化工进展 ›› 2021, Vol. 40 ›› Issue (5): 2719-2729.DOI: 10.16085/j.issn.1000-6613.2020-1165
生物大分子介导仿生矿化制备磁性纳米粒子的研究进展
- 华侨大学化工学院,福建 厦门 361021
-
收稿日期:2020-06-23出版日期:2021-05-06发布日期:2021-05-24 -
通讯作者:张光亚 -
作者简介:周雁红(1996—),女,硕士研究生,研究方向为纳米材料的仿生制备。E-mail:zhouyh1026@126.com 。 -
基金资助:国家自然科学基金(21376103);福建省自然科学基金(2020J01079);华侨大学研究生科研创新能力培育计划
Research progress of biomacromolecular-mediated biomimetic mineralization for the preparation of magnetic nanoparticles
ZHOU Yanhong( ), LI Xialan, ZHANG Guangya(
), LI Xialan, ZHANG Guangya( )
)
- College of Chemical Engineering, Huaqiao University, Xiamen 361021, Fujian, China
-
Received:2020-06-23Online:2021-05-06Published:2021-05-24 -
Contact:ZHANG Guangya
摘要:
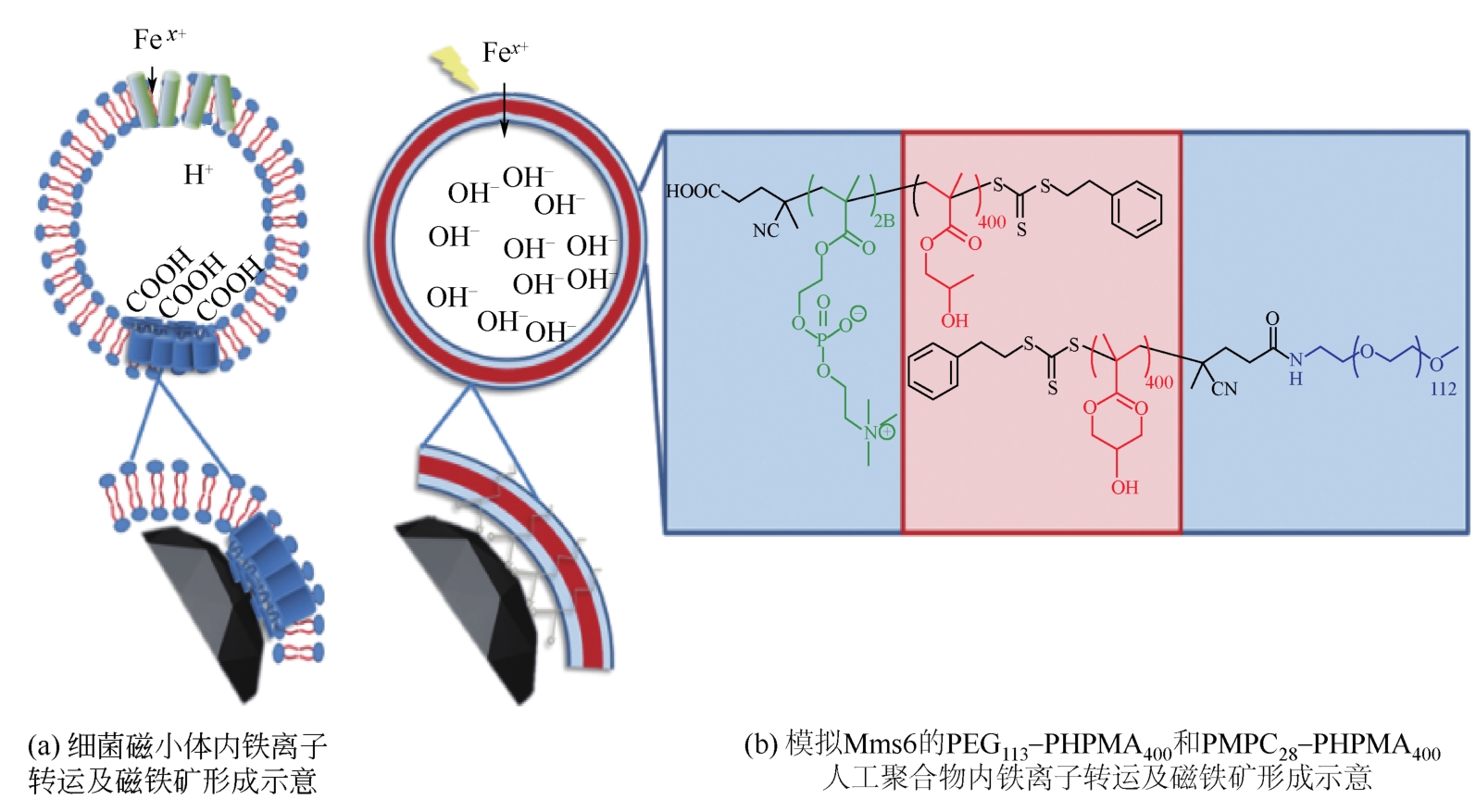
