化工进展 ›› 2024, Vol. 43 ›› Issue (S1): 209-224.DOI: 10.16085/j.issn.1000-6613.2024-0510
金属-掺杂氧化铈体系H2/CO电化学反应机理研究进展
- 1.北京科技大学能源与环境工程学院,北京 100083
2.北京怀柔实验室,北京 101400
-
收稿日期:2024-03-28修回日期:2024-06-08出版日期:2024-11-20发布日期:2024-12-06 -
通讯作者:包成 -
作者简介:林梅洁(1998—),女,硕士研究生,研究方向为Ni-GDC阳极电催化机理。E-mail:m202120230@xs.ustb.edu.cn。 -
基金资助:国家重点研发计划(2021YFB2500401);国家自然科学基金面上项目(51976009)
Research progress of H2 and CO electrochemical oxidation mechanisms in metal and doped ceria system
LIN Meijie1( ), MI Shuodong1, BAO Cheng1,2(
), MI Shuodong1, BAO Cheng1,2( )
)
- 1.School of Energy and Environmental Engineering, University of Science and Technology Beijing, Beijing 100083, China
2.Beijing Huairou Laboratory, Beijing 101400, China
-
Received:2024-03-28Revised:2024-06-08Online:2024-11-20Published:2024-12-06 -
Contact:BAO Cheng
