化工进展 ›› 2024, Vol. 43 ›› Issue (9): 5063-5078.DOI: 10.16085/j.issn.1000-6613.2023-1424
• 材料科学与技术 • 上一篇
硅基介孔材料的合成、功能化及对金属的吸附研究进展
刘丽1,2( ), 冯博1,2,3(
), 冯博1,2,3( ), 文洋1,2, 古启雄1,2
), 文洋1,2, 古启雄1,2
- 1.江西理工大学矿冶环境污染防控江西省重点实验室,江西 赣州 341000
2.江西理工大学资源与环境工程学院,江西 赣州 341000
3.江西理工大学稀有稀土资源开发与利用省部共建教育部协同创新中心,江西 赣州 341000
-
收稿日期:2023-08-15修回日期:2023-10-15出版日期:2024-09-15发布日期:2024-09-30 -
通讯作者:冯博 -
作者简介:刘丽(1997—),女,博士研究生,研究方向为稀土废水处理。E-mail:ll9888520@163.com。 -
基金资助:国家自然科学基金(52174248);江西省双千计划(jxsq2019201115);江西省研究生创新计划(YC2023-B216)
Research progress in synthesis, functionalization and metal adsorption of silica-based mesoporous materials
LIU Li1,2( ), FENG Bo1,2,3(
), FENG Bo1,2,3( ), WEN Yang1,2, GU Qixiong1,2
), WEN Yang1,2, GU Qixiong1,2
- 1.Jiangxi Provincial Key Laboratory of Environmental Pollution Prevention and Control in Mining and Metallurgy, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi, China
2.School of Resources and Environment Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi, China
