化工进展 ›› 2025, Vol. 44 ›› Issue (5): 2407-2420.DOI: 10.16085/j.issn.1000-6613.2024-2127
• 合成生物制造 • 上一篇
靶向治疗蛋白与肽的工程化设计优化及应用进展
江奇逸( ), 邓鑫月, 袁艳婷, 张雅倩, 杨敏, 李伟娜(
), 邓鑫月, 袁艳婷, 张雅倩, 杨敏, 李伟娜( ), 范代娣(
), 范代娣( )
)
- 西北大学化工学院,陕西 西安 710127
-
收稿日期:2024-12-31修回日期:2025-03-31出版日期:2025-05-25发布日期:2025-05-20 -
通讯作者:李伟娜,范代娣 -
作者简介:江奇逸(2002—),女,硕士研究生,研究方向为合成生物学。E-mail:j625982@163.com。 -
基金资助:国家自然科学基金(22378330)
Advances in engineering design, optimization and application of targeted therapeutic proteins and peptides
JIANG Qiyi( ), DENG Xinyue, YUAN Yanting, ZHANG Yaqian, YANG Min, LI Weina(
), DENG Xinyue, YUAN Yanting, ZHANG Yaqian, YANG Min, LI Weina( ), FAN Daidi(
), FAN Daidi( )
)
- College of Chemical Engineering, Northwest University, Xi’an 710127, Shaanxi, China
-
Received:2024-12-31Revised:2025-03-31Online:2025-05-25Published:2025-05-20 -
Contact:LI Weina, FAN Daidi
摘要:
靶向性治疗蛋白和肽在精准医学中发挥着越来越重要的作用,尤其在癌症、免疫性疾病等治疗领域,展示了显著的潜力。与传统的小分子药物相比,靶向性蛋白和肽具有更高的特异性和较低的副作用,能够精准靶向疾病相关的分子。然而,它们的临床应用仍然面临一系列挑战,如稳定性差、免疫原性高、药代动力学差等问题。为了克服这些挑战,工程化设计优化已经成为提升其药效、安全性和临床应用前景的关键。本文首先介绍了靶向性治疗蛋白和肽的工程化设计的步骤;简述了肽类药物、蛋白质药物的优化设计方法;探索了相关药物递送策略,列举部分药物实例;分析了靶向性治疗蛋白和肽的临床应用现状、面临的挑战和发展前景;最后指出随着技术的不断发展,靶向性治疗蛋白和肽将在精准医学中发挥更重要的作用,为多种复杂疾病提供更高效的治疗方案。
中图分类号:
引用本文
江奇逸, 邓鑫月, 袁艳婷, 张雅倩, 杨敏, 李伟娜, 范代娣. 靶向治疗蛋白与肽的工程化设计优化及应用进展[J]. 化工进展, 2025, 44(5): 2407-2420.
JIANG Qiyi, DENG Xinyue, YUAN Yanting, ZHANG Yaqian, YANG Min, LI Weina, FAN Daidi. Advances in engineering design, optimization and application of targeted therapeutic proteins and peptides[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2407-2420.
| 靶标 | 相关疾病 | 靶向药物名称 | 作用机制 | 参考文献 |
|---|---|---|---|---|
| HER2受体 | HER2阳性乳腺癌、胃癌 | 赫赛汀(曲妥珠单抗) | 阻断HER2受体,抑制肿瘤细胞增殖并促进免疫系统攻击肿瘤细胞 | [ |
| PD-1/PD-L1受体 | 黑色素瘤、非小细胞肺癌、肾癌 | 帕博利珠单抗、纳武单抗 | 解除免疫抑制,增强T细胞抗肿瘤活性 | [ |
| VEGF受体 | 结直肠癌、肾癌、湿性黄斑变性 | 贝伐珠单抗 | 阻断VEGF,抑制肿瘤血管生成 | [ |
| TNF-α | 类风湿性关节炎、银屑病、克罗恩病 | 阿达木单抗、依那西普 | 阻断TNF-α,减轻免疫反应和炎症 | [ |
| CD20 | B细胞淋巴瘤、慢性淋巴细胞白血病(CLL) | 利妥昔单抗 | 与CD20结合,激活免疫系统清除肿瘤细胞 | [ |
| EGFR | 非小细胞肺癌、头颈癌、结直 肠癌 | 西妥昔单抗、吉非替尼 | 阻断EGFR受体,抑制肿瘤细胞增殖与侵袭 | [ |
| CD19 | 急性淋巴细胞白血病(ALL)、大B细胞淋巴瘤 | 替萨根微核素、axi-cel | 通过基因改造T细胞,识别并攻击CD19阳性肿瘤细胞 | [ |
| C5补体成分 | 阵发性睡眠性血红蛋白尿症(PNH) | 依库珠单抗 | 抑制C5,减少补体系统对红细胞的攻击,防止溶血 | [ |
表1 常见靶标相关疾病及药物实例
| 靶标 | 相关疾病 | 靶向药物名称 | 作用机制 | 参考文献 |
|---|---|---|---|---|
| HER2受体 | HER2阳性乳腺癌、胃癌 | 赫赛汀(曲妥珠单抗) | 阻断HER2受体,抑制肿瘤细胞增殖并促进免疫系统攻击肿瘤细胞 | [ |
| PD-1/PD-L1受体 | 黑色素瘤、非小细胞肺癌、肾癌 | 帕博利珠单抗、纳武单抗 | 解除免疫抑制,增强T细胞抗肿瘤活性 | [ |
| VEGF受体 | 结直肠癌、肾癌、湿性黄斑变性 | 贝伐珠单抗 | 阻断VEGF,抑制肿瘤血管生成 | [ |
| TNF-α | 类风湿性关节炎、银屑病、克罗恩病 | 阿达木单抗、依那西普 | 阻断TNF-α,减轻免疫反应和炎症 | [ |
| CD20 | B细胞淋巴瘤、慢性淋巴细胞白血病(CLL) | 利妥昔单抗 | 与CD20结合,激活免疫系统清除肿瘤细胞 | [ |
| EGFR | 非小细胞肺癌、头颈癌、结直 肠癌 | 西妥昔单抗、吉非替尼 | 阻断EGFR受体,抑制肿瘤细胞增殖与侵袭 | [ |
| CD19 | 急性淋巴细胞白血病(ALL)、大B细胞淋巴瘤 | 替萨根微核素、axi-cel | 通过基因改造T细胞,识别并攻击CD19阳性肿瘤细胞 | [ |
| C5补体成分 | 阵发性睡眠性血红蛋白尿症(PNH) | 依库珠单抗 | 抑制C5,减少补体系统对红细胞的攻击,防止溶血 | [ |
| 合成方法 | 概述 | 优点 | 局限性 | 参考文献 |
|---|---|---|---|---|
| 固相合成法(SPPS) | 在固相载体上逐步合成肽链,通过肽键连接氨基酸 | 高效,自动化程度高,适用于短肽合成,获得高纯度肽 | 对于长肽或大规模生产成本高,可能出现肽链折叠问题 | [ |
| 液相合成法(LPPS) | 在溶液中进行肽合成,通过酰胺化反应形成肽链 | 适合特殊反应和大规模合成 | 合成周期长,纯化难度较大 | [ |
| 重组DNA技术 | 通过将目标蛋白或肽的基因导入宿主细胞进行表达,生成目的蛋白 | 能够高效表达大量蛋白,适合复杂蛋白和大规模生产 | 对于某些复杂蛋白或肽,可能需要特殊的后期修饰(如糖基化) | [ |
| 化学合成与后期修饰 | 在化学合成的基础上进行后期修饰(如PEG化、脂化等),提高药物活性和稳定性 | 改善肽或蛋白的稳定性、溶解度和免疫原性 | 后期修饰增加合成难度和成本 | [ |
表2 靶向性治疗蛋白和肽的合成方法
| 合成方法 | 概述 | 优点 | 局限性 | 参考文献 |
|---|---|---|---|---|
| 固相合成法(SPPS) | 在固相载体上逐步合成肽链,通过肽键连接氨基酸 | 高效,自动化程度高,适用于短肽合成,获得高纯度肽 | 对于长肽或大规模生产成本高,可能出现肽链折叠问题 | [ |
| 液相合成法(LPPS) | 在溶液中进行肽合成,通过酰胺化反应形成肽链 | 适合特殊反应和大规模合成 | 合成周期长,纯化难度较大 | [ |
| 重组DNA技术 | 通过将目标蛋白或肽的基因导入宿主细胞进行表达,生成目的蛋白 | 能够高效表达大量蛋白,适合复杂蛋白和大规模生产 | 对于某些复杂蛋白或肽,可能需要特殊的后期修饰(如糖基化) | [ |
| 化学合成与后期修饰 | 在化学合成的基础上进行后期修饰(如PEG化、脂化等),提高药物活性和稳定性 | 改善肽或蛋白的稳定性、溶解度和免疫原性 | 后期修饰增加合成难度和成本 | [ |
| 药物类别 | 融合蛋白功能域 | 作用机制 | 应用领域 | 融合药物及应用疾病 | 参考文献 |
|---|---|---|---|---|---|
| Fc融合蛋白 | Fc段(人类IgG Fc) | 延长半衰期,增加稳定性,增强免疫系统识别和靶向性 | 疫苗、免疫治疗、抗体药物偶联物 | 依那西普:融合TNF-α受体与Fc段,用于治疗类风湿性关节炎 | [ |
| 抗体药物偶联物(ADCs) | Fc段与细胞毒性药物结合 | 精准递送细胞毒性药物到靶细胞,减少对正常细胞的影响 | 癌症治疗 | 曲妥珠单抗:HER2靶向药物,用于HER2阳性乳腺癌治疗 | [ |
| 免疫检查点抑制剂融合蛋白 | Fc段与免疫抑制因子结合 | 解除免疫抑制,增强T细胞抗肿瘤活性 | 癌症免疫治疗 | 阿特朱单抗:结合PD-L1抗体与Fc段,用于治疗非小细胞肺癌和其他癌症 | [ |
| 抗体-酶融合蛋白 | Fc段与酶(如酶活性蛋白) | 通过Fc段增加酶的稳定性和靶向递送,提升治疗效果 | 代谢性疾病、癌症治疗 | α葡糖糖苷酶:用于治疗庞贝病的酶替代疗法 | [ |
| 细胞因子融合蛋白 | Fc段与细胞因子(如IL-2、GM-CSF等) | 提高细胞因子的稳定性,增强免疫反应和细胞活性 | 免疫治疗、癌症、炎症性疾病 | 非格司亭:与Fc融合的G-CSF,用于治疗化疗引起的白细胞减少症 | [ |
