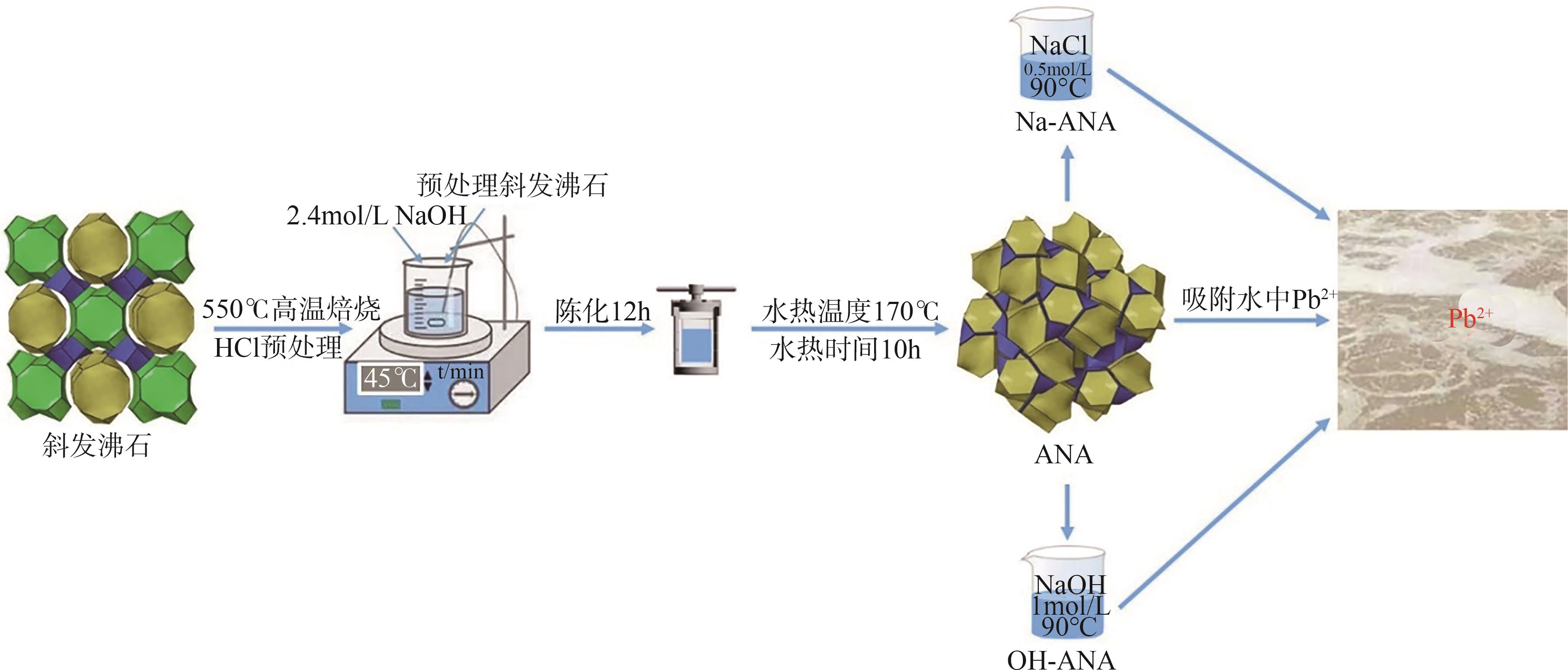
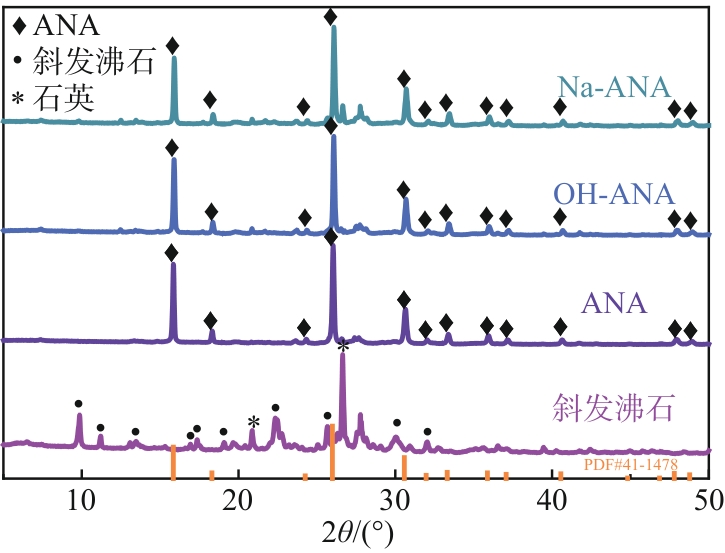
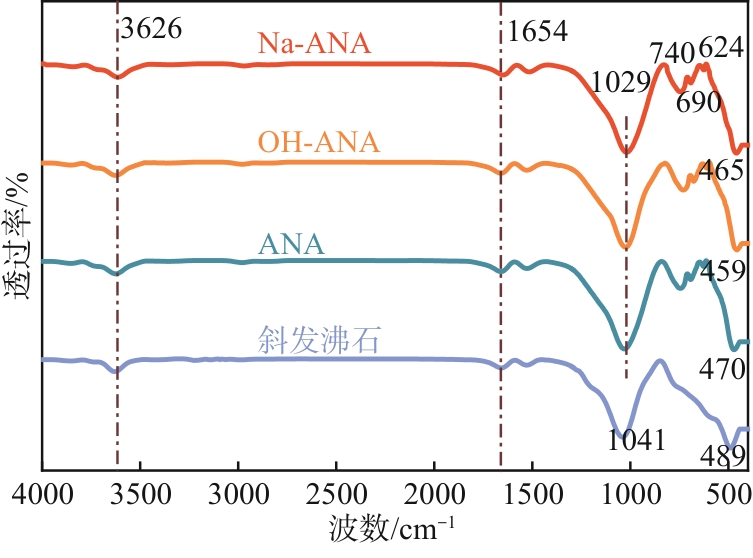
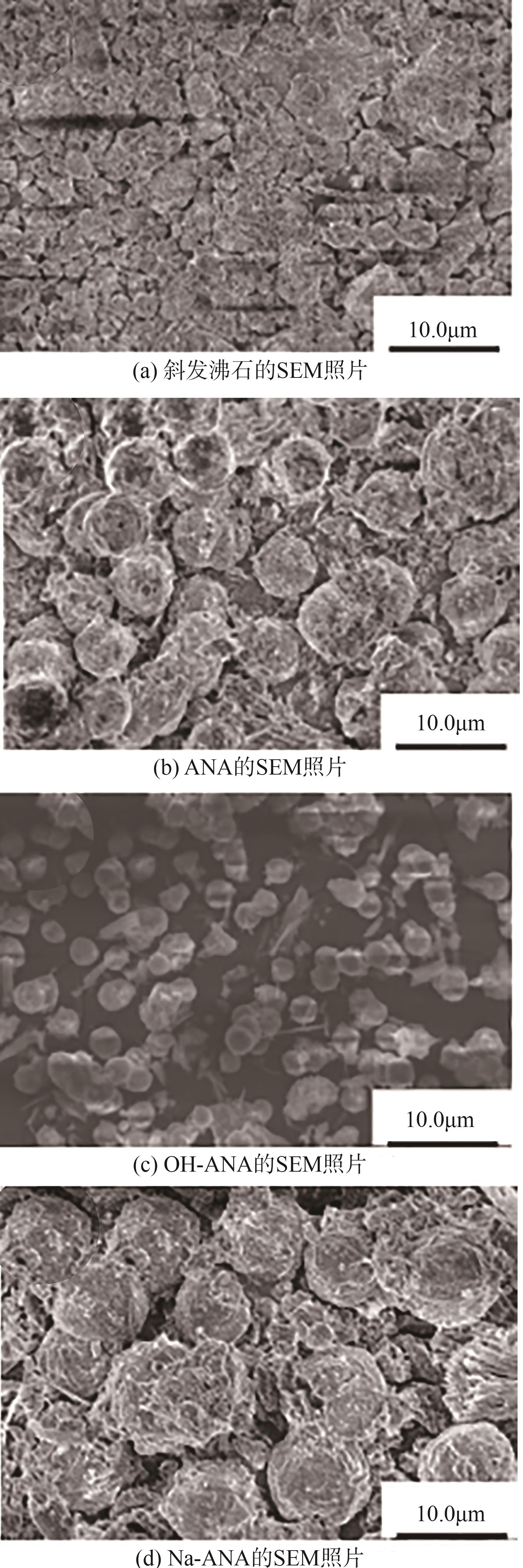
| 1 |
ZHAI Shimin, LI Min, PENG Hongyun, et al. Cost-effective resource utilization for waste biomass: A simple preparation method of photo-thermal biochar cakes (BCs) toward dye wastewater treatment with solar energy[J]. Environmental Research, 2021, 194: 110720.