摘要:
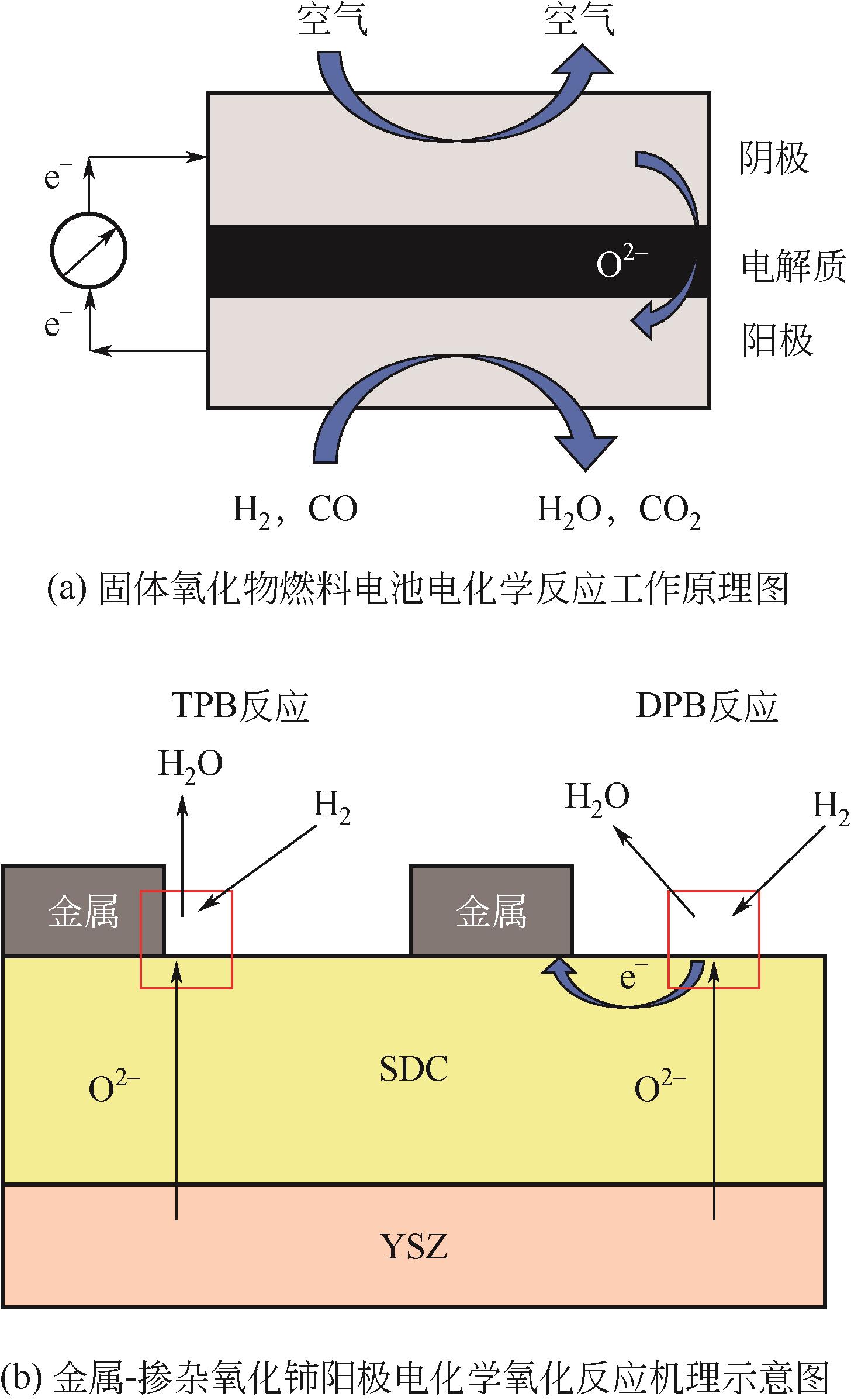
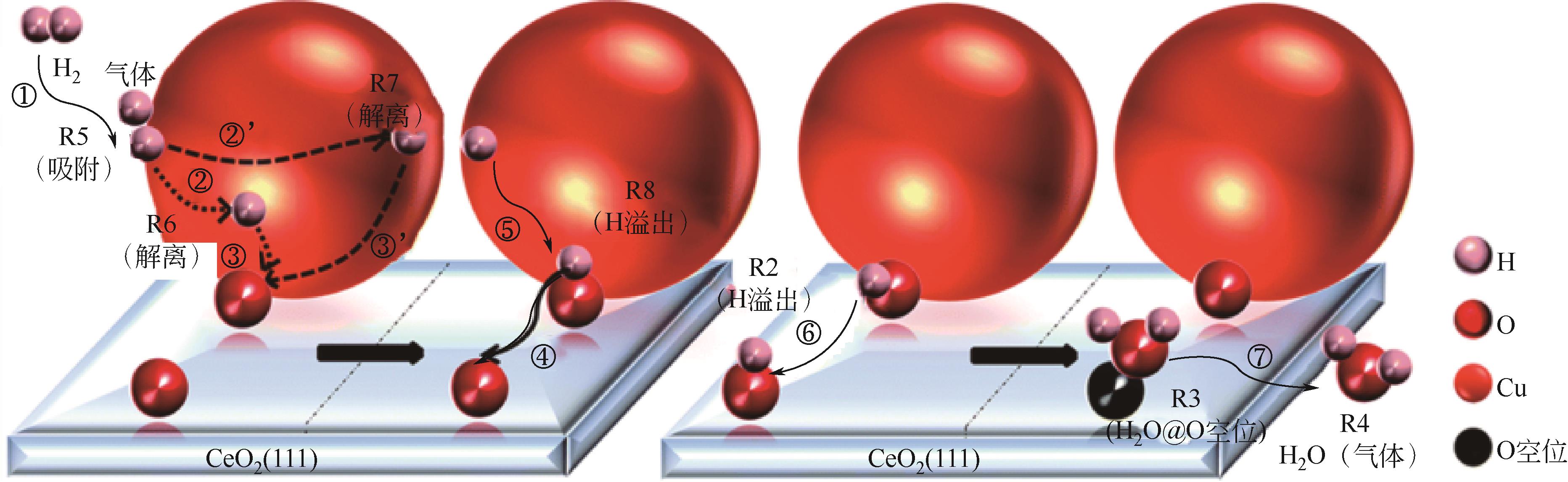
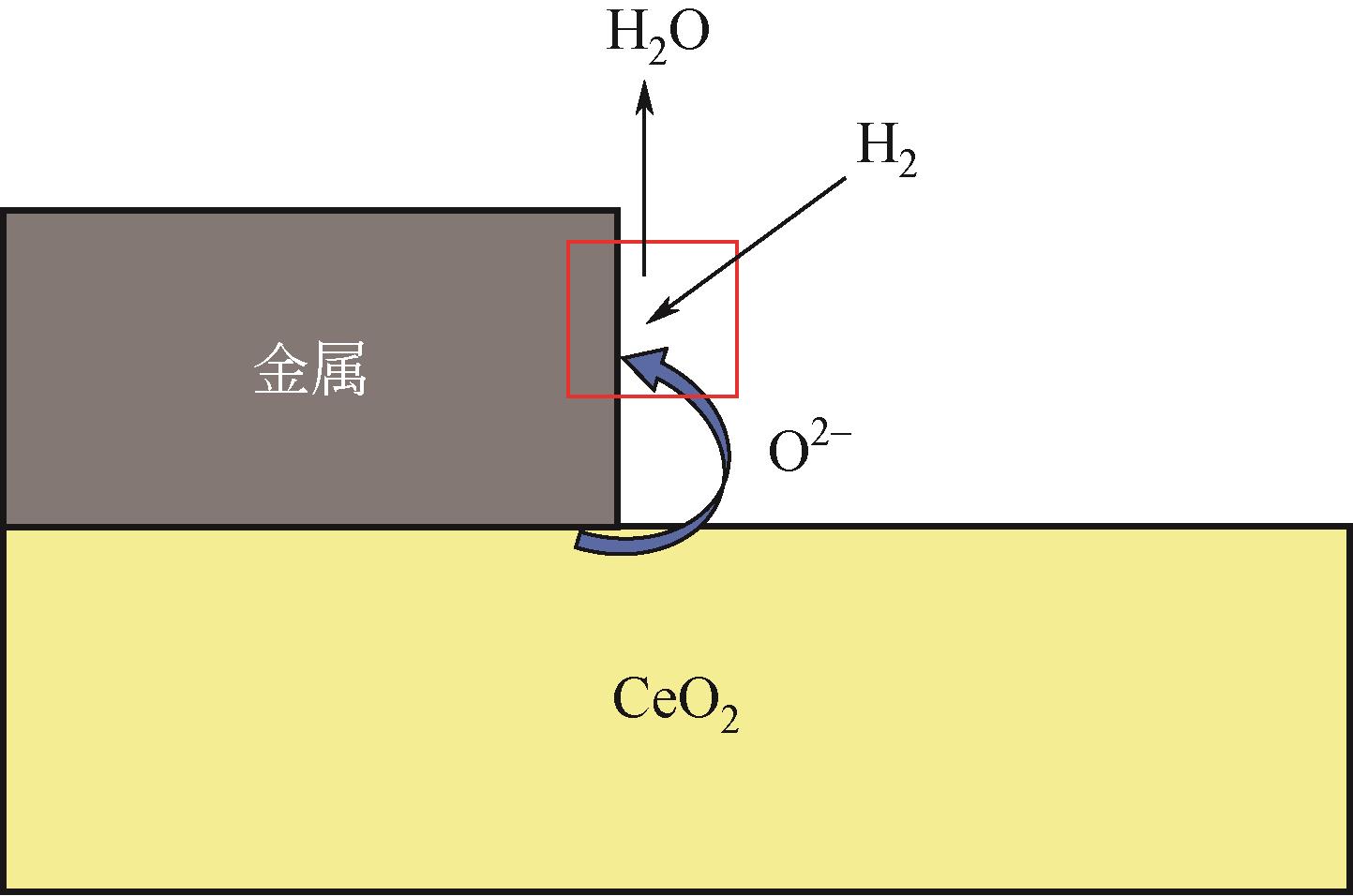
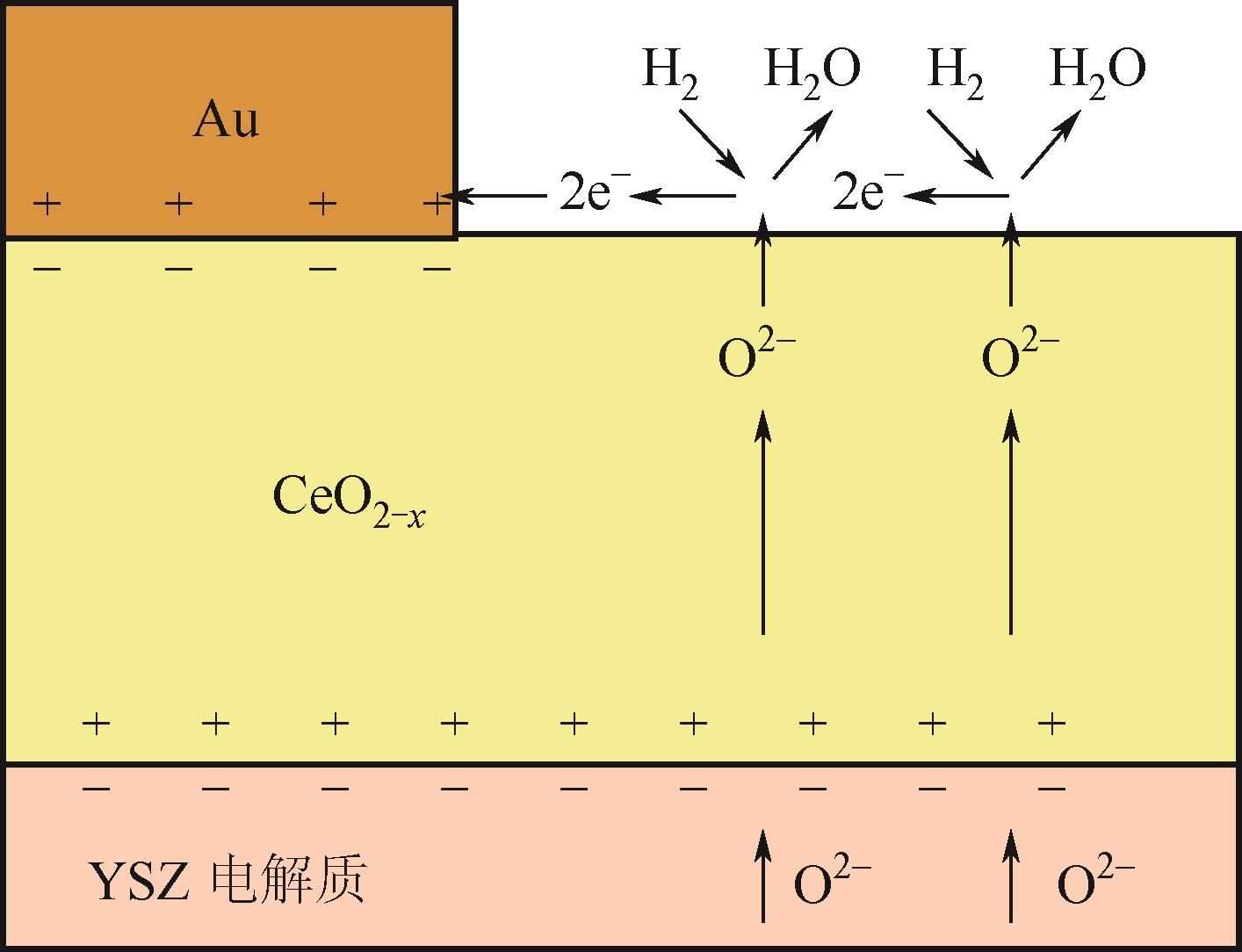
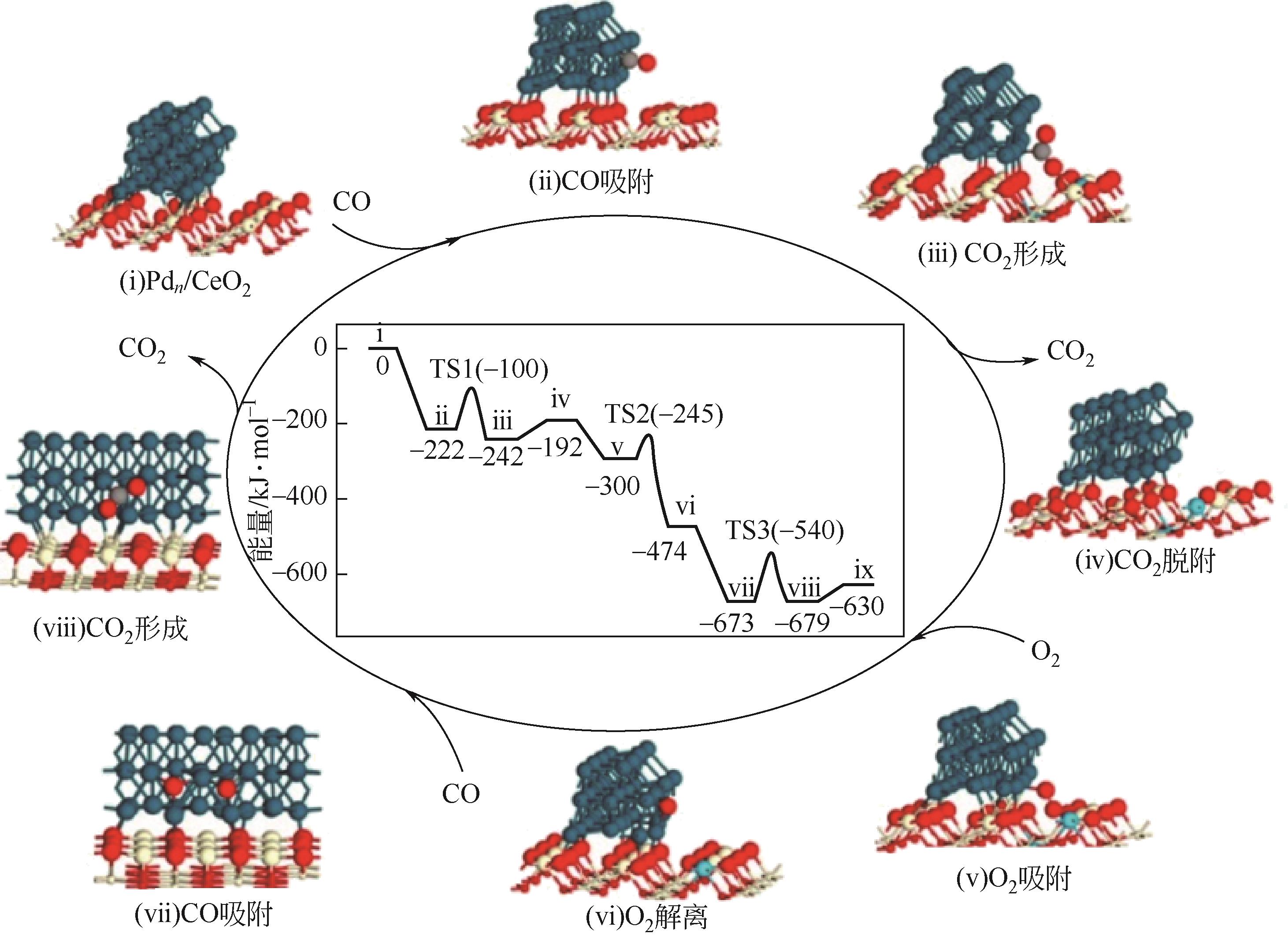
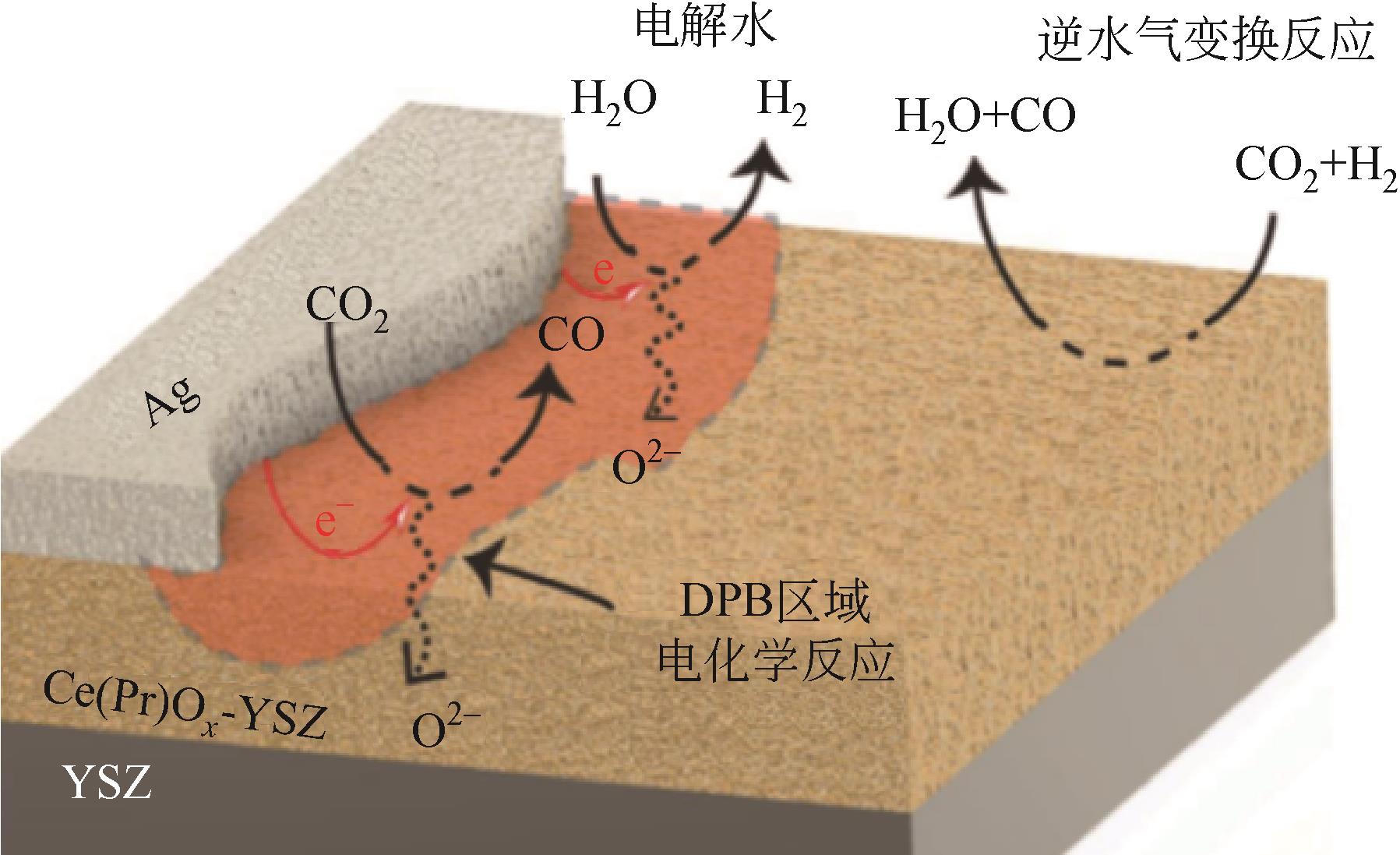
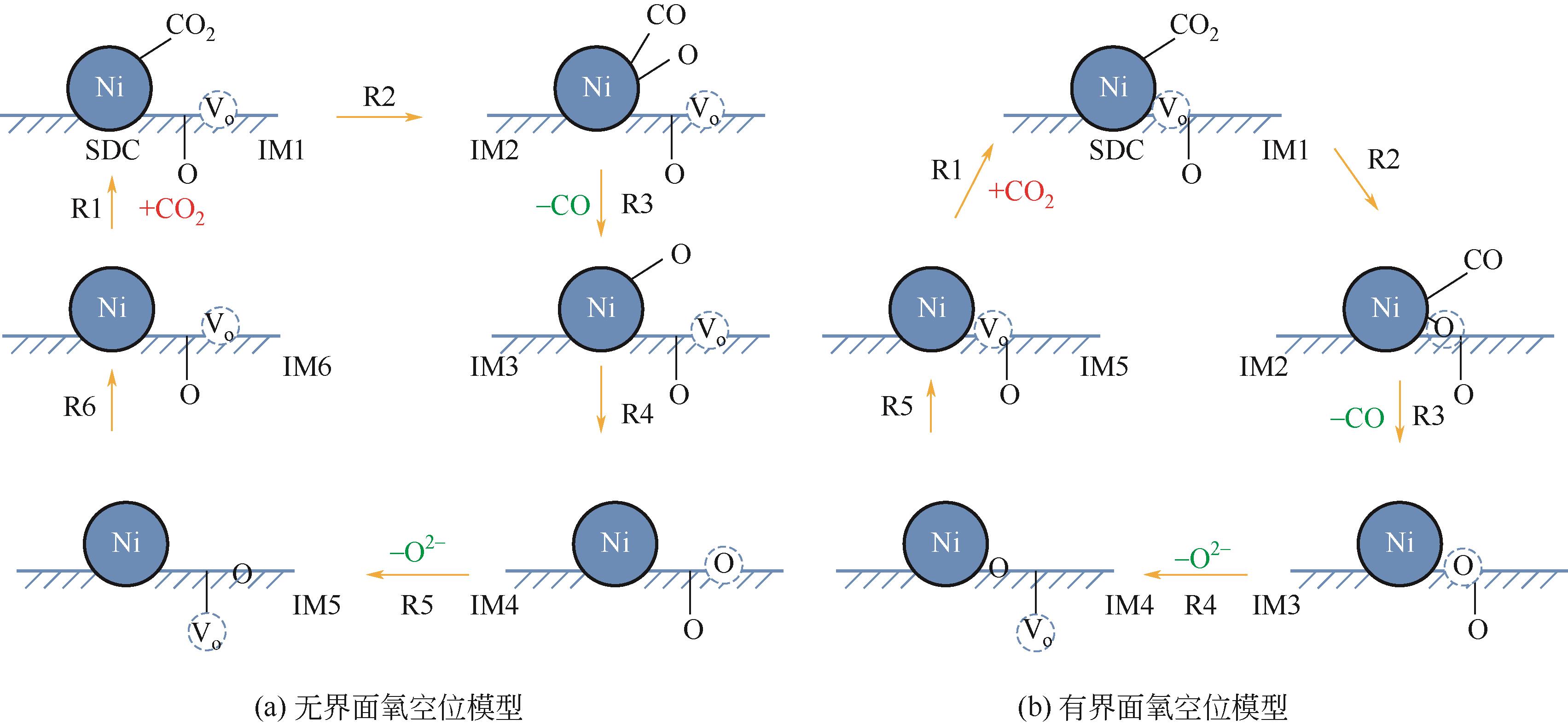
应用金属-稀土元素掺杂氧化铈阳极是固体氧化物燃料电池(SOFC)中低温化的重要策略之一。掺杂氧化铈自身的混合离子电子导体(MIEC)特性拓展了反应界面,也使得反应机理更为复杂。本文综述了金属-掺杂氧化铈体系H2和CO的电化学氧化反应机理,指出在H2电化学反应中类Ni/YSZ型H溢出机理占主导地位;对于CO电化学反应,结合在CO催化反应中主要的Marse-van Krevelen(MvK)机制和CO2电化学还原反应逆过程,预测其电荷转移步骤主要发生在氧空位形成和CO2形成反应。在MIEC型反应机理中,H2氧化反应路径的主要区别在于H2解离吸附位点的不同;而CO氧化反应路径根据吸附位点可分为与CeO2晶格氧反应直接生成CO2或者生成碳酸盐中间体,电荷转移步骤为碳酸盐形成和CO2形成反应。综上,H2电化学氧化以H溢出为主,CO电化学氧化的主导反应机理尚不明确,亟待深入研究。本文工作对明晰H2和CO乃至H2/CO混合燃料体系类Ni/YSZ型和MIEC型反应机理具有一定的指导意义。
中图分类号:
引用本文
林梅洁, 米烁东, 包成. 金属-掺杂氧化铈体系H2/CO电化学反应机理研究进展[J]. 化工进展, 2024, 43(S1): 209-224.
