化工进展 ›› 2021, Vol. 40 ›› Issue (S1): 334-343.DOI: 10.16085/j.issn.1000-6613.2020-2100
酿酒酵母钙通道膜蛋白单克隆抗体制备及鉴定
- 大连大学生命科学与技术学院,辽宁 大连 116622
-
收稿日期:2020-10-19修回日期:2021-01-25出版日期:2021-10-25发布日期:2021-11-09 -
作者简介:董晓宇(1973—),女,博士,教授,硕士生导师,研究方向为代谢调控。E-mail:dongxiaoyu@dlu.edu.cn 。 -
基金资助:国家自然科学基金面上项目(21476032)
Preparation and identification of monoclonal antibodies of calcium channel membrane proteins in Saccharomyces cerevisiae
- School of Life Science and Biotechnology, Dalian University, Dalian 116622, Liaoning, China
-
Received:2020-10-19Revised:2021-01-25Online:2021-10-25Published:2021-11-09
摘要:
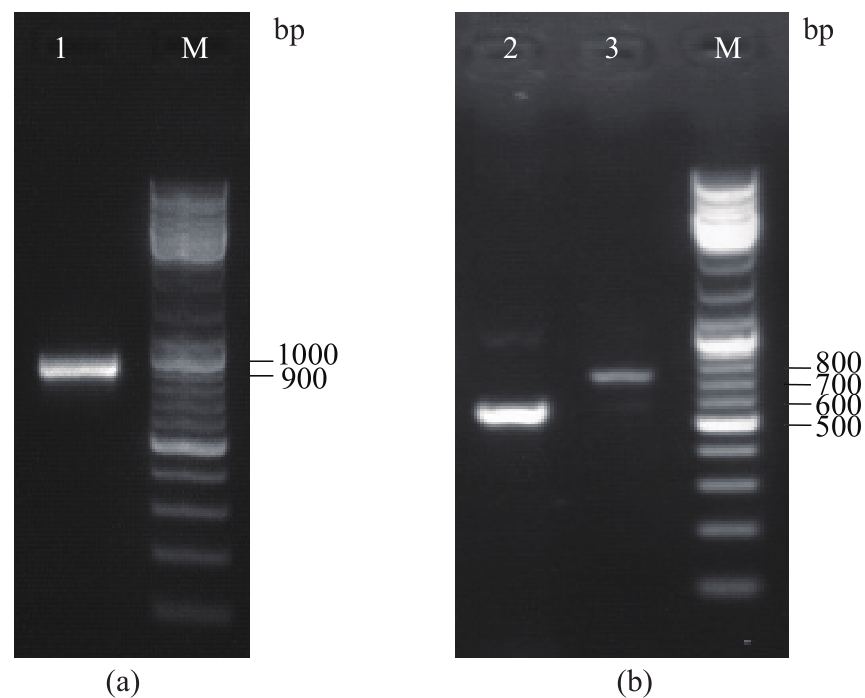
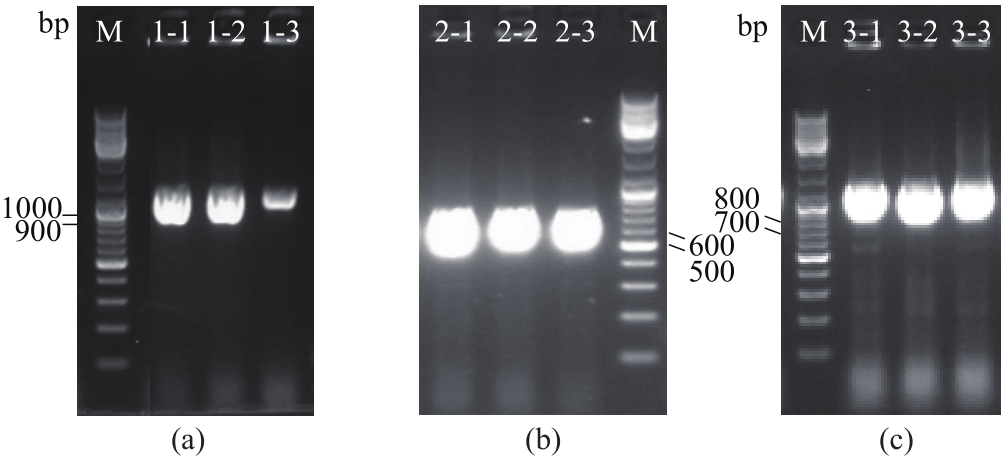
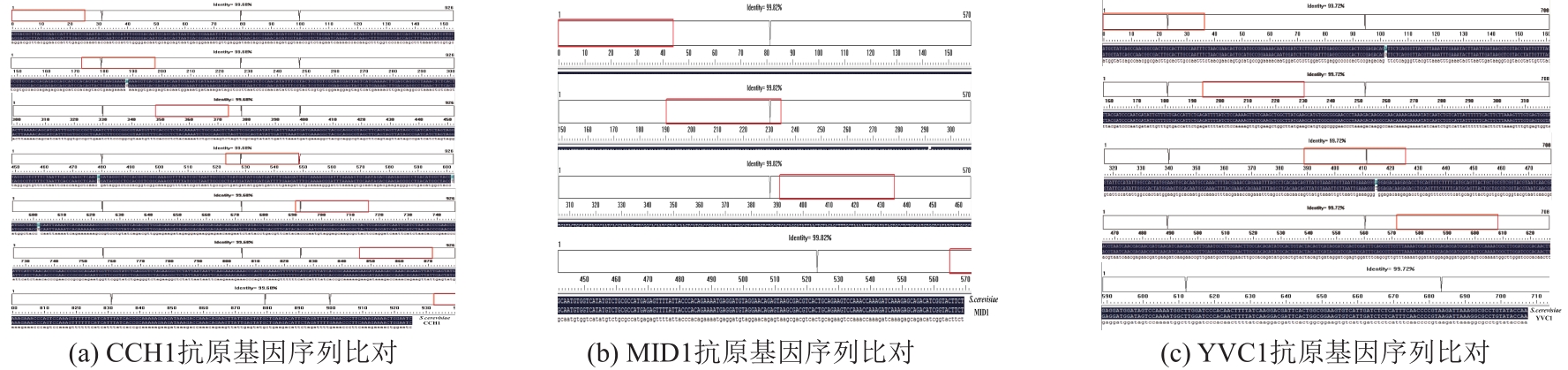
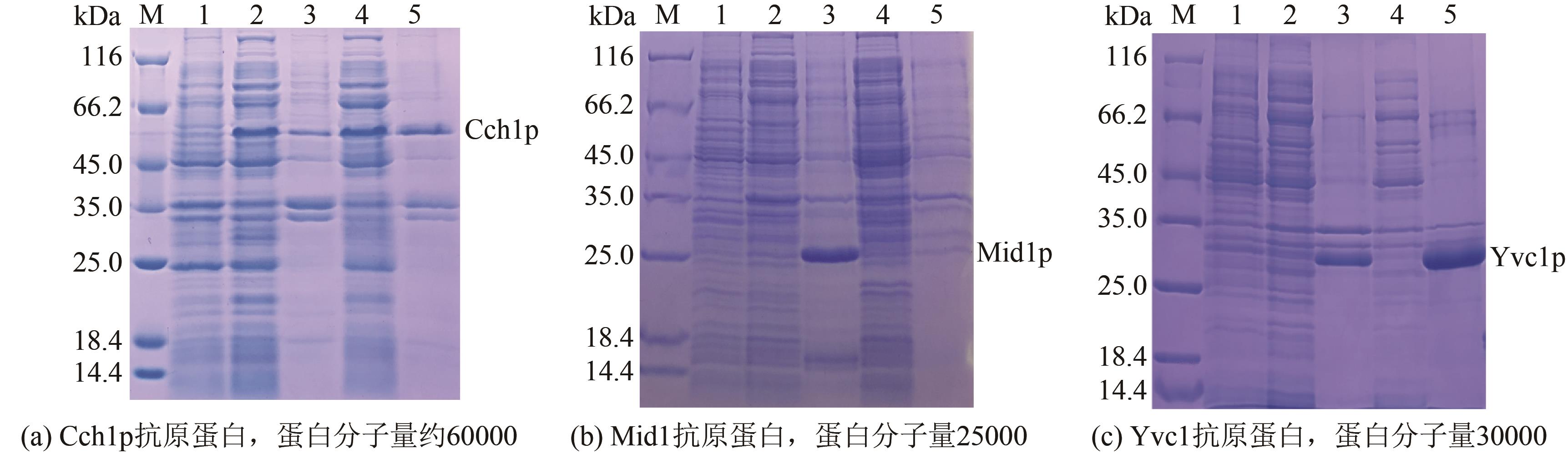
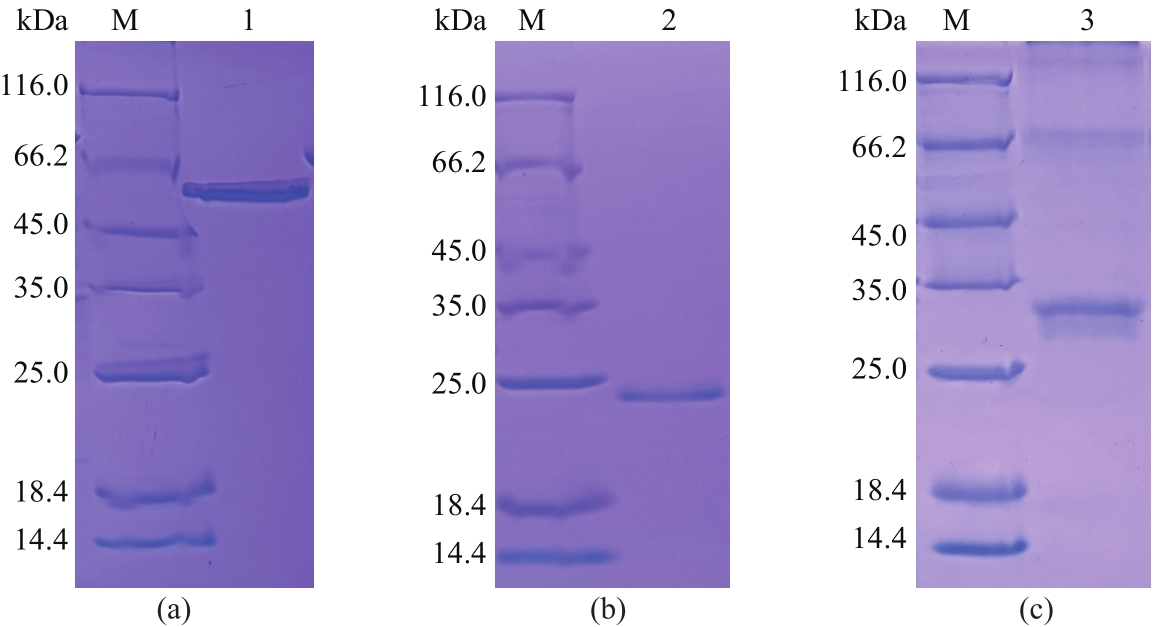
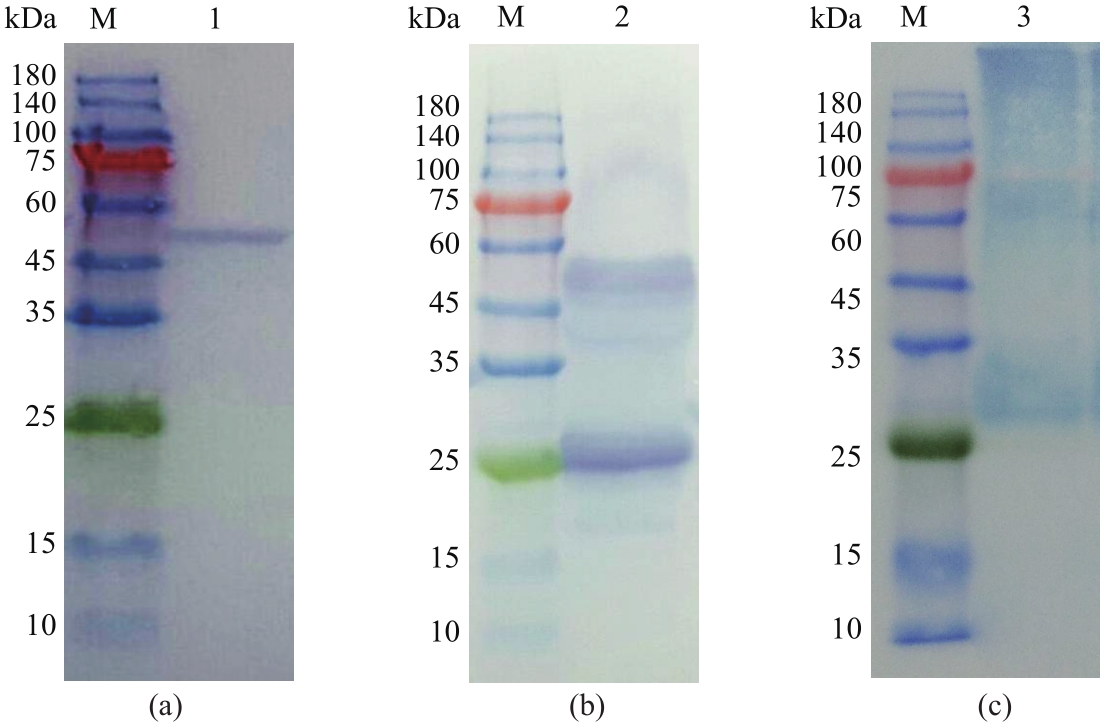
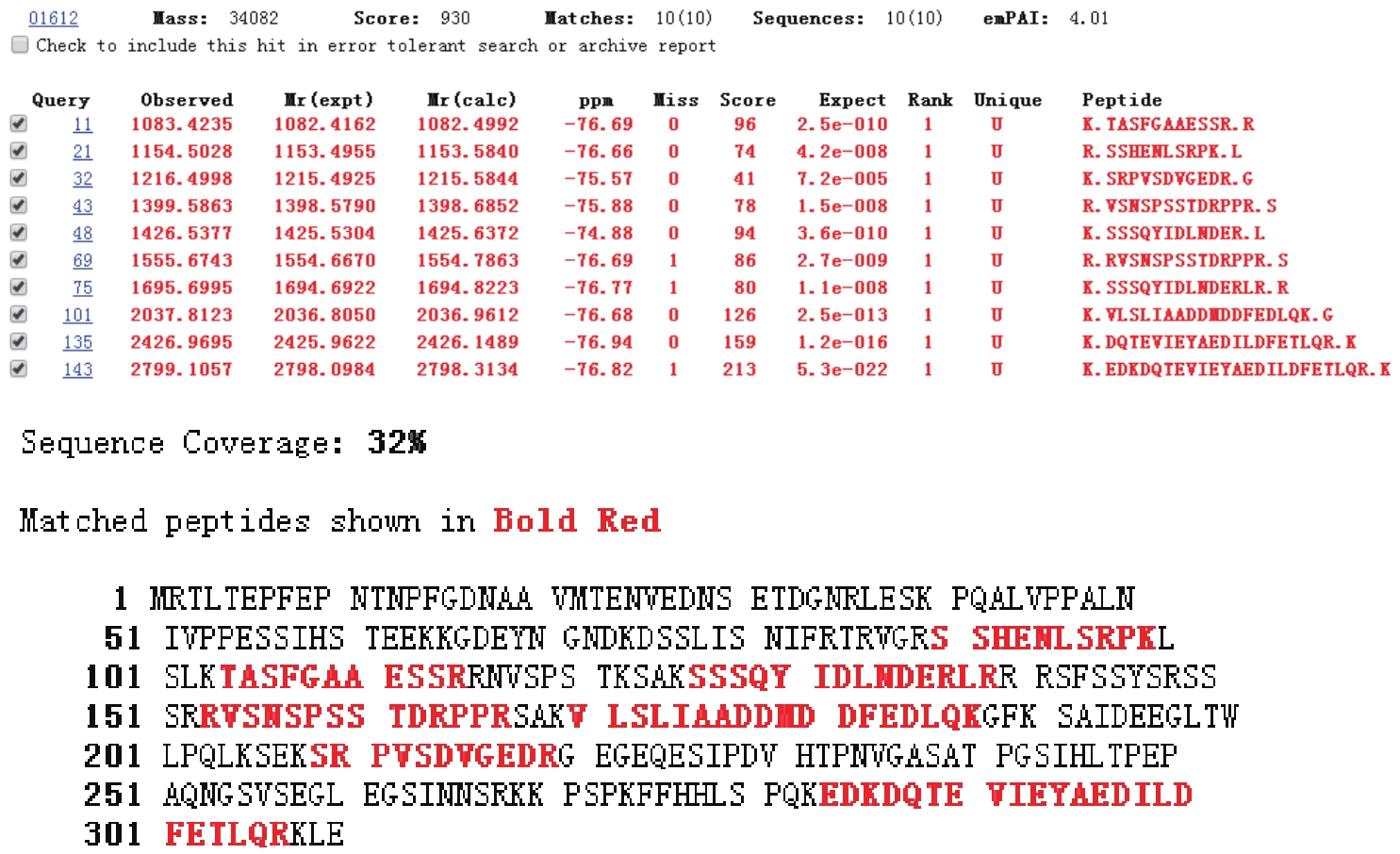
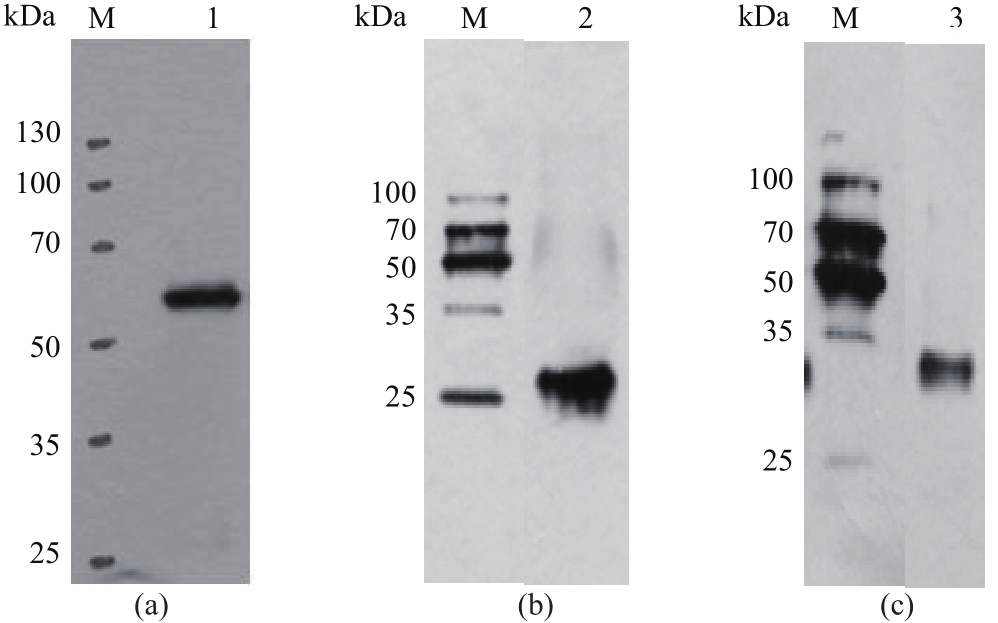
以酿酒酵母电压门控钙通道膜蛋白(Cch1p)、牵张敏感性钙通道膜蛋白(Mid1p)和瞬时受体电位钙通道膜蛋白(Yvc1p)为研究材料,制备其单克隆抗体。采用生物信息学方法确定3种膜蛋白抗原表位,根据分析结果克隆抗原基因,并进行原核表达和表达产物分析鉴定,通过Ni2+-NTA树脂亲和层析技术获得重组抗原蛋白,免疫小鼠后细胞融合技术制备单克隆抗体,酶联免疫吸附测定(ELISA)检测抗体效价,免疫印迹技术检测单克隆抗体对重组纯化抗原和天然酿酒酵母钙通道膜蛋白的反应性和特异性。生物信息学分析结果表明,Cch1p、Mid1p和Yvc1p抗原表位可能分别位于1~300位氨基酸残基、359~548位氨基酸残基、1~236位氨基酸残基;克隆目的基因条带大小分别为926bp、570bp和708bp,与预期结果一致;原核表达抗原蛋白分子量分别为60000、25000和30000,Western blot检测条带正确;重组纯化抗原免疫BALB/c小鼠,细胞融合技术制备单克隆抗体,ELISA检测显示单克隆抗体效价分别高达1∶256000、1∶128000和1∶64000,Western blot检测到3种重组纯化抗原和天然酿酒酵母钙通道膜蛋白Cch1p、Mid1p和Yvc1p。这些结果说明本文制备的单克隆抗体可以成功用于检测酿酒酵母钙通道膜蛋白Cch1p、Mid1p和Yvc1p表达的相关研究。