|
| 2 |
DENG Rui, HUANG Danlian, ZENG Guangming, et al. Decontamination of lead and tetracycline from aqueous solution by a promising carbonaceous nanocomposite: Interaction and mechanisms insight[J]. Bioresource Technology, 2019, 283: 277-285.
|
| 3 |
ROSHANFEKR RAD Leila, ANBIA Mansoor. Zeolite-based composites for the adsorption of toxic matters from water: A review[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106088.
|
| 4 |
王路星, 周新涛, 罗中秋, 等. 农林废弃物吸附废水中重金属Pb2+的性能及机理研究进展[J]. 材料导报, 2020, 34(17): 17115-17123.
|
|
WANG Luxing, ZHOU Xintao, LUO Zhongqiu, et al. Research progress of the properties and mechanism of heavy metal Pb2+ absorbed by agricultural and forestry waste in wastewater[J]. Materials Reports, 2020, 34(17): 17115-17123.
|
| 5 |
路广军, 韩晋钢, 陈英, 等. 镁渣基多孔材料的制备及其对废水中Pb2+的吸附性能[J]. 化工进展, 2024, 43(4): 2126-2134.
|
|
LU Guangjun, HAN Jingang, CHEN Ying, et al. Preparation of magnesium slag-based porous materials and their performance for Pb2+ adsorption in wastewater[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2126-2134.
|
| 6 |
陆艳, 罗中秋, 周新涛, 等. 铜渣铁基类沸石地质聚合物吸附Pb2+、Cu2+、Zn2+性能及机理[J]. 精细化工, 2023, 40(12): 2739-2751.
|
|
LU Yan, LUO Zhongqiu, ZHOU Xintao, et al. Adsorption properties and mechanism of Pb2+, Cu2+ and Zn2+ by iron base zeolite-like geopolymer derived from copper slag[J]. Fine Chemicals, 2023, 40(12): 2739-2751.
|
| 7 |
KHAKSARFARD Yasaman, BAGHERI Ahmad, RAFATI Amir Abbas. Synergistic effects of binary surfactant mixtures in the adsorption of diclofenac sodium drug from aqueous solution by modified zeolite[J]. Journal of Colloid and Interface Science, 2023, 644: 186-199.
|
| 8 |
宋学锋, 陆伟宁. 转化方式对粉煤灰地聚物原位转化沸石及其Pb2+吸附性能的影响[J]. 材料导报, 2023, 37(6): 236-242.
|
|
SONG Xuefeng, LU Weining. Influence of conversion method on in-situ conversion of fly ash geopolymer to zeolite and its Pb2+ adsorption performance[J]. Materials Reports, 2023, 37(6): 236-242.
|
| 9 |
ERDEM E, KARAPINAR N, DONAT R. The removal of heavy metal cations by natural zeolites[J]. Journal of Colloid and Interface Science, 2004, 280(2): 309-314.