LIN Meijie, MI Shuodong, BAO Cheng. Research progress of H2 and CO electrochemical oxidation mechanisms in metal and doped ceria system[J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 209-224.
| 双羟基路径(DH)[ | 均相解离路径[ | 非均相路径 (在PDC、NDC、SDC和GDC上掺杂金属参与的过程)[ | 非均相路径 (在含有氧空位的GDC上,O空位参与的过程)[ |
|---|---|---|---|
表1 纯CeO2和掺杂CeO2体系H2氧化均相解离和非均相解离反应路径
| 双羟基路径(DH)[ | 均相解离路径[ | 非均相路径 (在PDC、NDC、SDC和GDC上掺杂金属参与的过程)[ | 非均相路径 (在含有氧空位的GDC上,O空位参与的过程)[ |
|---|---|---|---|
| LH反应机理 | MvK反应机理 | Au-CeO2界面作为反应分子的结合位点 |
|---|---|---|
表2 Au/CeO2及Au/CeO2-x 体系CO氧化反应机理[64]
| LH反应机理 | MvK反应机理 | Au-CeO2界面作为反应分子的结合位点 |
|---|---|---|
| Ni/SDC体系CO2电化学还原反应机理[ | 对应电荷转移步骤的电化学表达式 | 推测CO电化学氧化反应机理 |
|---|---|---|
| 步骤1: | ||
| 步骤2: | ||
| 步骤3: | ||
表3 Ni/SDC体系CO2RR反应机理和对应的电荷转移步骤以及CO电化学氧化反应机理预测
| Ni/SDC体系CO2电化学还原反应机理[ | 对应电荷转移步骤的电化学表达式 | 推测CO电化学氧化反应机理 |
|---|---|---|
| 步骤1: | ||
| 步骤2: | ||
| 步骤3: | ||
| CO2在Pt底部吸附 | CO2在Pt顶部吸附 | 推测CO电化学氧化反应机理 |
|---|---|---|
表4 Pt/SDC体系及Pt/CeO2-x 体系CO2RR反应机理[80]
| CO2在Pt底部吸附 | CO2在Pt顶部吸附 | 推测CO电化学氧化反应机理 |
|---|---|---|
| CO直接(电)氧化不形成碳酸盐结构 | CO形成碳酸盐结构 | ||
|---|---|---|---|
| 氧化反应[ | 电化学氧化[ | 氧化反应[ | 电化学氧化[ |
表5 CeO2体系CO(电)化学反应机理
| CO直接(电)氧化不形成碳酸盐结构 | CO形成碳酸盐结构 | ||
|---|---|---|---|
| 氧化反应[ | 电化学氧化[ | 氧化反应[ | 电化学氧化[ |
| 1 | DUAN Chuancheng, TONG Jianhua, SHANG Meng, et al. Readily processed protonic ceramic fuel cells with high performance at low temperatures[J]. Science, 2015, 349(6254): 1321-1326. |
| 2 | 李小勇, 宁小亮, 徐传伟, 等. SOFC氧离子导体电解质材料的研究进展及性能优化策略[J]. 现代技术陶瓷, 2023, 44(1): 33-42. |
| LI Xiaoyong, NING Xiaoliang, XU Chuanwei, et al. Research progress and performance optimization strategies of SOFC oxygen ion conductor electrolyte materials[J]. Advanced Ceramics, 2023, 44(1): 33-42. | |
| 3 | MONTINI Tiziano, MELCHIONNA Michele, MONAI Matteo, et al. Fundamentals and catalytic applications of CeO2-based materials[J]. Chemical Reviews, 2016, 116(10): 5987-6041. |
| 4 | FAN Liangdong, WANG Chengyang, CHEN Mingming, et al. Recent development of ceria-based (nano)composite materials for low temperature ceramic fuel cells and electrolyte-free fuel cells[J]. Journal of Power Sources, 2013, 234: 154-174. |
| 5 | IDERIS Asmida, CROISET Eric, PRITZKER Mark. Ni-Samaria-doped ceria (Ni-SDC) anode-supported solid oxide fuel cell (SOFC) operating with CO[J]. International Journal of Hydrogen Energy, 2017, 42(14): 9180-9187. |
| 6 | YAO Xueli, LI Ping, YU Baolong, et al. Hydrothermally synthesized NiO-samarium doped ceria nano-composite as an anode material for intermediate-temperature solid oxide fuel cells[J]. International Journal of Hydrogen Energy, 2017, 42(34): 22192-22200. |
| 7 | SHI Nan, YU Shancheng, CHEN Sainan, et al. Dense thin YSZ electrolyte films prepared by a vacuum slurry deposition technique for SOFCs[J]. Ceramics International, 2017, 43(1): 182-186. |
| 8 | ABDULWAHAB Khadijat Olabisi, KHAN Mohammad Mansoob, JENNINGS James Robert. Doped ceria nanomaterials: Preparation, properties, and uses[J]. ACS Omega, 2023, 8(34): 30802-30823. |
| 9 | KANNAN Karthik, RADHIKA D, NESARAJ A S, et al. A simple chemical precipitation of ceria based (Sm doped-CGO) nanocomposite: Structural and electrolytic behaviour for LT-SOFCs[J]. SN Applied Sciences, 2020, 2(7): 1220. |
| 10 | DENG Tian, ZHANG Chongrong, XIAO Yuyuan, et al. One-step synthesis of samarium-doped ceria and its CO catalysis[J]. Bulletin of Materials Science, 2015, 38(5): 1149-1154. |
| 11 | 张伟, 魏嘉璐, JOSÉ Antonio Alonso, 等. 碳基固体氧化物燃料电池研究进展[J]. 洁净煤技术, 2024, 30(1): 239-264. |
| ZHANG Wei, WEI Jialu, JOSé Antonio Alonso, et al. Recent advances in hydrocarbon-fueled solid oxide fuel cells[J]. Clean Coal Technology, 2024, 30(1): 239-264. | |
| 12 | YUN Jeong Woo, YOON Sung Pil, KIM Hee Su, et al. Effect of Sm0.2Ce0.8O1.9 on the carbon coking in Ni-based anodes for solid oxide fuel cells running on methane fuel[J]. International Journal of Hydrogen Energy, 2012, 37(5): 4356-4366. |
| 13 | ARSHAD Muhammad Sarfraz, RAZA Rizwan, Ashfaq AHMAD M, et al. An efficient Sm and Ge co-doped ceria nanocomposite electrolyte for low temperature solid oxide fuel cells[J]. Ceramics International, 2018, 44(1): 170-174. |
| 14 | ARTINI Cristina. Rare-earth-doped ceria systems and their performance as solid electrolytes: A puzzling tangle of structural issues at the average and local scale[J]. Inorganic Chemistry, 2018, 57(21): 13047-13062. |
| 15 | NAKAMURA Takashi, YASHIRO Keiji, KAIMAI Atsushi, et al. Determination of the reaction zone in gadolinia-doped ceria anode for solid oxide fuel cell[J]. Journal of the Electrochemical Society, 2008, 155(12): B1244. |
| 16 | LING Yihan, WANG Xinxin, MA Zhenkai, et al. Review of experimental and modelling developments for ceria-based solid oxide fuel cells free from internal short circuits[J]. Journal of Materials Science, 2020, 55(1): 1-23. |
| 17 | BAO Cheng, WANG Ying, FENG Daili, et al. Macroscopic modeling of solid oxide fuel cell (SOFC) and model-based control of SOFC and gas turbine hybrid system[J]. Progress in Energy and Combustion Science, 2018, 66: 83-140. |
| 18 | MI Shuodong, BAO Cheng, Xin LYU. ReaxFF reactive molecular dynamics study on electrochemistry of H2/CO hybrid fuel in Ni/YSZ anode[J]. Fuel, 2023, 332: 125989. |
