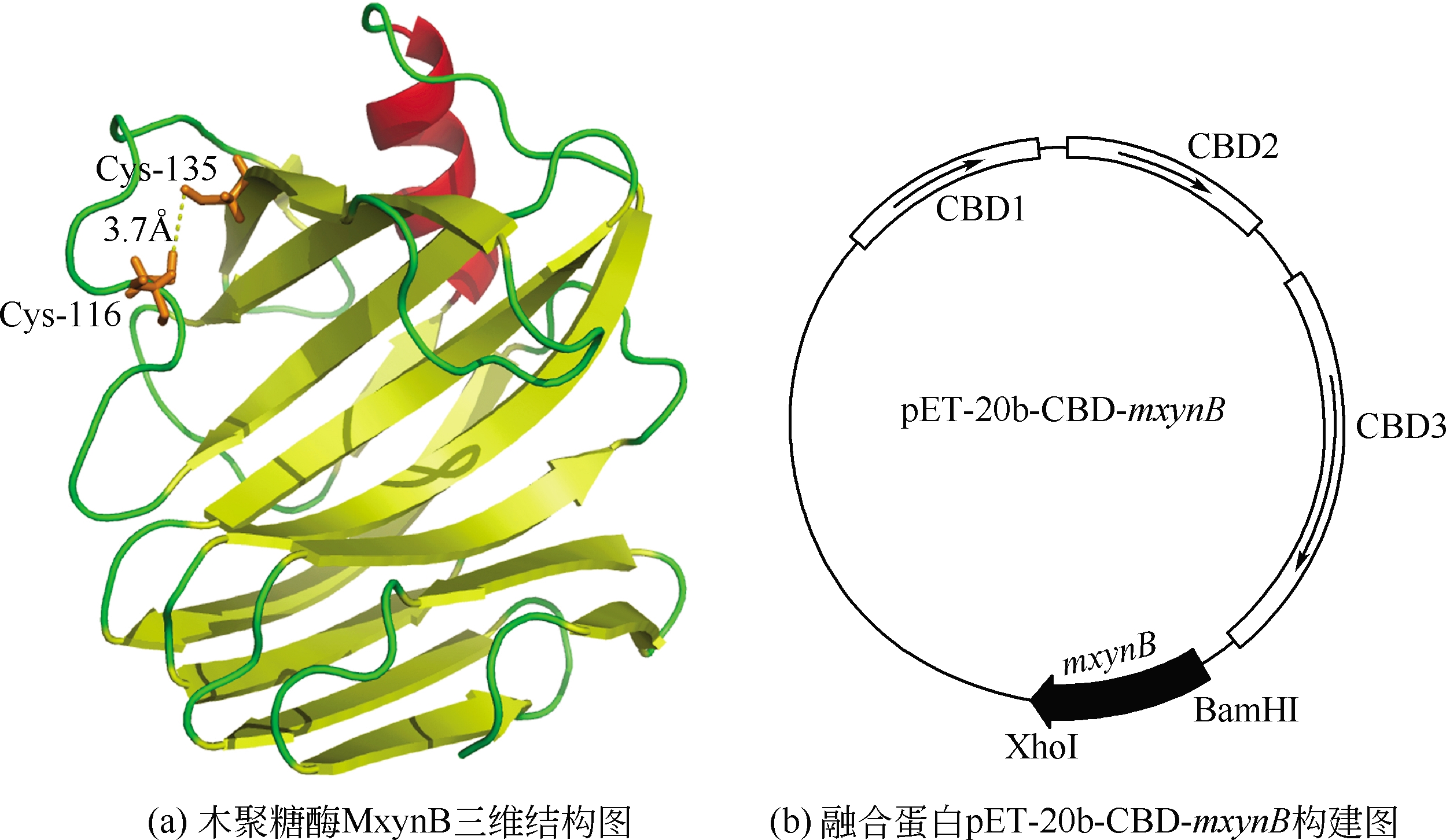
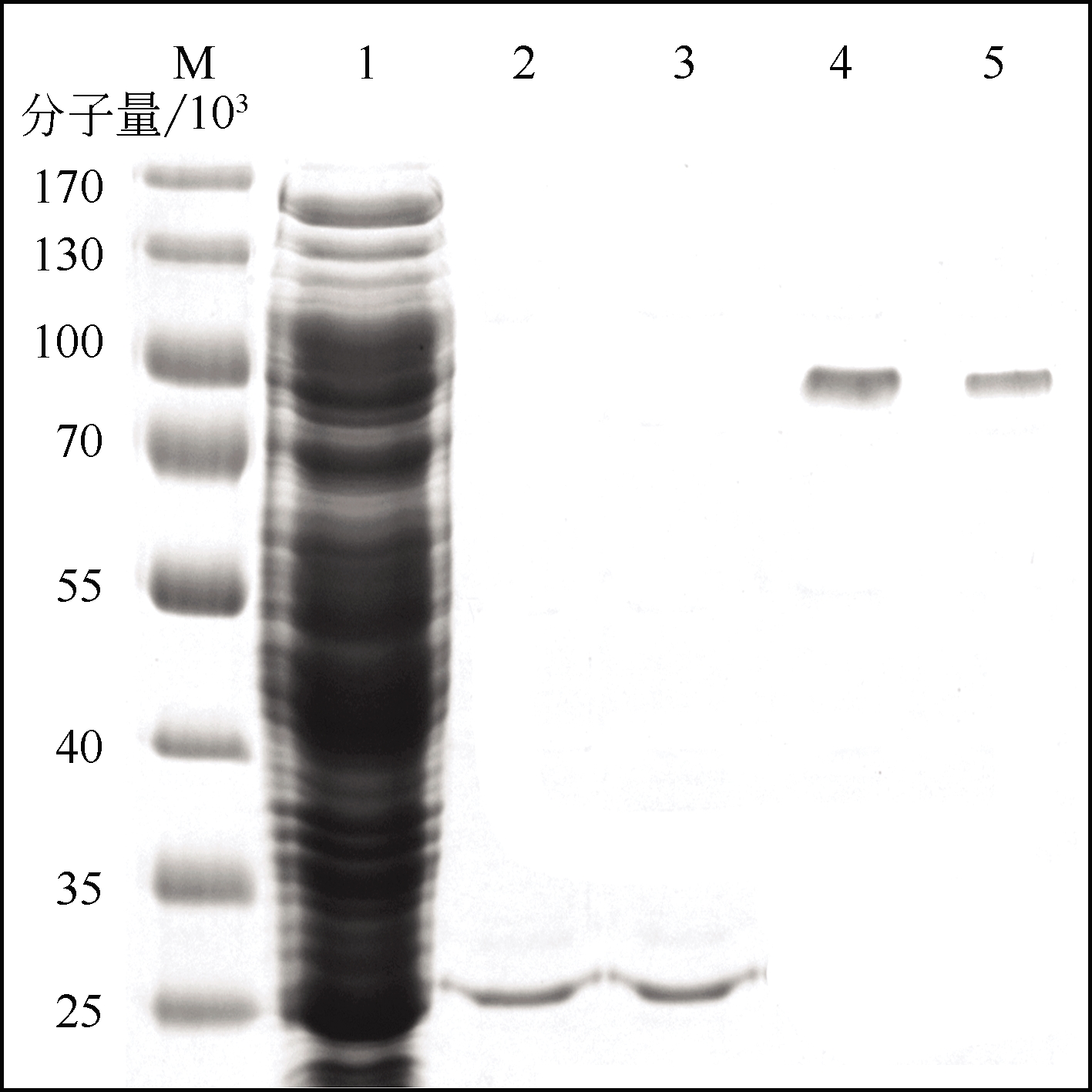
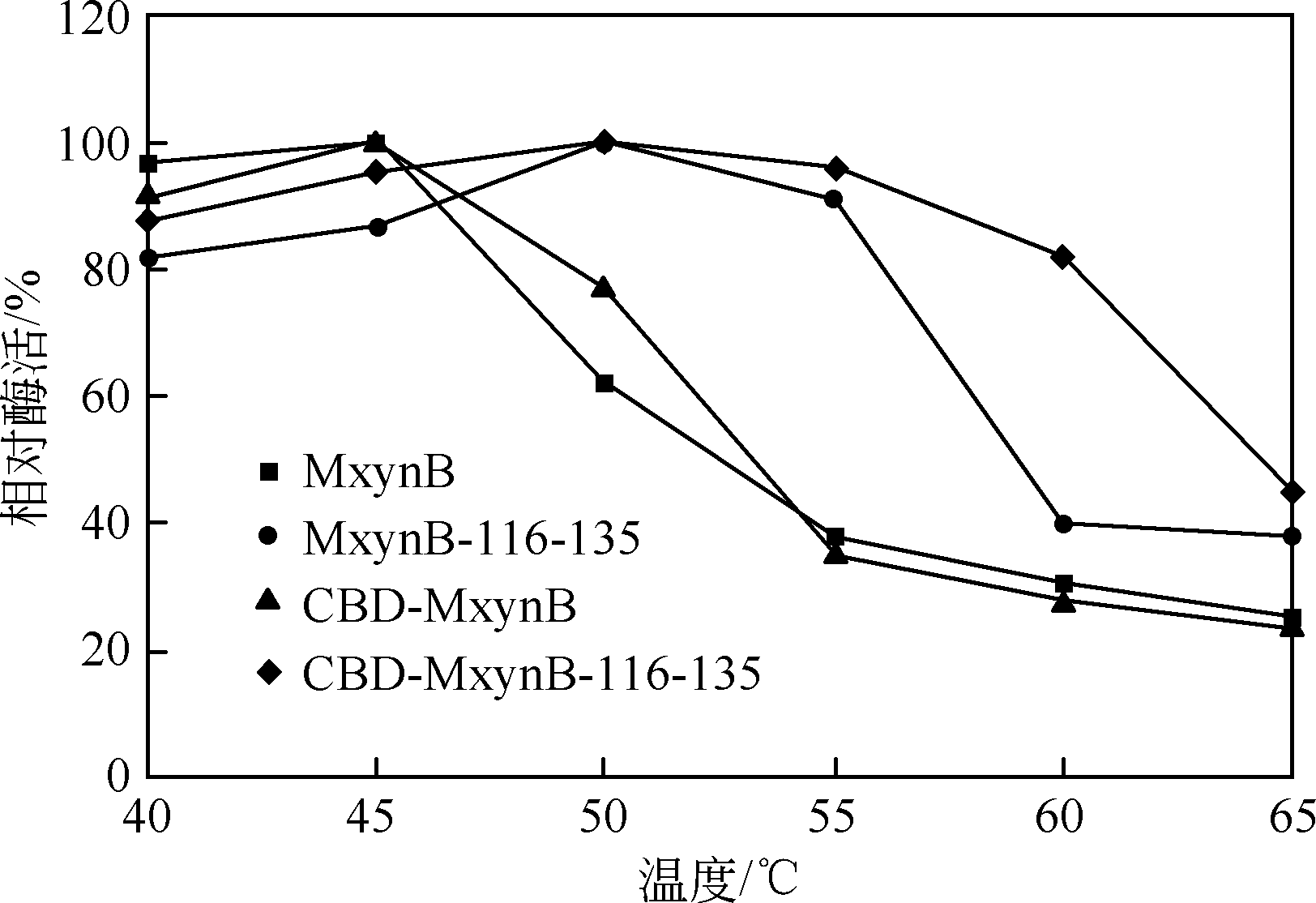
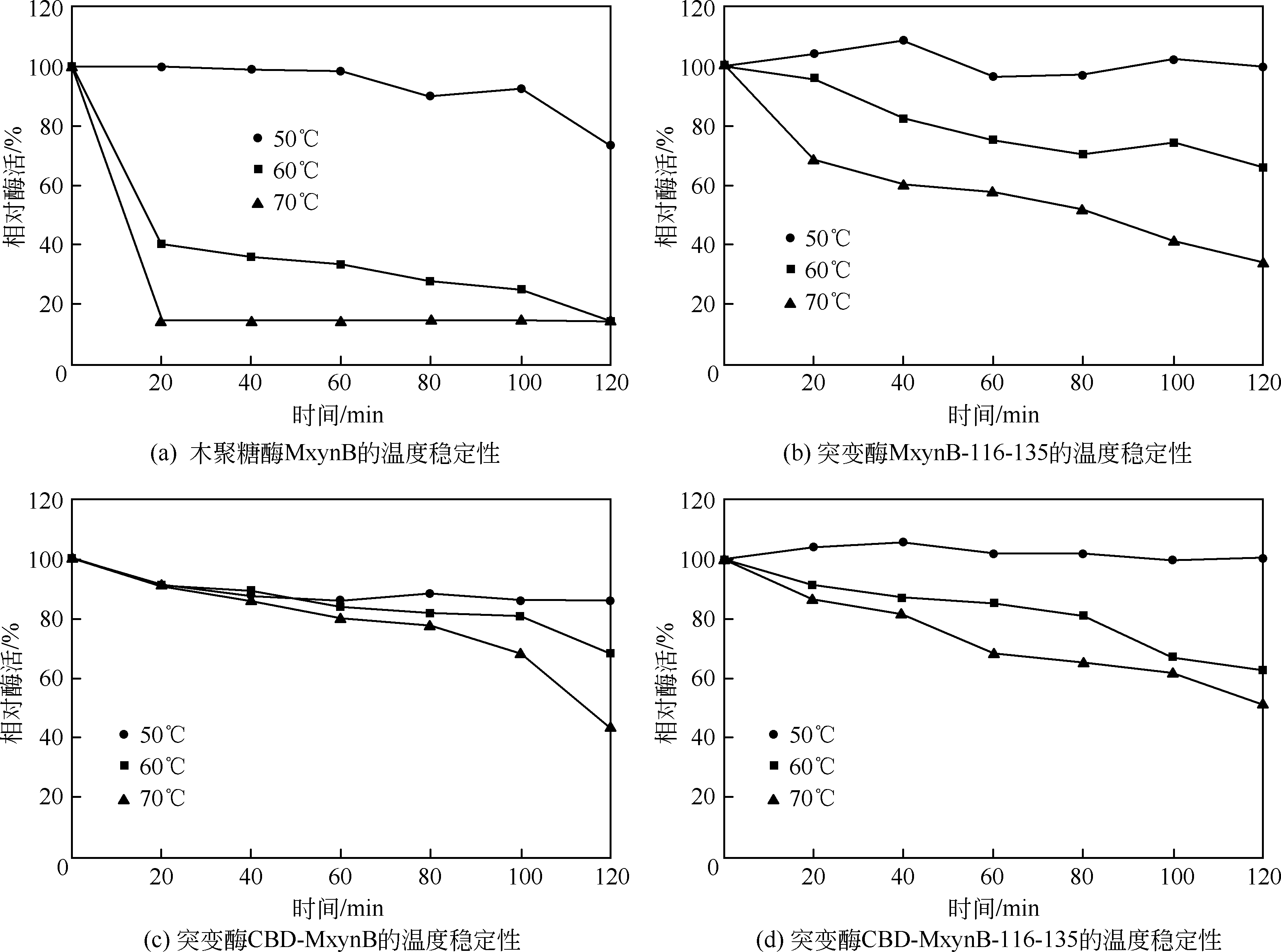
| 1 |
JIANG Z , CONG Q , YAN Q , et al . Characterization of a thermostable xylanase from Chaetomium sp. and its application in Chinese steamed bread[J]. Food Chem., 2010, 120(2): 457-462.
|
| 2 |