| 抗体-抗体融合 蛋白 | Fc段与另一个抗体(如抗肿瘤抗体) | 通过增强免疫系统的联合作用,提高对肿瘤细胞的免疫反应 | 癌症治疗、免疫疗法 | 博纳吐单抗:融合抗CD19与CD3抗体,用于治疗急性淋巴细胞白血病 | [ |
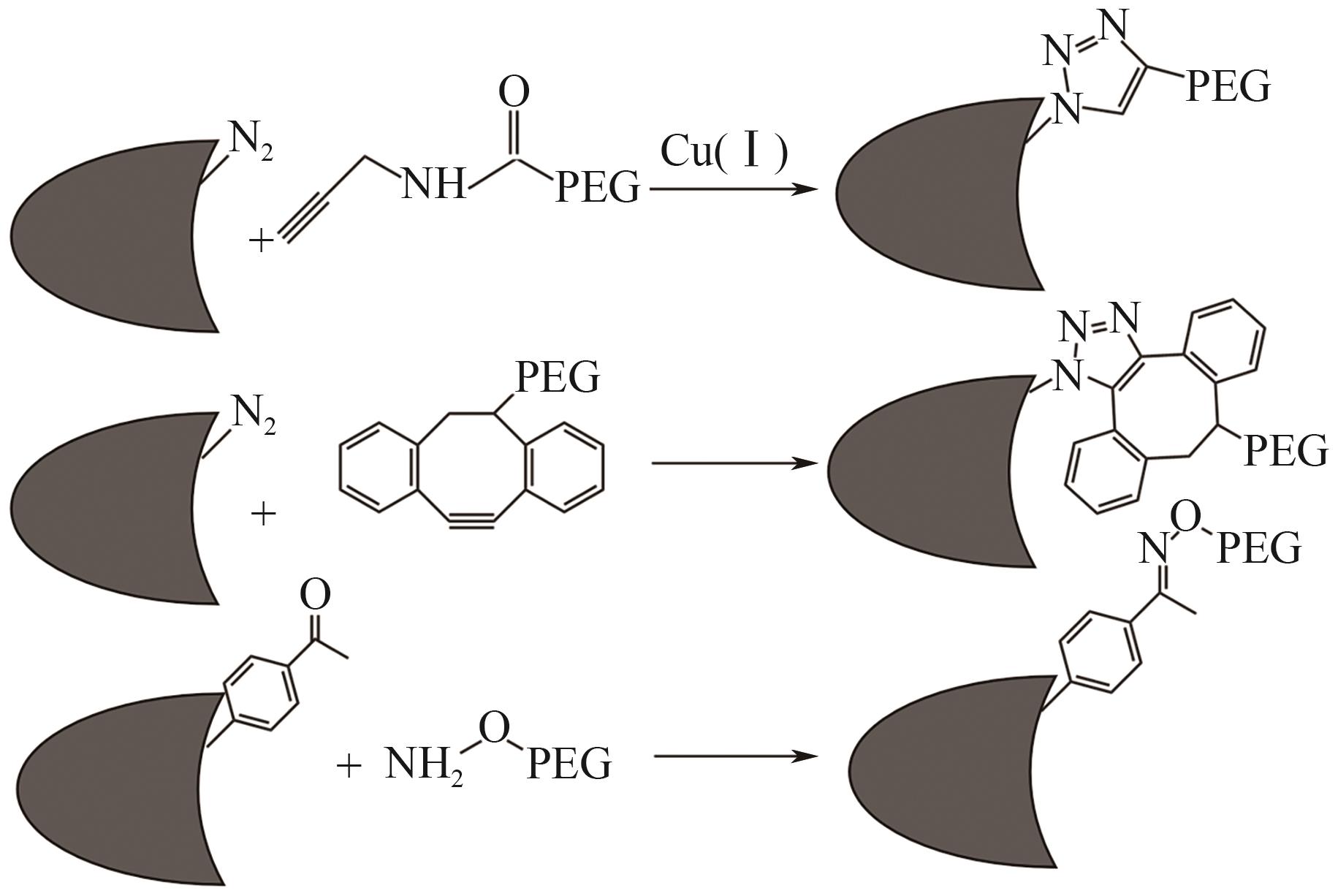
| Fc融合蛋白疫苗 | Fc段与抗原结合 | 通过Fc段促进抗原呈递,增强免疫反应,提高疫苗效果 | 疫苗开发 | Cervarix:HPV疫苗,采用Fc融合蛋白增加免疫反应 | [ |
表3 通过基因融合与Fc结合的肽和蛋白质药物
| 药物类别 | 融合蛋白功能域 | 作用机制 | 应用领域 | 融合药物及应用疾病 | 参考文献 |
|---|---|---|---|---|---|
| Fc融合蛋白 | Fc段(人类IgG Fc) | 延长半衰期,增加稳定性,增强免疫系统识别和靶向性 | 疫苗、免疫治疗、抗体药物偶联物 | 依那西普:融合TNF-α受体与Fc段,用于治疗类风湿性关节炎 | [ |
| 抗体药物偶联物(ADCs) | Fc段与细胞毒性药物结合 | 精准递送细胞毒性药物到靶细胞,减少对正常细胞的影响 | 癌症治疗 | 曲妥珠单抗:HER2靶向药物,用于HER2阳性乳腺癌治疗 | [ |
| 免疫检查点抑制剂融合蛋白 | Fc段与免疫抑制因子结合 | 解除免疫抑制,增强T细胞抗肿瘤活性 | 癌症免疫治疗 | 阿特朱单抗:结合PD-L1抗体与Fc段,用于治疗非小细胞肺癌和其他癌症 | [ |
| 抗体-酶融合蛋白 | Fc段与酶(如酶活性蛋白) | 通过Fc段增加酶的稳定性和靶向递送,提升治疗效果 | 代谢性疾病、癌症治疗 | α葡糖糖苷酶:用于治疗庞贝病的酶替代疗法 | [ |
| 细胞因子融合蛋白 | Fc段与细胞因子(如IL-2、GM-CSF等) | 提高细胞因子的稳定性,增强免疫反应和细胞活性 | 免疫治疗、癌症、炎症性疾病 | 非格司亭:与Fc融合的G-CSF,用于治疗化疗引起的白细胞减少症 | [ |
| 抗体-抗体融合 蛋白 | Fc段与另一个抗体(如抗肿瘤抗体) | 通过增强免疫系统的联合作用,提高对肿瘤细胞的免疫反应 | 癌症治疗、免疫疗法 | 博纳吐单抗:融合抗CD19与CD3抗体,用于治疗急性淋巴细胞白血病 | [ |
| Fc融合蛋白疫苗 | Fc段与抗原结合 | 通过Fc段促进抗原呈递,增强免疫反应,提高疫苗效果 | 疫苗开发 | Cervarix:HPV疫苗,采用Fc融合蛋白增加免疫反应 | [ |
| 类别 | 技术/方法 | 优点 | 相关研究与应用 |
|---|---|---|---|
| 聚合物纳米粒 | 可生物降解的聚合物纳米粒 | 良好的生物相容性与生物降解性,能够有效保护蛋白质,延长半衰期 | 通过自组装法交联ε-聚赖氨酸形成的核壳纳米粒,可延长胰岛素的降血糖作用时间 |
| 胰岛素包覆在磷脂复合物-聚合物纳米粒中,可显著降低糖尿病大鼠血糖水平 | |||
| 蛋白质药物的有效转运与释放 | |||
| 无机纳米粒 | 金纳米粒、介孔二氧化硅纳米粒 | 载药量大、稳定性好,适合多肽药物与疫苗递送 | 金纳米粒递送卵清蛋白多肽抗原和CpG佐剂,提高抗肿瘤效果 |
| 介孔二氧化硅纳米粒避免内吞作用,延长多肽药物作用时间 | |||
| pH敏感纳米粒 | 环境响应性聚合物纳米粒 | 可响应环境刺激(pH、温度等),优化药物释放动力学 | 硫醇化Eudragit L100用于口服胰岛素递送,改性后载药量更高,性能更佳 |
| 壳聚糖-海藻酸盐纳米粒,口服胰岛素递送显著提高降血糖效果 | |||
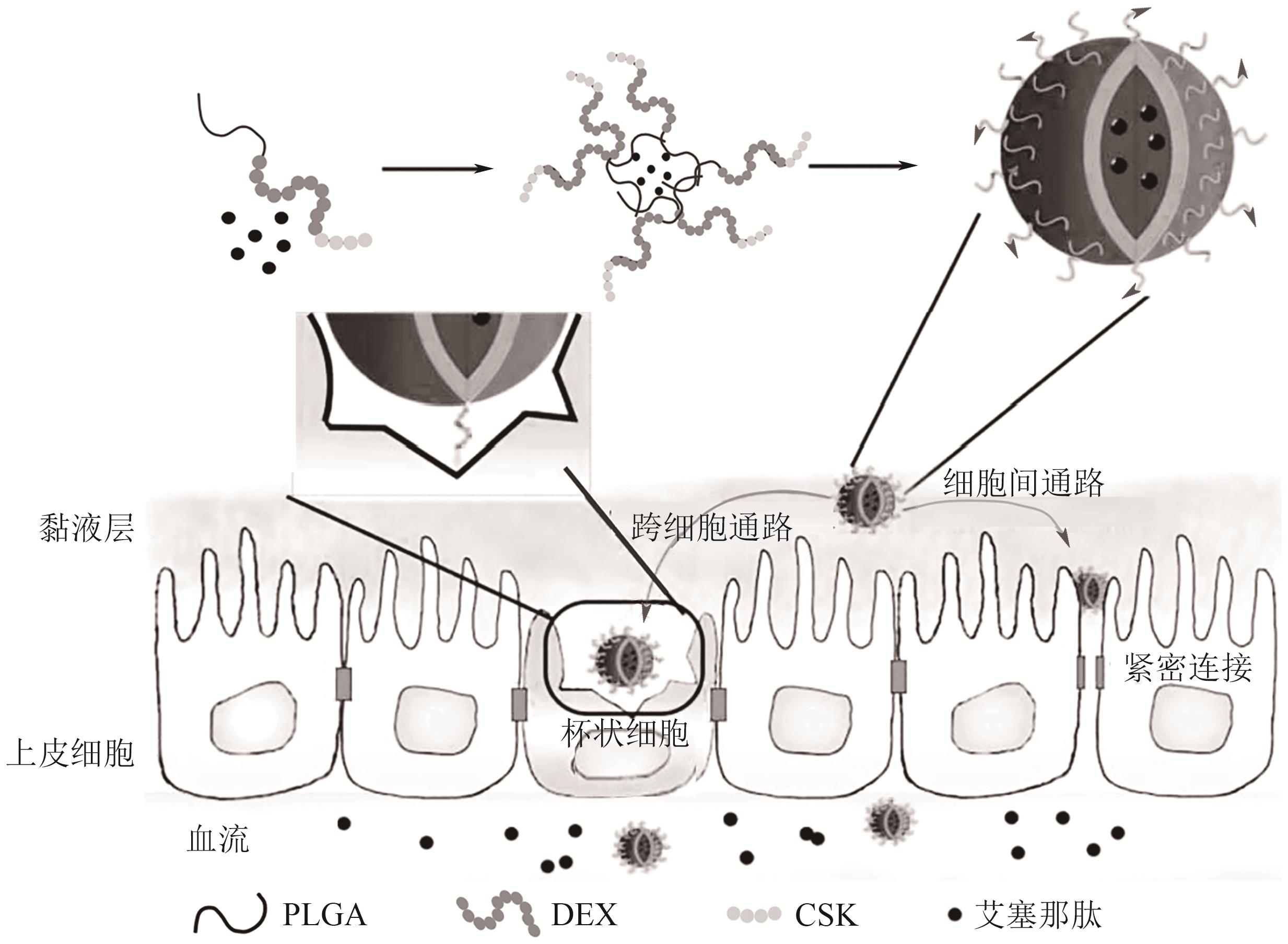
| 靶向纳米粒 | 带有肽配体的靶向纳米粒 | 提高药物的肠道靶向吸收效率与稳定性 | CSK肽修饰的DEX-PLGA纳米粒显著提高艾塞那肽的口服生物利用度 |
| CSK-DEX-PLGA纳米粒在小肠的吸收效率增加,糖尿病大鼠降血糖反应持续 |
表4 新型纳米载体技术在蛋白质药物递送中的应用及相关研究的总结
| 类别 | 技术/方法 | 优点 | 相关研究与应用 |
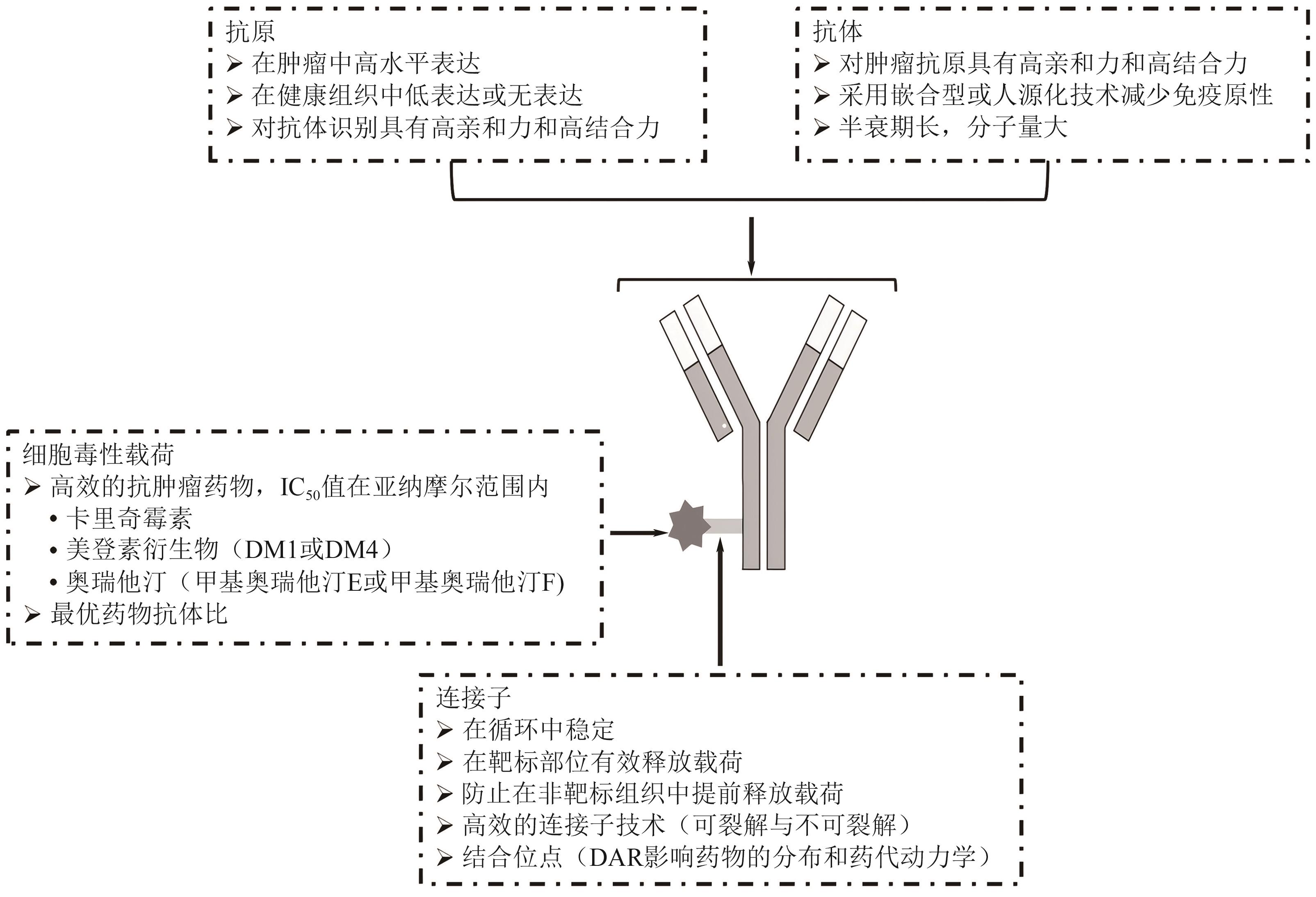
|---|---|---|---|
| 聚合物纳米粒 | 可生物降解的聚合物纳米粒 | 良好的生物相容性与生物降解性,能够有效保护蛋白质,延长半衰期 | 通过自组装法交联ε-聚赖氨酸形成的核壳纳米粒,可延长胰岛素的降血糖作用时间 |
| 胰岛素包覆在磷脂复合物-聚合物纳米粒中,可显著降低糖尿病大鼠血糖水平 | |||
| 蛋白质药物的有效转运与释放 | |||
| 无机纳米粒 | 金纳米粒、介孔二氧化硅纳米粒 | 载药量大、稳定性好,适合多肽药物与疫苗递送 | 金纳米粒递送卵清蛋白多肽抗原和CpG佐剂,提高抗肿瘤效果 |
| 介孔二氧化硅纳米粒避免内吞作用,延长多肽药物作用时间 | |||
| pH敏感纳米粒 | 环境响应性聚合物纳米粒 | 可响应环境刺激(pH、温度等),优化药物释放动力学 | 硫醇化Eudragit L100用于口服胰岛素递送,改性后载药量更高,性能更佳 |
| 壳聚糖-海藻酸盐纳米粒,口服胰岛素递送显著提高降血糖效果 | |||
| 靶向纳米粒 | 带有肽配体的靶向纳米粒 | 提高药物的肠道靶向吸收效率与稳定性 | CSK肽修饰的DEX-PLGA纳米粒显著提高艾塞那肽的口服生物利用度 |
| CSK-DEX-PLGA纳米粒在小肠的吸收效率增加,糖尿病大鼠降血糖反应持续 |
| 药物类型 | 药物名称 | 靶标 | 适应症 | 作用机制 | 参考文献 |
|---|---|---|---|---|---|
| 单克隆抗体 | 赫赛汀(曲妥珠单抗) | HER2受体 | HER2阳性乳腺癌、胃癌 | 阻断HER2信号通路并激活抗体依赖性细胞介导的细胞毒性(ADCC) | [ |
| 派姆单抗 | PD-1受体 | 黑色素瘤、非小细胞肺癌、头颈癌 | 解除免疫抑制,增强T细胞抗肿瘤活性 | [ | |
| 贝伐珠单抗 | VEGF | 结直肠癌、湿性黄斑变性 | 阻断VEGF,抑制肿瘤血管生成 | [ | |
| 融合蛋白 | 依那西普 | TNF-α | 类风湿性关节炎、银屑病 | 阻断TNF-α信号,缓解炎症 | [ |
| 阿柏西普 | VEGF-A和PIGF | 湿性黄斑变性 | 阻断血管生成因子,抑制病变血管形成 | [ | |
| 酶类药物 | α-葡萄糖苷酶 | 糖原累积病相关糖原 | 庞贝病 | 降解细胞内糖原,纠正代谢缺陷 | [ |
| 瑞替普酶 | 纤维蛋白 | 急性心肌梗死 | 溶解血栓,恢复血流 | [ | |
| 激素类蛋白 | 甘精胰岛素 | 胰岛素受体 | 糖尿病 | 调节葡萄糖代谢,降低血糖 | [ |
| 特立帕肽 | 甲状旁腺激素受体 | 骨质疏松症 | 刺激成骨细胞形成,增强骨密度 | [ | |
| 细胞因子 | 干扰素α | 病毒感染相关基因 | 乙型肝炎、丙型肝炎、某些癌症 | 激活抗病毒基因,增强免疫识别 | [ |
| 白介素-2 | T细胞 | 转移性肾癌、黑色素瘤 | 刺激T细胞和自然杀伤细胞增殖,增强抗肿瘤活性 | [ | |
| 肽类药物 | 布舍瑞林 | GnRH受体 | 前列腺癌、子宫内膜异位症 | 调节激素水平,抑制疾病进展 | [ |
| 利拉鲁肽 | GLP-1受体 | 2型糖尿病、肥胖症 | 延缓胃排空,抑制胰高血糖素分泌,降低血糖 | [ |
表5 靶向性治疗蛋白和肽药物的分类及临床应用实例
| 药物类型 | 药物名称 | 靶标 | 适应症 | 作用机制 | 参考文献 |
|---|---|---|---|---|---|
| 单克隆抗体 | 赫赛汀(曲妥珠单抗) | HER2受体 | HER2阳性乳腺癌、胃癌 | 阻断HER2信号通路并激活抗体依赖性细胞介导的细胞毒性(ADCC) | [ |
| 派姆单抗 | PD-1受体 | 黑色素瘤、非小细胞肺癌、头颈癌 | 解除免疫抑制,增强T细胞抗肿瘤活性 | [ | |
| 贝伐珠单抗 | VEGF | 结直肠癌、湿性黄斑变性 | 阻断VEGF,抑制肿瘤血管生成 | [ | |
| 融合蛋白 | 依那西普 | TNF-α | 类风湿性关节炎、银屑病 | 阻断TNF-α信号,缓解炎症 | [ |
| 阿柏西普 | VEGF-A和PIGF | 湿性黄斑变性 | 阻断血管生成因子,抑制病变血管形成 | [ | |
| 酶类药物 | α-葡萄糖苷酶 | 糖原累积病相关糖原 | 庞贝病 | 降解细胞内糖原,纠正代谢缺陷 | [ |
| 瑞替普酶 | 纤维蛋白 | 急性心肌梗死 | 溶解血栓,恢复血流 | [ | |
| 激素类蛋白 | 甘精胰岛素 | 胰岛素受体 | 糖尿病 | 调节葡萄糖代谢,降低血糖 | [ |
| 特立帕肽 | 甲状旁腺激素受体 | 骨质疏松症 | 刺激成骨细胞形成,增强骨密度 | [ | |
| 细胞因子 | 干扰素α | 病毒感染相关基因 | 乙型肝炎、丙型肝炎、某些癌症 | 激活抗病毒基因,增强免疫识别 | [ |
| 白介素-2 | T细胞 | 转移性肾癌、黑色素瘤 | 刺激T细胞和自然杀伤细胞增殖,增强抗肿瘤活性 | [ | |
| 肽类药物 | 布舍瑞林 | GnRH受体 | 前列腺癌、子宫内膜异位症 | 调节激素水平,抑制疾病进展 | [ |
| 利拉鲁肽 | GLP-1受体 | 2型糖尿病、肥胖症 | 延缓胃排空,抑制胰高血糖素分泌,降低血糖 | [ |
| 110 | 黄思思, 吴建辉. 肾细胞癌治疗药物的研发进展[J]. 现代肿瘤医学, 2024, 32(12): 2298-2303. |