3.Collaborative Innovation Center for Development and Utilization of Rare Metal Resources Co-sponsored by Ministry of Education and Jiangxi Province, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi, China
-
Received:2023-08-15Revised:2023-10-15Online:2024-09-15Published:2024-09-30 -
Contact:FENG Bo
摘要:
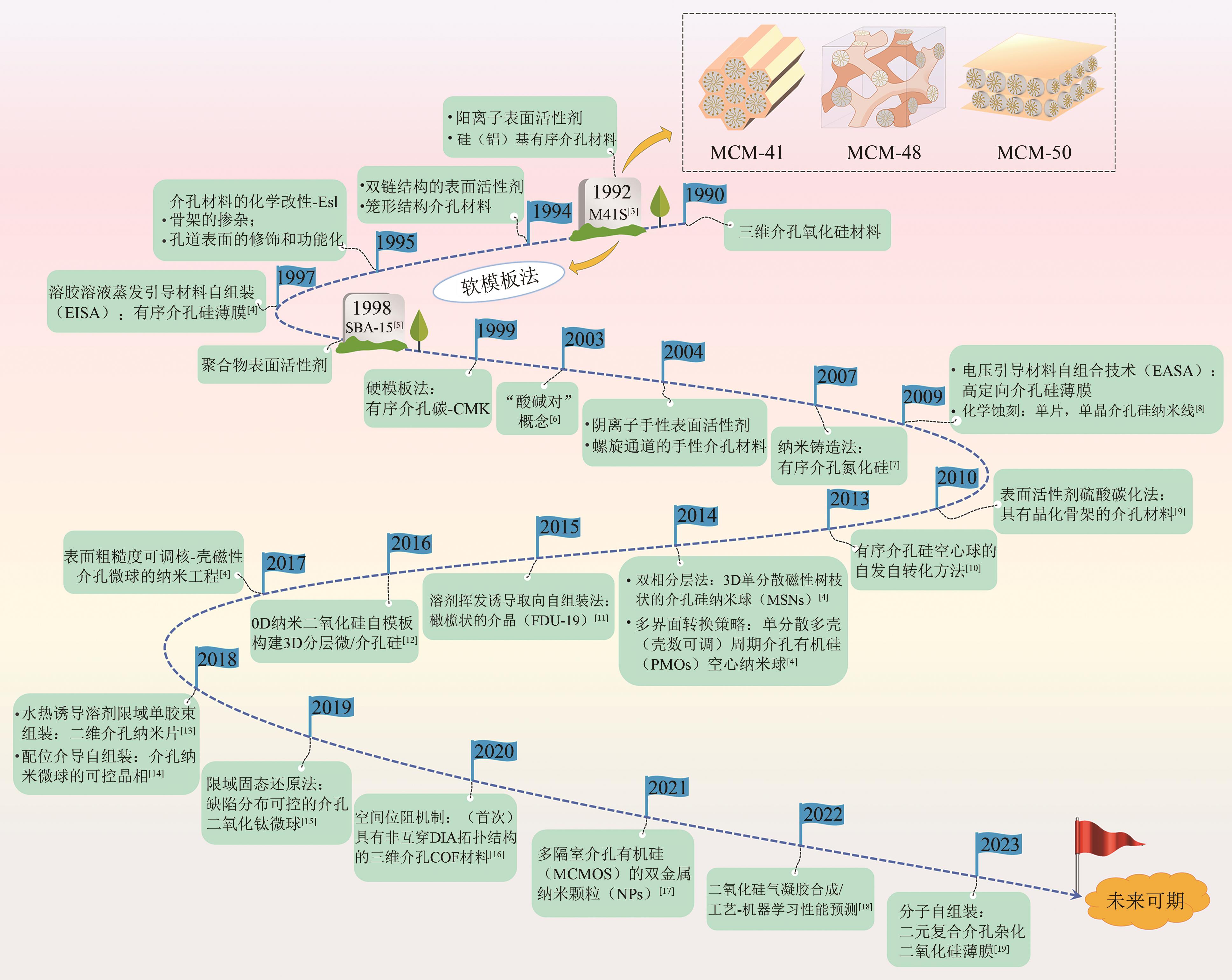
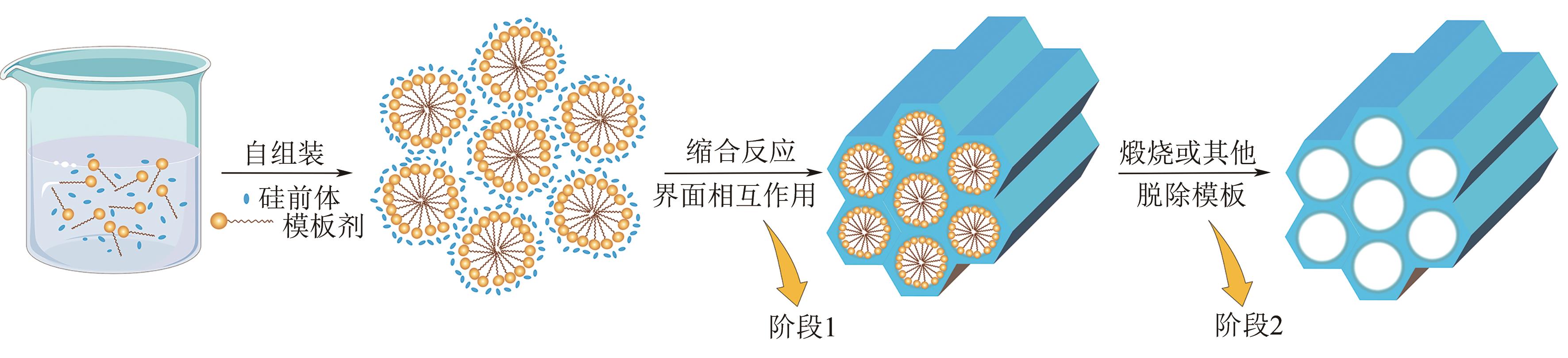
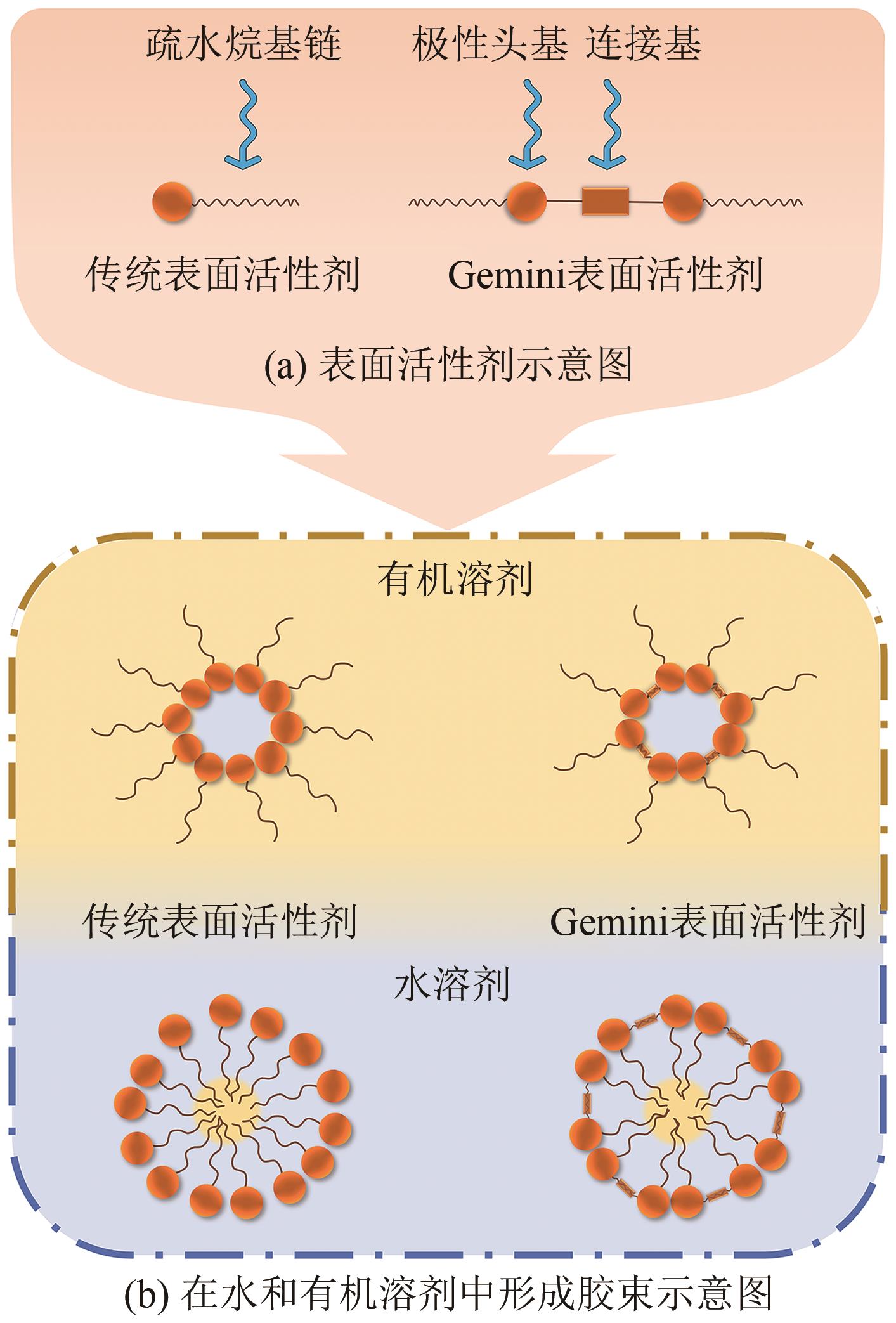
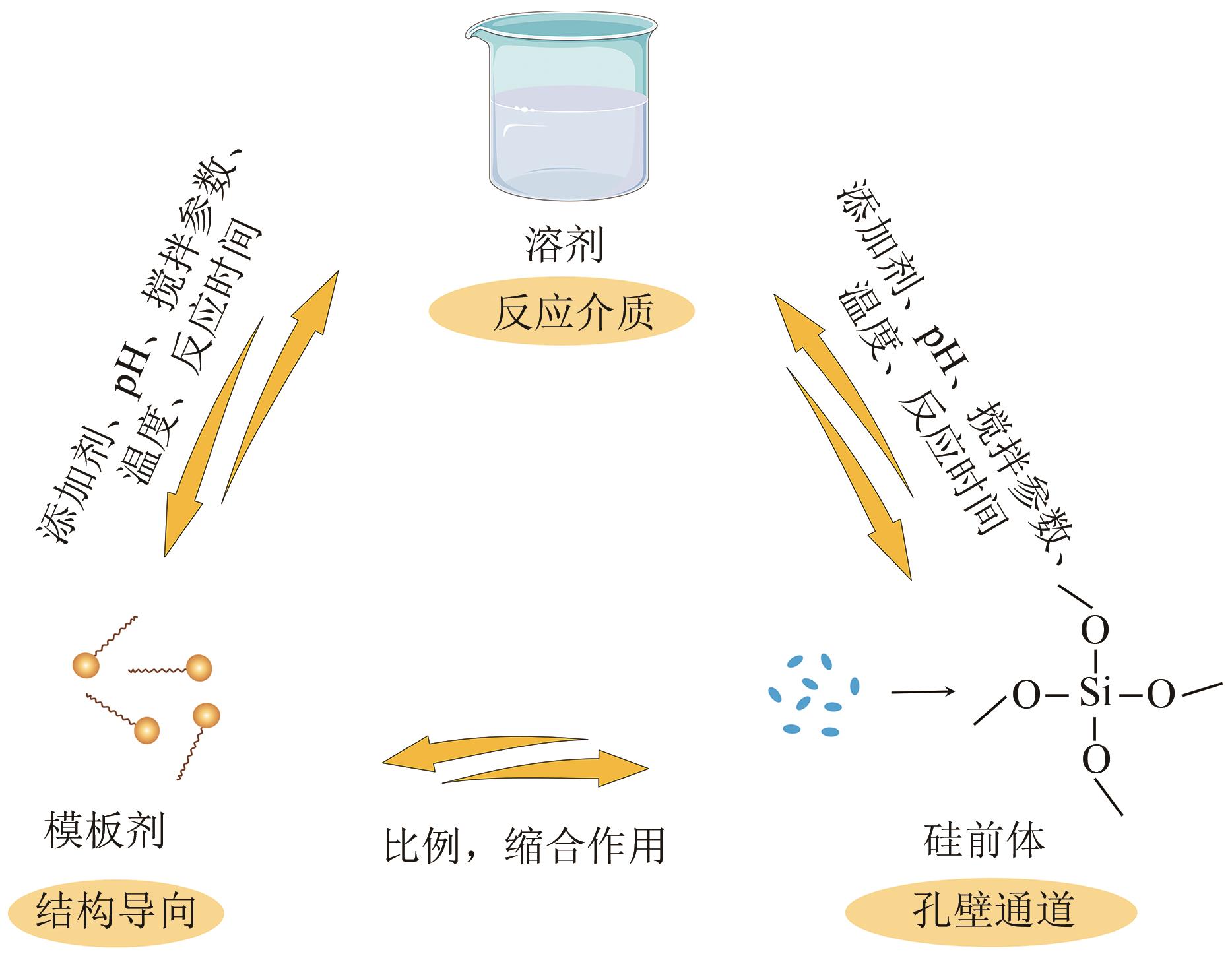
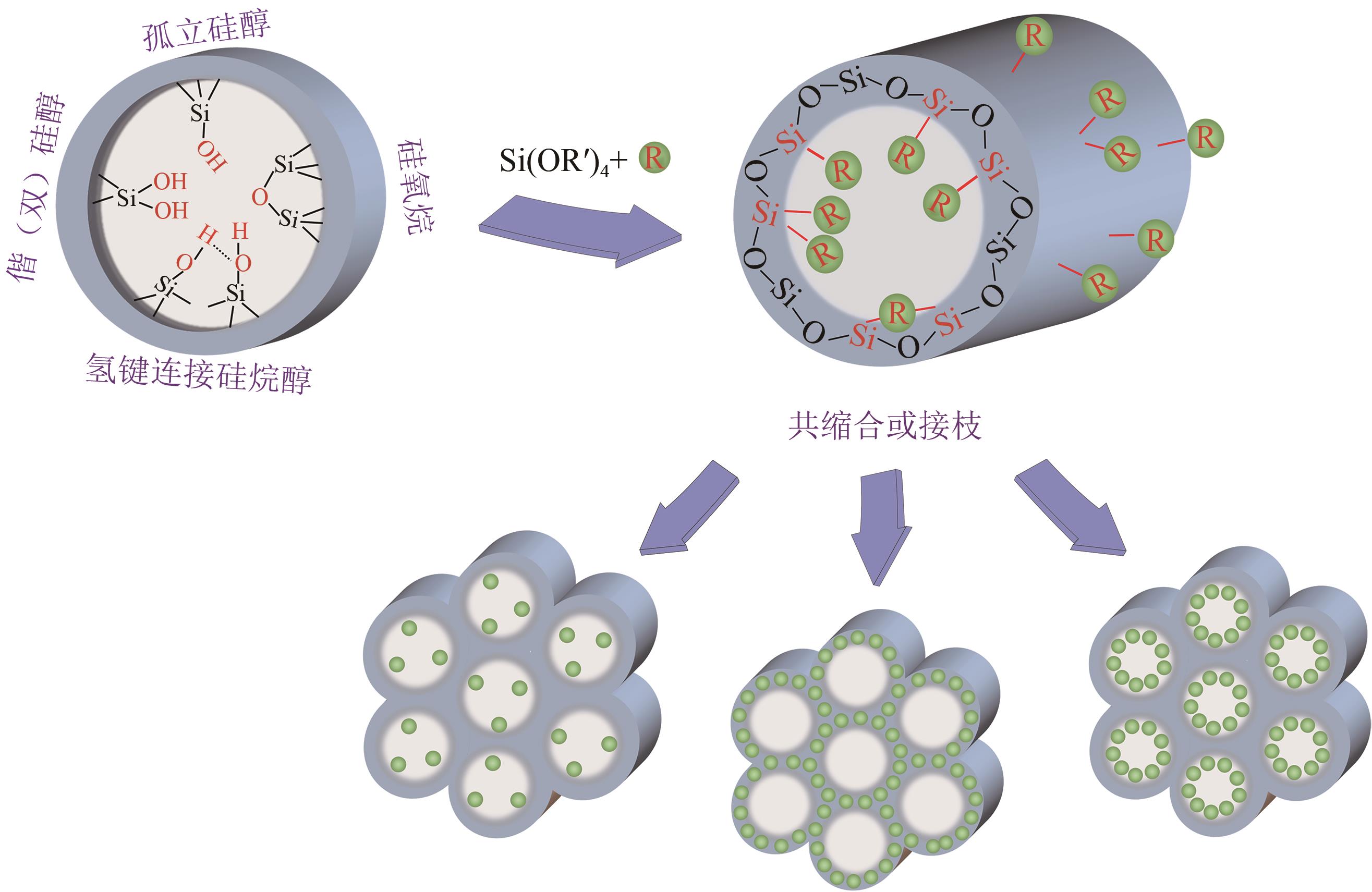
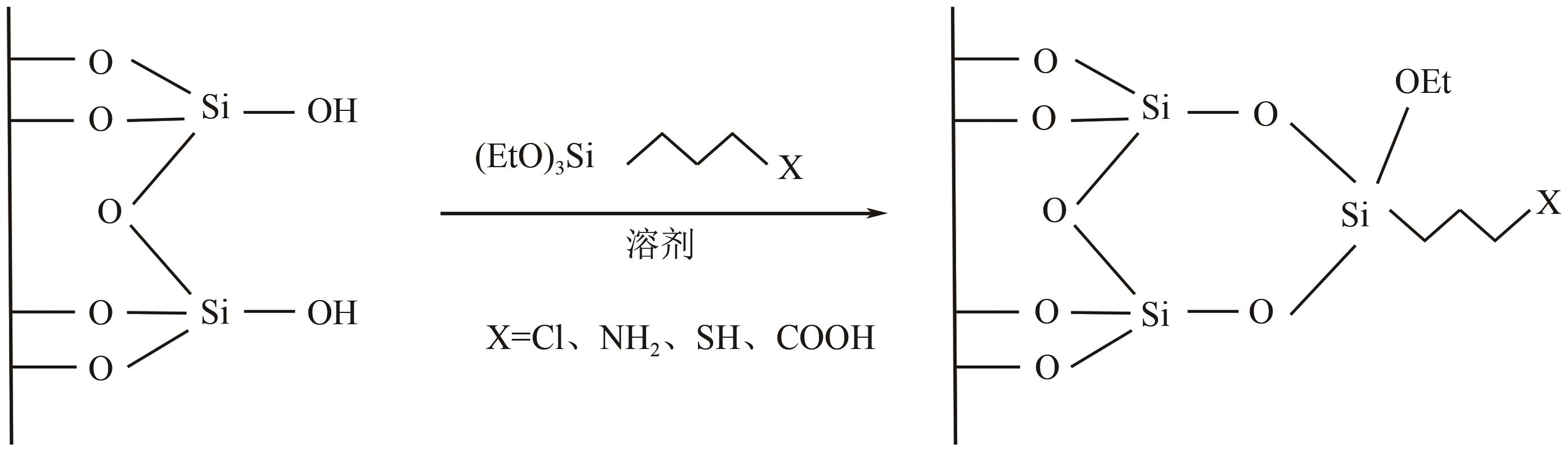
硅基介孔材料是一类具有“里程碑式”意义的新兴材料,其具有可调孔径、高比表面积、有序孔体系、易于功能化的稳定骨架等独特的结构和性能。发展至今,这些材料应用领域仍在不断扩展,尤其在吸附废水中的污染物和金属分离方面有着显著的优势。本文首先简要介绍了硅基介孔材料的研究工作,其中M41S和SBA系列介孔材料的研究最为广泛;综述了硅基介孔材料的制备要素,包括模板剂、硅源、合成条件,以及三者之间的相互作用对材料合成的影响;材料改性是研究的热点,对无机、有机和其他改性三个部分的研究工作做了总结,阐述了功能化修饰在材料的结构和性能方面的作用;最后探讨了这些材料在金属吸附方面的应用及其对金属的吸附特性和机理,并展望了硅基介孔材料在合成和应用方面的发展方向,为其在实际中的工程应用提供理论依据。
中图分类号:
引用本文
刘丽, 冯博, 文洋, 古启雄. 硅基介孔材料的合成、功能化及对金属的吸附研究进展[J]. 化工进展, 2024, 43(9): 5063-5078.
LIU Li, FENG Bo, WEN Yang, GU Qixiong. Research progress in synthesis, functionalization and metal adsorption of silica-based mesoporous materials[J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5063-5078.