COLLINS T , GERDAY C , FELLER G . Xylanases, xylanase families and extremophilic xylanases[J]. FEMS Microbiol. Rev., 2005, 29(1): 3-23.
|
| 3 |
唐伟, 王立东, 张丽萍 . 超声波前处理去麸皮中木聚糖提取率的影响[J]. 食品科技, 2010, 35(5): 191-194.
|
|
TANG W , WANG L D , ZHANG L P . Promotional effect of ultrasonic wave on extraction of xylan from wheat bran[J]. Food Science and Technology, 2010, 35(5): 191-194.
|
| 4 |
BEG Q K, KAPOOR M , MAHAJAN L , et al . Microbiol xylanases and their industrial applications: a review[J]. Appl. Microbiol. Biotechnol., 2001, 56(3/4): 326-338.
|
| 5 |
LIAB K , AZADI P , COLLINS R , et al . Relationships between activities of xylanases and xylan structures[J]. Enzyme Microb. Technol., 2000, 27(1/2): 89-94.
|
| 6 |
LOMBARD V , GOLACONDA R H , DRULA E , et al . The carbohydrate-active enzymes database (CAZy) in 2013[J]. Nucleic Acids Research, 2013, 42(D1): D490-D495.
|
| 7 |
JUTURU V , WU J C . Microbial xylanases: engineering, production and industrial applications[J]. Biotechnology Advances, 2012, 30(6): 1219-1227.
|
| 8 |
何敏超, 张宇, 许敬亮, 等 . 木聚糖酶高产菌筛选研究进展[J]. 化工进展, 2013, 32(1):145-150.
|
|
HE M C , ZHANG Y , XU J L , et al . Research progress of screening of high xylanase-producing strain[J]. Chemical Industry and Engineering Progress, 2013, 32(1): 145-150.