| 19 | CHUEH William C, HAO Yong, JUNG WooChul, et al. High electrochemical activity of the oxide phase in model ceria-Pt and ceria-Ni composite anodes[J]. Nature Materials, 2011, 11(2): 155-161. |
| 20 | NOLAN Michael, PARKER Stephen C, WATSON Graeme W. CeO2 catalysed conversion of CO, NO2 and NO from first principles energetics[J]. Physical Chemistry Chemical Physics, 2006, 8(2): 216-218. |
| 21 | ANDERSSON D A, SIMAK S I, JOHANSSON B, et al. Modeling of CeO2, Ce2O3, and CeO2- x in the LDA + U formalism[J]. Physical Review B, 2007, 75(3): 035109. |
| 22 | PATEL H C, TABISH A N, COMELLI F, et al. Oxidation of H2, CO and syngas mixtures on ceria and nickel pattern anodes[J]. Applied Energy, 2015, 154: 912-920. |
| 23 | TANG Yuanhao, ZHANG Hua, CUI Lixia, et al. First-principles investigation on redox properties of M-doped CeO2 (M=Mn, Pr, Sn, Zr)[J]. Physical Review B, 2010, 82(12): 125104. |
| 24 | SHI H, HUSSAIN T, AHUJA R, et al. Role of vacancies, light elements and rare-earth metals doping in CeO2 [J]. Scientific Reports, 2016, 6: 31345. |
| 25 | LAWRENCE Neil J, BREWER Joseph R, WANG Lu, et al. Defect engineering in cubic cerium oxide nanostructures for catalytic oxidation[J]. Nano Letters, 2011, 11(7): 2666-2671. |
| 26 | TIAN Dong, LI Kongzhai, WEI Yonggang, et al. DFT insights into oxygen vacancy formation and CH4 activation over CeO2 surfaces modified by transition metals (Fe, Co and Ni)[J]. Physical Chemistry Chemical Physics, 2018, 20(17): 11912-11929. |
| 27 | CHEN Meina, GAO Huiying, ZHANG Lei, et al. Unlocking the nature of the co-doping effect on the ionic conductivity of CeO2-based electrolyte[J]. Ceramics International, 2019, 45(3): 3977-3985. |
| 28 | VAYSSILOV Georgi N, LYKHACH Yaroslava, MIGANI Annapaola, et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles[J]. Nature Materials, 2011, 10(4): 310-315. |
| 29 | BARON M, BONDARCHUK O, STACCHIOLA D, et al. Interaction of gold with cerium oxide supports: CeO2(111) thin films vs CeO x nanoparticles[J]. The Journal of Physical Chemistry C, 2009, 113(15): 6042-6049. |
| 30 | ZHANG Changjun, MICHAELIDES Angelos, JENKINS Stephen J. Theory of gold on ceria[J]. Physical Chemistry Chemical Physics, 2011, 13(1): 22-33. |
| 31 | LIU Zongyuan, LUSTEMBERG Pablo, GUTIÉRREZ Ramón A, et al. In situ investigation of methane dry reforming on metal/ceria(111) surfaces: Metal-support interactions and C—H bond activation at low temperature[J]. Angewandte Chemie International Edition, 2017, 56(42): 13041-13046. |
| 32 | HANNA J, LEE W Y, SHI Y, et al. Fundamentals of electro- and thermochemistry in the anode of solid-oxide fuel cells with hydrocarbon and syngas fuels[J]. Progress in Energy and Combustion Science, 2014, 40: 74-111. |
| 33 | SHISHKIN M, ZIEGLER T. The electronic structure and chemical properties of a Ni/CeO2 Anode in a solid oxide fuel cell: A DFT +U Study[J]. The Journal of Physical Chemistry C, 2010, 114(49): 21411-21416. |
| 34 | WANG Lei. Model development for gadolinia-doped ceria-based anodes in solid oxde fuel cells[D]. Maryland: University of Maryland, 2014. |
| 35 | BESSLER Wolfgang G, Jürgen WARNATZ, GOODWIN David G. The influence of equilibrium potential on the hydrogen oxidation kinetics of SOFC anodes[J]. Solid State Ionics, 2007, 177(39/40): 3371-3383. |
| 36 | SHARMA Vaneet, CROZIER Peter A, SHARMA Renu, et al. Direct observation of hydrogen spillover in Ni-loaded Pr-doped ceria[J]. Catalysis Today, 2012, 180(1): 2-8. |
| 37 | MAHER Robert C, SHEARING Paul R, BRIGHTMAN Edward, et al. Reduction dynamics of doped ceria, nickel oxide, and cermet composites probed using in situ Raman spectroscopy[J]. Advanced Science, 2015, 3(1): 1500146. |
| 38 | WANG Shuang, ZHENG Minghao, LI Mei, et al. Synergistic effects towards H2 oxidation on the Cu-CeO2 electrode: A combination study with DFT calculations and experiments[J]. Journal of Materials Chemistry A, 2016, 4(15): 5745-5754. |
| 39 | ZHENG Minghao, WANG Shuang, LI Mei, et al. H2 and CO oxidation process at the three-phase boundary of Cu-ceria cermet anode for solid oxide fuel cell[J]. Journal of Power Sources, 2017, 345: 165-175. |
| 40 | JIANG Yunan, WANG Shuang, XU Jun, et al. Hydrogen oxidation pathway over Ni-ceria electrode: Combined study of DFT and experiment[J]. Frontiers in Chemistry, 2021, 8: 591322. |
| 41 | WANG Lei, YU Yi, GASKELL Karen J, et al. In operando X-ray photoelectron spectroscopy studies of H2 oxidation and H2O electrolysis on gadolinia-doped ceria electrodes[J]. Journal of Physics: Energy, 2021, 3(1): 014004. |
| 42 | KIM Hyunjoong, YOO Ji Mun, CHUNG Dong Young, et al. Design of a metal/oxide/carbon interface for highly active and selective electrocatalysis[J]. ACS Nano, 2022, 16(10): 16529-16538. |
| 43 | TANG Chunmei, YAO Yao, WANG Ning, et al. Green hydrogen production by intermediate-temperature protonic solid oxide electrolysis cells: Advances, challenges, and perspectives[J]. InfoMat, 2024, 6(3): e12515. |
| 44 | MIN YOU Hyo, NAGASAWA Tsuyoshi, LEE Jae WOO, et al. Mechanistic study of oxygen reduction reaction on a Pd/CeO2-ZrO2 catalyst[J]. Applied Surface Science, 2024, 648: 159045. |