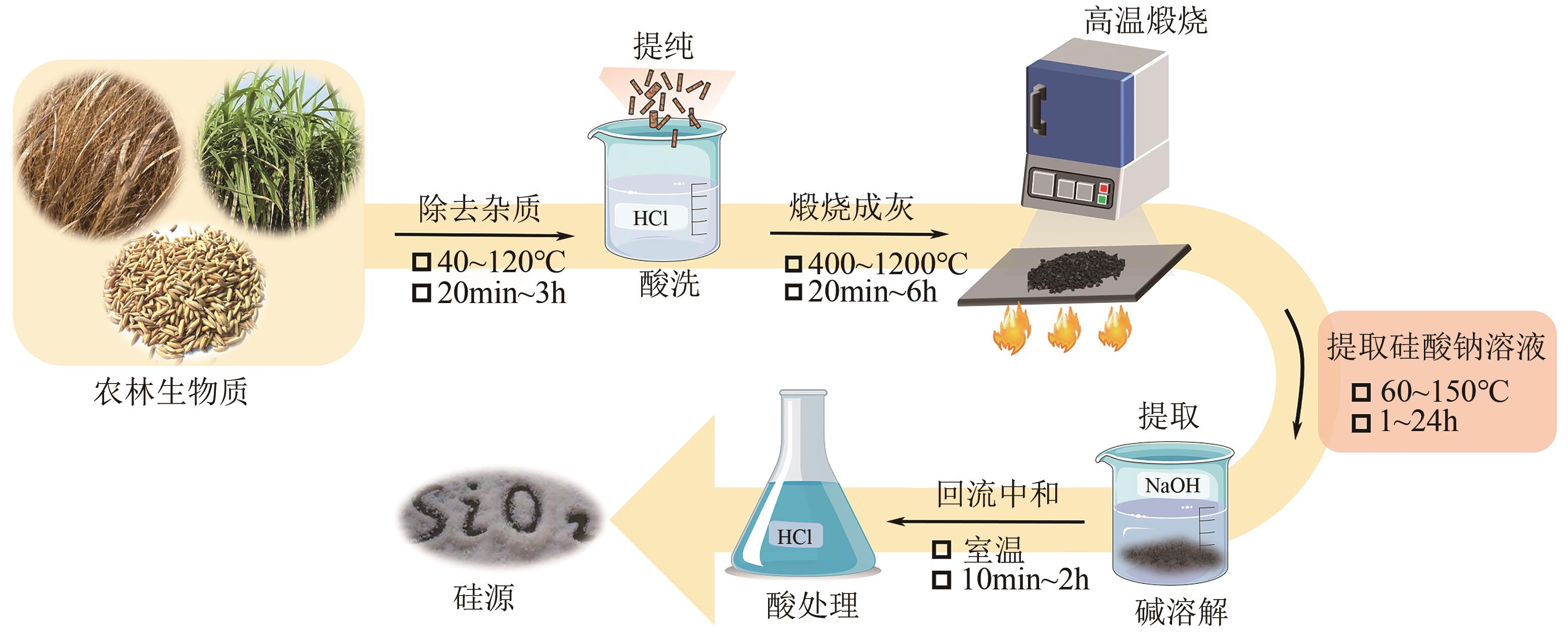
| 硅源 | 硅前体提取方法 | 硅质量 分数/% | 介孔材料 | 参考文献 |
|---|---|---|---|---|
| 玉米芯灰 | 碱提酸沉 | 50.4 | 提取SiO2 | [ |
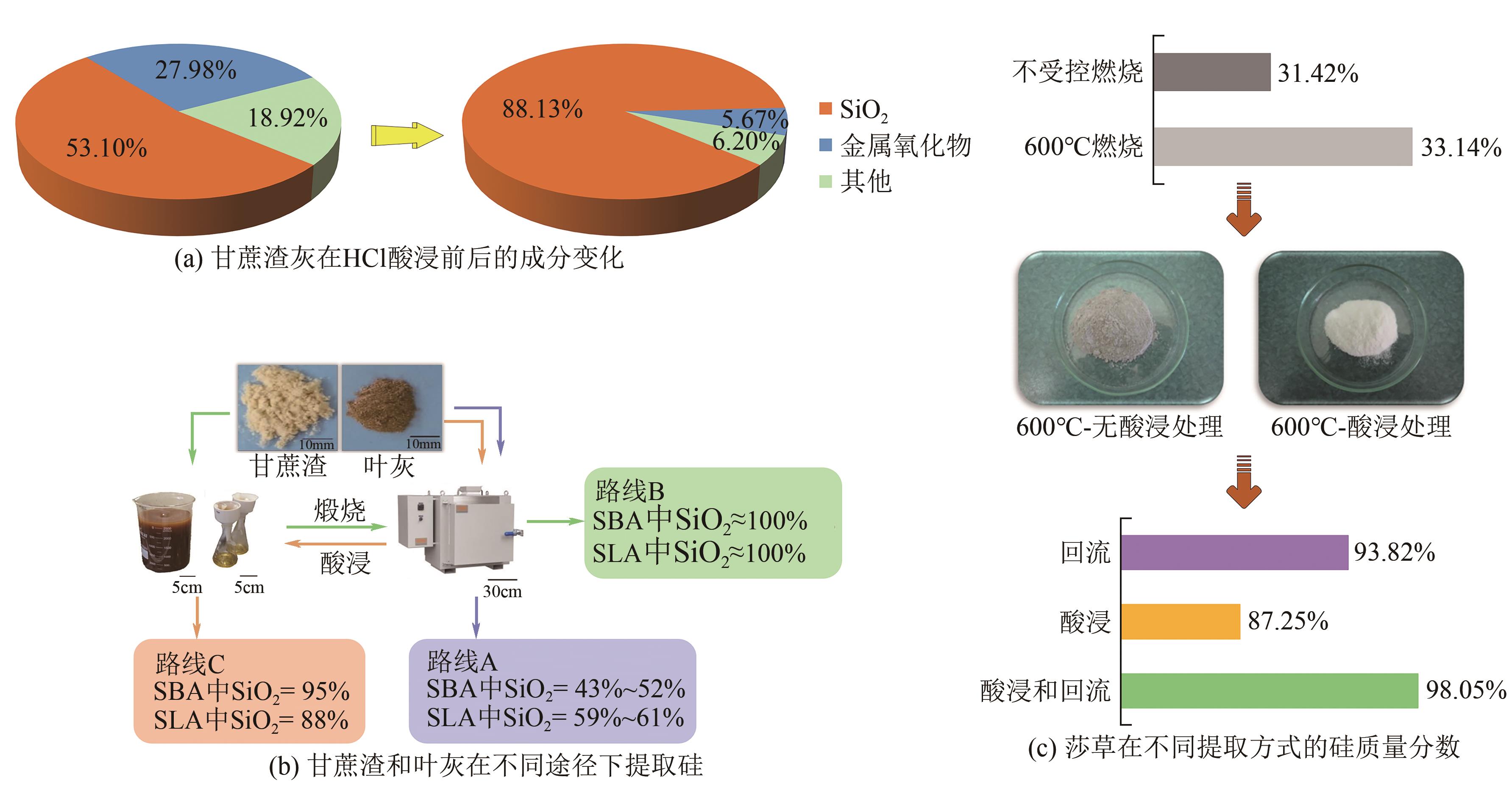
| 甘蔗渣和叶灰 | 煅烧和酸浸 | 88.0~95.0 | SiNPs | [ |
| 麦秆灰 | 碱回流 | 99.0 | MCM-41 | [ |
| 麦秸灰 | 碱回流 | >90.0 | MCM-41 | [ |
| 稻壳灰 | 碱熔/酸浸 | 88.5/95.4 | KCC-1 | [ |
| 棕榈油燃料灰 | 碱熔/酸浸 | 47.7/85.5 | SBA-15 | [ |
| 粉煤灰 | 碱熔 | 46.2~54.8 | MCM-41 | [ |
| 硅灰 | — | 96.9 | SiNPs | [ |
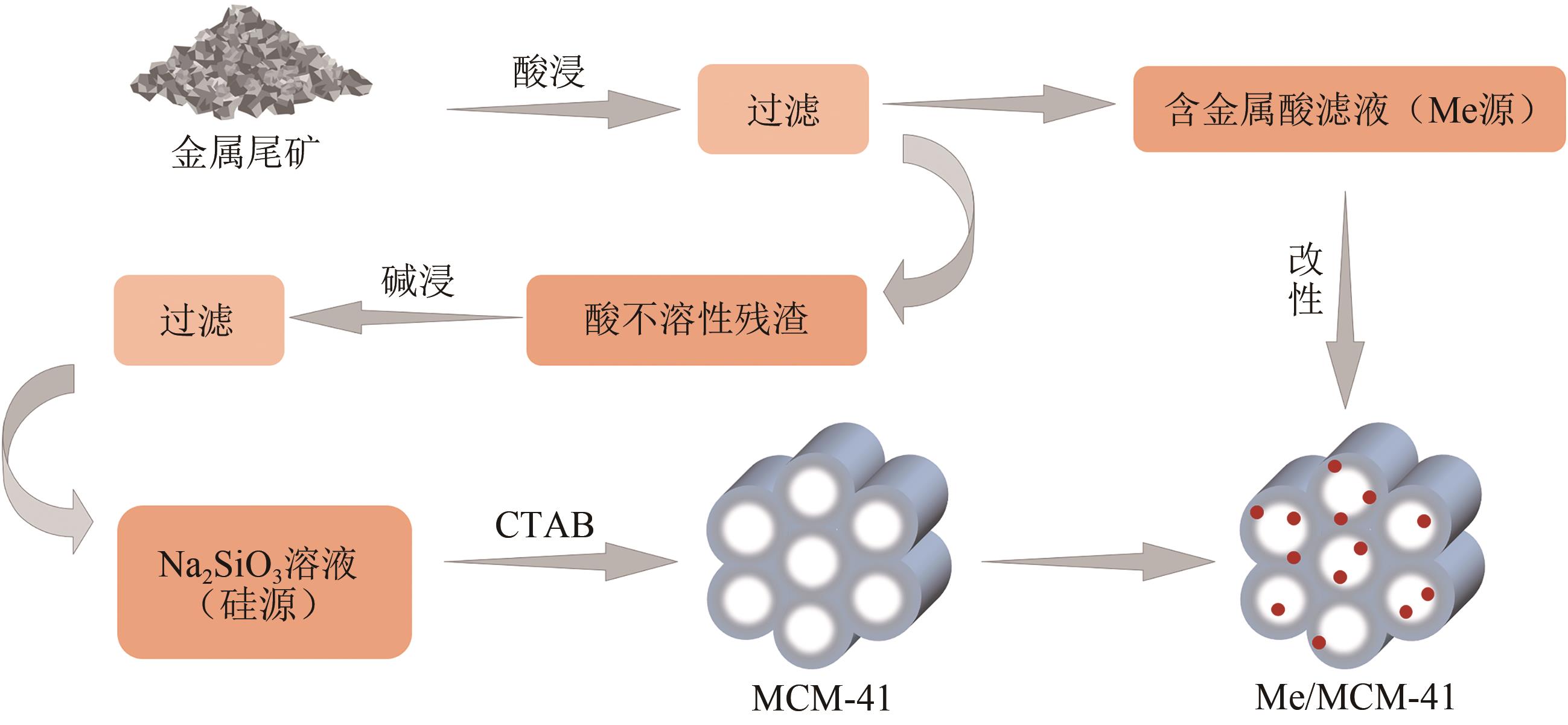
| 铜矿石尾矿 | 碱性熔盐 | 68.2 | MCM-41 | [ |
| 金矿尾矿 | 碱熔 | 36.4/77.7 | MSU-3/SBA-15 | [ |
| 铁矿尾矿 | 碱熔 | 71.3 | MS | [ |
| 再生玻璃 | 碱熔 | 97.8 | MCM-41 | [ |
| 电子垃圾 | 碱抽提 | 78.0 | MCM-48 | [ |
| 坡缕石 | 碱煅烧 | 50.0 | MCM-41 | [ |
| 阿尔及利亚 膨润土 | 碱熔 | 60.5 | MCM-41/SBA-15 | [ |
| 高岭土 | 煅烧和酸浸 | 95.17 | MCM-41 | [ |
| 埃洛石 | 碱老化 | 32.3 | SBA-15 | [ |
| 硅藻土 | 碱抽提 | 92.8 | MCM-41 | [ |
| 累托石 | 碱抽提 | 40.4 | MCM-41 | [ |
| 珍珠岩 | 酸浸和碱抽提 | 70.8 | MCM-41 | [ |
表1 部分工农业和天然矿物的硅源提取方法、硅质量分数和制备材料
| 硅源 | 硅前体提取方法 | 硅质量 分数/% | 介孔材料 | 参考文献 |
|---|---|---|---|---|
| 玉米芯灰 | 碱提酸沉 | 50.4 | 提取SiO2 | [ |
| 甘蔗渣和叶灰 | 煅烧和酸浸 | 88.0~95.0 | SiNPs | [ |
| 麦秆灰 | 碱回流 | 99.0 | MCM-41 | [ |
| 麦秸灰 | 碱回流 | >90.0 | MCM-41 | [ |
| 稻壳灰 | 碱熔/酸浸 | 88.5/95.4 | KCC-1 | [ |
| 棕榈油燃料灰 | 碱熔/酸浸 | 47.7/85.5 | SBA-15 | [ |
| 粉煤灰 | 碱熔 | 46.2~54.8 | MCM-41 | [ |
| 硅灰 | — | 96.9 | SiNPs | [ |
| 铜矿石尾矿 | 碱性熔盐 | 68.2 | MCM-41 | [ |
| 金矿尾矿 | 碱熔 | 36.4/77.7 | MSU-3/SBA-15 | [ |
| 铁矿尾矿 | 碱熔 | 71.3 | MS | [ |
| 再生玻璃 | 碱熔 | 97.8 | MCM-41 | [ |
| 电子垃圾 | 碱抽提 | 78.0 | MCM-48 | [ |
| 坡缕石 | 碱煅烧 | 50.0 | MCM-41 | [ |
| 阿尔及利亚 膨润土 | 碱熔 | 60.5 | MCM-41/SBA-15 | [ |
| 高岭土 | 煅烧和酸浸 | 95.17 | MCM-41 | [ |
| 埃洛石 | 碱老化 | 32.3 | SBA-15 | [ |
| 硅藻土 | 碱抽提 | 92.8 | MCM-41 | [ |
| 累托石 | 碱抽提 | 40.4 | MCM-41 | [ |
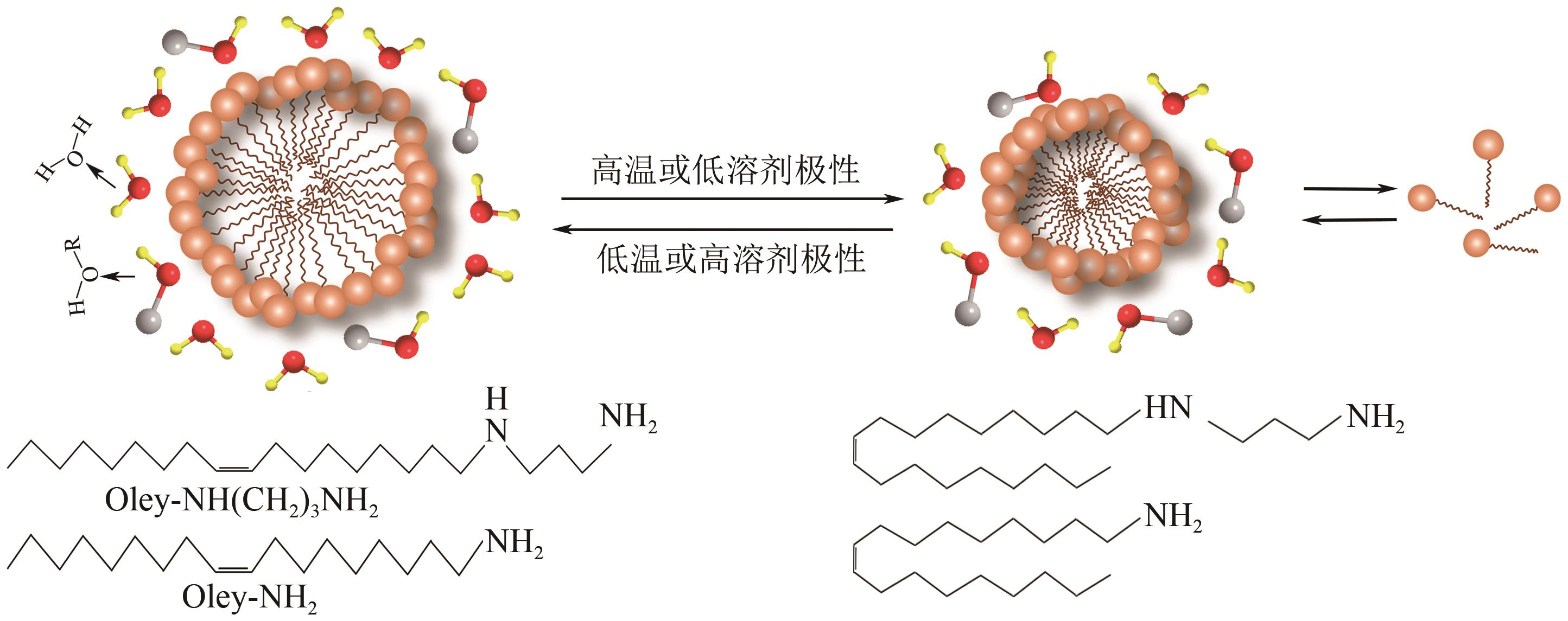
| 珍珠岩 | 酸浸和碱抽提 | 70.8 | MCM-41 | [ |
| 结构 | 代表性材料 | 代表性结构 |
|---|---|---|
| 核-壳结构 | Fe3O4@nSiO2@mSiO2球体 Fe3O4@mSiO2球体(单核) Fe3O4-x@mSiO2球体(多核) | 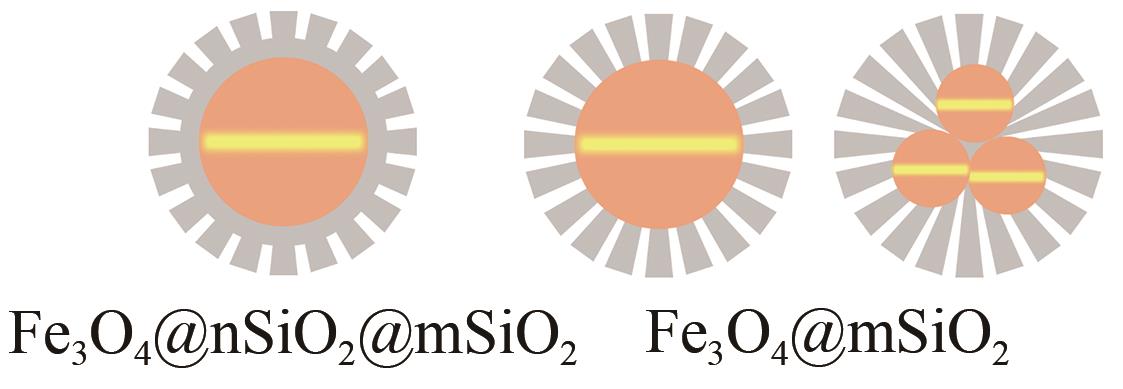 |
| 嵌式结构 | Fe x O y -SBA-15米粒状颗粒 Fe x O y -MCM-41球状颗粒 Fe2O3/FeO x -SBA-16 Fe3O4/Fe2O3-mSiO2球体/纳米棒 Fe3O4/FMSMs球体 | 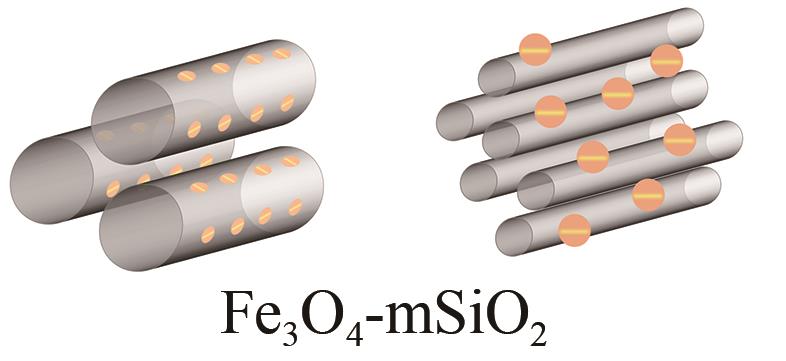 |
| 摇铃式中空结构 | Fe3O4/Fe2O3@mSiO2椭圆体 Fe3O4/Fe2O3@mSiO2椭圆体(双介孔壳) Fe3O4/Fe2O3@mSiO2球体(模板法) Fe3O4/Fe2O3@mSiO2球体(多壳可调) Fe3O4@mSiO2球体 (水热腐蚀法) | 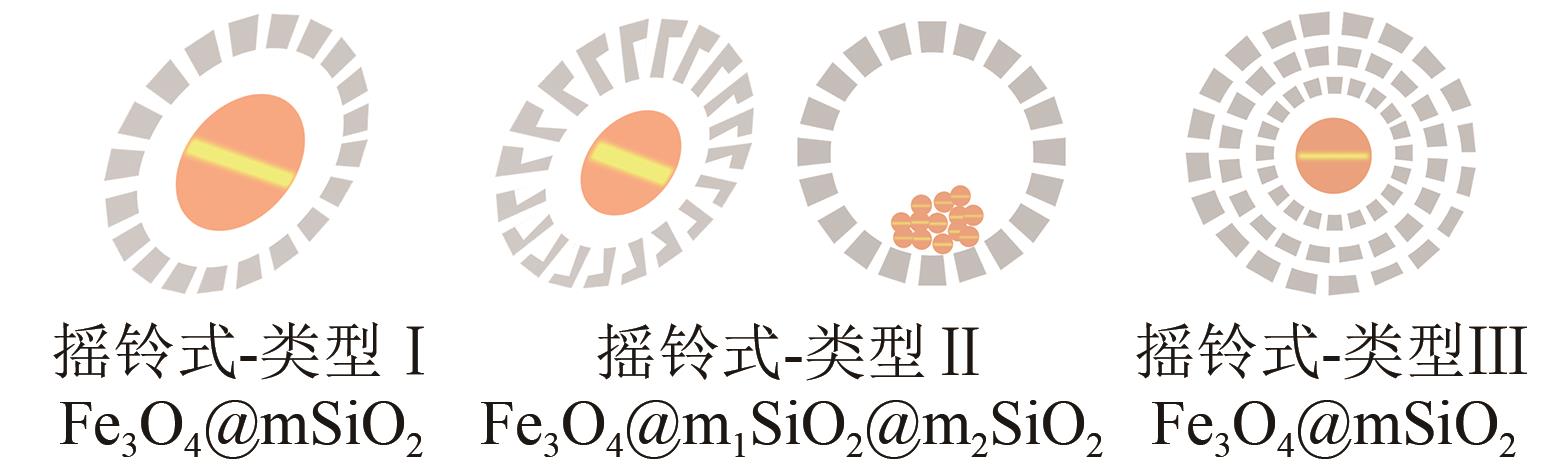 |
| mSiO2封装Fe3O4 | mSiO2封装Fe3O4 | — |
表2 Fe3O4/Fe2O3介孔二氧化硅颗粒的功能化结构[84]
| 结构 | 代表性材料 | 代表性结构 |
|---|---|---|
| 核-壳结构 | Fe3O4@nSiO2@mSiO2球体 Fe3O4@mSiO2球体(单核) Fe3O4-x@mSiO2球体(多核) |  |
| 嵌式结构 | Fe x O y -SBA-15米粒状颗粒 Fe x O y -MCM-41球状颗粒 Fe2O3/FeO x -SBA-16 Fe3O4/Fe2O3-mSiO2球体/纳米棒 Fe3O4/FMSMs球体 | 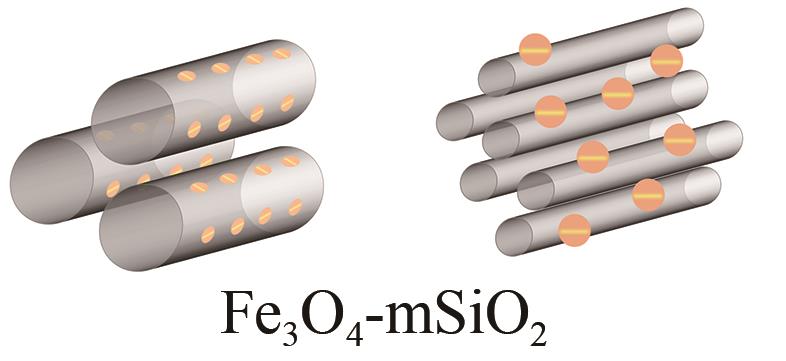 |
| 摇铃式中空结构 | Fe3O4/Fe2O3@mSiO2椭圆体 Fe3O4/Fe2O3@mSiO2椭圆体(双介孔壳) Fe3O4/Fe2O3@mSiO2球体(模板法) Fe3O4/Fe2O3@mSiO2球体(多壳可调) Fe3O4@mSiO2球体 (水热腐蚀法) |  |
| mSiO2封装Fe3O4 | mSiO2封装Fe3O4 | — |
| 吸附材料 | 金属 | 条件 | 吸附容量/mg·g-1 | 等温模型 | 动力学模型 | 参考文献 | |
|---|---|---|---|---|---|---|---|
| pH | T/K | ||||||
| Al-MCM-41 | Cs(Ⅰ) | 7 | RT | 84.0 | Freundlich | — | [ |
| MCM-41/NH2-NTAA-MCM-41 | MnO4(Ⅰ) | 3 | RT | 23.3/164.5 | Langmuir | PSO | [ |
| Pb(Ⅱ) | 5 | RT | 37.8/147.5 | ||||
| 胍-SBA-16 | Pb(Ⅱ) | 8 | 303 | 289.9 | Langmuir | PSO | [ |
| Hg(Ⅱ) | 5 | 308 | 259.9 | ||||
| Cd(Ⅱ) | 5 | 313 | 228.8 | ||||
| 氨基丙基-SBA-15/ 氨基丙基-PVP-SBA-15 | Ni(Ⅱ) | 6.66 | 298 | 19.2/72.4 | Langmuir | PFO | [ |
| Cu(Ⅱ) | 27.3/128.2 | ||||||
| Pb(Ⅱ) | 65.8/175.4 | ||||||
| 壳聚糖-MCM-41 | Be(Ⅱ) | 6 | RT | — | Langmuir和Freundlich | PSO | [ |
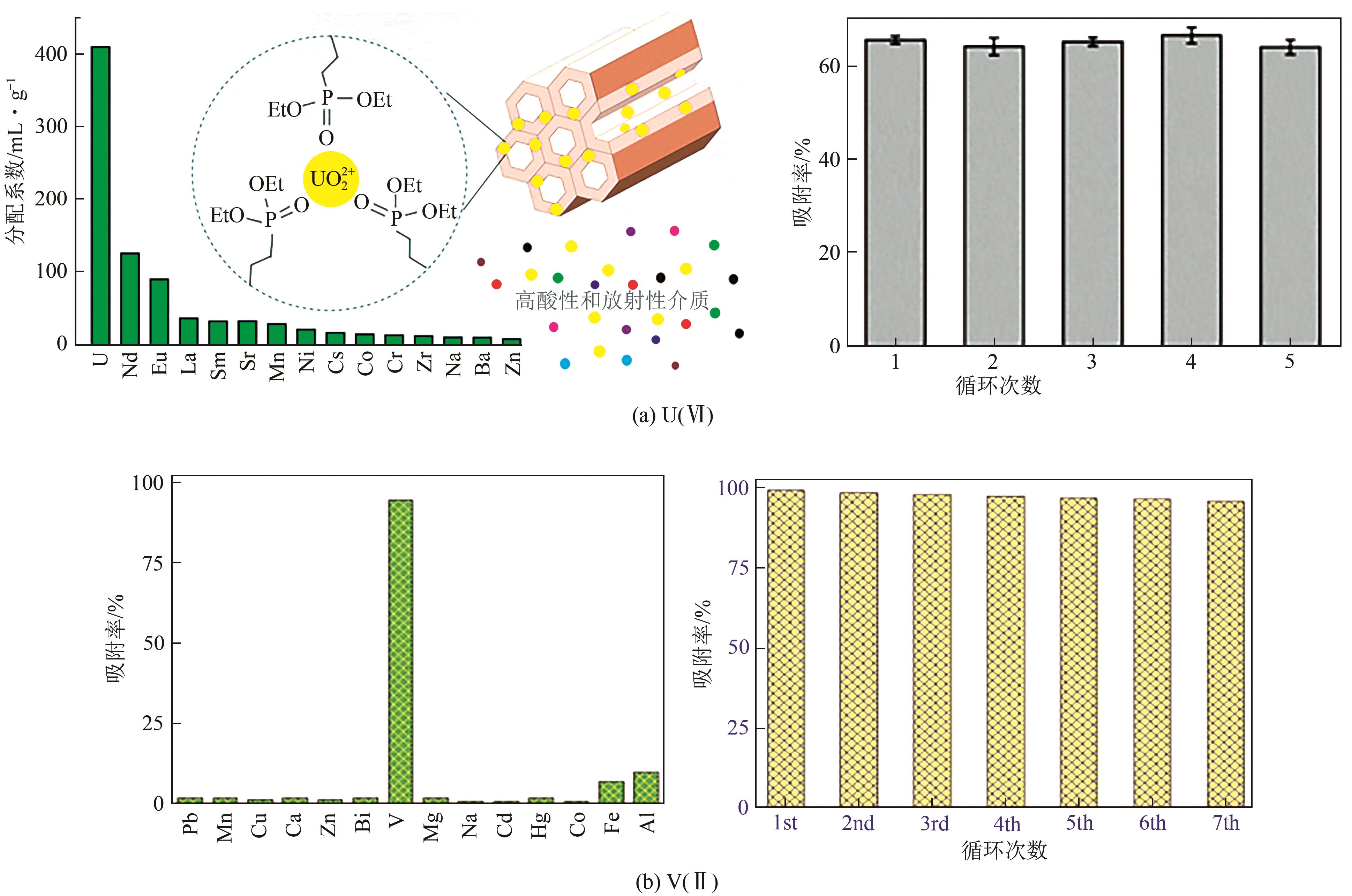
| 8-羟基喹啉-MSNs | V(Ⅱ) | 4 | RT | 492.6 | Langmuir | — | [ |
| 聚羧酸气凝胶 | Pd(Ⅱ) | 2.3 | RT | 369.0 | Sips | — | [ |
| 二硫代草酰胺-SBA-15 | Co(Ⅱ) | 10 | 318 | 2500.0 | Freundlich | PSO | [ |
| Sr(Ⅱ)印迹聚合物 | Sr(Ⅱ) | 6.5 | 298/308 | 47.3/89.1 | Langmuir | PSO | [ |
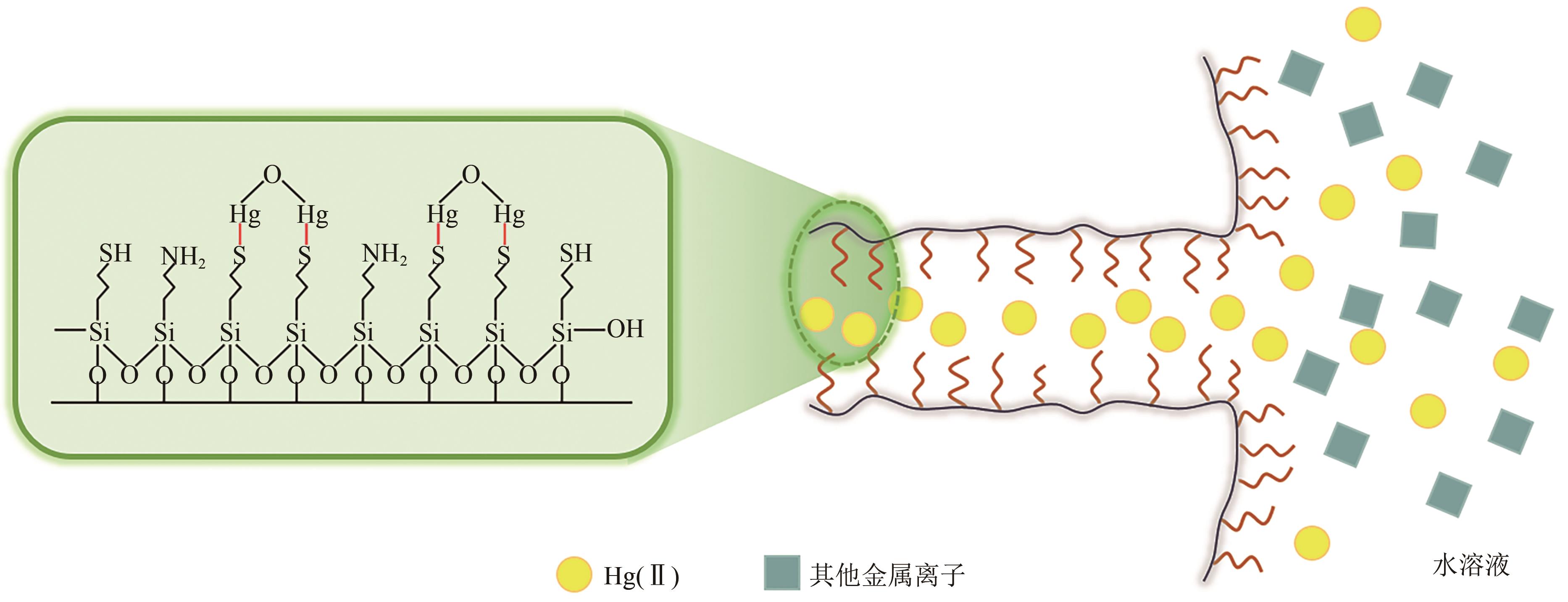
| 壳聚糖-PAA-MCM-41 | Hg(Ⅱ) | 4 | 298 | 164.0 | Langmuir | PSO | [ |
| 硫杂冠醚@SBA-15 | Au(Ⅲ) | 2 | 303 | 129.1 | Langmuir | PSO和PFO | [ |
| MnFe2O4-MCM-41-SH | Sb(Ⅲ) | 7 | RT | 164.8 | Langmuir | PSO | [ |
| EDTA-MSF | Nd(Ⅲ) | 6 | 298 | 117.0 | Langmuir | PSO | [ |
| Dy(Ⅲ)印迹IMS | Dy(Ⅲ) | 2 | 298 | 22.3 | Langmuir | PSO | [ |
| 硫醇-SBA-15 | Pt(Ⅳ) | 1~4 | RT | 222.0 | Langmuir | — | [ |
| 铀酰离子印迹-MS | U(Ⅵ) | 5.2 | 298 | 80.0 | Langmuir | PSO | [ |
| 氨基丙基-MCM-41 | Cr(Ⅵ) | 2 | 298 | 78.8 | Langmuir | — | [ |
| 氨基丙基-CH3-MCM-41 | 29.2 | ||||||
| 乙醇胺-KIT-6 | Re(Ⅶ) | 3 | 303 | 111.4 | Langmuir | — | [ |
表3 部分改性介孔硅基材料对金属的吸附
| 吸附材料 | 金属 | 条件 | 吸附容量/mg·g-1 | 等温模型 | 动力学模型 | 参考文献 | |
|---|---|---|---|---|---|---|---|
| pH | T/K | ||||||
| Al-MCM-41 | Cs(Ⅰ) | 7 | RT | 84.0 | Freundlich | — | [ |
| MCM-41/NH2-NTAA-MCM-41 | MnO4(Ⅰ) | 3 | RT | 23.3/164.5 | Langmuir | PSO | [ |
| Pb(Ⅱ) | 5 | RT | 37.8/147.5 | ||||
| 胍-SBA-16 | Pb(Ⅱ) | 8 | 303 | 289.9 | Langmuir | PSO | [ |
| Hg(Ⅱ) | 5 | 308 | 259.9 | ||||
| Cd(Ⅱ) | 5 | 313 | 228.8 | ||||
| 氨基丙基-SBA-15/ 氨基丙基-PVP-SBA-15 | Ni(Ⅱ) | 6.66 | 298 | 19.2/72.4 | Langmuir | PFO | [ |
| Cu(Ⅱ) | 27.3/128.2 | ||||||
| Pb(Ⅱ) | 65.8/175.4 | ||||||
| 壳聚糖-MCM-41 | Be(Ⅱ) | 6 | RT | — | Langmuir和Freundlich | PSO | [ |
| 8-羟基喹啉-MSNs | V(Ⅱ) | 4 | RT | 492.6 | Langmuir | — | [ |
| 聚羧酸气凝胶 | Pd(Ⅱ) | 2.3 | RT | 369.0 | Sips | — | [ |
| 二硫代草酰胺-SBA-15 | Co(Ⅱ) | 10 | 318 | 2500.0 | Freundlich | PSO | [ |
| Sr(Ⅱ)印迹聚合物 | Sr(Ⅱ) | 6.5 | 298/308 | 47.3/89.1 | Langmuir | PSO | [ |
| 壳聚糖-PAA-MCM-41 | Hg(Ⅱ) | 4 | 298 | 164.0 | Langmuir | PSO | [ |
| 硫杂冠醚@SBA-15 | Au(Ⅲ) | 2 | 303 | 129.1 | Langmuir | PSO和PFO | [ |
| MnFe2O4-MCM-41-SH | Sb(Ⅲ) | 7 | RT | 164.8 | Langmuir | PSO | [ |