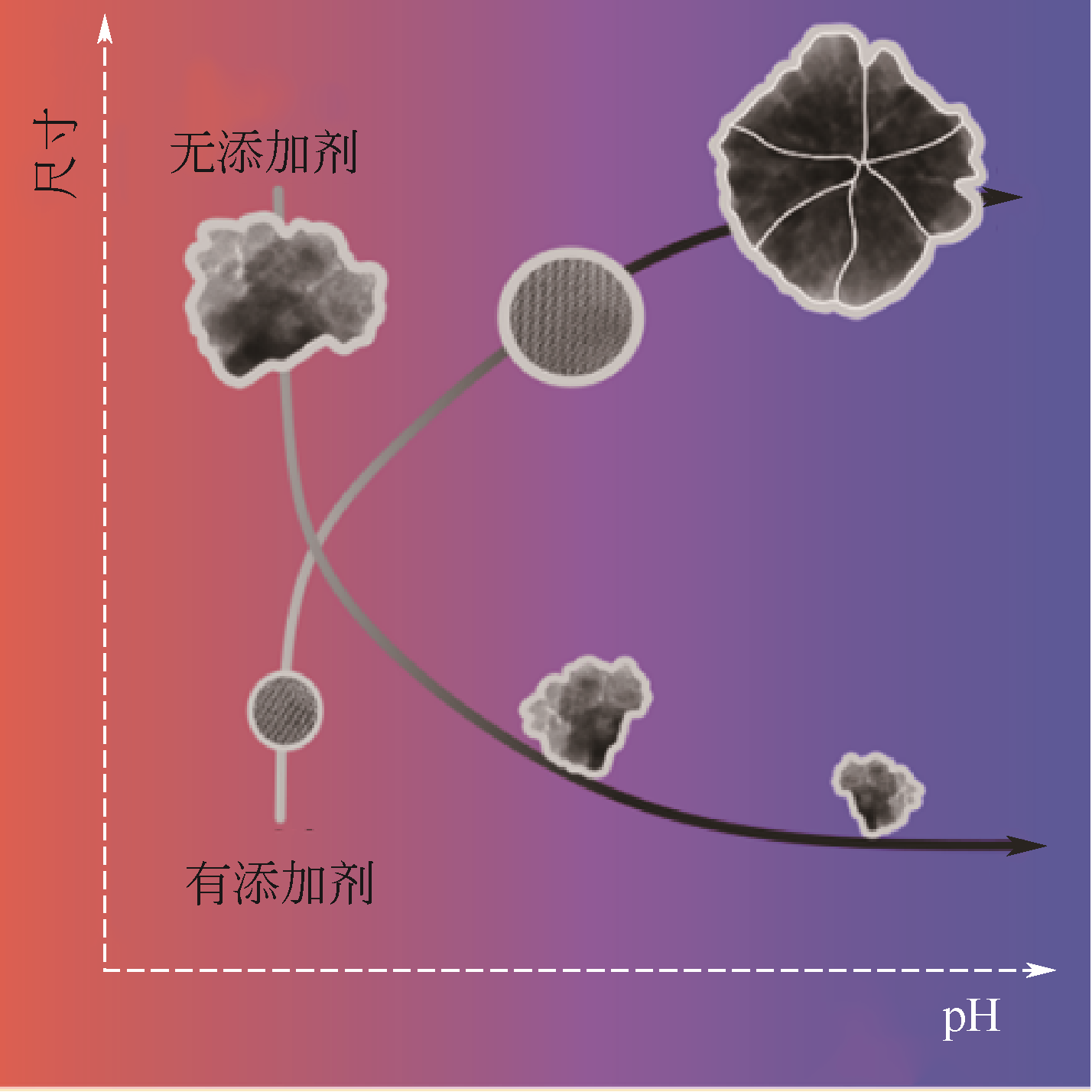
相比其他纳米材料,磁铁矿(Fe3O4)纳米粒子由于具有磁响应性而被广泛应用于酶固定化、定向给药及核酸提取等方面。不同大小和形状的磁铁矿纳米颗粒可用于不同的领域,如晶体尺寸越小的Fe3O4对人体副作用越小,有望用于疾病高效、靶向治疗。近年来,控制Fe3O4纳米粒子大小和形貌的新方法研究逐步成为热点。因此,本文回顾了传统的共沉淀制备磁性纳米颗粒的方法,这些方法需要使用有机溶剂或高温等条件控制,介绍了这些方法存在的环境污染和安全性问题。在此基础上,本文深入介绍了近年来出现的一种受自然界生物矿化启发的生物大分子介导的仿生矿化制备磁性纳米粒子的新趋势,综述了生物大分子蛋白质(或多肽)介导的仿生矿化的最新研究进展,阐释了该方法在磁铁矿(Fe3O4)纳米粒子的大小和形貌控制方面的优缺点,并对其应用前景及面临的挑战进行了展望。
中图分类号:
引用本文
周雁红, 李夏兰, 张光亚. 生物大分子介导仿生矿化制备磁性纳米粒子的研究进展[J]. 化工进展, 2021, 40(5): 2719-2729.
ZHOU Yanhong, LI Xialan, ZHANG Guangya. Research progress of biomacromolecular-mediated biomimetic mineralization for the preparation of magnetic nanoparticles[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2719-2729.