| 45 | MIZUSAKI Junichiro, TAGAWA Hiroaki, SAITO Takatoshi, et al. Preparation of nickel pattern electrodes on YSZ and their electrochemical properties in H2-H2O atmospheres[J]. Journal of the Electrochemical Society, 2019, 141(8): 2129-2134. |
| 46 | HAPPEL M, MYSLIVEČEK J, JOHÁNEK V, et al. Adsorption sites, metal-support interactions, and oxygen spillover identified by vibrational spectroscopy of adsorbed CO: A model study on Pt/ceria catalysts[J]. Journal of Catalysis, 2012, 289: 118-126. |
| 47 | BERNAL S, CALVINO J J, CIFREDO G A, et al. The key role of highly dispersed rhodium in the chemistry of hydrogen-ceria systems[J]. Journal of the Chemical Society, Chemical Communications, 1992(6): 460-462. |
| 48 | BRUCE Linda A, HOANG Manh, HUGHES Anthony E, et al. Surface area control during the synthesis and reduction of high area ceria catalyst supports[J]. Applied Catalysis A: General, 1996, 134(2): 351-362. |
| 49 | CHEN Hsin-Tsung, CHOI Yong Man, LIU Meilin, et al. A theoretical study of surface reduction mechanisms of CeO2(111) and (110) by H2 [J]. ChemPhysChem, 2007, 8(6): 849-855. |
| 50 | DECALUWE Steven C, GRASS Michael E, ZHANG Chunjuan, et al. In situ characterization of ceria oxidation states in high-temperature electrochemical cells with ambient pressure XPS[J]. The Journal of Physical Chemistry C, 2010, 114(46): 19853-19861. |
| 51 | ZHANG Chunjuan, YU Yi, GRASS Michael E, et al. Mechanistic studies of water electrolysis and hydrogen electro-oxidation on high temperature ceria-based solid oxide electrochemical cells[J]. Journal of the American Chemical Society, 2013, 135(31): 11572-11579. |
| 52 | PATEL H C, TABISH A N, ARAVIND P V. Modelling of elementary kinetics of H2 and CO oxidation on ceria pattern cells[J]. Electrochimica Acta, 2015, 182: 202-211. |
| 53 | Delia FERNÁNDEZ-TORRE, CARRASCO Javier, Verónica GANDUGLIA-PIROVANO M, et al. Hydrogen activation, diffusion, and clustering on CeO2(111): A DFT+U study[J]. The Journal of Chemical Physics, 2014, 141(1): 014703. |
| 54 | ZHU Houyu, HOU Yongchun, REN Hao, et al. Theoretical investigation on H2 oxidation mechanisms over pristine and Sm-doped CeO2(111) surfaces[J]. Applied Surface Science, 2020, 511: 145388. |
| 55 | LIU Dongyuan, ZHU Houyu, YUAN Saifei, et al. Understanding the oxygen-vacancy-related catalytic cycle for H2 oxidation on ceria-based SOFC anode and the promotion effect of lanthanide doping from theoretical perspectives[J]. Applied Surface Science, 2022, 576: 151803. |
| 56 | SCHWEKE D, SHELLY L, DAVID R BEN, et al. Comprehensive study of the ceria–H2 system: Effect of the reaction conditions on the reduction extent and intermediates[J]. The Journal of Physical Chemistry C, 2020, 124(11): 6180-6187. |
| 57 | CHEN Bohao, MA Yunsheng, DING Liangbing, et al. Reactivity of hydroxyls and water on a CeO2(111) thin film surface: The role of oxygen vacancy[J]. The Journal of Physical Chemistry C, 2013, 117(11): 5800-5810. |
| 58 | RAWADIEH Saleh E, ALTARAWNEH Mohammednoor, ALTARAWNEH Ibrahem S, et al. A kinetic model for evolution of H2 and CO over Zr-doped ceria[J]. Molecular Catalysis, 2020, 498: 111256. |
| 59 | WANG Jenshi B, TSAI De-Hao, HUANG Ta-Jen. Synergistic catalysis of carbon monoxide oxidation over copper oxide supported on samaria-doped ceria[J]. Journal of Catalysis, 2002, 208(2): 370-380. |
| 60 | ZHU Huaqing, QIN Zhangfeng, SHAN Wenjuan, et al. Low-temperature oxidation of CO over Pd/CeO2-TiO2 catalysts with different pretreatments[J]. Journal of Catalysis, 2005, 233(1): 41-50. |
| 61 | LUO Jinyong, MENG Ming, ZHA Yuqing, et al. Identification of the active sites for CO and C3H8 total oxidation over nanostructured CuO-CeO2 and Co3O4-CeO2 catalysts[J]. The Journal of Physical Chemistry C, 2008, 112(23): 8694-8701. |
| 62 | JIA Aiping, JIANG Shiyu, LU Jiqing, et al. Study of catalytic activity at the CuO-CeO2 interface for CO oxidation[J]. The Journal of Physical Chemistry C, 2010, 114(49): 21605-21610. |
| 63 | ROYER Sébastien, DUPREZ Daniel. Catalytic oxidation of carbon monoxide over transition metal oxides[J]. ChemCatChem, 2011, 3(1): 24-65. |
| 64 | KIM Hyun You, LEE Hyuck Mo, HENKELMAN Graeme. CO oxidation mechanism on CeO2-supported Au nanoparticles[J]. Journal of the American Chemical Society, 2012, 134(3): 1560-1570. |
| 65 | GHOSH Prasenjit, FARNESI CAMELLONE Matteo, FABRIS Stefano. Fluxionality of Au clusters at ceria surfaces during CO oxidation: Relationships among reactivity, size, cohesion, and surface defects from DFT simulations[J]. The Journal of Physical Chemistry Letters, 2013, 4(14): 2256-2263. |
| 66 | SONG Weiyu, JANSEN Antonius P J, HENSEN Emiel J M. A computational study of the influence of the ceria surface termination on the mechanism of CO oxidation of isolated Rh atoms[J]. Faraday Discussions, 2013, 162: 281-292. |
| 67 | SONG Weiyu, SU Yaqiong, HENSEN Emiel J M. A DFT study of CO oxidation at the Pd-CeO2(110) interface[J]. The Journal of Physical Chemistry C, 2015, 119(49): 27505-27511. |