| HUANG Sisi, WU Jianhui. Research advances in therapeutic drugs for renal cell carcinoma[J]. Journal of Modern Oncology, 2024, 32(12): 2298-2303. | |
| 111 | TAMBARE Rashmi S, SHAHI Sadhana R, GURUMUKHI Vishal C, et al. Quality by design (QbD) based development and validation of RP-HPLC method for buserelin acetate in polymeric nanoparticles: Release study[J]. Heliyon, 2024, 10(20): e39172. |
| 112 | LIU Xiaoyun, Laurentiu M POP, VITETTA Ellen S. Engineering therapeutic monoclonal antibodies[J]. Immunological Reviews, 2008, 222(1): 9-27. |
| 113 | NEUHAUS Claudia S, GABERNET Gisela, STEUER Christian, et al. Simulated molecular evolution for anticancer peptide design[J]. Angewandte Chemie International Edition, 2019, 58(6): 1674-1678. |
| 114 | Soumya DEO, TURTON Kristi L, KAINTH Tajinder, et al. Strategies for improving antimicrobial peptide production[J]. Biotechnology Advances, 2022, 59: 107968. |
| 115 | 龙心怡, 胡荣. 急性髓系白血病靶向治疗的研究进展[J]. 现代肿瘤医学, 2022, 30(15): 2847-2850. |
| LONG Xinyi, HU Rong. Research progress of targeted therapy for acute myeloid leukemia[J]. Journal of Modern Oncology, 2022, 30(15): 2847-2850. | |
| 116 | MORENO Carol, MUNIR Talha, OWEN Carolyn, et al. First-line fixed-duration ibrutinib plus venetoclax (Ibr+Ven) versus chlorambucil plus obinutuzumab (Clb+O): 55-month follow-up from the glow study[J]. Blood, 2023, 142: 634. |
| 117 | MIURA Shogo, KOBUNE Masayoshi, IBATA Soushi, et al. CD34/EPO-R double positive MDS cells produce erythroferrone in response to erythropoietin[J]. Blood, 2016, 128(22): 2455. |
| 118 | Hanny AL-SAMKARI, KUTER David J, GOODARZI Katayoon, et al. Romiplostim for treatment and prevention of chemotherapy-associated thrombocytopenia[J]. Blood, 2016, 128(22): 3748. |
| 119 | HAN Xiao, WANG Yao, WEI Jianshu, et al. Multi-antigen-targeted chimeric antigen receptor T cells for cancer therapy[J]. Journal of Hematology & Oncology, 2019, 12(1): 128. |
| 1 | Antonio GÓMEZ-OUTES, SUÁREZ-GEA Ma Luisa, Gonzalo CALVO-ROJAS, et al. Discovery of anticoagulant drugs: A historical perspective[J]. Current Drug Discovery Technologies, 2012, 9(2): 83-104. |
| 2 | IMMING Peter, SINNING Christian, MEYER Achim. Drugs, their targets and the nature and number of drug targets[J]. Nature Reviews Drug Discovery, 2006, 5(10): 821-834. |
| 3 | 邹健平, 姚新欣, 温淳, 等. 肿瘤靶向性遮蔽抗体研究进展[J]. 中国药理学与毒理学杂志, 2025, 39(1): 58-68. |
| ZOU Jianping, YAO Xinxin, WEN Chun, et al. Research progress in tumor-targeted masked antibodies[J]. Chinese Journal of Pharmacology and Toxicology, 2025, 39(1): 58-68. | |
| 4 | JIN Shijie, SUN Yanping, LIANG Xiao, et al. Emerging new therapeutic antibody derivatives for cancer treatment[J]. Signal Transduction and Targeted Therapy, 2022, 7(1): 39. |
| 5 | FINE Justyn, MEKSIRIPORN Bunyarit, TAN Jiacheng, et al. Mechanism-driven design of multispecific antibodies for targeted disease treatment[J]. Annual Review of Chemical and Biomolecular Engineering, 2024(15): 105-138. |
| 6 | CAO Shujun, Zhiqiang LYU, GUO Su, et al. An update—Prolonging the action of protein and peptide drugs[J]. Journal of Drug Delivery Science and Technology, 2021, 61: 102124. |
| 7 | SANTOS Rita, URSU Oleg, GAULTON Anna, et al. A comprehensive map of molecular drug targets[J]. Nature Reviews Drug Discovery, 2017, 16(1): 19-34. |
| 8 | REICHERT Janice M. Marketed therapeutic antibodies compendium[J]. mAbs, 2012, 4(3): 413-415. |
| 9 | CARTER P. Improving the efficacy of antibody-based cancer therapies[J]. Nature Reviews Cancer, 2001, 1(2): 118-129. |
| 10 | HAO Yue, YU Xinchao, BAI Yonghong, et al. Cryo-EM structure of HER2-trastuzumab-pertuzumab complex[J]. PLoS One, 2019, 14(5): e0216095. |
| 11 | LEE Kaping, LUO Rongzhen, WANG Shusen, et al. Fragement C Gamma receptor IIIa polymorphism is predictive of trastuzumab efficacy in the neoadjuvant setting of HER2-positive breast cancer patients[J]. Journal of Clinical Oncology, 2022,40:e12607. |
| 12 | BURMESTER Gerd R, PANACCIONE Remo, GORDON Kenneth B, et al. Adalimumab: Long-term safety in 23458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease[J]. Annals of the Rheumatic Diseases, 2013, 72(4): 517-524. |
| 13 | SILVER Aliyah B, LEONARD Elissa K, GOULD Joseph R, et al. Engineered antibody fusion proteins for targeted disease therapy[J]. Trends in Pharmacological Sciences, 2021, 42(12): 1064-1081. |