| EDTA-MSF | Nd(Ⅲ) | 6 | 298 | 117.0 | Langmuir | PSO | [ |
| Dy(Ⅲ)印迹IMS | Dy(Ⅲ) | 2 | 298 | 22.3 | Langmuir | PSO | [ |
| 硫醇-SBA-15 | Pt(Ⅳ) | 1~4 | RT | 222.0 | Langmuir | — | [ |
| 铀酰离子印迹-MS | U(Ⅵ) | 5.2 | 298 | 80.0 | Langmuir | PSO | [ |
| 氨基丙基-MCM-41 | Cr(Ⅵ) | 2 | 298 | 78.8 | Langmuir | — | [ |
| 氨基丙基-CH3-MCM-41 | 29.2 | ||||||
| 乙醇胺-KIT-6 | Re(Ⅶ) | 3 | 303 | 111.4 | Langmuir | — | [ |
| 1 | SALIMIAN S, ZADHOUSH A, MOHAMMADI A. A review on new mesostructured composite materials: Part Ⅰ. Synthesis of polymer-mesoporous silica nanocomposite[J]. Journal of Reinforced Plastics and Composites, 2018, 37(7): 441-459. |
| 2 | 郭程, 张威, 唐云. 有序介孔材料: 历史、现状与发展趋势[J]. 高等学校化学学报, 2022, 43(8): 25-41. |
| GUO Cheng, ZHANG Wei, TANG Yun. Ordered mesoporous materials: History, progress and perspective[J]. Chemical Journal of Chinese Universities, 2022, 43(8): 25-41. | |
| 3 | KRESGE C T, LEONOWICZ M E, ROTH W J, et al. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism[J]. Nature, 1992, 359: 710-712. |
| 4 | QIU Pengpeng, MA Bing, HUNG Chin-Te, et al. Spherical mesoporous materials from single to multilevel architectures[J]. Accounts of Chemical Research, 2019, 52(10): 2928-2938. |
| 5 | ZHAO Dongyuan, HUO Qisheng, FENG Jianglin, et al. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures[J]. Journal of the American Chemical Society, 1998, 120(24): 6024-6036. |
| 6 | TIAN Bozhi, LIU Xiaoying, TU Bo, et al. Self-adjusted synthesis of ordered stable mesoporous minerals by acid-base pairs[J]. Nature Materials, 2003, 2(3): 159-163. |
| 7 | SHI Yifeng, WAN Ying, TU Bo, et al. Nanocasting synthesis of ordered mesoporous silicon nitrides with a high nitrogen content[J]. The Journal of Physical Chemistry C, 2008, 112(1): 112-116. |
| 8 | HOCHBAUM Allon I, GARGAS Daniel, HWANG Yun Jeong, et al. Single crystalline mesoporous silicon nanowires[J]. Nano Letters, 2009, 9(10): 3550-3554. |
| 9 | ZHANG Renyuan, TU Bo, ZHAO Dongyuan. Synthesis of highly stable and crystalline mesoporous anatase by using a simple surfactant sulfuric acid carbonization method[J]. Chemistry—A European Journal, 2010, 16(33): 9977-9981. |
| 10 | TENG Zhaogang, SU Xiaodan, ZHENG Yuanyi, et al. Mesoporous silica hollow spheres with ordered radial mesochannels by a spontaneous self-transformation approach[J]. Chemistry of Materials, 2013, 25(1): 98-105. |
| 11 | LIU Yong, LUO Yongfeng, ELZATAHRY Ahmed A, et al. Mesoporous TiO2 mesocrystals: Remarkable defects-induced crystallite-interface reactivity and their in situ conversion to single crystals[J]. ACS Central Science, 2015, 1(7): 400-408. |
| 12 | ZUO Xiuxia, XIA Yonggao, JI Qing, et al. Self-templating construction of 3D hierarchical macro-/mesoporous silicon from 0D silica nanoparticles[J]. ACS Nano, 2017, 11(1): 889-899. |
| 13 | LAN Kun, LIU Yao, ZHANG Wei, et al. Uniform ordered two-dimensional mesoporous TiO2 nanosheets from hydrothermal-induced solvent-confined monomicelle assembly[J]. Journal of the American Chemical Society, 2018, 140(11): 4135-4143. |
| 14 | ZHANG Wei, HE Haili, TIAN Yong, et al. Synthesis of uniform ordered mesoporous TiO2 microspheres with controllable phase junctions for efficient solar water splitting[J]. Chemical Science, 2018, 10(6): 1664-1670. |
| 15 | ZHANG Wei, HE Haili, TIAN Yong, et al. Defect-engineering of mesoporous TiO2 microspheres with phase junctions for efficient visible-light driven fuel production[J]. Nano Energy, 2019, 66: 104113. |
| 16 | WANG Yujie, LIU Yaozu, LI Hui, et al. Three-dimensional mesoporous covalent organic frameworks through steric hindrance engineering[J]. Journal of the American Chemical Society, 2020, 142(8): 3736-3741. |
| 17 | ZOU Houbing, DAI Jinyu, SUO Jinquan, et al. Dual metal nanoparticles within multicompartmentalized mesoporous organosilicas for efficient sequential hydrogenation[J]. Nature Communications, 2021, 12(1): 4968. |
| 18 | WALKER Rebecca C, HYER Andres P, GUO Haiquan, et al. Silica aerogel synthesis/process-property predictions by machine learning[J]. Chemistry of Materials, 2023, 35(13): 4897-4910. |
| 19 | KUSZ Jakub, BOISSIERE Cédric, IHIAWAKRIM Dris, et al. Insight into the molecular self-assembly and structural organization of mesoporous hybrid silica films with binary composition[J]. Chemistry of Materials, 2023, 35(18): 7671-7682. |
| 20 | AKTI Filiz. The effect of potassium modification on structural properties and catalytic activity of copper and iron containing SBA-16 catalysts for selective oxidation of ethanol[J]. Materials Chemistry and Physics, 2019, 227: 21-28. |
| 21 | KIPKEMBOI Pius, FOGDEN Andrew, ALFREDSSON Viveka, et al. Triblock copolymers as templates in mesoporous silica formation: Structural dependence on polymer chain length and synthesis temperature[J]. Langmuir, 2001, 17(17): 5398-5402. |
| 22 | COSTA José Arnaldo S, PARANHOS Caio M. Mitigation of silica-rich wastes: An alternative to the synthesis eco-friendly silica-based mesoporous materials[J]. Microporous and Mesoporous Materials, 2020, 309: 110570. |
| 23 | COSTA José Arnaldo S, DE JESUS Roberta A, SANTOS Danilo O, et al. Recent progresses in the adsorption of organic, inorganic, and gas compounds by MCM-41-based mesoporous materials[J]. Microporous and Mesoporous Materials, 2020, 291: 109698. |