| 68 | HINOKUMA Satoshi, YAMASHITA Noriko, KATSUHARA Yasuo, et al. CO oxidation activity of thermally stable Fe-Cu/CeO2 catalysts prepared by dual-mode arc-plasma process[J]. Catalysis Science & Technology, 2015, 5(8): 3945-3952. |
| 69 | CENTENO Miguel, RAMÍREZ REINA Tomás, IVANOVA Svetlana, et al. Au/CeO2 catalysts: Structure and CO oxidation activity[J]. Catalysts, 2016, 6(10): 158. |
| 70 | YUAN Kun, GUO Yu, HUANG Ling, et al. Tunable electronic metal-support interactions on ceria-supported noble-metal nanocatalysts in controlling the low-temperature CO oxidation activity[J]. Inorganic Chemistry, 2021, 60(7): 4207-4217. |
| 71 | HUANG Min, FABRIS Stefano. CO adsorption and oxidation on ceria surfaces from DFT+U calculations[J]. The Journal of Physical Chemistry C, 2008, 112(23): 8643-8648. |
| 72 | YANG Zongxian, Tom K WOO, BAUDIN Micael, et al. Atomic and electronic structure of unreduced and reduced CeO2 surfaces: A first-principles study[J]. The Journal of Chemical Physics, 2004, 120(16): 7741-7749. |
| 73 | NOLAN Michael, WATSON Graeme W. The surface dependence of CO adsorption on ceria[J]. The Journal of Physical Chemistry B, 2006, 110(33): 16600-16606. |
| 74 | CHEN Fendy, LIU Di, ZHANG Jie, et al. A DFT+U study of the lattice oxygen reactivity toward direct CO oxidation on the CeO2(111) and (110) surfaces[J]. Physical Chemistry Chemical Physics, 2012, 14(48): 16573-16580. |
| 75 | POLYCHRONOPOULOU Kyriaki, ALKHOORI Ayesha A, EFSTATHIOU Angelos M, et al. Design aspects of doped CeO2 for low-temperature catalytic CO oxidation: Transient kinetics and DFT approach[J]. ACS Applied Materials & Interfaces, 2021, 13(19): 22391-22415. |
| 76 | VENÂNCIO Selma Aparecida, DE MIRANDA Paulo Emilio Valadão. Ni-free SOFC anode material with thermal and redox stabilities for the direct utilization of ethanol[J]. Catalysts, 2023, 13(1): 134. |
| 77 | KAMBOJ Vipin, RAYCHOWDHURY Soham, SHIVAM Shivam, et al. Enhancement in CO2 electroreduction upon Pr infiltration of ceria electrodes[J]. ChemRxiv, 2024. DOI: 10.26434/chemrxiv-2024-swljj . |
| 78 | HAHN Konstanze R, SEITSONEN Ari P, IANNUZZI Marcella, et al. Functionalization of CeO2(111) by deposition of small Ni clusters: Effects on CO2 adsorption and O vacancy formation[J]. ChemCatChem, 2015, 7(4): 625-634. |
| 79 | REN Bohua, LI Jingde, WEN Guobin, et al. First-principles based microkinetic modeling of CO2 reduction at the Ni/SDC cathode of a solid oxide electrolysis cell[J]. The Journal of Physical Chemistry C, 2018, 122(37): 21151-21161. |
| 80 | YANG Yi, WANG Shuang, JIANG Yunan, et al. CO2 activation and reduction on Pt-CeO2-based catalysts[J]. The Journal of Physical Chemistry C, 2019, 123(28): 17092-17101. |
| 81 | YU Yi, MAO Baohua, GELLER Aaron, et al. CO2 activation and carbonate intermediates: An operando AP-XPS study of CO2 electrolysis reactions on solid oxide electrochemical cells[J]. Physical Chemistry Chemical Physics, 2014, 16(23): 11633-11639. |
| 82 | FENG Zhuoluo A, MACHALA Michael L, CHUEH William C. Surface electrochemistry of CO2 reduction and CO oxidation on Sm-doped CeO2- x : Coupling between Ce3+ and carbonate adsorbates[J]. Physical Chemistry Chemical Physics, 2015, 17(18): 12273-12281. |
| 83 | SHAUR Ahmad, DRAZKOWSKI Michel, ZHU Shaochen, et al. A single-phase gadolinium-doped ceria cathode for highly efficient CO2 electrolysis[J]. Journal of Materials Chemistry A, 2023, 11(45): 25020-25030. |
| 84 | SALA Elena Marzia, MAZZANTI Nicola, CHIABRERA Francesco M, et al. Unravelling the role of dopants in the electrocatalytic activity of ceria towards CO2 reduction in solid oxide electrolysis cells[J]. Physical Chemistry Chemical Physics, 2023, 25(4): 3457-3471. |
| [1] | 李新月, 李振京, 韩沂杭, 郭永强, 闫瑜, 哈力米热·卡热木拉提, 赵会吉, 柴永明, 刘东, 殷长龙. 油脂加氢脱氧生产绿色柴油催化剂的研究进展[J]. 化工进展, 2024, 43(S1): 351-364. |
| [2] | 邹鹏翔, 张明阳, 朱文杰, 郭耀骏, 程婕, 赵妍舒, 袁迎春. 基于CFD的脂肪酸甲酯环氧化用液-液撞击流旋流反应器入口结构优化[J]. 化工进展, 2024, 43(S1): 166-173. |
| [3] | 李帅哲, 聂懿宸, PHIDSAVARD Keomeesay, 顾雯, 张伟, 刘娜, 徐高翔, 刘莹, 李兴勇, 陈玉保. 非贵金属催化生物质加氢脱氧制备烃基生物燃料的研究进展[J]. 化工进展, 2024, 43(S1): 225-242. |
| [4] | 熊磊, 丁飞燕, 李聪, 王群乐, 吕起, 翟晓娜, 刘峰. 金属Pt负载型非均相催化剂研究进展[J]. 化工进展, 2024, 43(S1): 295-304. |
| [5] | 宋财城, 陈晓贞, 刘丽, 杨成敏, 郑步梅, 尹晓莹, 孙进, 姚运海, 段为宇. 碳基载体负载加氢脱硫催化剂的研究进展[J]. 化工进展, 2024, 43(S1): 305-314. |
| [6] | 韩洪晶, 车宇, 田宇轩, 王海英, 张亚男, 陈彦广. 木质素催化氢解催化剂及溶剂的研究进展[J]. 化工进展, 2024, 43(S1): 315-324. |
| [7] | 胡兴, 刘易, 杜泽学. 3-氯丙烯直接合成环氧氯丙烷催化剂研究进展[J]. 化工进展, 2024, 43(S1): 325-334. |
| [8] | 于梦洁, 吴语童, 罗发祥, 豆义波. 低浓度二氧化碳还原光催化剂结构设计的研究进展[J]. 化工进展, 2024, 43(S1): 335-350. |
| [9] | 张浩, 刘世钰, 沈卫华, 方云进. Ca-ZSM-5催化尿素脱水制备单氰胺[J]. 化工进展, 2024, 43(S1): 365-373. |
| [10] | 何世坤, 张荣花, 李昊阳, 潘晖, 冯君锋. 脱铝分子筛固体酸催化葡萄糖制备5-羟甲基糠醛[J]. 化工进展, 2024, 43(S1): 374-381. |
| [11] | 张日东, 吕建华, 刘继东, 郭豹, 李文松. Ru-K-NaY催化草酸二甲酯脱羰基制备碳酸二甲酯[J]. 化工进展, 2024, 43(S1): 382-390. |
| [12] | 马桂璇, 徐子桐, 肖志华, 宁国庆, 魏强, 徐春明. 氧硫双掺杂CNTs水系导电剂辅助构筑高性能石墨/SiO负极[J]. 化工进展, 2024, 43(S1): 443-456. |
| [13] | 高聪志, 张雅萱, 林璐, 邓晓婷, 殷霞, 丁一刚, 肖艳华, 杜治平. 新戊二醇的合成工艺[J]. 化工进展, 2024, 43(S1): 469-478. |
| [14] | 陈高祥, 王荣昌, 蒋佳承. 微生物电合成系统阴极电子传递机制和氢介导强化措施[J]. 化工进展, 2024, 43(S1): 504-516. |
| [15] | 万震, 王绍庆, 李志合, 赵天生. HZSM-5分子筛催化木质素热解制芳烃研究进展[J]. 化工进展, 2024, 43(S1): 517-532. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||