| 14 | MURER Patrizia, NERI Dario. Antibody-cytokine fusion proteins: A novel class of biopharmaceuticals for the therapy of cancer and of chronic inflammation[J]. New Biotechnology, 2019, 52: 42-53. |
| 15 | LIU Liming. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins[J]. Protein & Cell, 2018, 9(1): 15-32. |
| 16 | DHILLON Sohita, LYSENG-WILLIAMSON Katherine A, SCOTT Lesley J. Etanercept: A review of its use in the management of rheumatoid arthritis[J]. Drugs, 2007, 67(8): 1211-1241. |
| 17 | 孙凤艳, 冯红卫, 刘振, 等. 依那西普联合来氟米特治疗老年类风湿关节炎的疗效及对骨代谢指标、炎症因子及红细胞沉降率的影响[J]. 临床和实验医学杂志, 2024, 23(2): 165-169. |
| SUN Fengyan, FENG Hongwei, LIU Zhen, et al. Efficacy of Etanercept combined with Leflunomide in the treatment of elderly rheumatoid arthritis and its effects on bone metabolic indexes, inflammatory factors and erythrocyte precipitation rate[J]. Journal of Clinical and Experimental Medicine, 2024, 23(2): 165-169. | |
| 18 | CAO Xuan, SANCHEZ Jaron Castillo, PATEL Tapan P, et al. Aflibercept is more effective than bevacizumab at weaning neovascular age-related macular degeneration patients off therapy[J]. The Journal of clinical investigation, 2022,133(2): 1-12. |
| 19 | FOSGERAU Keld, HOFFMANN Torsten. Peptide therapeutics: Current status and future directions[J]. Drug Discovery Today, 2015, 20(1): 122-128. |
| 20 | WANG Lei, WANG Nanxi, ZHANG Wenping, et al. Therapeutic peptides: Current applications and future directions[J]. Signal Transduction and Targeted Therapy, 2022, 7: 48. |
| 21 | DRUCKER Daniel J, DRITSELIS Argyris, KIRKPATRICK Peter. Liraglutide[J]. Nature Reviews Drug Discovery, 2010, 9(4): 267-268. |
| 22 | WISEMAN Shari. Liraglutide and learning[J]. Nature Neuroscience, 2023, 26(10): 1660. |
| 23 | LIU Jianhao, ZHAO Ruogang, JIANG Xiaowen, et al. Progress on the application of Bortezomib and Bortezomib-based nanoformulations[J]. Biomolecules, 2021, 12(1): 51. |
| 24 | COOK Joselle, JOHNSON Isla, HIGGINS Alexandra, et al. Outcomes with different administration schedules of Bortezomib in bortezomib, lenalidomide and dexamethasone (VRd) as first-line therapy in multiple myeloma[J]. American Journal of Hematology, 2021, 96(3): 330-337. |
| 25 | 展鹏, 王学顺, 刘新泳. “精准医疗” 背景下的分子靶向药物研究——精准药物设计策略浅析[J]. 化学进展, 2016, 28(9): 1363-1386. |
| ZHAN Peng, WANG Xueshun, LIU Xinyong. Contemporary molecular targeted drug in the context of “precision medicine”: An attempting discussion of “precision drug design”[J]. Progress in Chemistry, 2016, 28(9): 1363-1386. | |
| 26 | CAMILLE Georges Wermuth, DAVID Aldous, PIERRE Raboisson, et al. The practice of medicinal chemistry[M]. New York: Elsevier, 2015. |
| 27 | Akshaya SIMHA N, PATIL Shashank M, CHAGALAMARI Akhila, et al. Protocol to identify multiple protein targets and therapeutic compounds using an in silico polypharmacological approach[J]. STAR Protocols, 2023, 4(3): 102440. |
| 28 | WEATHERALD Jason, BONDEELLE Louise, CHAUMAIS Marie-Camille, et al. Pulmonary complications of Bcr-Abl tyrosine kinase inhibitors[J]. The European Respiratory Journal, 2020, 56(4): 2000279. |
| 29 | RECKEL Sina, GEHIN Charlotte, TARDIVON Delphine, et al. Structural and functional dissection of the DH and PH domains of oncogenic Bcr-Abl tyrosine kinase[J]. Nature Communications, 2017, 8(1): 2101. |
| 30 | ROSS CAMIDGE D, DAVIES Kurtis D. MET copy number as a secondary driver of epidermal growth factor receptor tyrosine kinase inhibitor resistance in EGFR-mutant non-small-cell lung cancer[J]. Journal of Clinical Oncology, 2019, 37(11): 855-857. |
| 31 | TANG Jie, LIU Xiaoling, GONG Youling, et al. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) impact on immune microenvironment in non-small cell lung cancer (NSCLC)[J]. Journal of Clinical Oncology, 2018, 36(15): e21154. |
| 32 | TOSKA Eneda. Epigenetic mechanisms of cancer progression and therapy resistance in estrogen-receptor (ER+) breast cancer[J]. Biochimica et Biophysica Acta (BBA)—Reviews on Cancer, 2024, 1879(3): 189097. |
| 33 | GOEL Shom, DECRISTO Molly J, WATT April C, et al. CDK4/6 inhibition triggers anti-tumour immunity[J]. Nature, 2017, 548(7668): 471-475. |
| 34 | YANG Yilan, LUO Jurui, CHEN Xingxing, et al. CDK4/6 inhibitors: A novel strategy for tumor radiosensitization[J]. Journal of Experimental & Clinical Cancer Research, 2020, 39(1): 188. |