| 24 | LIU Chunyan, WANG Suyan, RONG Zhihong, et al. Synthesis of structurally stable MCM-48 using mixed surfactants as co-template and adsorption of vitamin B12 on the mesoporous MCM-48[J]. Journal of Non-Crystalline Solids, 2010, 356(25/26/27): 1246-1251. |
| 25 | WEI Yen, JIN Danliang, DING Tianzhong, et al. A non-surfactant templating route to mesoporous silica materials[J]. Advanced Materials, 1998, 10(4): 313-316. |
| 26 | THOMAS Bejoy, BABONNEAU Florence, CORADIN Thibaud, et al. One-step introduction of broad-band mesoporosity in silica particles using a stimuli-responsive bioderived glycolipid[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(3): 512-522. |
| 27 | AHMADY Azin Rashidy, HOSSEINZADEH Pakshid, SOLOUK Atefeh, et al. Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: A review[J]. Advances in Colloid and Interface Science, 2022, 299: 102581. |
| 28 | COLLART O, VAN DER VOORT P, VANSANT E F, et al. A high-yield reproducible synthesis of MCM-48 starting from fumed silica[J]. The Journal of Physical Chemistry B, 2001, 105(51): 12771-12777. |
| 29 | JIAO Jian, WANG Lei, WU Guangli, et al. Effects of framework structure and coupling modification on the properties of mesoporous silica/poly(methyl methacrylate) composites[J]. Journal of Reinforced Plastics and Composites, 2015, 34(3): 222-231. |
| 30 | HU Jun, GAO Feng, SHANG Yazhuo, et al. One-step synthesis of micro/mesoporous material templated by CTAB and imidazole ionic liquid in aqueous solution[J]. Microporous and Mesoporous Materials, 2011, 142(1): 268-275. |
| 31 | CHEN Fengxi, HUANG Lin, YANG Xiaojun, et al. Synthesis of Al-substituted MCM-41 and MCM-48 solid acids with mixed cationic-anionic surfactants as templates[J]. Materials Letters, 2013, 109: 299-301. |
| 32 | AHMED Sohail, RAMLI Anita, YUSUP Suzana. CO2 adsorption study on primary, secondary and tertiary amine functionalized Si-MCM-41[J]. International Journal of Greenhouse Gas Control, 2016, 51: 230-238. |
| 33 | MIAO Chao, LIANG Lixing, ZHANG Fan, et al. Review of the fabrication and application of porous materials from silicon-rich industrial solid waste[J]. International Journal of Minerals, Metallurgy and Materials, 2022, 29(3): 424-438. |
| 34 | VELMURUGAN Palanivel, SHIM Jaehong, LEE Kui-Jae, et al. Extraction, characterization, and catalytic potential of amorphous silica from corn cobs by sol-gel method[J]. Journal of Industrial and Engineering Chemistry, 2015, 29: 298-303. |
| 35 | BORTOLOTTO TEIXEIRA Luyza, GUZI DE MORAES Elisângela, PAOLINELLI SHINHE Giovanna, et al. Obtaining biogenic silica from sugarcane bagasse and leaf ash[J]. Waste and Biomass Valorization, 2021, 12(6): 3205-3221. |
| 36 | SOHRABNEZHAD Sh, JAFARZADEH A, POURAHMAD A. Synthesis and characterization of MCM-41 ropes[J]. Materials Letters, 2018, 212: 16-19. |
| 37 | MA Ying, CHEN Hui, SHI Yuanchang, et al. Low cost synthesis of mesoporous molecular sieve MCM-41 from wheat straw ash using CTAB as surfactant[J]. Materials Research Bulletin, 2016, 77: 258-264. |
| 38 | HASAN R, CHONG C C, BUKHARI S N, et al. Effective removal of Pb(Ⅱ) by low-cost fibrous silica KCC-1 synthesized from silica-rich rice husk ash[J]. Journal of Industrial and Engineering Chemistry, 2019, 75: 262-270. |
| 39 | ABDULLAH N, CHONG C C, RAZAK H A, et al. Synthesis of Ni/SBA-15 for CO2 reforming of CH4: Utilization of palm oil fuel ash as silica source[J]. Materials Today: Proceedings, 2018, 5(10): 21594-21603. |
| 40 | XU Chenglong, FENG Yali, LI Haoran, et al. Adsorption of heavy metal ions by iron tailings: Behavior, mechanism, evaluation and new perspectives[J]. Journal of Cleaner Production, 2022, 344: 131065. |
| 41 | ZHOU Chunyu, YAN Chunjie, ZHAO Junjie, et al. Rapid synthesis of morphology-controlled mesoporous silica nanoparticles from silica fume[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 62: 307-312. |
| 42 | FU Pingfeng, YANG Tianwen, FENG Jie, et al. Synthesis of mesoporous silica MCM-41 using sodium silicate derived from copper ore tailings with an alkaline molted-salt method[J]. Journal of Industrial and Engineering Chemistry, 2015, 29: 338-343. |
| 43 | SARI YILMAZ Muge, KARAKAS Sevil Begum. Low-cost synthesis of organic-inorganic hybrid MSU-3 from gold mine waste for CO2 adsorption[J]. Water, Air, & Soil Pollution, 2018, 229(10): 326. |
| 44 | HAN Xiaoyu, WANG Yaping, ZHANG Na, et al. Facile synthesis of mesoporous silica derived from iron ore tailings for efficient adsorption of methylene blue[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 617: 126391. |
| 45 | RAMÍREZ-ARÉVALO Marina S, Tezozomoc PÉREZ-LÓPEZ, Patricia QUINTANA-OWEN, et al. Comparative study of physicochemical properties of MCM-41 silica nanoparticles obtained from recycled glass and TEOS[J]. Silicon, 2023, 15(6): 2653-2661. |
| 46 | LIOU Tzong-Horng, JHENG Jhu-Yin. Synthesis of high-quality ordered mesoporous carbons using a sustainable way from recycling of E-waste as a silica template source[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(5): 6507-6517. |
| 47 | GUAN Yuan, WANG Shaomang, WANG Xin, et al. Preparation of mesoporous Al-MCM-41 from natural palygorskite and its adsorption performance for hazardous aniline dye-basic fuchsin[J]. Microporous and Mesoporous Materials, 2018, 265: 266-274. |
| 48 | SAHEL Fatma, SEBIH Fatiha, BELLAHOUEL Salima, et al. Synthesis and characterization of highly ordered mesoporous nanomaterials Al-MCM-41 and Al-SBA-15 from bentonite as efficient catalysts for the production of biodiesel MELA and EELA[J]. Research on Chemical Intermediates, 2020, 46(1): 133-148. |
