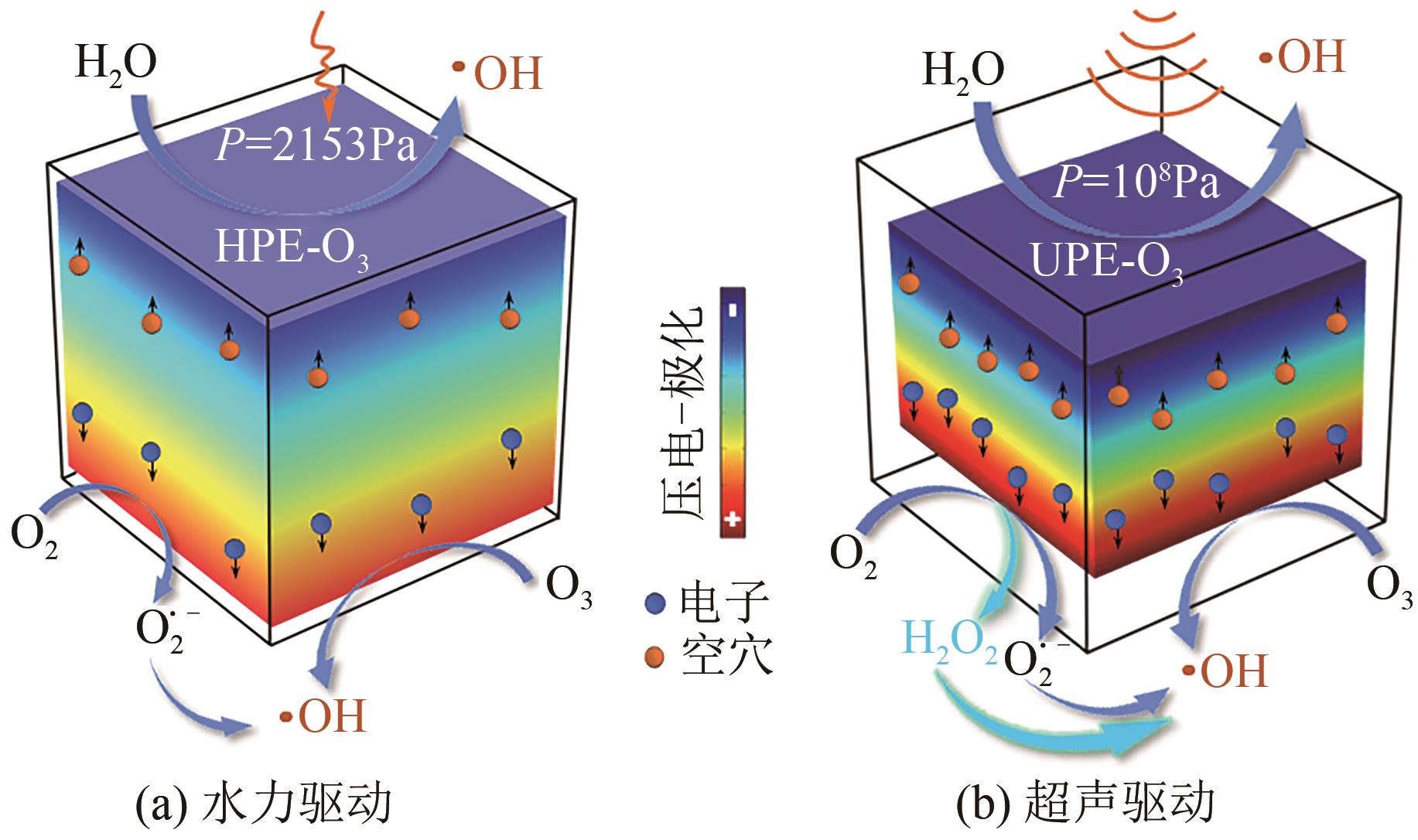
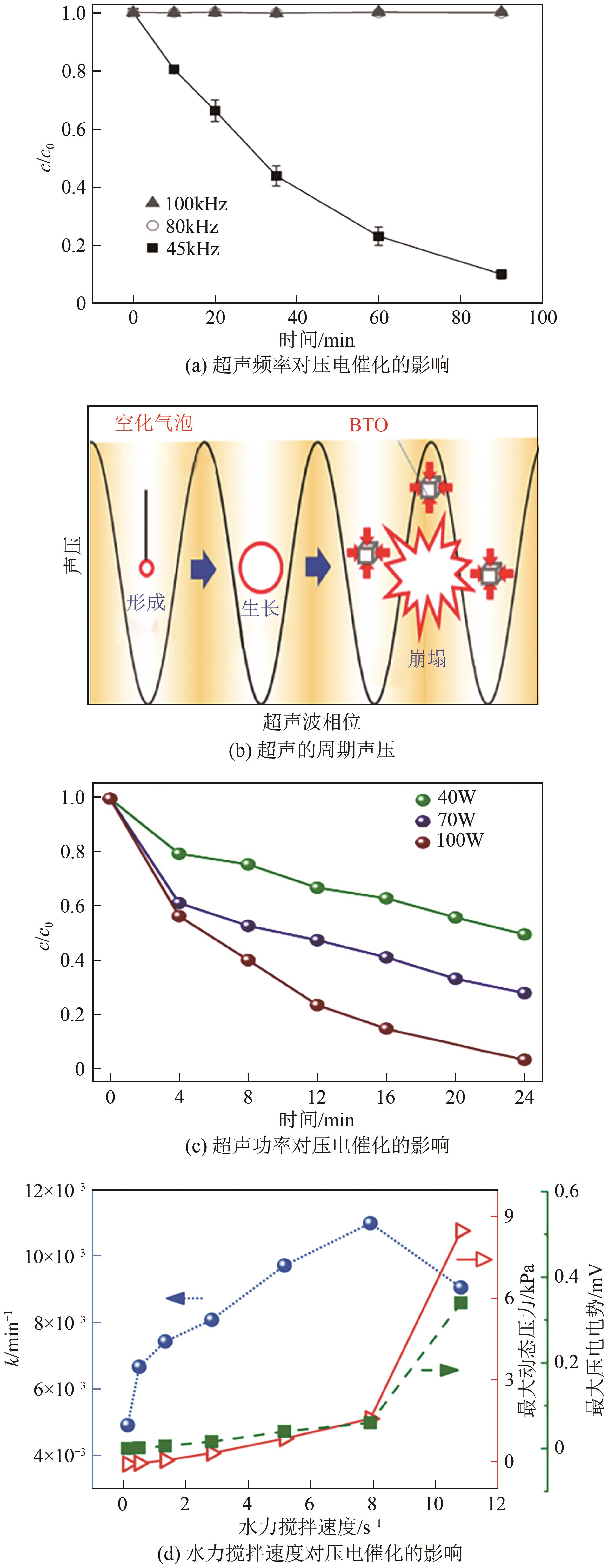
化工进展 ›› 2024, Vol. 43 ›› Issue (10): 5723-5733.DOI: 10.16085/j.issn.1000-6613.2023-1509
• 资源与环境化工 • 上一篇
压电催化降解水中有机污染物的增效研究进展
- 1.东华理工大学水资源与环境工程学院,江西 南昌 330013
2.重庆大学环境与生态学院,重庆 400030
-
收稿日期:2023-08-30修回日期:2023-10-11出版日期:2024-10-15发布日期:2024-10-29 -
通讯作者:李寻 -
作者简介:郑莹(1993—),女,博士,讲师,研究方向为高级氧化技术、水污染控制技术。E-mail:yingzheng@ecut.edu.cn。 -