| 35 | 高峰, 洪亚珍, 陈晨, 等. 癌症治疗的新兴免疫靶点及相关研究进展[J]. 中国肿瘤临床, 2020, 47(19): 1001-1006. |
| GAO Feng, HONG Yazhen, CHEN Chen, et al. Current status of emerging targets for cancer immunotherapy[J]. Chinese Journal of Clinical Oncology, 2020, 47(19): 1001-1006. | |
| 36 | SAWYERS Charles L. Herceptin: A first assault on oncogenes that launched a revolution[J]. Cell, 2019, 179(1): 8-12. |
| 37 | GIL Ziv, BILLAN Salem. Pembrolizumab-chemotherapy for advanced oesophageal cancer[J]. Lancet, 2021, 398(10302): 726-727. |
| 38 | READY Neal E, Patrick A OTT, HELLMANN Matthew D, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: Results from the CheckMate 032 randomized cohort[J]. Journal of Thoracic Oncology, 2020, 15(3): 426-435. |
| 39 | SIDAWAY Peter. SUNLIGHT illuminates the activity of bevacizumab[J]. Nature Reviews Clinical Oncology, 2023, 20(8): 504. |
| 40 | BILLI Allison C, GUDJONSSON Johann E. Adalimumab in psoriasis: How much is enough?[J]. Journal of Investigative Dermatology, 2019, 139(1): 19-22. |
| 41 | ROMERO Diana. Haematological cancer: Extended EFS with rituximab[J]. Nature Reviews Clinical Oncology, 2017, 14(12): 714. |
| 42 | MARTINELLI E, TROIANI T, CARDONE C, et al. 668TiP Phase Ⅱ study of avelumab in combination with cetuximab as a rechallenge strategy in pre-treated RAS wild type metastatic colorectal cancer patients: CAVE (cetuximab-avelumab) colon[J]. Annals of Oncology, 2019, 30: v251. |
| 43 | MIYAUCHI Eisaku, MORITA Satoshi, NAKAMURA Atsushi, et al. Updated analysis of NEJ009: Gefitinib-alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated EGFR[J]. Journal of Clinical Oncology, 2022, 40(31): 3587-3592. |
| 44 | STIRRUPS Robert. CAR T-cells for relapsed B-cell ALL in children and young adults[J]. Lancet Oncology, 2018, 19(3): e144. |
| 45 | ZHAO Weili, WU Depei, HU Yu. Axicabtagene ciloleucel in large B-cell lymphoma[J]. The New England Journal of Medicine, 2023, 389(12): 1152-1153. |
| 46 | DINGLI David, MATOS Joana E, LEHRHAUPT Kerri, et al. The burden of illness in patients with paroxysmal nocturnal hemoglobinuria receiving treatment with the C5-inhibitors eculizumab or ravulizumab: Results from a US patient survey[J]. Annals of Hematology, 2022, 101(2): 251-263. |
| 47 | GUO Yanjun, PAN Weiwei, LIU Shengbing, et al. ERK/MAPK signalling pathway and tumorigenesis[J]. Experimental and Therapeutic Medicine, 2020, 19(3): 1997-2007. |
| 48 | GIORDANO Federica, VAIRA Valentina, CORTINOVIS Diego, et al. p65BTK is a novel potential actionable target in KRAS-mutated/EGFR-wild type lung adenocarcinoma[J]. Journal of Experimental & Clinical Cancer Research, 2019, 38(1): 260. |
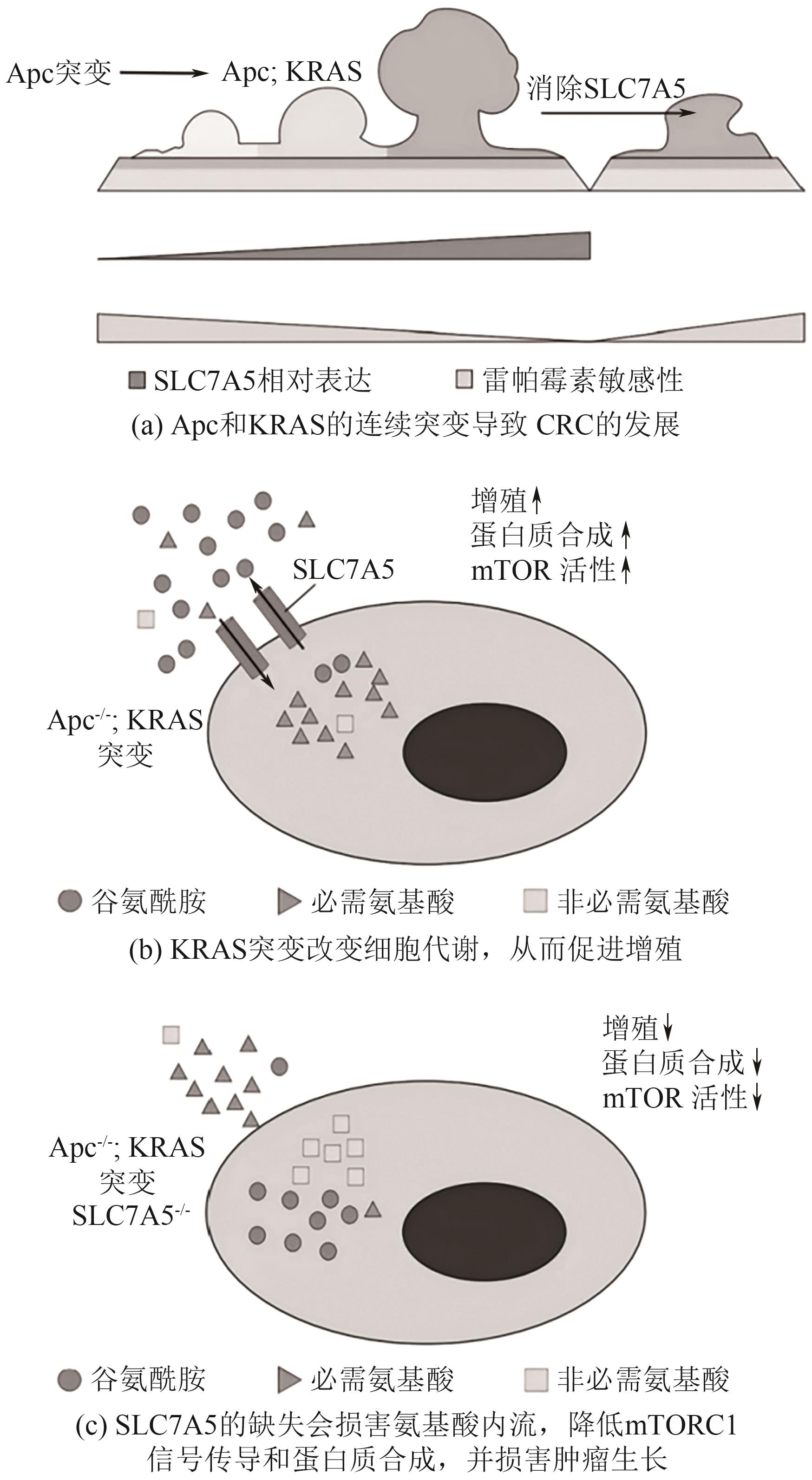
| 49 | KIMMELMAN Alec C. Metabolic dependencies in RAS-driven cancers[J]. Clinical Cancer Research, 2015, 21(8): 1828-1834. |
| 50 | LIU Chang, ZHANG Li, LIU Hao, et al. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications[J]. Journal of Controlled Release, 2017, 266: 17-26. |
| 51 | GAO Shan, YANG Chen, JIANG Shan, et al. Applications of RNA interference high-throughput screening technology in cancer biology and virology[J]. Protein & Cell, 2014, 5(11): 805-815. |
| 52 | SAHA Pranay, MOITRA Parikshit, BHATTACHARJEE Urmimala, et al. Selective pathological and intracellular detection of human serum albumin by photophysical and electrochemical techniques using a FRET-based molecular probe[J]. Biosensors and Bioelectronics, 2022, 203: 114007. |
| 53 | BOOTSMA Sanne, VAN NEERVEN Sanne M, VERMEULEN Louis. Exploiting KRAS-mediated metabolic reprogramming as a therapeutic target[J]. Nature Genetics, 2021, 53(1): 9-10. |
| 54 | Adetola OKE, SAHIN Deniz, CHEN Xin, et al. High throughput screening for drug discovery and virus detection[J]. Combinatorial Chemistry & High Throughput Screening, 2022, 25(9): 1518-1533. |
| 55 | ZHOU Yuanzhe, JIANG Yangwei, CHEN Shijie. RNA-ligand molecular docking: Advances and challenges[J]. Wiley Interdisciplinary Reviews: Computational Molecular Science, 2022, 12(3): e1571. |
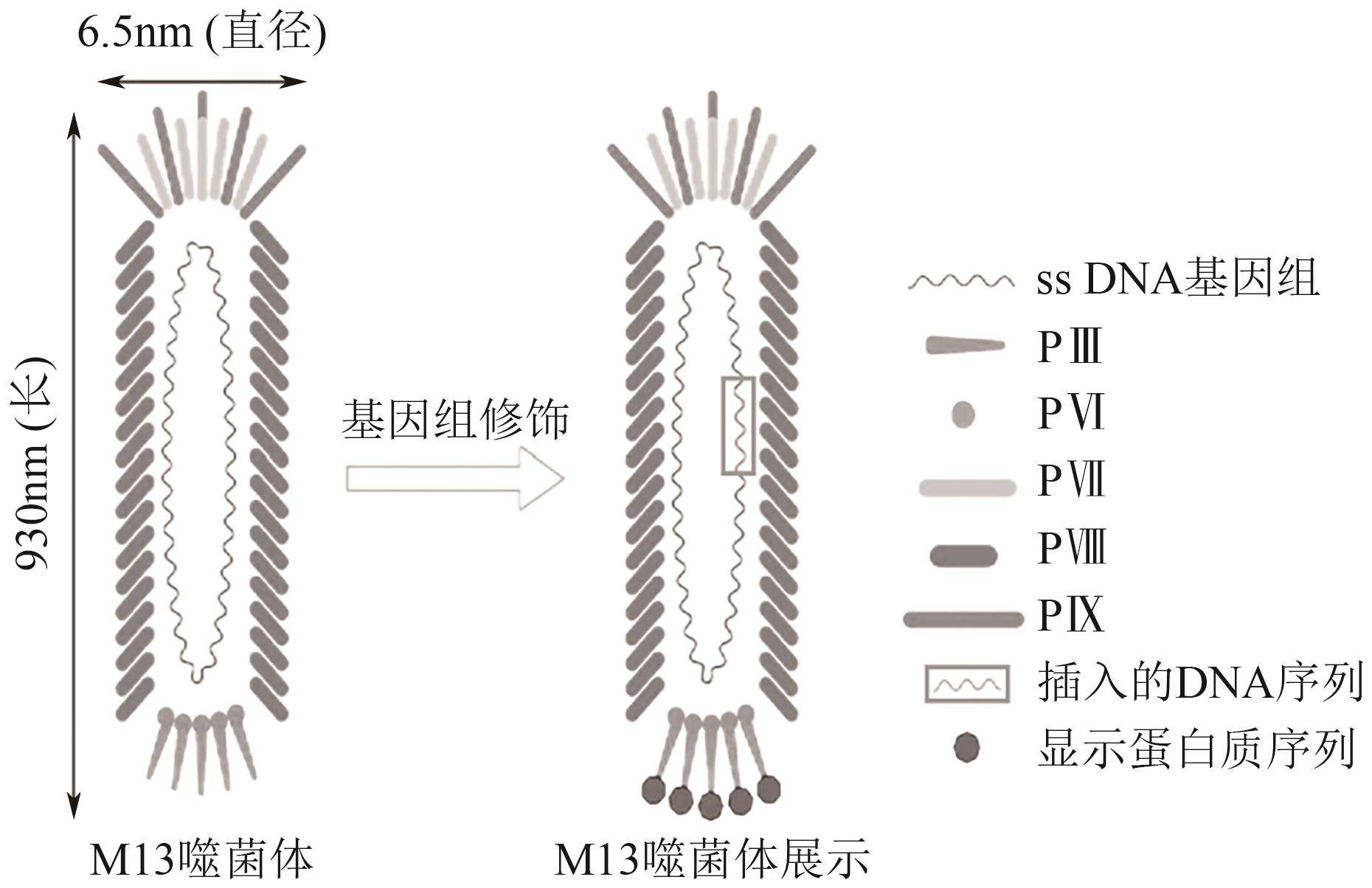
| 56 | SMITH G P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface[J]. Science, 1985, 228(4705): 1315-1317. |