中图分类号:
引用本文
董晓宇. 酿酒酵母钙通道膜蛋白单克隆抗体制备及鉴定[J]. 化工进展, 2021, 40(S1): 334-343.
DONG Xiaoyu. Preparation and identification of monoclonal antibodies of calcium channel membrane proteins in Saccharomyces cerevisiae[J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 334-343.
| 引物名称 | 引物序列 |
|---|---|
| RT427-CCH1-16F | AGGACGCTTACGGAACCATT |
| RT427-CCH1-941R | GATTCCAGTTTTCTTTGAAGGGTT |
| RT427-MID1-1F | AGAAGTACCGATGTCTGCTCTTTG |
| RT427-MID1-570R | CGTATCGTCCAATGGATGAATTAC |
| RT427-YVC1-1F | ATGGTATCAGCCAACGGCG |
| RT427-YVC1-708R | TTGGTATACAGGCGCCTTTAATC |
表1 引物信息
| 引物名称 | 引物序列 |
|---|---|
| RT427-CCH1-16F | AGGACGCTTACGGAACCATT |
| RT427-CCH1-941R | GATTCCAGTTTTCTTTGAAGGGTT |
| RT427-MID1-1F | AGAAGTACCGATGTCTGCTCTTTG |
| RT427-MID1-570R | CGTATCGTCCAATGGATGAATTAC |
| RT427-YVC1-1F | ATGGTATCAGCCAACGGCG |
| RT427-YVC1-708R | TTGGTATACAGGCGCCTTTAATC |
| 引物名称 | 引物序列 |
|---|---|
| XA4965F | GACACGACAC |
| XA4965R | GACAC |
| XA1209FXOF | GCCTGGTGCCGCGCGGCAGC |
| XA1209FXOR | CAGTGGTGGTGGTGGTGGTG |
| XA1628F | GACACGACAC |
| XA1628R | GACAC |
表2 引物信息
| 引物名称 | 引物序列 |
|---|---|
| XA4965F | GACACGACAC |
| XA4965R | GACAC |
| XA1209FXOF | GCCTGGTGCCGCGCGGCAGC |
| XA1209FXOR | CAGTGGTGGTGGTGGTGGTG |
| XA1628F | GACACGACAC |
| XA1628R | GACAC |
| 单克隆抗体 | 蛋白浓度/mg·mL-1 | 不同抗体稀释率下的吸光值 | 空白对照 | 阴性对照 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1000① | 2000 | 4000 | 8000 | 16000 | 32000 | 64000 | 128000 | 256000 | ||||