| 49 | SANTOS Evânia, COSTA Luelc, OLIVEIRA Edipo, et al. Al-MCM-41 synthesized from Kaolin via hydrothermal route: Structural characterization and use as an efficient adsorbent of methylene blue[J]. Journal of the Brazilian Chemical Society, 2018: 29: 2378-2386. |
| 50 | PHAM Xuan Nui, NGUYEN Manh B, DOAN Huan V. Direct synthesis of highly ordered Ti-containing Al-SBA-15 mesostructured catalysts from natural halloysite and its photocatalytic activity for oxidative desulfurization of dibenzothiophene[J]. Advanced Powder Technology, 2020, 31(8): 3351-3360. |
| 51 | FU Yong, HUANG Yue, HU Jianshe. Preparation of chitosan/ MCM-41-PAA nanocomposites and the adsorption behaviour of Hg(Ⅱ) ions[J]. Royal Society Open Science, 2018, 5(3): 171927. |
| 52 | CHEN Hongyun, YANG Huaming, XI Yunfei. Highly ordered and hexagonal mesoporous silica materials with large specific surface from natural rectorite mineral[J]. Microporous and Mesoporous Materials, 2019, 279: 53-60. |
| 53 | CHEN Hongyun, FU Siyao, FU Liangjie, et al. Simple synthesis and characterization of hexagonal and ordered Al-MCM-41 from natural perlite[J]. Minerals, 2019, 9(5): 264. |
| 54 | FALK G, SHINHE G P, TEIXEIRA L B, et al. Synthesis of silica nanoparticles from sugarcane bagasse ash and nano-silicon via magnesiothermic reactions[J]. Ceramics International, 2019, 45(17): 21618-21624. |
| 55 | SEPTEMBER Lyle A, KHESWA Ntombizonke, SEROKA Ntalane S, et al. Green synthesis of silica and silicon from agricultural residue sugarcane bagasse ash—A mini review[J]. RSC Advances, 2023, 13(2): 1370-1380. |
| 56 | NORSURAYA S, FAZLENA H, NORHASYIMI R. Sugarcane bagasse as a renewable source of silica to synthesize santa barbara amorphous-15 (SBA-15)[J]. Procedia Engineering, 2016, 148: 839-846. |
| 57 | LIOU Tzong-Horng, WANG Sheng-Yeh, LIN Yen-Tung, et al. Sustainable utilization of rice husk waste for preparation of ordered nanostructured mesoporous silica and mesoporous carbon: Characterization and adsorption performance[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 636: 128150. |
| 58 | YU Lei, FARINMADE Azeem, AJUMOBI Oluwole, et al. One-step hydropyrolysis and hydrotreating tandem reactions of miscanthus×giganteus using Ni impregnated ZSM-5/MCM-41 composites[J]. Energy & Fuels, 2021, 35(24): 20117-20130. |
| 59 | GHORBANI Farshid, YOUNESI Habibollah, MEHRABAN Zahra, et al. Preparation and characterization of highly pure silica from sedge as agricultural waste and its utilization in the synthesis of mesoporous silica MCM-41[J]. Journal of the Taiwan Institute of Chemical Engineers, 2013, 44(5): 821-828. |
| 60 | AZIZI Seyed Naser, GHASEMI Shahram, Olia RANGRIZ-ROSTAMI. Synthesis of MCM-41 nanoparticles from stem of common reed ash silica and their application as substrate in electrooxidation of methanol[J]. Bulletin of Materials Science, 2018, 41(3): 88. |
| 61 | CHONG Chi cheng, ABDULLAH Nornasuha, BUKHARI Syahida Nasuha, et al. Hydrogen production via CO2 reforming of CH4 over low-cost Ni/SBA-15 from silica-rich palm oil fuel ash (POFA) waste[J]. International Journal of Hydrogen Energy, 2019, 44(37): 20815-20825. |
| 62 | ARUMUGAM A, KARUPPASAMY Gopinath, JEGADEESAN Gautham B. Synthesis of mesoporous materials from bamboo leaf ash and catalytic properties of immobilized lipase for hydrolysis of rubber seed oil[J]. Materials Letters, 2018, 225: 113-116. |
| 63 | PANEK R, WDOWIN M, FRANUS W, et al. Fly ash-derived MCM-41 as a low-cost silica support for polyethyleneimine in post-combustion CO2 capture[J]. Journal of CO2 Utilization, 2017, 22: 81-90. |
| 64 | ZHANG Xu, DU Tao, JIA He. Efficient activation of coal fly ash for silica and alumina leaches and the dependence of Pb(Ⅱ) removal capacity on the crystallization conditions of Al-MCM-41[J]. International Journal of Molecular Sciences, 2021, 22(12): 6540. |
| 65 | XU Yusheng, HU Enzheng, XU Dongyan, et al. Activation of peroxymonosulfate by bimetallic CoMn oxides loaded on coal fly ash-derived SBA-15 for efficient degradation of Rhodamine B[J]. Separation and Purification Technology, 2021, 274: 119081. |
| 66 | ABUKHADRA M R, SHABAN M. Recycling of different solid wastes in synthesis of high-order mesoporous silica as adsorbent for safranin dye[J]. International Journal of Environmental Science and Technology, 2019, 16(11): 7573-7582. |
| 67 | KHAN Afzal Husain, LÓPEZ-MALDONADO Eduardo Alberto, ALAM Shah Saud, et al. Municipal solid waste generation and the current state of waste-to-energy potential: State of art review[J]. Energy Conversion and Management, 2022, 267: 115905. |
| 68 | DENG Yanxi, XU Xiaodong, WANG Rong, et al. Characterization and photocatalytic evaluation of Fe-loaded mesoporous MCM-41 prepared using iron and silicon sources extracted from iron ore tailing[J]. Waste and Biomass Valorization, 2020, 11(4): 1491-1498. |
| 69 | QIANG Zhiqin, SHEN Xianjiang, GUO Min, et al. A simple hydrothermal synthesis of zeolite X from bauxite tailings for highly efficient adsorbing CO2 at room temperature[J]. Microporous and Mesoporous Materials, 2019, 287: 77-84. |
| 70 | MORI Yutaka, PINNAVAIA Thomas J. Optimizing organic functionality in mesostructured silica: Direct assembly of mercaptopropyl groups in wormhole framework structures[J]. Chemistry of Materials, 2001, 13(6): 2173-2178. |