| 57 | ZHANG Yi Wolf, ZHENG Nan, CHOU Danny Hung-Chieh. Serine-mediated hydrazone ligation displaying insulin-like peptides on M13 phage pⅢ[J]. Organic & Biomolecular Chemistry, 2023, 21(44): 8902-8909. |
| 58 | JAROSZEWICZ W, MORCINEK-ORŁOWSKA J, PIERZYNOWSKA K, et al. Phage display and other peptide display technologies[J]. FEMS Microbiol Rev, 2022, 46(2): fuab052. |
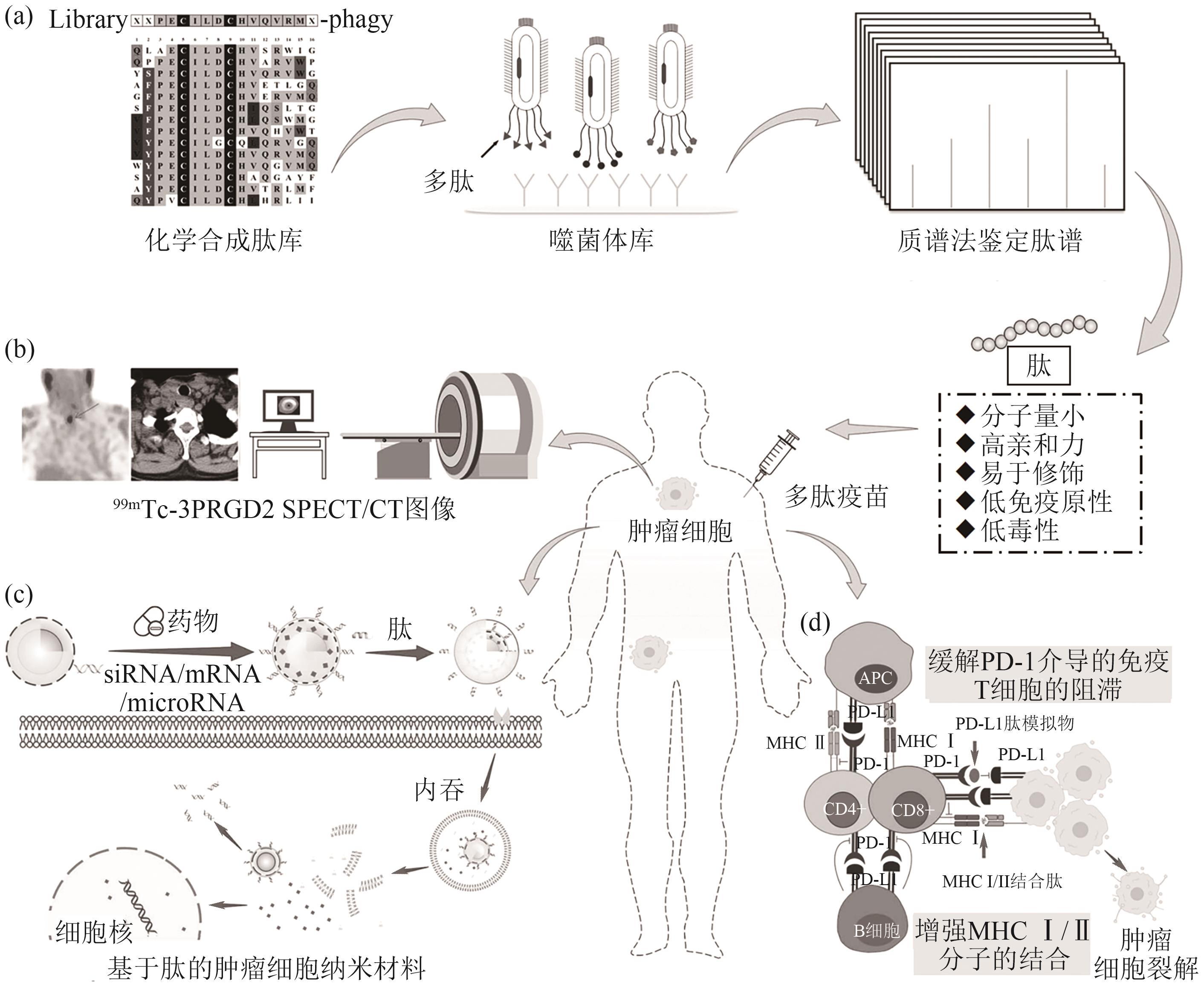
| 59 | 王睿. 基于噬菌体展示技术筛选靶向HER2的肽类分子探针[D]. 遵义: 遵义医科大学, 2023. |
| WANG Rui. Screening of peptide molecular probes targeting HER2 based on phage display technology[D]. Zunyi: Zunyi Medcial University, 2023. | |
| 60 | Robert BÜCKER, Pascal HOGAN-LAMARRE, MEHRABI Pedram, et al. Serial protein crystallography in an electron microscope[J]. Nature Communications, 2020, 11(1): 996. |
| 61 | PLATZER Gerald, MAYER Moriz, BEIER Andreas, et al. PI by NMR: Probing CH-π interactions in protein-ligand complexes by NMR spectroscopy[J]. Angewandte Chemie International Edition, 2020, 59(35): 14861-14868. |
| 62 | GATSOGIANNIS Christos, BALOGH Dora, MERINO Felipe, et al. Cryo-EM structure of the ClpXP protein degradation machinery[J]. Nature Structural & Molecular Biology, 2019, 26(10): 946-954. |
| 63 | CUI Jing, FENG Yongwei, YANG Ting, et al. Computer-aided designing peptide inhibitors of human hematopoietic prostaglandin D2 synthase combined molecular docking and molecular dynamics simulation[J]. Molecules, 2023, 28(15): 5933. |
| 64 | SMYRLAKI Ioanna, SHAW Alan, YANG Yunshi, et al. Solid phase synthesis of DNA nanostructures in heavy liquid[J]. Small, 2023, 19(4): 2204513. |
| 65 | SHARMA Anamika, KUMAR Ashish, DE LA TORRE Beatriz G, et al. Liquid-phase peptide synthesis (LPPS): A third wave for the preparation of peptides[J]. Chemical Reviews, 2022, 122(16): 13516-13546. |
| 66 | CARROLL W L. Introduction to recombinant-DNA technology[J]. The American Journal of Clinical Nutrition, 1993, 58(2): 249S-258S. |
| 67 | LAU Jolene L, DUNN Michael K. Therapeutic peptides: Historical perspectives, current development trends, and future directions[J]. Bioorganic & Medicinal Chemistry, 2018, 26(10): 2700-2707. |
| 68 | CAO Shujun, XU Shuo, WANG Huiming, et al. Nanoparticles: Oral delivery for protein and peptide drugs[J]. AAPS PharmSciTech, 2019, 20(5): 190. |
| 69 | SAMEC Timothy, BOULOS Jessica, GILMORE Serena, et al. Peptide-based delivery of therapeutics in cancer treatment[J]. Materials Today Bio, 2022, 14: 100248. |
| 70 | WU Tingjuan, CHEN Kexin, HE Shuangyan, et al. Drug development through modification of small molecular drugs with monodisperse poly(ethylene glycol)s[J]. Organic Process Research & Development, 2020, 24(8): 1364-1372. |
| 71 | VERONESE Francesco M, MERO Anna. The impact of PEGylation on biological therapies[J]. BioDrugs, 2008, 22(5): 315-329. |
| 72 | VAN WITTELOOSTUIJN Søren B, PEDERSEN Søren L, JENSEN Knud J. Half-life extension of biopharmaceuticals using chemical methods: Alternatives to PEGylation[J]. ChemMedChem, 2016, 11(22): 2474-2495. |
| 73 | SCHLAPSCHY Martin, BINDER Uli, Claudia BÖRGER, et al. PASylation: A biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins[J]. Protein Engineering, Design & Selection, 2013, 26(8): 489-501. |
| 74 | BREIBECK Joscha, SKERRA Arne. The polypeptide biophysics of proline/alanine-rich sequences (PAS): Recombinant biopolymers with PEG-like properties[J]. Biopolymers, 2018, 109(1): e23069. |
| 75 | MORATH Volker, BOLZE Florian, SCHLAPSCHY Martin, et al. PASylation of murine leptin leads to extended plasma half-life and enhanced in vivo efficacy[J]. Molecular Pharmaceutics, 2015, 12(5): 1431-1442. |
| 76 | AHMADPOUR Sajjad, HOSSEINIMEHR Seyed Jalal. PASylation as a powerful technology for improving the pharmacokinetic properties of biopharmaceuticals[J]. Current Drug Delivery, 2018, 15(3): 331-341. |
| 77 | MENDLER Claudia T, FRIEDRICH Lars, LAITINEN Iina, et al. High contrast tumor imaging with radio-labeled antibody Fab fragments tailored for optimized pharmacokinetics via PASylation[J]. mAbs, 2015, 7(1): 96-109. |
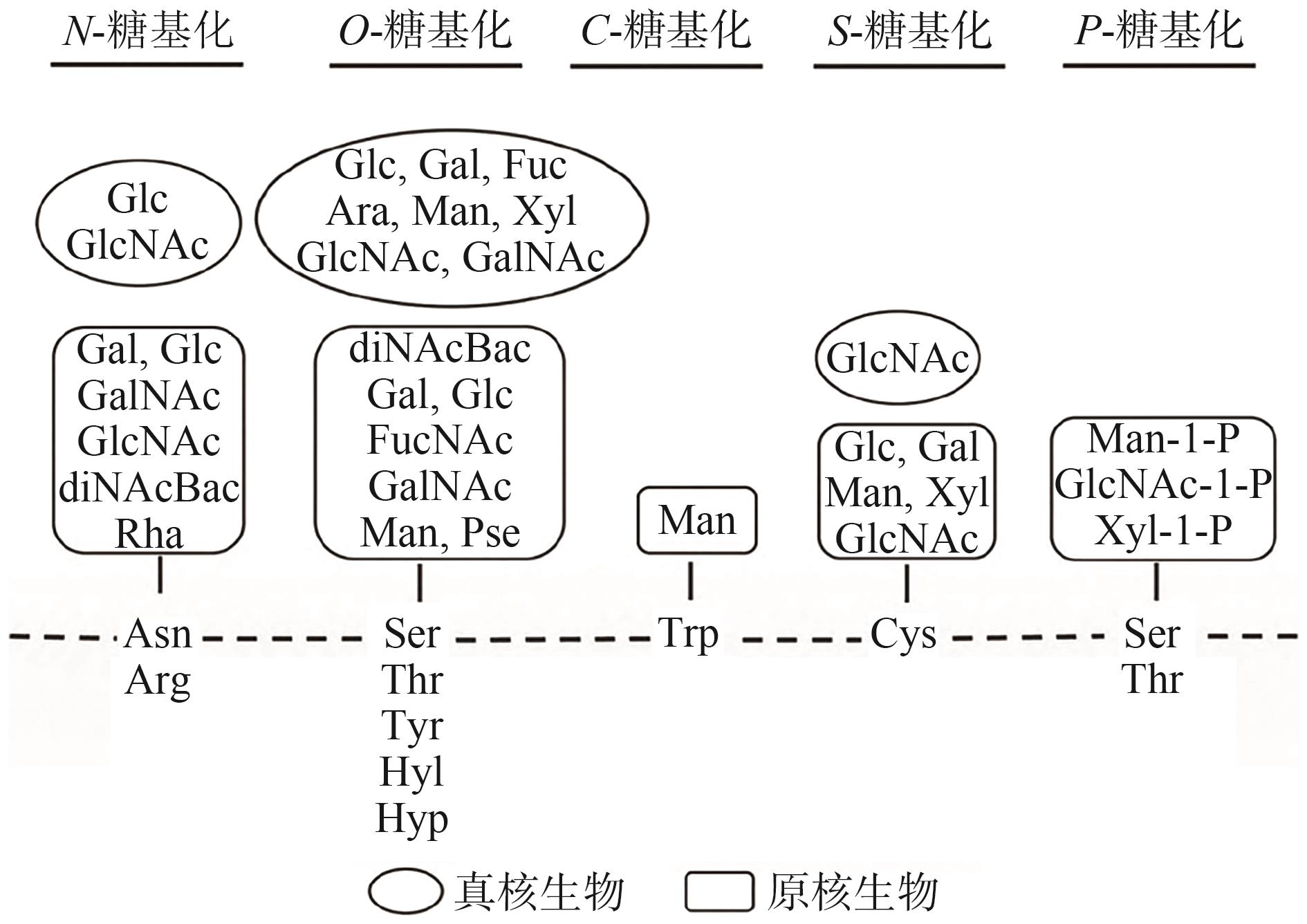
| 78 | 郑小菊, 刘长松, 王凤山. 糖基化修饰对蛋白质和肽性质的影响及修饰方法[J]. 生命的化学, 2024, 44(7): 1291-1297. |
| ZHENG Xiaoju, LIU Changsong, WANG Fengshan. The effects of glycosylation modification on protein and peptide properties and modification methods[J]. Chemistry of Life, 2024, 44(7): 1291-1297. | |