| 抗Cch1p 抗体 | 2.6 | 0.934 | 0.903 | 0.849 | 0.809 | 0.661 | 0.494 | 0.319 | 0.227 | 0.159 | 0.051 | 0.042 |
| 抗Mid1p 抗体 | 1.0 | 0.994 | 0.985 | 0.871 | 0.738 | 0.551 | 0.368 | 0.260 | 0.196 | 0.129 | 0.071 | 0.076 |
| 抗Yvc1p 抗体 | 5.5 | 1.944 | 1.860 | 1.677 | 0.910 | 0.790 | 0.393 | 0.268 | 0.160 | 0.121 | 0.076 | 0.069 |
表3 单克隆抗体浓度和效价
| 单克隆抗体 | 蛋白浓度/mg·mL-1 | 不同抗体稀释率下的吸光值 | 空白对照 | 阴性对照 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1000① | 2000 | 4000 | 8000 | 16000 | 32000 | 64000 | 128000 | 256000 | ||||
| 抗Cch1p 抗体 | 2.6 | 0.934 | 0.903 | 0.849 | 0.809 | 0.661 | 0.494 | 0.319 | 0.227 | 0.159 | 0.051 | 0.042 |
| 抗Mid1p 抗体 | 1.0 | 0.994 | 0.985 | 0.871 | 0.738 | 0.551 | 0.368 | 0.260 | 0.196 | 0.129 | 0.071 | 0.076 |
| 抗Yvc1p 抗体 | 5.5 | 1.944 | 1.860 | 1.677 | 0.910 | 0.790 | 0.393 | 0.268 | 0.160 | 0.121 | 0.076 | 0.069 |
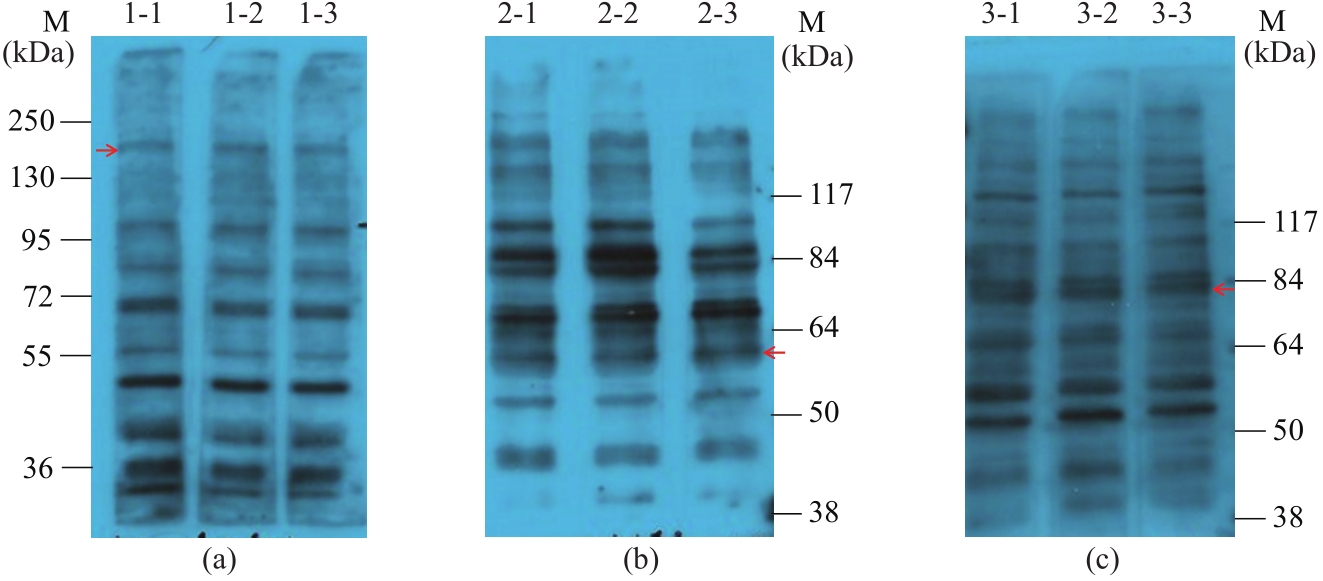
图9 酿酒酵母天然钙通道膜蛋白与单克隆抗体的Western blot分析M—蛋白质标准品(36000~250000;38000~117000);1-1~3—天然Cch1蛋白,蛋白分子量234600;2-1~3—天然Mid1蛋白,蛋白分子量61500;3-1~3—天然Yvc1蛋白,蛋白分子量78000
| 1 | VEYS K, LABRO A J, SCHUTTER E De, et al. Quantitative single-cell ion-channel gene expression profiling through an improved qRT-PCR technique combined with whole cell patch clamp[J]. Journal of Neuroscience Methods, 2012, 209(1): 227-234. |
| 2 | TADA T, OHMORI M, IIDA H. Molecular dissection of the hydrophobic segments H3 and H4 of the yeast Ca2+ channel component Mid1[J]. Journal of Biological Chemistry, 2003, 278(11): 9647-9654. |