| 71 | COSTA José Arnaldo S, DE JESUS Roberta A, SANTOS Danilo O, et al. Synthesis, functionalization, and environmental application of silica-based mesoporous materials of the M41S and SBA-n families: A review[J]. Journal of Environmental Chemical Engineering, 2021, 9(3): 105259. |
| 72 | ANDRADE Gracielle Ferreira, SOARES Daniel Cristian Ferreira, DE SOUSA ALMEIDA Ramon Kenned, et al. Mesoporous silica SBA-16 functionalized with alkoxysilane groups: Preparation, characterization, and release profile study[J]. Journal of Nanomaterials, 2012, 2012: 816496. |
| 73 | 吴冰峰, 杨丽娜, 李剑, 等. 生物质模板剂制备介孔材料研究进展[J]. 化工进展, 2018, 37(7): 2686-2693. |
| WU Bingfeng, YANG Lina, LI Jian, et al. Application of biomass templates in the preparation of mesoporous materials[J]. Chemical Industry and Engineering Progress, 2018, 37(7): 2686-2693. | |
| 74 | CANLAS Christian P, PINNAVAIA Thomas J. Bio-derived oleylsurfactants as porogens for the sustainable synthesis of micelle-templated mesoporous silica[J]. RSC Advances, 2012, 2(19): 7449-7455. |
| 75 | BJÖRK Emma M, Peter MÄKIE, Lina ROGSTRÖM, et al. Formation of block-copolymer-templated mesoporous silica[J]. Journal of Colloid and Interface Science, 2018, 521: 183-189. |
| 76 | ZHANG Siqian, RAVI Seenu, LEE Yuri, et al. Fly ash-derived mesoporous silica foams for CO2 capture and aqueous Nd3+ adsorption[J]. Journal of Industrial and Engineering Chemistry, 2019, 72: 241-249. |
| 77 | LASMI Sofiane, ZOUKRAMI Fouzia, MARCOS-FERNÁNDEZ Ángel Antonio, et al. Influence of modified mesoporous silica SBA-15 and compatibilizer on the properties and structure of ethylene-vinyl acetate copolymer-based nanocomposites[J]. Polymer-Plastics Technology and Materials, 2020, 59(18): 2003-2017. |
| 78 | MEOTO Silo, KENT Niall, NIGRA Michael M, et al. Effect of stirring rate on the morphology of FDU-12 mesoporous silica particles[J]. Microporous and Mesoporous Materials, 2017, 249: 61-66. |
| 79 | LI Dandan, MIN Hongyang, JIANG Xu, et al. One-pot synthesis of aluminum-containing ordered mesoporous silica MCM-41 using coal fly ash for phosphate adsorption[J]. Journal of Colloid and Interface Science, 2013, 404: 42-48. |
| 80 | RAJALAKSHMI Rajamanickam, SRINIVASAN Vinju Vasudevan, PACHAMUTHU Muthusamy P, et al. Characterizations of tin (SnO2) doped KIT-5 by direct synthesis[J]. Materials Chemistry and Physics, 2015, 154: 164-169. |
| 81 | Dolores GARRIDO M, HASKOURI Jamal EL, David VIE, et al. Generalized “one-pot” preparative strategy to obtain highly functionalized silica-based mesoporous spherical particles[J]. Microporous and Mesoporous Materials, 2022, 337: 111942. |
| 82 | ELÍAS Verónica R, FERRERO Gabriel O, OLIVEIRA Rafael G, et al. Improved stability in SBA-15 mesoporous materials as catalysts for photo-degradation processes[J]. Microporous and Mesoporous Materials, 2016, 236: 218-227. |
| 83 | FU Xingrui, LIU Yue, YAO Weiyuan, et al. One-step synthesis of bimetallic Pt-Pd/MCM-41 mesoporous materials with superior catalytic performance for toluene oxidation[J]. Catalysis Communications, 2016, 83: 22-26. |
| 84 | YANG Piaoping, GAI Shili, LIN Jun. Functionalized mesoporous silica materials for controlled drug delivery[J]. Chemical Society Reviews, 2012, 41(9): 3679-3698. |
| 85 | WU Zhangxiong, ZHAO Dongyuan. Ordered mesoporous materials as adsorbents[J]. Chemical Communications, 2011, 47(12): 3332-3338. |
| 86 | ZHAO Lingyu, ZHU Qingyun, MAO Li, et al. Preparation of thiol- and amine-bifunctionalized hybrid monolithic column via “one-pot” and applications in speciation of inorganic arsenic[J]. Talanta, 2019, 192: 339-346. |
| 87 | ZHAO Hongwei, HAN Hui. Synthesis and characterization of functionalized SBA-15 silica through template removal[J]. Journal of Solid State Chemistry, 2020, 282: 121074. |
| 88 | AHMED Sohail, RAMLI Anita, YUSUP Suzana. Development of polyethylenimine-functionalized mesoporous Si-MCM-41 for CO2 adsorption[J]. Fuel Processing Technology, 2017, 167: 622-630. |
| 89 | XU Xiang, HE Junjie, ZENG Yanning, et al. Controllable surface-initiated metal-free atom transfer radical polymerization of methyl methacrylate on mesoporous SBA-15 via reductive quenching[J]. European Polymer Journal, 2020, 131: 109724. |
| 90 | LIU Yan, CHEN Rui, YUAN Dandan, et al. Thermal-responsive ion-imprinted polymer based on magnetic mesoporous silica SBA-15 for selective removal of Sr(Ⅱ) from aqueous solution[J]. Colloid and Polymer Science, 2015, 293(1): 109-123. |
| 91 | Joanna DOBRZYŃSKA, Marzena DĄBROWSKA, OLCHOWSKI Rafał, et al. An ion-imprinted thiocyanato-functionalized mesoporous silica for preconcentration of gold(Ⅲ) prior to its quantitation by slurry sampling graphite furnace AAS[J]. Microchimica Acta, 2018, 185(12): 564. |
| 92 | YANG Sen, QIAN Jun, KUANG Liangju, et al. Ion-imprinted mesoporous silica for selective removal of uranium from highly acidic and radioactive effluent[J]. ACS Applied Materials & Interfaces, 2017, 9(34): 29337-29344. |
| 93 | GLOTOV Aleksandr, LEVSHAKOV Nikolay, STAVITSKAYA Anna, et al. Templated self-assembly of ordered mesoporous silica on clay nanotubes[J]. Chemical Communications, 2019, 55(38): 5507-5510. |
| 94 | GAO Li, SHI Zhiyuan, ETIM Ubong Jerome, et al. Superior catalytic performance of micro-mesoporous Beta-SBA-15 composite with a high indexed isomerization factor in hydroisomerization of n-heptane[J]. Fuel, 2019, 252: 653-665. |