| 79 | HOU Huiwen, WANG Juan, WANG Jie, et al. A review of bioactive peptides: Chemical modification, structural characterization and therapeutic applications[J]. Journal of Biomedical Nanotechnology, 2020, 16(12): 1687-1718. |
| 80 | BALAKRISHNAN Anagha, MISHRA Saurav Kumar, SHARMA Kanchan, et al. Intersecting peptidomics and bioactive peptides in drug therapeutics[J]. Current Bioinformatics, 2025, 20(2): 103-119. |
| 81 | GOTH Christoffer K, MEHTA Akul Y, MCQUILLAN Alyssa M, et al. Chemokine binding to PSGL-1 is controlled by O-glycosylation and tyrosine sulfation[J]. Cell Chemical Biology, 2023, 30(8): 893-905.e7. |
| 82 | KOBAYASHI Satoshi, FUKUSHIMA Taito, UENO Makoto, et al. Progression pattern and post-progression survival following atezolizumab and bevacizumab treatment in advanced hepatocellular carcinoma[J]. Liver International, 2024, 44(6): 1343-1350. |
| 83 | BLAIR Hannah A. Cipaglucosidase Alfa: First approval[J]. Drugs, 2023, 83(8): 739-745. |
| 84 | WANG Xingtong, GUO Wei, ZHAO Yangzhi, et al. The combination of pegfilgrastim and filgrastim improves the outcomes of mobilization and engraftment after transplantation of autologous hematopoietic stem cells for the treatment of lymphoma[J]. Blood, 2023, 142: 4891. |
| 85 | ALDOSS Ibrahim, SONG Joo Y. Extramedullary relapse of KMT2A(MLL)-rearranged acute lymphoblastic leukemia with lineage switch following blinatumomab[J]. Blood, 2018, 131(22): 2507. |
| 86 | COOMBES R. Cervarix: Definitely not the new MMR[J]. Bmj, 2009, 339(oct071): b4124. |
| 87 | ZHANG Yu, ZHANG Yujie, GUO Qin, et al. Trained macrophage bioreactor for penetrating delivery of fused antitumor protein[J]. ACS Applied Materials & Interfaces, 2019, 11(26): 23018-23025. |
| 88 | POLLARO Lisa, HEINIS Christian. Strategies to prolong the plasma residence time of peptide drugs[J]. MedChemComm, 2010, 1(5): 319-324. |
| 89 | BRANTLY Mark L, KUHN Brooks T, FARAH Humam W, et al. Recombinant alpha-1 antitrypsin-Fc fusion protein INBRX-101 in adults with alpha-1 antitrypsin deficiency: A phase 1 study[J]. Chronic Obstructive Pulmonary Diseases, 2024, 11(3): 282-292. |
| 90 | DE Xinqi, GAO Mingchun, JIA Zheng, et al. A novel oral vaccine delivery system for enhancing stability and immune protection: Bacterium-like particle with functional coating[J]. Frontiers in Microbiology, 2024, 15: 1481514. |
| 91 | SHOJAEI Shirin, POURMADADI Mehrab, HOMAYOONFAL Mina, et al. Revolutionizing lung cancer treatment: Nanotechnology-driven advances in targeted drug delivery and novel therapeutic strategies[J]. Journal of Drug Delivery Science and Technology, 2024, 101: 106186. |
| 92 | SONG Yina, SHI Yanan, ZHANG Liping, et al. Synthesis of CSK-DEX-PLGA nanoparticles for the oral delivery of exenatide to improve its mucus penetration and intestinal absorption[J]. Molecular Pharmaceutics, 2019, 16(2): 518-532. |
| 93 | 张敏娜, 唐仁明, 钟鸣, 等. 靶向纳米药物在胶质瘤治疗中的研究进展[J]. 现代肿瘤医学, 2024, 32(9): 1749-1755. |
| ZHANG Minna, TANG Renming, ZHONG Ming, et al. Research progress of targeted nanomedicine in the treatment of glioma[J]. Journal of Modern Oncology, 2024, 32(9): 1749-1755. | |
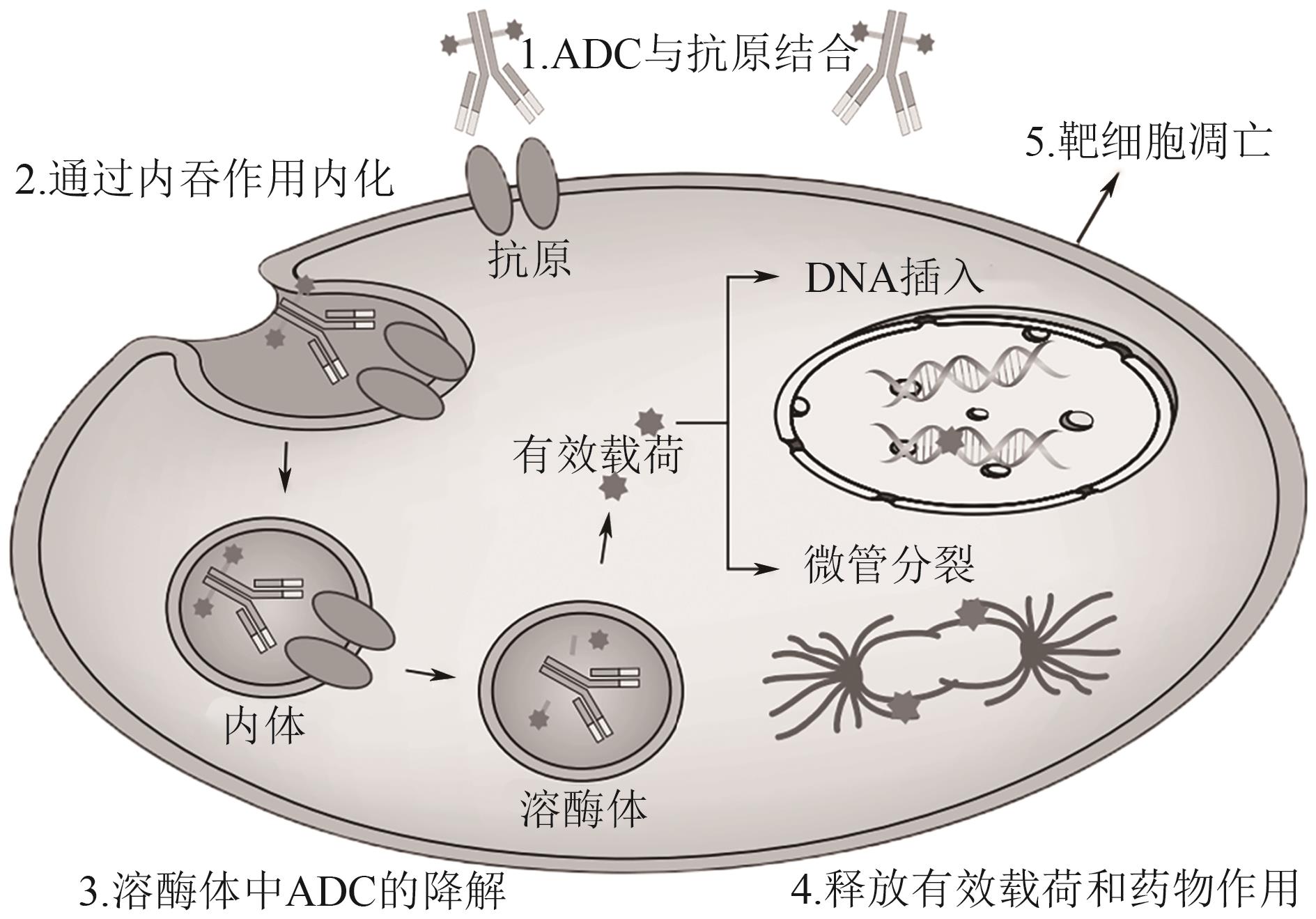
| 94 | CHAU Cindy H, STEEG Patricia S, FIGG William D. Antibody–drug conjugates for cancer[J]. The Lancet, 2019, 394(10200): 793-804. |
| 95 | KHERA Eshita, THURBER Greg M. Pharmacokinetic and immunological considerations for expanding the therapeutic window of next-generation antibody-drug conjugates[J]. BioDrugs, 2018, 32(5): 465-480. |
| 96 | 贾艳丽, 李小钰, 范厚武, 等. 抗体-药物偶联物毒性的发生机制与优化方法研究进展[J]. 肿瘤防治研究, 2024, 51(7): 606-612. |
| JIA Yanli, LI Xiaoyu, FAN Houwu, et al. Research progress on the mechanism and optimization method of antibody-drug conjugate toxicity[J]. Cancer Research on Prevention and Treatment, 2024, 51(7): 606-612. | |
| 97 | CARTER Paul J, QUARMBY Valerie. Immunogenicity risk assessment and mitigation for engineered antibody and protein therapeutics[J]. Nature Reviews Drug Discovery, 2024, 23(12): 898-913. |
| 98 | LIANG Shide, ZHANG Chi. Prediction of immunogenicity for humanized and full human therapeutic antibodies[J]. PLoS One, 2020, 15(8): e0238150. |