| 3 | SU Z, ANISHKIN A, KUNG C, SAIMI Y. The core domain as the force sensor of the yeast mechanosensitive TRP channel[J]. Journal of General Physiology, 2011, 138(6): 627-640. |
| 4 | 贾炜娇, 代广斌, 耿国帅, 等. 膜片钳技术在细胞电生理研究方面的最新应用[J]. 高校化学工程学报, 2018, 32(4): 767-778. |
| JIA Weijiao, DAI Guangbin, GENG Guosuai, et al. Recent studies on the application of patch-clamp technique in cellular electrophysiology[J]. Journal of Chemical Engineering of Chinese Universities, 2018, 32(4): 767-778. | |
| 5 | CHEN P, ZHANG W, ZHOU J, et al. Development of planar patch clamp technology and its application in the analysis of cellular electrophysiology[J]. Progress in Natural Science, 2009, 19(2): 153-160. |
| 6 | FOURATI Z, HOWARD R J, HEUSSER S A, et al. Structural basis for a bimodal allosteric mechanism of general anesthetic modulation in pentameric ligand-gated ion channels[J]. Cell Reports, 2018, 23(4): 993-1004. |
| 7 | THONGHIN N, KARGAS V, CLEWS J, et al. Cryo-electron microscopy of membrane proteins[J]. Methods, 2018, 147: 176-186. |
| 8 | PATINO-GARCIA D, ROCHA-PEREZ N, MORENO R D, et al. Antigen retrieval by citrated solution improves western blot signal[J]. MethodsX, 2019, 6: 464-468. |
| 9 | SUURS F V, HOOGE M N L, VRIES E G E D, et al. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges[J]. Pharmacology & Therapeutics, 2019, 201: 103-119. |