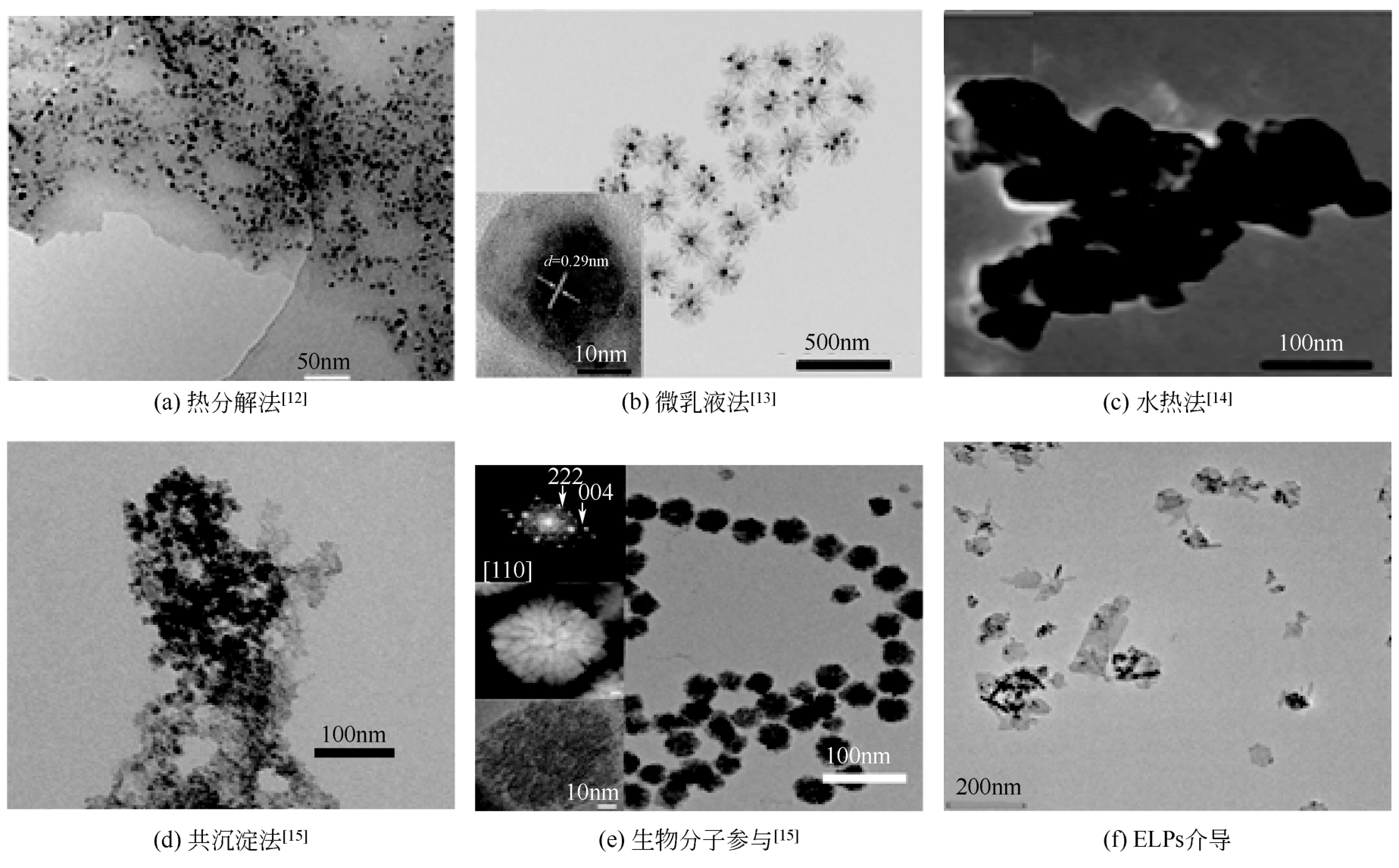
| 制备方法 | 反应条件 | 产物特点 | 形态 | 参考文献 |
|---|---|---|---|---|
| 热分解法 | 高温,需要添加稳定剂,操作复杂 | 高单分散性,粒径分布很窄,平均尺寸一般小于30nm | 由试剂比例、分解温度及熟化时间决定,形态可控,见 | [ |
| 微乳液法 | 需要有机复合物,操作复杂 | 单分散性,粒径分布较窄,平均尺寸一般大于30nm | 形态可控,见 | [ |
| 水热法 | 高温高压,操作简单 | 高单分散性,粒径分布很窄,平均尺寸一般大于100nm | 形态可控,见 | [ |
| 化学沉淀法 | 操作简单,在空气中反应 | 粒径分布较均匀,大小可控 | 由所用盐的种类、反应温度、Fe2+/Fe3+比例、pH及介质的离子强度决定,形态不易控制,见 | [ |
表1 常见合成Fe3O4纳米粒子方法的比较
| 制备方法 | 反应条件 | 产物特点 | 形态 | 参考文献 |
|---|---|---|---|---|
| 热分解法 | 高温,需要添加稳定剂,操作复杂 | 高单分散性,粒径分布很窄,平均尺寸一般小于30nm | 由试剂比例、分解温度及熟化时间决定,形态可控,见 | [ |
| 微乳液法 | 需要有机复合物,操作复杂 | 单分散性,粒径分布较窄,平均尺寸一般大于30nm | 形态可控,见 | [ |
| 水热法 | 高温高压,操作简单 | 高单分散性,粒径分布很窄,平均尺寸一般大于100nm | 形态可控,见 | [ |
| 化学沉淀法 | 操作简单,在空气中反应 | 粒径分布较均匀,大小可控 | 由所用盐的种类、反应温度、Fe2+/Fe3+比例、pH及介质的离子强度决定,形态不易控制,见 | [ |
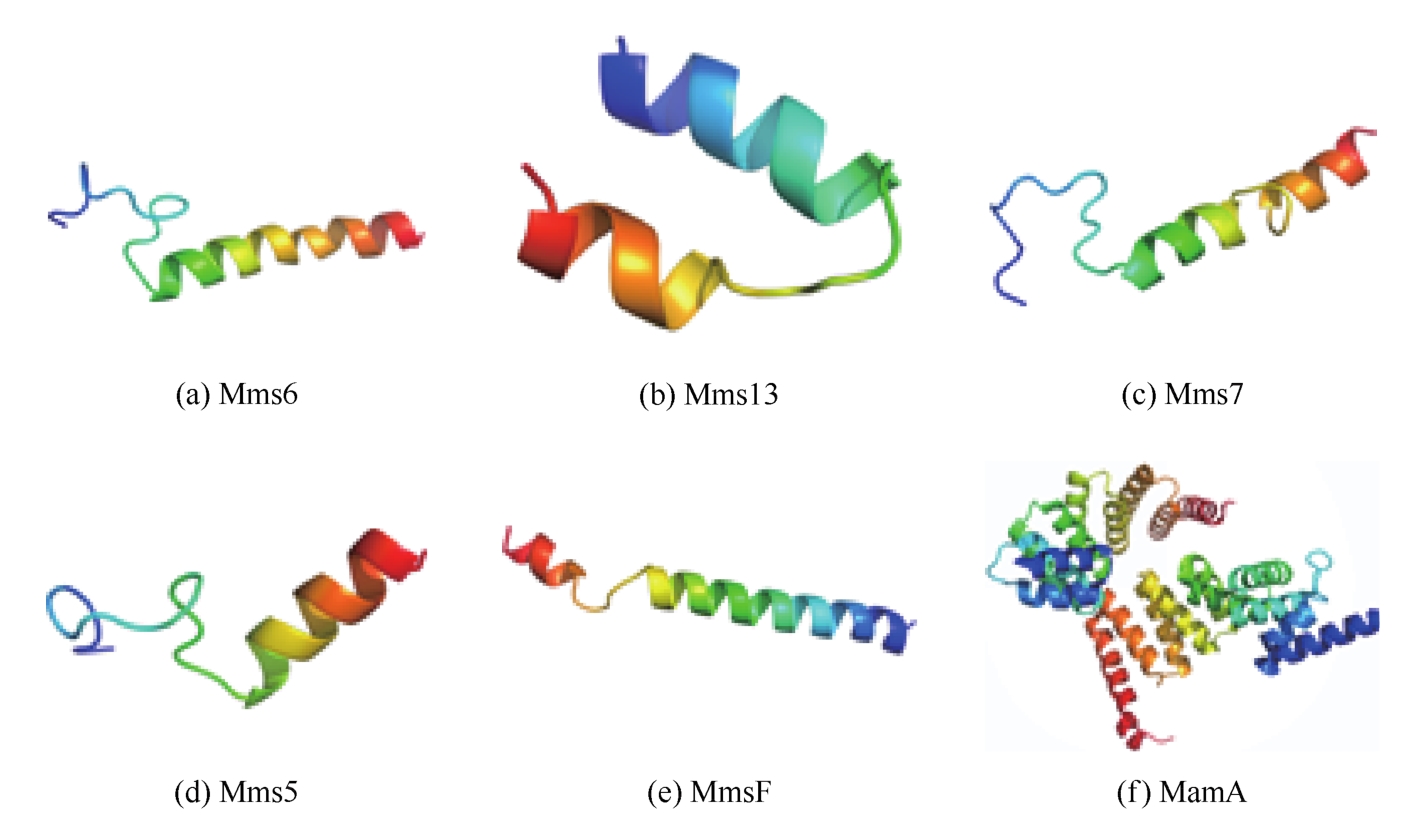
| 蛋白名称 | 结构特点 | 氨基酸组成 | 作用 | 参考文献 |
|---|---|---|---|---|
| Mms6 | 小分子膜蛋白(12500, pI 4.5),包括一个跨膜螺旋,如 | 主要由一个N端疏水区和含有多个酸性氨基酸的C端亲水区组成。C末端区域含有紧密的羧基和羟基,与铁离子结合。这些蛋白质N端具有共同的序列LGLGLGAWGPXXLGXXGXAGA。此外,中间和C端之间的区域含有一些共同的氨基酸,如赖氨酸(Lys)、酪氨酸(Tyr)和精氨酸(Arg) | 定位于磁小体,只存在于趋磁细菌中,与细菌磁铁矿晶体紧密结合。可以与铁离子结合,在成核和磁铁矿晶体生长过程中具有重要作用。其中,以重组蛋白形式表达的MamC和MamD会影响磁铁矿晶体的尺寸,缺失MamC和MamD会导致磁铁矿晶体尺寸的适度减小。 | [ |
| MamC(Mms13) | 小分子膜蛋白(12400, pI 7.2)有两个跨膜螺旋,由一个长度为21个残基的小肽连接,如 | [ | ||
| MamD(Mms7) | 小分子膜蛋白,7000,pI 5.9,如 | [ | ||
| MamG(Mms5) | 小分子膜蛋白,5000,pI 6.1,如 | [ | ||
| MmsF | MmsF包括3个跨膜螺旋,N端在细胞质中,而C端面对磁小体,如 | 磁小体内的环(C端和第一和第二螺旋之间的环)含有高度保守的残基 | 除MmsF会导致产生更小且畸形的磁小体,在控制形态方面至关重要。MmsF中的环状结构可以介导MmsF与矿物质或其他生物矿化蛋白的相互作用 | [ |
| Mms16(BMP膜特异性GTP酶) | — | — | BMP膜特异性Mms16启动细胞质膜的内陷,形成细胞内囊泡 | [ |
表2 生物矿化相关蛋白结构及功能特征
| 蛋白名称 | 结构特点 | 氨基酸组成 | 作用 | 参考文献 |
|---|---|---|---|---|
| Mms6 | 小分子膜蛋白(12500, pI 4.5),包括一个跨膜螺旋,如 | 主要由一个N端疏水区和含有多个酸性氨基酸的C端亲水区组成。C末端区域含有紧密的羧基和羟基,与铁离子结合。这些蛋白质N端具有共同的序列LGLGLGAWGPXXLGXXGXAGA。此外,中间和C端之间的区域含有一些共同的氨基酸,如赖氨酸(Lys)、酪氨酸(Tyr)和精氨酸(Arg) | 定位于磁小体,只存在于趋磁细菌中,与细菌磁铁矿晶体紧密结合。可以与铁离子结合,在成核和磁铁矿晶体生长过程中具有重要作用。其中,以重组蛋白形式表达的MamC和MamD会影响磁铁矿晶体的尺寸,缺失MamC和MamD会导致磁铁矿晶体尺寸的适度减小。 | [ |
| MamC(Mms13) | 小分子膜蛋白(12400, pI 7.2)有两个跨膜螺旋,由一个长度为21个残基的小肽连接,如 | [ | ||
| MamD(Mms7) | 小分子膜蛋白,7000,pI 5.9,如 | [ | ||
| MamG(Mms5) | 小分子膜蛋白,5000,pI 6.1,如 | [ | ||
| MmsF | MmsF包括3个跨膜螺旋,N端在细胞质中,而C端面对磁小体,如 | 磁小体内的环(C端和第一和第二螺旋之间的环)含有高度保守的残基 | 除MmsF会导致产生更小且畸形的磁小体,在控制形态方面至关重要。MmsF中的环状结构可以介导MmsF与矿物质或其他生物矿化蛋白的相互作用 | [ |
| Mms16(BMP膜特异性GTP酶) | — | — | BMP膜特异性Mms16启动细胞质膜的内陷,形成细胞内囊泡 | [ |
| 1 | DAVID L M, JASON T A, MOISE N, et al. Toward a structure determination method for biomineral-associated protein using combined solid-state NMR and computational structure prediction[J]. Structure, 2010, 18(12): 1678-1687. |
| 2 | DARJA L, ALENKA M. Anisotropic magnetic nanoparticles: a review of their properties, syntheses and potential applications[J]. Prog. Mater. Sci., 2018, 95(5): 286-328. |