|
| 10 |
NAKHAEI Mohammad, MOKHTARI Hamid Reza, VATANPOUR Vahid, et al. Investigating the effectiveness of natural zeolite (clinoptilolite) for the removal of lead, cadmium, and cobalt heavy metals in the western parts of Iran’s varamin aquifer[J]. Water, Air, & Soil Pollution, 2023, 234(12): 746.
|
| 11 |
WANG Cheng, YU Jiale, FENG Kai, et al. Alkali treatment to transform natural clinoptilolite into zeolite Na-P: Influence of NaOH concentration[J]. Journal of Physics and Chemistry of Solids, 2022, 168: 110827.
|
| 12 |
HEGAZY Eman Z, EL MAKSOD Islam Hamdy Abd, ENIN RMM Abo EL. Preparation and characterization of Ti and V modified analcime from local Kaolin[J]. Applied Clay Science, 2010, 49(3): 149-155.
|
| 13 |
GALINDO VALBUENA Hugo Martín, MEDINA Andrés F, VARGAS Julio C, et al. Synthesis of zeolites Na-A, Na-X, and analcime from crushed stone waste and their applications in heavy metal removal in aqueous media[J]. Chemical Engineering Research and Design, 2023, 197: 159-172.
|
| 14 |
WANG Shiyao, TIAN Ren, HE Bo, et al. The success of dual-functional templating for synthesizing hierarchical analcime zeolite[J]. Applied Organometallic Chemistry, 2019, 33(4): e4711.
|
| 15 |
REVELLAME Emmanuel D, FORTELA Dhan Lord, SHARP Wayne, et al. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review[J]. Cleaner Engineering and Technology, 2020, 1: 100032.
|
| 16 |
Bo LYU, DENG Xiaowei, JIAO Feishuo, et al. Removal of Pb2+ in aqueous solutions using Na-type zeolite synthesized from coal gasification slag in a fluidized bed: Hydrodynamic and adsorption[J]. Process Safety and Environmental Protection, 2023, 174: 869-881.
|
| 17 |
INCE Olcay Kaplan, INCE Muharrem, YONTEN Vahap, et al. A food waste utilization study for removing lead(Ⅱ) from drinks[J]. Food Chemistry, 2017, 214: 637-643.
|
| 18 |
陈作义, 陈考昌, 李华辉, 等. CA/COSBC复合凝胶微球的制备及其对Pb(Ⅱ)的吸附性能[J/OL]. 精细化工,2024(11):109-118.
|
|
CHEN Zuoyi, CHEN Kaochang, LI Huahui, et al. Preparation of Pb(Ⅱ) adsorption performance of CA/COSBC composite gel microspheres[J/OL]. Fine Chemicals,2024(11):109-118.
|
| 19 |
Yunier GARCIA-BASABE, Inocente RODRIGUEZ-IZNAGA, DE MENORVAL Louis-Charles, et al. Step-wise dealumination of natural clinoptilolite: Structural and physicochemical characterization[J]. Microporous and Mesoporous Materials, 2010, 135(1/2/3): 187-196.
|
| 20 |
NOVEMBRE Daniela, GIMENO Domingo. Synthesis and characterization of analcime (ANA) zeolite using a kaolinitic rock[J]. Scientific Reports, 2021, 11(1): 13373.
|
| 21 |
WANG Jin, WU Xiuling, WANG Junxia, et al. Hydrothermal synthesis and characterization of alkali-activated slag-fly ash-metakaolin cementitious materials[J]. Microporous and Mesoporous Materials, 2012, 155: 186-191.
|
| 22 |
仉铭坤, 杨红薇, 杜明阳, 等. 改性沸石对二级生化出水中氨氮的吸附特性[J]. 环境工程学报, 2020, 14(4): 896-905.
|
|
ZHANG Mingkun, YANG Hongwei, DU Mingyang, et al. Adsorption characteristics of ammonia nitrogen in secondary effluent on modified zeolite[J]. Chinese Journal of Environmental Engineering, 2020, 14(4): 896-905.
|
| 23 |
BAYRAKDAR ATES Ezgi. Exploring the impact of NaOH pre-treatment for H2 and CO2 adsorption on clinoptilolite[J]. International Journal of Hydrogen Energy, 2024, 50: 990-1003.