| 10 | SINGH A. Antibodies: monoclonal and polyclonal[M]//CHAUDHARY S, AGARWAL A, VERMA A S. Animal Biotechnology. Salt Lake: Academic Press, 2014: 265-287. |
| 11 | 任建委, 小扎桑. 单克隆抗体技术的基本原理、改进及应用[J]. 高原科学研究, 2018(4): 110-115. |
| REN Jianwei, XIAO Zasang. Basic principle, improvement and application of monoclonal antibody technology[J]. Plateau Science Research, 2018(4): 110-115. | |
| 12 | ROSENSTEIN S, VAISMAN-MENTESH A, LEVY L, et al. Production of F(ab’)2 from monoclonal and polyclonal antibodies[J]. Current Protocols in Molecular Biology, 2020, 131(1): e119. |
| 13 | PAIDHUNGAT M, GARRETT S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1 (Ts) growth defect[J]. Molecular and Cellular Biology, 1997, 17(11): 6339-6347. |
| 14 | KANZAKI M, NAGASAWA M, KOJIMAL I, et al. Molecular identification of a eukaryotic, stretch-activated nonselective cation channel[J]. Science, 1999, 285(5429): 882-886. |
| 15 | PALMER C P, ZHOU X L, LIN J, et al. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane[J]. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(14): 7801-7805. |
| 16 | AMINI M, WANG H, BELKACEMI A, et al. Identification of inhibitory Ca2+ binding sites in the upper vestibule of the yeast vacuolar TRP channel[J]. iScience, 2019, 11: 1-12. |
| 17 | TENG J, IIDA K, IMAI A, et al. Hyperactive and hypoactive mutations in Cch1, a yeast homologue of the voltage-gated calcium-channel pore-forming subunit[J]. Microbiology, 2013, 159(Pt 5): 970-979. |