| 3 | NUDELMAN F, SOMMERDIJK N A. Biomineralization as an inspiration for materials chemistry[J]. Angew. Chem. Int. Ed., 2012, 51(27): 6582-6596. |
| 4 | MALDONADO C L, UNNI M, RINALDI C. Magnetic characterization of iron oxide nanoparticles for biomedical applications[J]. Methods Mol. Biol., 2017, 1570: 47-71. |
| 5 | AHN J M, SUNG H J, YOON Y H, et al. Integrated glycoproteomics demonstrates fucosylated serum paraoxonase 1 alterations in small cell lung cancer[J]. Mol. Cell. Proteomics., 2014, 13(1): 30-48. |
| 6 | XIA S, KANCHANAWONG P. Nanoscale mechanobiology of cell adhesions[J]. Semin. Cell Dev. Biol., 2017, 71: 53-67. |
| 7 | LUO Z, ZHENG K, XIE J. Engineering ultrasmall water-soluble gold and silver nanoclusters for biomedical applications[J]. Chem. Commun., 2014, 50(40): 5143-5155. |
| 8 | VALVERDE T C, MONTALBAN L M, PEREZ G T, et al. Size control of in vitro synthesized magnetite crystals by the MamC protein of Magnetococcus marinusstrain MC-1[J]. Appl. Microbiol. Biotechnol., 2015, 99(12): 5109-5121. |
| 9 | LENDERS J M, ZOPE H R, YAMAGISHI A, et al. Bioinspired magnetite crystallization directed by random copolypeptides[J]. Adv. Funct. Mater., 2015, 25(5):711-719. |
| 10 | SHEN L, QIAO Y, GUO Y, et al. Facile co-precipitation synthesis of shape-controlled magnetite nanoparticles[J]. Ceram. Int., 2014, 40(1):1519-1524. |
| 11 | AKBARZADEH A, SAMIEI M, DAVARAN S. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine[J]. Nanoscale Res. Lett., 2012, 7(1): 144-150. |
| 12 | THIMIRI G R D B, KHAN NA, VENKATACHALAM S, et al. Step-by-step protocol for superparamagnetic nanoparticle-based endosome and lysosome isolation from eukaryotic cell[J]. Methods Mol. Biol., 2020, 2125: 167-172. |
| 13 | DAI Y, YANG D, YU D, et al. Engineering of monodisperse core-shell up-conversion dendritic mesoporous silica nanocomposites with a tunable pore size[J]. Nanoscale, 2020, 12(8): 5075-5083. |
| 14 | TANIGUCHI T, WATANEBE T, ICHINOHE S, et al. Nanoscale heterogeneities in CeO2-ZrO2 nanocrystals highlighted by UV-resonant Raman spectroscopy[J]. Nanoscale, 2010, 2(8): 1426-1428. |
| 15 | XIE Y, ZENG P, SIEGEL R A, et al. Magnetic deposition of aerosols composed of aggregated superparamagnetic nanoparticles[J]. Pharm. Res., 2010, 27(5): 855-865. |
| 16 | LI W, FORTNER J D. (Super)paramagnetic nanoparticles as platform materials for environmental applications: from synthesis to demonstration[J]. Front. Environ. Sci. Eng., 2020, 14(5): 77. |
| 17 | ASAB G, ZEREFFA E A, ABDO S T. Synthesis of silica-coated Fe3O4 nanoparticles by microemulsion method: characterization and evaluation of antimicrobial activity[J]. Int. J. Biomater., DOI: 10.1155/2020/4783612. |