基金资助:江西省自然科学基金(20232BAB213031);江西省教育厅科学技术研究项目(GJJ2200766);东华理工大学博士科研启动基金(DHBK2022014);东华理工大学实验技术研究开发项目(DHSY-202305)
Research progress in enhancing the efficiency of piezoelectric catalytic degradation of organic pollutants from water
ZHENG Ying1( ), LI Xun1(
), LI Xun1( ), LI Zebing1, GAO Zhe1, ZHAO Chun2
), LI Zebing1, GAO Zhe1, ZHAO Chun2
- 1.School of Water Resources and Environmental Engineering, East China University of Technology, Nanchang 330013, Jiangxi, China
2.College of Environment and Ecology, Chongqing University, Chongqing 400045, China
-
Received:2023-08-30Revised:2023-10-11Online:2024-10-15Published:2024-10-29 -
Contact:LI Xun
摘要:
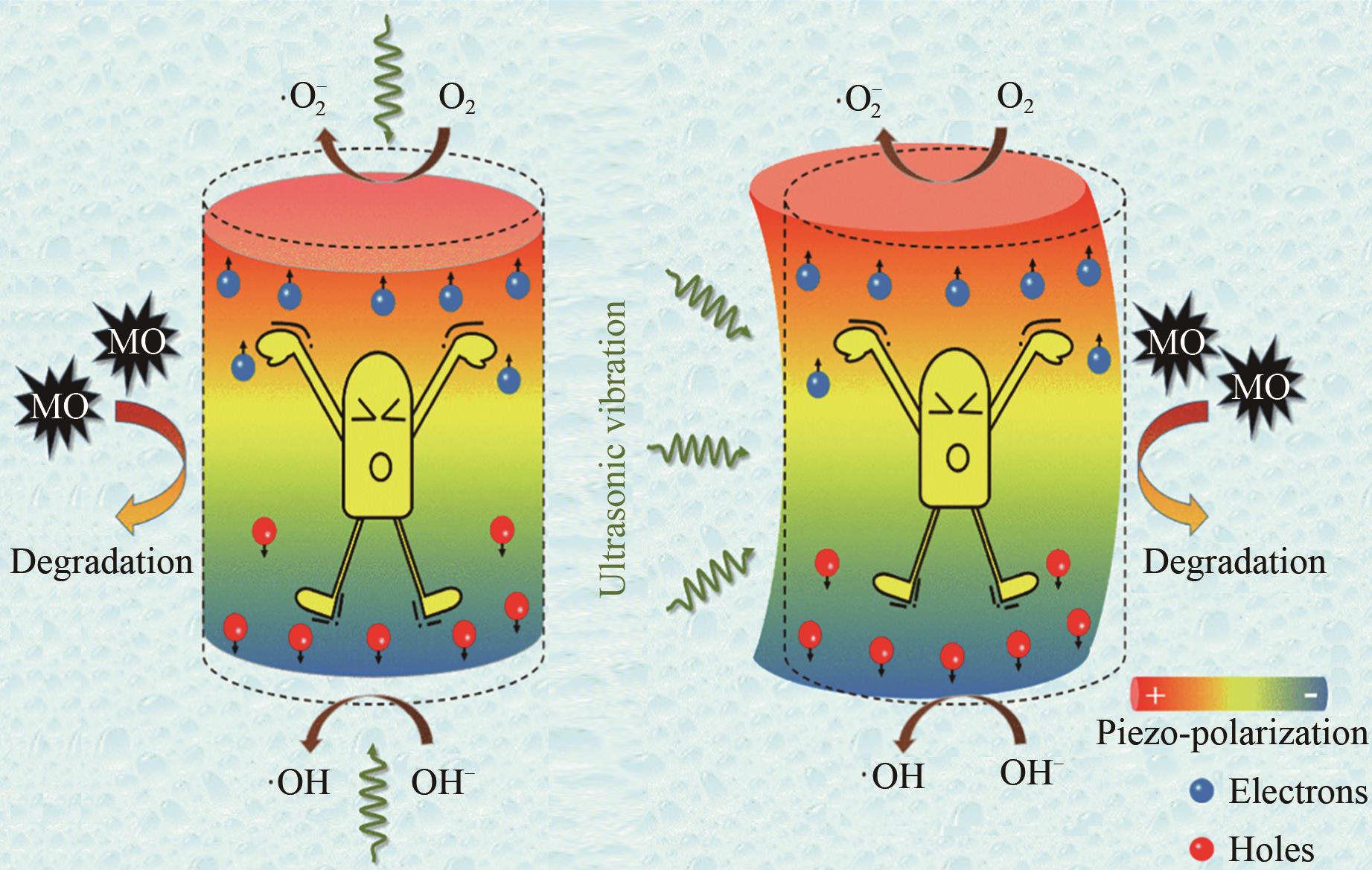
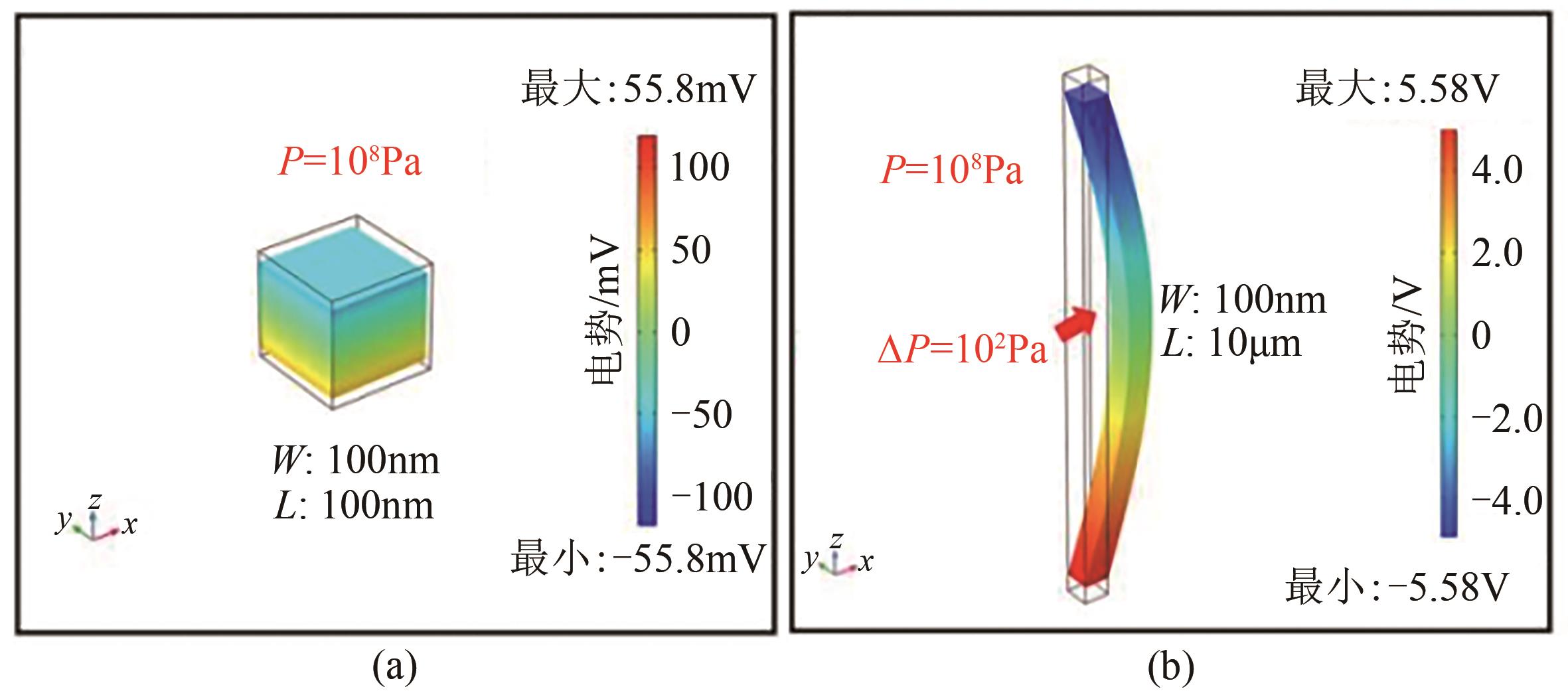
水污染和能源紧缺作为全球性的问题,受到广泛关注。压电催化能够将外部机械能转化为化学能,是一种绿色有效、节能产能的新技术,在解决水污染和能源紧缺问题上具有潜力。然而,简单压电催化体系氧化能力有限,目标污染物多限于水中低浓度易降解有机污染物。针对上述问题,科研工作者采用多种方法强化了压电体系的氧化能力。文章综述了目前常用的三类压电催化强效方法,包括压电材料性能调控、压电-化学药剂耦合、压电驱动方式优化,分析了压电催化技术的能源和环境意义,并对该领域未来的发展方向进行了展望。
中图分类号:
引用本文
郑莹, 李寻, 李泽兵, 高哲, 赵纯. 压电催化降解水中有机污染物的增效研究进展[J]. 化工进展, 2024, 43(10): 5723-5733.
ZHENG Ying, LI Xun, LI Zebing, GAO Zhe, ZHAO Chun. Research progress in enhancing the efficiency of piezoelectric catalytic degradation of organic pollutants from water[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5723-5733.