| 18 | CHANDEL A, DAS K K, BACHHAWAT A K. Glutathione depletion activates the yeast vacuolar transient receptor potential channel, Yvc1p, by reversible glutathionylation of specific cysteines[J]. Molecular Biology of the Cell, 2016, 27(24): 3913-3925. |
| 19 | YOSHIMURA H, TADA T, IIDA H. Subcellular localization and oligomeric structure of the yeast putative stretch-activated Ca2+ channel component Mid1[J]. Experimental Cell Research, 2004, 293(2): 185-195. |
| 20 | CHANDEL A, BACHHAWAT A K. Redox regulation of the yeast voltage-gated Ca2+ channel homology Cch1p, by glutathionylation of specific cysteine residues[J]. Journal of Cell Science, 2017, 130(14): 2317-2328. |
| 21 | MARTIN D C, KIM H, MACKIN N A, et al. New regulators of a high affinity Ca2+ influx system revealed through a genome-wide screen in yeast[J]. Journal of Biological Chemistry, 2011, 286(12): 10744-10754. |
| 22 | HAMAMOTO S, MORI Y, YABE I, et al. In vitro and in vivo characterization of modulation of the vacuolar cation channel TRPY1 from Saccharomyces cerevisiae[J]. The FEBS Journal, 2018, 285(6): 1146-1161. |
| 23 | LIAO J L, HUANG Y J. Evaluation of protocols used in 2-D electrophoresis for proteome analysis of young rice caryopsis[J]. Genomics Proteomics and Bioinformatics, 2011, 9(6): 229-237. |
| 24 | VU K, BAUTOS J, HONG M P, et al. The functional expression of toxic gene: lessons learned from molecular cloning of CCH1, a high-affinity Ca2+ channel[J]. Analytical Biochemistry, 2009, 393(2): 234-241. |