| 18 | ANUSHREE C, PHILIP J. A novel approach to enhance high temperature thermal stability of superparamagnetic Fe3O4 nanoparticles[J]. J. Nanosci. Nanotechnol., 2019, 19(9): 5624-5632. |
| 19 | JIANG Z, LIU Q, DEKKERS M J, et al. Control of earth-like magnetic fields on the transformation of ferrihydrite to hematite and goethite[J]. Sci. Rep., 2016, 6: 30395. |
| 20 | HUANG S, CAO P, LI Y, et al. Nucleation and crystallization kinetics of a multicomponent lithium disilicate glass by in situ and real-time synchrotron X-ray diffraction[J]. Cryst. Growth. Des., 2013, 13(9): 4031-4038. |
| 21 | ZHANG C, DU C, LIAO J Y, et al. Synthesis of magnetite hybrid nanocomplexes to eliminate bacteria and enhance biofilm disruption[J]. Biomater. Sci., 2019, 7(7): 2833-2840. |
| 22 | GRUSKIENE R, KRIVOROTOVA T, STANEVICIENE R, et al. Preparation and characterization of iron oxide magnetic nanoparticles functionalized by nisin[J]. Colloid Surfaces B, 2018, 169: 126-134. |
| 23 | GULIZ Ak, AKSU D, APKN E, et al. Delivery of pemetrexed by magnetic nanoparticles: design, characterization, in vitro and in vivo assessment[J]. Prep. Biochem. Biotechnol., 2020, 50(3): 215-225. |
| 24 | AHN T, KIM J H, YANG H M, et al. Formation pathways of magnetite nanoparticles by coprecipitation method[J]. J. Phys. Chem. C, 2012, 116(10): 6069-6076. |
| 25 | LIN J, WANG X, TANG R. Regulations of organism by materials: a new understanding of biological inorganic chemistry[J]. J. Biol. Inorg. Chem., 2019, 24(4): 467-481. |
| 26 | MURSHED M. Mechanism of bone mineralization[J]. Cold Spring Harb. Perspect. Med., 2018, 8(12): a031229. |
| 27 | PAWOLSKI D, HEINTZE C, MEY I, et al. Reconstituting the formation of hierarchically porous silica patterns using diatom biomolecules[J]. J. Struct. Biol., 2018, 204(1): 64-74. |
| 28 | KOTZSCH A, GROGER P, PAWOLSKI D, et al. Silicanin-1 is a conserved diatom membrane protein involved in silica biomineralization[J]. BMC Biol., 2017, 15(1): 65. |
| 29 | KRAJINA B A, PROCTOR A C, SCHOEN A P, et al. Biotemplated synthesis of inorganic materials: an emerging paradigm for nanomaterial synthesis inspired by nature[J]. Prog. Mater. Sci., 2018, 91(1): 1-23. |
| 30 | GILBERT P U P A, PPRTER S M, SUN C Y, et al. Biomineralization by particle attachment in early animals[J]. Proc. Natl. Acad. Sci. USA, 2019, 116(36): 17659-17665. |
| 31 | MCCAUSLAND HC, KOMEILI A. Magnetic genes: studying the genetics of biomineralization in magnetotactic bacteria[J]. PLoS Genet., 2020, 16(2): e1008499. |
| 32 | MAEDA K, KOBAYASHI Y, KOIDE M, et al. The regulation of bone metabolism and disorders by wnt signaling[J]. Int. J. Mol. Sci., 2019, 20(22): 5525. |
| 33 | POHL A, BERGER F, SULLAN R M A, et al. Decoding biomineralization: interaction of a Mad10-derived peptide with magnetite thin films[J]. Nano Lett., 2019, 19(11): 8207-8215. |