| 95 | MARTIN Pedro, RAFTI Matías, MARCHETTI Sergio, et al. MCM-41-based composite with enhanced stability for Cr(Ⅵ) removal from aqueous media[J]. Solid State Sciences, 2020, 106: 106300. |
| 96 | SUN Chunyan, ZHANG Feng, WANG Xiao, et al. Facile preparation of ammonium molybdophosphate/Al-MCM-41 composite material from natural clay and its use in cesium ion adsorption[J]. European Journal of Inorganic Chemistry, 2015, 2015(12): 2125-2131. |
| 97 | CHEN Feiyun, HONG Mingzhu, YOU Weijie, et al. Simultaneous efficient adsorption of Pb2+ and MnO4 - ions by MCM-41 functionalized with amine and nitrilotriacetic acid anhydride[J]. Applied Surface Science, 2015, 357: 856-865. |
| 98 | GUPTA Radha, GUPTA Sunil Kumar, PATHAK Devendra Deo. Selective adsorption of toxic heavy metal ions using guanine-functionalized mesoporous silica [SBA-16-g] from aqueous solution[J]. Microporous and Mesoporous Materials, 2019, 288: 109577. |
| 99 | BETIHA M A, MOUSTAFA Y M, EL-SHAHAT M F, et al. Polyvinylpyrrolidone-aminopropyl-SBA-15 schiff base hybrid for efficient removal of divalent heavy metal cations from wastewater[J]. Journal of Hazardous Materials, 2020, 397: 122675. |
| 100 | MORSI Rania E, ELSHERIEF Mohamed A, SHABAAN M, et al. Chitosan/MCM-41 nanocomposites for efficient beryllium separation[J]. Journal of Applied Polymer Science, 2018, 135(13): e46040. |
| 101 | SHAHAT Ahmed, HASSAN Hassan M A, EL-SHAHAT M F, et al. A ligand-anchored optical composite material for efficient vanadium(Ⅱ) adsorption and detection in wastewater[J]. New Journal of Chemistry, 2019, 43(26): 10324-10335. |
| 102 | HERMAN Petra, Krisztián MOLDOVÁN, PAUL Geo, et al. Selective and reversible surface complexation of aqueous palladium(Ⅱ) by polycarboxylate (pyromellitic acid) functionalized hybrid aerogel sorbent[J]. Applied Surface Science, 2023, 613: 156026. |
| 103 | SADEGHI Mohammad Mehdi, Ali Shokuhi RAD, ARDJMAND Mehdi, et al. Functionalization of SBA-15 by dithiooxamide towards removal of Co (Ⅱ) ions from real samples: Isotherm, thermodynamic and kinetic studies[J]. Advanced Powder Technology, 2019, 30(9): 1823-1834. |
| 104 | FISSAHA Hiluf T, TORREJOS Rey Eliseo C, KIM Hern, et al. Thia-crown ether functionalized mesoporous silica (SBA-15) adsorbent for selective recovery of gold (Au3+) ions from electronic waste leachate[J]. Microporous and Mesoporous Materials, 2020, 305: 110301. |
| 105 | LI Weibin, FU Fenglian. Incorporating MnFe2O4 onto the thiol-functionalized MCM-41 for effective capturing of Sb(Ⅲ) in aqueous media[J]. Microporous and Mesoporous Materials, 2020, 298: 110060. |
| 106 | ZHENG Xudong, LIU Enli, ZHANG Fusheng, et al. Efficient adsorption and separation of dysprosium from NdFeB magnets in an acidic system by ion imprinted mesoporous silica sealed in a dialysis bag[J]. Green Chemistry, 2016, 18(18): 5031-5040. |
| 107 | BARCZAK Mariusz, Joanna DOBRZYŃSKA, OSZUST Monika, et al. Synthesis and application of thiolated mesoporous silicas for sorption, preconcentration and determination of platinum[J]. Materials Chemistry and Physics, 2016, 181: 126-135. |
| 108 | GAO Jing, ZHANG Danyang, WANG Yuejiao, et al. Ethanolamine modified ordered mesoporous silica KIT-6: One-pot and rapid microwave synthesis, and efficient recovery for rhenium(Ⅶ)[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 656: 130337. |
| 109 | PETROVA Petranka, CHOCHKOVA Maya, KARADJOV Metody. Adsorption of Pd(Ⅱ) in N and S-modified silica sorbents[J]. Journal of Chemical Technology and Metallurgy, 2022, 57(5): 962-970. |
| 110 | LEE Byunghwan, KIM Younghun, LEE Hyunjoo, et al. Synthesis of functionalized porous silicas via templating method as heavy metal ion adsorbents: The introduction of surface hydrophilicity onto the surface of adsorbents[J]. Microporous and Mesoporous Materials, 2001, 50(1): 77-90. |
| 111 | GE Shaobing, HE Xiaowei, ZHAO Jiawei, et al. Removal of cationic dyes, heavy metal ions, and CO2 capture by adsorption on mesoporous silica HMS[J]. Water, Air, & Soil Pollution, 2017, 228(12): 460. |
| [1] | 慕铭, 赵伟伟, 陈光孟, 刘小青. 基于激光诱导石墨烯的应变传感器研究进展[J]. 化工进展, 2024, 43(9): 4970-4979. |
| [2] | 申纯宇, 李翠利, 汤建伟, 刘咏, 刘鹏飞, 丁俊祥, 申博, 王保明. 纳米氢氧化镁制备及其阻燃应用进展[J]. 化工进展, 2024, 43(9): 4980-4995. |
| [3] | 卞维柏, 张睿轩, 潘建明. 无机金属锂离子筛材料制备方法研究进展[J]. 化工进展, 2024, 43(8): 4173-4186. |
| [4] | 张叶素, 权燕红, 丁欣欣, 任军. 链状MFI型分子筛的合成与应用[J]. 化工进展, 2024, 43(8): 4382-4392. |
| [5] | 王嘉, 李文翠, 吴凡, 高新芊, 陆安慧. NiMo/Al2O3催化剂活性组分分布调控及其加氢脱硫应用[J]. 化工进展, 2024, 43(8): 4393-4402. |
| [6] | 龙涛, 周锋, 张伟, 吴泓, 王建, 陈霖. CO-CO2体系制备氘代甲醇催化剂的合成与改性[J]. 化工进展, 2024, 43(8): 4411-4420. |
| [7] | 孙忻茹, 张秋怡, 卓建坤, 杨润, 姚强. CaCl2复合热化学储热材料的研究进展[J]. 化工进展, 2024, 43(8): 4506-4515. |
| [8] | 郑云香, 高艺伦, 李宴汝, 刘青霖, 张浩腾, 王向鹏. 氨基三乙酸酐改性多孔双网络水凝胶的制备及吸附性能[J]. 化工进展, 2024, 43(8): 4542-4549. |
| [9] | 刘玉灿, 高中鲁, 徐心怡, 纪现国, 张岩, 孙洪伟, 王港. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 2024, 43(8): 4630-4641. |
| [10] | 胡君杰, 黄兴俊, 雷成, 杨敏, 兰元宵, 罗建洪. 页岩气采出水中小分子有机物的深度处理[J]. 化工进展, 2024, 43(8): 4674-4680. |
| [11] | 武哲, 曲树光, 冯练享, 曾湘楚. 海藻酸钠/微晶纤维素复合水凝胶对水中甲基橙和亚甲基蓝的吸附性能与机理[J]. 化工进展, 2024, 43(8): 4681-4693. |
| [12] | 黄鸿, 欧阳浩民, 杨依静, 李昌霖, 陈烁娜. 硫化零价铁-微生物复合吸附剂对磷酸三(2-氯乙基)酯的吸附-降解机制[J]. 化工进展, 2024, 43(8): 4704-4713. |
| [13] | 郭长滨, 李蒙蒙, 冯梦晗, 原田, 张克强, 罗艳丽, 王风. 铈掺杂镧基钙钛矿制备及对水体磷酸盐和植酸的吸附性能[J]. 化工进展, 2024, 43(8): 4748-4756. |
| [14] | 毛华恺, 余洋, 张悦, 夏广坤, 吴赟韬, 楼乐瑶, 牛文娟, 刘念. 生物炭光催化氧化-吸附协同降解亚硝酸盐[J]. 化工进展, 2024, 43(8): 4757-4765. |
| [15] | 王丽娜, 武金升. 共价有机框架材料的合成与应用研究进展[J]. 化工进展, 2024, 43(7): 3834-3856. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||