|
| 9 |
裴建军, 李杰, 李琦, 等 . Thermoanaerobacterium saccharolyticum JW/SL-YS485来源的GH11家族木聚糖酶的克隆表达及其应用[J]. 化工进展, 2018, 37(4):1544-1551.
|
|
PEI J J , LI J , LI Q , et al . Expression and characterization of a GH11 xylanase from Thermoanaerobacterium saccharolyticum JW/SL-YS485 and its application in xylooligosaccharide production[J]. Chemical Industry and Engineering Progress, 2018, 37(4):1544-1551.
|
| 10 |
SUBRAMANIYAN S , PREMA P . Biotechnology of microbial xylanases: enzymology, molecular biology, and application[J]. Crit. Rev. Biotechnol., 2002, 22(1):33-64.
|
| 11 |
李同彪, 周晨妍, 王丹丹 . 木聚糖酶热稳定性分子改造的研究进展[J]. 中国生物制品学杂志, 2014, 27(11):1493-1496.
|
|
LI T B , ZHOU C Y , WANG D D . Progress in research on molecular modification of xylanase thermostability[J]. Chin. J. Biologicals, 2014, 27(11): 1493-1496.
|
| 12 |
SUN J Y , LIU M Q , XU Y L , et al . Improvement of the thermostability and catalytic activity of a mesophilic family 11 xylanase by N-terminus replacement[J]. Protein Expression and Purification, 2005, 42(1): 122-130.
|
| 13 |
FONSECAMALDONADO R , VIEIRA D S , ALPONTI J S , et al . Engineering the pattern of protein glycosylation modulates the thermostability of a GH11 xylanase[J]. Journal of Biological Chemistry, 2013, 288(35):25522-25534.
|
| 14 |
刘晓彤, 邬敏辰, 殷欣, 等 . 二硫键对提高木聚糖酶AoXyn11A热稳定性的作用[J]. 食品与生物技术学报, 2014, 33(10):1038-1043.
|
|
LIU X T , WU M C , YIN X , et al . Effect of disulfide bridge on thermostability improvement of the xylanase AoXyn11A[J]. Journal of Food Science and Biotechnology, 2014, 33(10): 1038-1043.
|
| 15 |
XUE H , ZHOU J , YOU C , et al . Amino acid substitutions in the N-terminus, cord and α-helix domains improved the thermostability of a family 11 xylanase XynR8[J]. Journal of Industrial Microbiology and Biotechnology, 2012, 39(9): 1279-1288.
|
| 16 |
LIU L W , CHENG J , CHEN H , et al . Directed evolution of a mesophilic fungal xylanase by fusion of a thernmophilic bacterial carbohydrate-binding module[J]. Process Biochem., 2011, 46(1): 395-398.
|
| 17 |
LI F , YANG S Y , ZHAO L G , et al . Synonymous condon usage bias and overexpression of a synthetic xynB gene from Aspergillus niger NL-1 in Pichia pastoris [J]. Bioresources, 2012, 7(2): 2330-2343.
|
| 18 |
MILLER G L . Use of dinitrosalicylic acid reagent for determination of reducing sugar[J]. Analytical Chem., 1959, 31(3): 426-428.
|
| 19 |
李琦, 陈明真, 赵林果 . 热木聚糖酶基因的克隆表达及其在酶解玉米芯木聚糖中的应用[J]. 林业工程学报, 2017, 2(4): 63-69.
|
|
LI Q , CHEN M Z , ZHAO L G . Cloning and expression of a thermophile GH11 xylanase and its application in xylooligosaccharide production[J]. Journal of Forestry Engineering, 2017, 2(4): 63-69.
|
| 20 |
许正宏, 熊筱晶, 陶文沂 . 低聚木糖的生产及应用研究进展[J]. 食品与发酵工业, 2002, 28(1): 56-59.
|
|
XU Z H , XIONG X J , TAO W Y . Research progress of production and application of xylooligosaccharide[J]. Food and Fermentation Industries, 2002, 28(1): 56-59.
|
 ),蒋雯怡1(
),蒋雯怡1( ),孙雨薇1,吴涛1,2,赵林果1,2(
),孙雨薇1,吴涛1,2,赵林果1,2( )
)
 ),Wenyi JIANG1(
),Wenyi JIANG1( ),Yuwei SUN1,Tao WU1,2,Linguo ZHAO1,2(
),Yuwei SUN1,Tao WU1,2,Linguo ZHAO1,2( )
)