| 34 | THOMAS J, WORCH H, KRUPPKE B, et al. Contribution to understand the biomineralization of bones[J]. J. Bone. Miner. Metab., 2020, 38(4): 456-468. |
| 35 | FANG P A, CONWAY J F, MARGOLIS H C, et al. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale[J]. Proc. Natl. Acad. Sci. USA, 2011, 108(34): 14097-14102. |
| 36 | PEIGNEUX A, JABALERA Y, VIVAS M A F, et al. Tuning properties of biomimetic magnetic nanoparticles by combining magnetosome associated proteins[J]. Sci. Rep, 2019, 9(1): 8804. |
| 37 | ARAKAKI A, WEBB J, MATSUNAGA T. A novel protein tightly bound to bacterial magnetic particles in Magnetospirillum magneticum strain AMB-1[J]. J. Biol. Chem., 2003, 278(10): 8745-8750. |
| 38 | YAMAGISHI A, NARUMIYA K, TANAKA M, et al. Core amino acid residues in the morphology-regulating protein, Mms6, for intracellular magnetite biomineralization[J]. Sci. Rep., 2016, 6(1): 35670. |
| 39 | LENDERS, JOS J M, BAWAZER, et al. Combinatorial evolution of biomimetic magnetite nanoparticles[J]. Adv. Funct. Mater., 2017, 27(10): 1604863. |
| 40 | RAWLINGS A E, SOMNER L A, FITZPATRICK M M, et al. Artificial coiled coil biomineralisation protein for the synthesis of magnetic nanoparticles[J]. Nat. Commun., 2019, 10(1): 2873. |
| 41 | WOOD R. Exploring the drivers of early biomineralization[J]. Emerg. Top. Life Sci., 2018, 2(2): 201-212. |
| 42 | BARBOSA P F, LAGOEIRO L. Crystallographic texture of the magnetite-hematite transformation: evidence for topotactic relationships in natural samples from Quadrilatero Ferrifero, Brazil[J]. Am. Mineral., 2010, 95(1): 118-125. |
| 43 | BAIN J, LEGGE C J, BEATTIE D L, et al. A biomimetic magnetosome: formation of iron oxide within carboxylic acid terminated polymersomes[J]. Nanoscale, 2019, 11(24): 11617-11625. |
| 44 | ARAKAKI A, MASUDA F, AMEMIYA Y, et al. Control of the morphology and size of magnetite particles with peptides mimicking the Mms6 protein from magnetotactic bacteria[J]. J. Colloid Interface Sci., 2010, 343(1): 65-70. |
| 45 | GALLOWAY J M, BRAMBLE J P, RAWLINGS A E, et al. Biotemplated magnetic nanoparticle arrays[J]. Small, 2012, 8(2): 204-208. |
| 46 | BIRD S M, GALLOWAY J M, RAWLINGS A E, et al. Taking a hard line with biotemplating: cobalt-doped magnetite magnetic nanoparticle arrays[J]. Nanoscale, 2015, 7(16): 7340-7351. |
| 47 | MURAT D, FALAHATI V, BERTINETTI L, et al. The magnetosome membrane protein, MmsF, is a major regulator of magnetite biomineralization in Magnetospirillum magneticum AMB-1[J]. Mol. Microbiol., 2012, 85(4): 684-699. |
| 48 | BERNSTEIN E F, SARKAS H W, BOLAND P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients[J]. J. Cosmet. Dermatol., 2020, 19(2): 407-415. |