| 25 | DONG X Y, YUAN X, WANG R J. Interaction of air cold plasma with Saccharomyces cerevisiae in the multi-scale microenvironment for improved ethanol yield[J]. Bioresource Technology, 2021, 323: 124621. |
| 26 | DONG X Y. Fuel ethanol production from sugarcane[M]. London: IntechOpen, 2019: 157-175. |
| 27 | DONG X Y, LIU T, XIONG Y. A novel approach to regulate cell membrane permeability for ATP and NADH formation in Saccharomyces cerevisiae induced by air cold plasma[J]. Plasma Science and Technology, 2017, 19: 024001. |
| 28 | COUCHESNE W E, VLASEK C, KLUKOVICH R, et al. Ethanol induces calcium influx via the Cch1-Mid1 transporter in Saccharomyces cerevisiae[J]. Archives of Microbiology, 2011, 193(5): 323-334. |
| 29 | FISCHER M, SCHNELL N, CHATTAWAY J, et al. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating[J]. FEBS Letters, 1997, 419(2/3): 259-262. |
| 30 | IIDA H, NAKAMURA H, ONO T, et al. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating[J]. Molecular and Cellular Biology, 1994, 14(12): 8259-8271. |
| 31 | BERRIDGE M, BOOTMAN M, RODERICK H. Calcium signaling: dynamics, homeostasis and remodeling[J]. Nature Reviews Molecular Cell Biology, 2003, 4(7): 517-529. |
| 32 | BERTL A, GRADMANN D, SLAYMAN C. Calcium- and voltage-dependent ion channels in Saccharomyces cerevisiae[J]. Philosophical Transactions of the Royal Society B, 1992, 338(1283): 63-72. |
| 33 | 董晓宇, 唐乾, 王仁军, 等. 酿酒酵母钙通道膜蛋白Cch1p、Mid1p和Yvc1p抗原、抗体、制备方法和应用: CN201910468785.8[P]. 2019-11-13. |
