 沈本贤, 等. 固定床双塔并联吸附分离石脑油中正构烷烃[J]. 石油炼制与化工, 2013, 44(4): 45-50. |
| CAO Xin, LIU Jichang, SHEN Benxian, et al. Adsorption of normal paraffins from naphtha using 5A molecular sieves in a double-column fixed-bed[J]. Petroleum Processing and Petrochemicals, 2013, 44(4): 45-50. | |
| 105 | 杨建锋. 正构烷烃吸附剂的合成与评价研究[D]. 青岛: 中国石油大学(华东), 2013. |
| YANG Jianfeng. Synthesis and evaluation of adsorbent for normal paraffins[D]. Qingdao: China University of Petroleum (East China), 2013. | |
| 106 | 刘军涛, 倪腾亚, 刘纪昌, 等. 基于分子管理的模拟移动床吸附分离石脑油中的正构烷烃[J]. 石油炼制与化工, 2014, 45(8): 71-76. |
| LIU Juntao, NI Tengya, LIU Jichang, et al. Adsorption separation of normal paraffins from naphtha by simulated moving bed based on molecule-scale management[J]. Petroleum Processing and Petrochemicals, 2014, 45(8): 71-76. | |
| 107 | 邵成勋. 分子筛吸附分离直馏汽油馏份正构烷烃[J]. 石油与天然气化工, 1991, 20(3): 1-6. |
| SHAO Chengxun. Separation of n-alkanes from straight-run gasoline fractions by molecular sieve adsorption[J]. Chemical Engineering of Oil and Gas, 1991, 20(3): 1-6. | |
| 108 | Pei Shi TIN, LIN Huey Yi, Rui Chin ONG, et al. Carbon molecular sieve membranes for biofuel separation[J]. Carbon, 2011, 49(2): 369-375. |
| [1] | 顾晟燊, 郭猛, 任秀秀, 潘阳, 靳栋梁, 钟璟. 微孔有机硅膜在CO2分离中的研究进展[J]. 化工进展, 2025, 44(5): 2846-2855. |
| [2] | 金少青, 范雪研, 唐智谋, 王衍力, 王达锐, 孙洪敏, 杨为民. 基于钛硅分子筛催化的绿色氧化技术进展[J]. 化工进展, 2025, 44(5): 2907-2918. |
| [3] | 陈彦君, 戴杰, 单军强, 张思欣, 计磊, 朱晨杰, 应汉杰. 我国纤维素乙醇的研究进展和发展趋势[J]. 化工进展, 2025, 44(5): 2541-2562. |
| [4] | 丁阿静, 周巧巧, 顾学红. 膜反应器中杨木催化气化制清洁合成气[J]. 化工进展, 2025, 44(5): 2716-2723. |
| [5] | 郑业, 何永清, 焦凤, 李欢. 倾斜壁面上液膜的流动不稳定性与烧干机制探究[J]. 化工进展, 2025, 44(4): 1876-1887. |
| [6] | 丁红兵, 柴旭天, 王世伟, 宋鑫宇, 孙宏军. 单液滴与多液滴撞击流动液膜的实验探究[J]. 化工进展, 2025, 44(4): 1888-1897. |
| [7] | 蔡瑞芸, 焦芮, 孙寒雪, 李安. 不对称浸润性Janus有机多孔膜的设计、制备及应用[J]. 化工进展, 2025, 44(4): 2057-2067. |
| [8] | 朱方龙, 冯倩倩. 红外透过型尼龙多孔膜的制备及光热性能[J]. 化工进展, 2025, 44(4): 2215-2224. |
| [9] | 薛立新, 董永平, 陈梦瑶, 高从堦. 十二烷基硫酸钠(SDS)和强碱(NaOH)对聚酰胺复合纳滤膜的协同调控机理[J]. 化工进展, 2025, 44(4): 2225-2237. |
| [10] | 王沛淦, 李乐利, 谢颂专, 宋冰冰, 孔巧平, 刘改革, 麻微微, 施雪卿. 污泥基FeCa-ALE复合材料对磷酸盐的吸附机制[J]. 化工进展, 2025, 44(4): 2365-2373. |
| [11] | 仇玉静, 刘畅, 罗国华, 董森, 李建华. 脱除苯中二硫化碳吸附剂的制备及其吸附性能[J]. 化工进展, 2025, 44(4): 2374-2382. |
| [12] | 何小龙, 康玉堂, 邹栋, 仲兆祥. 静电纺丝纳米纤维膜呼吸防护口罩研究进展[J]. 化工进展, 2025, 44(3): 1496-1504. |
| [13] | 陶金泉, 贾亦静, 白天瑜, 姚荣鹏, 黄文斌, 崔岩, 周亚松, 魏强. Silicalite-1分子筛的低成本合成及其MTP催化性能[J]. 化工进展, 2025, 44(3): 1550-1558. |
| [14] | 王文, 金央, 李军, 陈建钧, 陈明, 孟昕. 基于FeOOH原位生长制备用于CO2吸收的超疏水PVDF膜[J]. 化工进展, 2025, 44(3): 1570-1577. |
| [15] | 张伊, 么秋香, 孙鸣. 天然斜发沸石基方沸石及其改性对Pb2+的吸附性能[J]. 化工进展, 2025, 44(3): 1726-1738. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||