|
| 24 |
NGUYEN M L, TANNER C C. Ammonium removal from wastewaters using natural New Zealand zeolites[J]. New Zealand Journal of Agricultural Research, 1998, 41(3): 427-446.
|
| 25 |
ISAWI Heba. Using Zeolite/Polyvinyl alcohol/sodium alginate nanocomposite beads for removal of some heavy metals from wastewater[J]. Arabian Journal of Chemistry, 2020, 13(6): 5691-5716.
|
| 26 |
ZENDELSKA Afrodita, GOLOMEOVA Mirjana, GOLOMEOV Blagoj, et al. Removal of lead ions from acid aqueous solutions and acid mine drainage using zeolite bearing tuff[J]. Archives of Environmental Protection, 2018: 40(1): 87-96.
|
| 27 |
NGUYEN Thuy Chung, LOGANATHAN Paripurnanda, NGUYEN Tien Vinh, et al. Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies[J]. Chemical Engineering Journal, 2015, 270: 393-404.
|
| 28 |
ABDELRAHMAN Ehab A, ALHARBI Ahmed, SUBAIHI Abdu, et al. Facile fabrication of novel analcime/sodium aluminum silicate hydrate and zeolite Y/faujasite mesoporous nanocomposites for efficient removal of Cu(Ⅱ) and Pb(Ⅱ) ions from aqueous media[J]. Journal of Materials Research and Technology, 2020, 9(4): 7900-7914.
|
| 29 |
WANG Xiangxue, SHAO Dadong, HOU Guangshun, et al. Uptake of Pb(Ⅱ) and U(Ⅵ) ions from aqueous solutions by the ZSM-5 zeolite[J]. Journal of Molecular Liquids, 2015, 207: 338-342.
|
| 30 |
HAMOUDI Souhila Ait, KHELIFA Nedjma, NOURI Loubna, et al. Removal of Pb2+ and Cd2+ by adsorption onto Y zeolite and its selectivity of retention in an actual contaminated effluent[J]. Colloid and Polymer Science, 2023, 301(6): 631-645.
|
| 31 |
WANGI G M, OLUPOT P W, BYARUHANGA J, et al. Characterization of natural zeolite and determination of its ion-exchange potential for selected metal ions in water[J]. Environmental Processes, 2023, 10(4): 53.
|
| 32 |
MAYTA-ARMAS Angie F, Yamerson CANCHANYA-HUAMAN, Jemina POMALAYA-VELASCO, et al. Enhanced removal of As(Ⅴ) and Pb(Ⅱ) from drinking and irrigating water effluents using hydrothermally synthesized zeolite 5A[J]. Water, 2023, 15(10): 1892.
|
| 33 |
ZHANG Yunhui, ALESSI Daniel S, CHEN Ning, et al. Spectroscopic and modeling investigation of sorption of Pb(Ⅱ) to ZSM-5 zeolites[J]. ACS ES&T Water, 2021, 1(1): 108-116.
|
| 34 |
董颖博, 林海, 刘泉利. 化学改性对沸石去除水中碳、氮污染物的影响[J]. 四川大学学报(工程科学版), 2015, 47(3): 193-199.
|
|
DONG Yingbo, LIN Hai, LIU Quanli. Effects of chemical modification on removal of pollutants with carbon and nitrogen from aqueous solution by zeolite[J]. Journal of Sichuan University (Engineering Science Edition), 2015, 47(3): 193-199.
|
| 35 |
杨岳, 吴涛涛, 王闰民, 等. 沸石改性及对水中氨氮的吸附性能研究[J]. 环境与发展, 2020, 32(9): 118-120.
|
|
YANG Yue, WU Taotao, WANG Runmin, et al. Research on adsorption of ammonia nitrogen in water with modified zeolite[J]. Environment and Development, 2020, 32(9): 118-120.