| DONG Xiaoyu, TANG Qian, WANG Renjun, et al. Preparation and application of antigens and antibodies of Cch1p, Mid1p and Yvc1p of calcium channel in Saccharomyces cerevisiae: CN201910468785.8[P]. 2019-11-13. |
| [1] | 李琦, 蒋雯怡, 孙雨薇, 吴涛, 赵林果. Aspergillus niger NL-1来源的木聚糖酶的耐热性能改造及其在水解木聚糖中的应用[J]. 化工进展, 2019, 38(02): 1038-1044. |
| [2] | 常鹏程, 于洋, 王颖, 李春. 酿酒酵母高效合成萜类化合物的组合调控策略[J]. 化工进展, 2019, 38(01): 598-605. |
| [3] | 唐瑞琪, 熊亮, 程诚, 赵心清, 白凤武. 纤维素乙醇生产重组酿酒酵母菌株的构建与优化研究进展[J]. 化工进展, 2018, 37(08): 3119-3128. |
| [4] | 樊婧婧, 赵雨佳, 王晨, 李春, 周晓宏. 酿酒酵母乙酰辅酶A精细调控合成萜类化合物研究进展[J]. 化工进展, 2018, 37(07): 2773-2779. |
| [5] | 孙美莉, 刘虎虎, 邬文嘉, 任路静, 黄和, 纪晓俊. 代谢工程改造酵母生产多不饱和脂肪酸的研究进展[J]. 化工进展, 2016, 35(03): 872-878. |
| [6] | 白净, 张璐, 方书起, 陈俊英, 常春, 程晓辉, 杨富颖, 梁腾波. 糖基生物质生产食品化工产品研究进展[J]. 化工进展, 2015, 34(1): 212-218. |
| [7] | 张艳,卢文玉. 酿酒酵母细胞表达异源萜类化合物的研究进展[J]. 化工进展, 2014, 33(05): 1265-1270. |
| [8] | 张强,郭元,韩德明. 酿酒酵母乙醇耐受性的研究进展[J]. 化工进展, 2014, 33(01): 187-192. |
| [9] | 常 佳,费学宁,郝亚超,李彤鲜,朱慧芳. 污水厌氧生物处理监控技术研究进展[J]. 化工进展, 2013, 32(07): 1673-1677. |
| [10] | 吴作军,卢滇楠,张敏莲,刘 铮. 微生物分子生态学技术及其在石油污染土壤修复中的应用现状与展望 [J]. 化工进展, 2010, 29(5): 789-. |
| [11] | 郭金玲,穆晓清,徐 岩. 酵母耦合原位分离技术不对称合成(R)-扁桃酸甲酯 [J]. 化工进展, 2010, 29(3): 532-. |
| [12] | 刘媛媛,王 强,刘红芝. 酿酒酵母细胞壁多糖改性研究进展 [J]. 化工进展, 2009, 28(4): 686-. |
| [13] | 包莹玲,陈 砺,严宗诚,王红林. 高密度发酵在酒精生产中的应用进展 [J]. 化工进展, 2009, 28(11): 1996-. |
| [14] | 韩慧龙,刘 铮. 分子生物学技术在土壤生物修复中的应用及展望 [J]. 化工进展, 2007, 26(6): 782-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||