| 49 | TARTAJ P, MORALES M P, GONZALEZ C T, et al. The iron oxides strike back: from biomedical applications to energy storage devices and photoelectrochemical water splitting[J]. Adv. Mater., 2011, 23(44): 5243-5249. |
| 50 | CHATZIDAKIS M, PRABHUDEV S, SAIDI P, et al. Bulk immiscibility at the edge of the nanoscale[J]. ACS Nano, 2017, 11(11):10984-10991. |
| 51 | FANG G, CHEN H, ZHANG Y, et al. Immobilization of pectinase onto Fe3O4@SiO2-NH2 and its activity and stability[J]. Int. J. Biol. Macromol., 2016, 88: 189-195. |
| 52 | DEY A, LENDERS J J M, SOMMERDIJK N A J M. Bioinspired magnetite formation from a disordered ferrihydrite-derived precursor[J]. Faraday Discussions, 2015, 179: 215-225. |
| 53 | GORDON L M, ROMAN J K, EVERLY R M, et al. Selective formation of metastable ferrihydrite in the chiton tooth[J]. Angew. Chem. Int. Ed., 2014, 53(43): 11506-11509. |
| 54 | BAUMGARTNER J, DEY A, BOMANS P H, et al. Nucleation and growth of magnetite from solution[J]. Nat. Mater., 2013, 12(4): 310-314. |
| 55 | LIU L, PU X, YIN G, et al. Biomimetic mineralization of magnetic iron oxide nanoparticles mediated by bi-functional copolypeptides[J]. Molecules, 2019, 24(7): E1401. |
| 56 | LENDERS J J M, ALTAN C L, BOMANS P H H, et al. A bioinspired coprecipitation method for the controlled synthesis of magnetite nanoparticles[J]. Cryst. Growth Des., 2014, 14(11): 5561-5568. |
| 57 | KUHRTS L, MACIAS S E, TARAKINA N V, et al. Shaping magnetite with poly-L-arginine and pH: from small single crystals to large mesocrystals[J]. J. Phys. Chem., Lett., 2019, 10(18): 5514-5518. |
| 58 | WANG Y, ZHANG X Y, LUO Y L, et al. Dual stimuli-responsive Fe3O4 graft poly(acrylic acid)-block-poly(2-methacryloyloxyethyl ferrocenecarboxylate) copolymer micromicelles: surface RAFT synthesis, self-assembly and drug release applications[J]. J. Nanobiotechnology, 2017, 15(1): 76. |
| 59 | ZHANG K, WANG Z, LI Y, et al. Dual stimuli-responsive N-phthaloylchitosan-graft-(poly(N-isopropylacrylamide)-block-poly(acrylic acid)) copolymer prepared via RAFT polymerization[J]. Carbohydr. Polym., 2013, 92(1): 662-667. |
| 60 | LENDERS J J M, MIRABELLO G, SOMMERDIJK N A J M. Bioinspired magnetite synthesis via solid precursor phases[J]. Chem. Sci. 2016, 7(9): 5624-5634. |
| 61 | WANG X Z, GE H H, ZHANG D D, et al. Oligomerization triggered by foldon: a simple method to enhance the catalytic efficiency of lichenase and xylanase[J]. BMC Biotechnology, 2017, 17(1): 57. |
| 62 | LI C C, ZHANG G Y. The fusions of elastin-like polypeptides and xylanase self-assembled into insoluble active xylanase particles[J]. Journal of Biotechnology, 2014, 177: 60-66. |
| 63 | LIN Y Q, ZHANG G Y. Target-specific covalent immobilization of enzymes from cell lysate on SiO2 nanoparticles for biomass saccharification[J]. ACS Appl. Nano Mater., 2020, 3(1): 44-48. |