| 99 | ROSSOTTI Martin A, Kasandra BÉLANGER, HENRY Kevin A, et al. Immunogenicity and humanization of single-domain antibodies[J]. The FEBS Journal, 2022, 289(14): 4304-4327. |
| 100 | DONG Yawen, MA Tingting, XU Ting, et al. Characteristic roadmap of linker governs the rational design of PROTACs[J]. Acta Pharmaceutica Sinica B, 2024, 14(10): 4266-4295. |
| 101 | MCCOMBS Jessica R, OWEN Shawn C. Antibody drug conjugates: Design and selection of linker, payload and conjugation chemistry[J]. The AAPS Journal, 2015, 17(2): 339-351. |
| 120 | KECHAGIAS Konstantinos S, KALLIALA Ilkka, BOWDEN Sarah J, et al. Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: Systematic review and meta-analysis[J]. BMJ, 2022, 378: e070135. |
| 121 | ZHAO Hong, ZHOU Xiaoying, ZHOU Yihua. Hepatitis B vaccine development and implementation[J]. Human Vaccines & Immunotherapeutics, 2020, 16(7): 1533-1544. |
| 122 | DAY Patricia M, KINES Rhonda C, THOMPSON Cynthia D, et al. In vivo mechanisms of vaccine-induced protection against HPV infection[J]. Cell Host & Microbe, 2010, 8(3): 260-270. |
| 102 | CHYTIL Petr, Milada ŠÍROVÁ, Júlia KUDLÁČOVÁ, et al. Bloodstream stability predetermines the antitumor efficacy of micellar polymer-doxorubicin drug conjugates with pH-triggered drug release[J]. Molecular Pharmaceutics, 2018, 15(9): 3654-3663. |
| 103 | HIDALGO Alberto, Cristina GARCIA-MOUTON, AUTILIO Chiara, et al. Pulmonary surfactant and drug delivery: Vehiculization, release and targeting of surfactant/tacrolimus formulations[J]. Journal of Controlled Release, 2021, 329: 205-222. |
| 104 | 戴炜均, 张震坡, 宿凌, 等. 我国抗肿瘤药物临床试验研究热点[J]. 中国药业, 2024, 33(14): 43-47. |
| DAI Weijun, ZHANG Zhenpo, SU Ling, et al. Research hotspots of clinical trials of anti-tumor drugs in China[J]. China Pharmaceuticals, 2024, 33(14): 43-47. | |
| 105 | LI Shuya, GU Hongqiu, LI Hao, et al. Reteplase versus alteplase for acute ischemic stroke[J]. N Engl J Med, 2024, 390(24): 2264-2273. |
| 106 | PASQUEL Francisco J, CECILIA LANSANG M, KHOWAJA Ameer, et al. A randomized controlled trial comparing glargine U300 and glargine U100 for the inpatient management of medicine and surgery patients with type 2 diabetes: Glargine U300 hospital trial[J]. Diabetes Care, 2020, 43(6): 1242-1248. |
| 107 | ASIMUS Sara, Robert PALMÉR, ALBAYATY Muna, et al. Pharmacokinetics, pharmacodynamics and safety of the inverse retinoic acid-related orphan receptor γ agonist AZD0284[J]. British Journal of Clinical Pharmacology, 2020, 86(7): 1398-1405. |
| 108 | WANG Shuai, CHEN SHAN nan, SUN Zheng, et al. Four type Ⅰ IFNs, IFNa1, IFNa2, IFNb, IFNc, and their receptor usage in an osteoglossomorph fish, the Asian arowana, Scleropages formosus[J]. Fish & Shellfish Immunology, 2021, 117: 70-81. |
| 109 | GILLS Jessie, PARKER William P, PATE Scott, et al. The role of high dose interleukin-2 in the era of targeted therapy[J]. The Journal of Urology, 2017, 198(3): 538-545. |
| [1] | 曾泽阳, 徐偲, 金子杰, 王晓钟, 陈英奇, 戴立言. 基于琼脂糖微球金属螯合层析介质的制备及性能评价[J]. 化工进展, 2024, 43(10): 5693-5703. |
| [2] | 孙鲁芹, 卢会霞, 王建友. 电渗析/超滤内在耦合过程分离蛋清中溶菌酶[J]. 化工进展, 2023, 42(5): 2262-2271. |
| [3] | 吴霞, 蒋勋涛, 张余晓, 吕慧园, 黄方, 于筱溪. 基于液滴微流控技术的蛋白质结晶[J]. 化工进展, 2023, 42(4): 2024-2030. |
| [4] | 顾海洋, 王冬, 宗永忠, 付少海. 制革污泥蛋白质基生物质棉织物阻燃涂层的制备与阻燃性能[J]. 化工进展, 2023, 42(2): 641-649. |
| [5] | 谢力, 李秀芬. 胞外多糖含量对碱热水解法溶出污泥蛋白质及水解液固液分离性能的影响[J]. 化工进展, 2022, 41(8): 4580-4586. |
| [6] | 鲁泽平, 裴新华, 薛誉, 张晓光, 胡燚. 甜菜碱类离子液体化学修饰猪胰脂肪酶提升其酶学性能[J]. 化工进展, 2022, 41(11): 6045-6052. |
| [7] | 李宪秀, 何涛, 毛建卫, 沙如意. 减电荷聚甲基丙烯酸钠接枝介质的制备及蛋白质吸附性能[J]. 化工进展, 2022, 41(11): 6038-6044. |
| [8] | 董晓宇. 酿酒酵母钙通道膜蛋白单克隆抗体制备及鉴定[J]. 化工进展, 2021, 40(S1): 334-343. |
| [9] | 李梓泳, 马憬希, 赵明, 陈龙, 周红军, 周新华. 羧甲基纤维素-大豆分离蛋白农药缓释颗粒的制备及性能[J]. 化工进展, 2021, 40(5): 2739-2746. |
| [10] | 周雁红, 李夏兰, 张光亚. 生物大分子介导仿生矿化制备磁性纳米粒子的研究进展[J]. 化工进展, 2021, 40(5): 2719-2729. |
| [11] | 向燕, 谢锐, 巨晓洁, 汪伟, 刘壮, 褚良银. 盐响应型蛋白质吸附阳离子交换膜[J]. 化工进展, 2021, 40(4): 2234-2242. |
| [12] | 李学绅, 王词稼, 王康妮, 王伟云. 蛋白酶/热压力处理对污泥脱水性的影响[J]. 化工进展, 2019, 38(s1): 201-208. |
| [13] | 吕婷婷,安瑛,刘宇健,李好义,谭晶,杨卫民. 静电纺丝制备蛋清蛋白/聚氧化乙烯纳米纤维[J]. 化工进展, 2019, 38(12): 5487-5491. |
| [14] | 陈少奇, 邵媛媛, 马可颖, 郑莹, 祝京旭. 液固循环流化床的开发与应用——过程集成与强化[J]. 化工进展, 2019, 38(01): 122-137. |
| [15] | 张兰河, 王佳平, 陈子成, 郭静波, 贾艳萍, 张海丰, 李正. Ca2+对序批式生物反应器活性污泥性能的影响[J]. 化工进展, 2018, 37(09): 3675-3681. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||