|
| 36 |
郭宇, 佟民心, 吴红梅. 氨基功能化双醛淀粉吸附剂的制备及其对Pb(Ⅱ)的吸附行为[J]. 化工进展, 2023, 42(12): 6589-6599.
|
|
GUO Yu, TONG Minxin, WU Hongmei. Preparation of amino-functionalized dialdehyde starch adsorbent for adsorption of Pb(Ⅱ) ions[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6589-6599.
|
| 37 |
LV Yingwei, MA Baozhong, LIU Yubo, et al. Adsorption behavior and mechanism of mixed heavy metal ions by zeolite adsorbent prepared from lithium leach residue[J]. Microporous and Mesoporous Materials, 2022, 329: 111553.
|
| 38 |
PENG Jun, ZHANG Ziyue, WANG Ziwei, et al. Adsorption of Pb2+ in solution by phosphate-solubilizing microbially modified biochar loaded with Fe3O4 [J]. Journal of the Taiwan Institute of Chemical Engineers, 2024, 156: 105363.
|
| 39 |
吴红梅, 徐靖雯, 郭宇, 等. 席夫碱改性MCM-41分子筛的制备及其对铅离子的吸附性能[J]. 分析化学, 2024, 52(1): 102-112.
|
|
WU Hongmei, XU Jingwen, GUO Yu, et al. Characterization of schiff base modified MCM-41 molecular sieve for adsorption of lead ions from aqueous solution[J]. Chinese Journal of Analytical Chemistry, 2024, 52(1): 102-112.
|
| 40 |
KIM Gyuri, Yeonji YEA, NJARAMBA Lewis Kamande, et al. Synthesis, performance, and mechanisms of strontium ferrite-incorporated zeolite imidazole framework (ZIF-8) for the simultaneous removal of Pb(Ⅱ) and tetracycline[J]. Environmental Research, 2022, 212(Pt C): 113419.
|
| 41 |
LI Jing, LIN Guo, ZHONG Zhen, et al. A novel magnetic Ti-MOF/chitosan composite for efficient adsorption of Pb(Ⅱ) from aqueous solutions: Synthesis and investigation[J]. International Journal of Biological Macromolecules, 2024, 258: 129170.
|
| 42 |
田甜, 付义乐, 关丽, 等. 海藻酸钠-羧甲基纤维素-氧化石墨烯复合气凝胶的制备及其对Pb(Ⅱ)的吸附[J]. 复合材料学报, 2023, 40(10): 5792-5802.
|
|
TIAN Tian, FU Yile, GUAN Li, et al. Preparation of sodium alginate-carboxymethyl cellulose-graphene oxide composite aerogel for adsorption of Pb (Ⅱ) ion[J]. Acta Materiae Compositae Sinica, 2023, 40(10): 5792-5802.
|
| 43 |
HUANG Yongxiang, LUO Xiangping, LIU Chongmin, et al. Effective adsorption of Pb(Ⅱ) from wastewater using MnO2 loaded MgFe-LD(H)O composites: Adsorption behavior and mechanism[J]. RSC Advances, 2023, 13(28): 19288-19300.
|
| 44 |
LI Jing, HU Zehua, CHEN Yilin, et al. Removal of Pb(II) by adsorption of HCO-(Fe3O4) x composite adsorbent: Efficacy and mechanism[J]. Water, 2023, 15(10): 1857.
|
| 45 |
ZOU Yilong. Cu2+, Cd2+, and Pb2+ ions adsorption from wastewater using polysaccharide hydrogels made of oxidized carboxymethyl cellulose and chitosan grafted with catechol groups[J]. Iranian Polymer Journal, 2024, 33(1): 57-66.
|
| 46 |
AN Wenbo, LIU Yifan, CHEN He, et al. Oyster shell-modified lignite composite in globular shape as a low-cost adsorbent for the removal of Pb2+ and Cd2+ from AMD: Evaluation of adsorption properties and exploration of potential mechanisms[J]. Arabian Journal of Chemistry, 2024, 17(5): 105732.
|
 ), YAO Qiuxiang2, SUN Ming1(
), YAO Qiuxiang2, SUN Ming1( )
)