| 64 | 邱岳, 林源清, 罗少凡, 等. 类弹性蛋白多肽介导的二氧化硅仿生矿化[J]. 科学通报, 2018, 63(S2): 3037-3046. |
| QIU Y, LIN Y Q, LUO S F, et al. Elastase-like polypeptides mediated biomineralization of silica[J]. Chinese Science Bulletin, 2018, 63(S2): 3037-3046. | |
| 65 | QIU Y, LIN Y, ZHANG G Y. Unique silica biomimetic mineralization of acidic elastin-like polypeptides without hydroxyl and charged residues[J]. Int. J. Biol. Macromol., 2020, 153: 224-231. |
| 66 | LE D H T, SUGAWARA-NARUTAKI A. Elastin-like polypeptides as building motifs toward designing functional nanobiomaterials[J]. Mol. Syst. Des. Eng., 2019, 4: 545-565. |
| 67 | MOHAPATRA M, ANAND S. Synthesis and applications of nano-structured iron oxides/hydroxides—A review[J]. Int. J. Eng. Sci., 2011, 2: 127-146. |
| 68 | PATWARDHAN S V. Biomimetic and bioinspired silica: recent developments and applications[J]. Chem. Commun., 2011, 47(27): 7567-7582. |
| 69 | ABDELHAMID M A A, PACK S P. Biomimetic and bioinspired silicifications: recent advances for biomaterial design and applications[J]. Acta Biomater., 2021, 120: 38-56. |
| 70 | STREHL C, MAURIZI L, GABER T, et al. Modification of the surface of superparamagnetic iron oxide nanoparticles to enable their safe application in humans[J]. Int. J. Nanomed., 2016, 11: 5883-5896. |
| 71 | WANG L, HUANG J, CHEN H, et al. Exerting enhanced permeability and retention effect driven delivery by ultrafine iron oxide nanoparticles with T1-T2 switchable magnetic resonance imaging contrast[J]. ACS Nano, 2017, 11: 4582-4592. |
| 72 | DAS A, SINGH J, YOGALAKSHMI K N. Laccase immobilized magnetic iron nanoparticles: fabrication and its performance evaluation in chlorpyrifos degradation[J]. Int. Biodeterior. Biodegrad., 2017, 117: 183-189. |
| 73 | GUO M, WENG X, WANG T, Chen Z, et al. Biosynthesized iron-based nanoparticles used as a heterogeneous catalyst for the removal of 2,4-dichlorophenol[J]. Separ. Purif. Technol., 2017, 175: 222-228. |
| 74 | HUANG P H, ZHAO S, BACHMAN H, et al. Acoustofluidic synthesis of particulate nanomaterials[J]. Adv. Sci., 2019, 6(19): 1900913. |
| 75 | AJINKYA N, YU X F, KAITHAL P, et al. Magnetic iron oxide nanoparticle (IONP) synthesis to applications: present and future[J]. Materials, 2020, 13(20): 4644. |
| [1] | 蒋华义, 胡娟, 齐红媛, 游琰真, 王玉龙, 武哲. 磁性纳米粒子类型和质量浓度对微波热解含油污泥的影响[J]. 化工进展, 2022, 41(7): 3908-3914. |
| [2] | 孙娜娜, 孙会娜, 沈莉莎, 苏瑞宇, 赵超. 磁性纳米颗粒-微波辐射对稠油O/W乳状液的协同破乳[J]. 化工进展, 2022, 41(6): 3127-3137. |
| [3] | 郝好, 姚庆鑫, 高远, 谢建军. 酶催化超分子自组装及其在癌症诊疗中的应用研究进展[J]. 化工进展, 2020, 39(11): 4568-4574. |
| [4] | 高余良, 朱光明, 马拖拖. Fe3O4磁性纳米粒子及其生物医学应用研究进展[J]. 化工进展, 2017, 36(03): 973-980. |
| [5] | 强明辉,李云庆,王家喜. 磁性纳米负载金属催化剂的制备及其在甲苯催化氢化反应中的性能 [J]. 化工进展, 2010, 29(8): 1479-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||