| 压电材料 | 压电材料投加量/g·L-1 | 驱动方式 | 污染物 | 污染物浓度/mg·L-1 | 降解速率/min-1 | 参考文献 |
|---|---|---|---|---|---|---|
| BTO微枝晶 | 10.0 | 超声,40kHz | AO7染料 | 20 | 0.031 | |
| PZT纤维 | 12.5 | 超声,40kHz,80W | AO7染料 | 12 | 0.031 | |
| MoS2纳米花 | 文献未提及 | 超声,40kHz,250W | RhB染料 | 10 | 1.10 | |
| BaTiO3纳米颗粒 | 2.0 | 超声,40kHz,110W | 4-氯酚 | 25 | 0.011 | |
| BiFeO3微片 | 1 .0 | 超声,40kHz | RhB染料 | 10 | 0.034 | |
| Bi4Ti3O12微片 | 1.3 | 超声,40kHz,300W | MO染料 | 3 | 0.0042 | |
| BTO纳米纤维 | 0.1 | 超声,40kHz,80W | RhB染料 | 5 | 0.060 | |
| ZnO纳米棒 | 0.5 | 低频超声 | AO7染料 | 2 | 0.030 | |
| (Ba,Sr)TiO3纳米线 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.020 | |
| (Ba,Sr)TiO3纳米颗粒 | 1.0 | 超声,40kHz,0W | MO染料 | 5 | 0.0069 | |
| BTO纳米线 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.013 | |
| BTO纳米线 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.015 | |
| BTO纳米颗粒 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.0084 | |
| 商用BTO纳米颗粒 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.0036 | |
| BTO-800 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.019 | |
| NaNbO3纳米线 | 22.5 | 超声,40kHz | RhB染料 | 5 | 0.014 | |
| BTO纳米线 | 1.0 | 超声,40kHz,120W | MO染料 | 5 | 38.20min-1·mol-1 | |
| K0.5Na0.5NbO3-900 | 4.0 | 超声,40kHz,180W | RhB染料 | 5 | 0.020 | |
| BTO纳米颗粒 | 1.0 | 超声,40kHz,100W | RhB染料 | 5 | 0.0025 | |
| BTO纳米线 | 1.0 | 超声,40kH,100W | RhB染料 | 5 | 0.034 | |
| BTO纳米片 | 1.0 | 超声,40kHz,100W | RhB染料 | 5 | 0.13 | |
| m-Bi2O4 | 1.2.0 | 超声,40kHz,300W | 磺胺甲嘧啶 | 10 | 0.033 | |
| BTO纳米带 | 1.0 | 超声,50kHz,100W | RhB染料 | 10 | 0.015 | |
| 0.7BiFeO3-0.3BaTiO3 | 3.0 | 水力,0.2m/s | RhB染料 | 10 | 0.0018 | |
| 球形PZT | 5.0 | 水力,300r/min | RhB染料 | 10 | 0.010 | |
| BTO纳米线 | 1.0 | 水力,1000r/min | MO染料 | 5 | 0.58min-1·mol-1 |
表1 简单压电催化体系降解水中有机污染物
| 压电材料 | 压电材料投加量/g·L-1 | 驱动方式 | 污染物 | 污染物浓度/mg·L-1 | 降解速率/min-1 | 参考文献 |
|---|---|---|---|---|---|---|
| BTO微枝晶 | 10.0 | 超声,40kHz | AO7染料 | 20 | 0.031 | |
| PZT纤维 | 12.5 | 超声,40kHz,80W | AO7染料 | 12 | 0.031 | |
| MoS2纳米花 | 文献未提及 | 超声,40kHz,250W | RhB染料 | 10 | 1.10 | |
| BaTiO3纳米颗粒 | 2.0 | 超声,40kHz,110W | 4-氯酚 | 25 | 0.011 | |
| BiFeO3微片 | 1 .0 | 超声,40kHz | RhB染料 | 10 | 0.034 | |
| Bi4Ti3O12微片 | 1.3 | 超声,40kHz,300W | MO染料 | 3 | 0.0042 | |
| BTO纳米纤维 | 0.1 | 超声,40kHz,80W | RhB染料 | 5 | 0.060 | |
| ZnO纳米棒 | 0.5 | 低频超声 | AO7染料 | 2 | 0.030 | |
| (Ba,Sr)TiO3纳米线 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.020 | |
| (Ba,Sr)TiO3纳米颗粒 | 1.0 | 超声,40kHz,0W | MO染料 | 5 | 0.0069 | |
| BTO纳米线 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.013 | |
| BTO纳米线 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.015 | |
| BTO纳米颗粒 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.0084 | |
| 商用BTO纳米颗粒 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.0036 | |
| BTO-800 | 1.0 | 超声,40kHz,80W | MO染料 | 5 | 0.019 | |
| NaNbO3纳米线 | 22.5 | 超声,40kHz | RhB染料 | 5 | 0.014 | |
| BTO纳米线 | 1.0 | 超声,40kHz,120W | MO染料 | 5 | 38.20min-1·mol-1 | |
| K0.5Na0.5NbO3-900 | 4.0 | 超声,40kHz,180W | RhB染料 | 5 | 0.020 | |
| BTO纳米颗粒 | 1.0 | 超声,40kHz,100W | RhB染料 | 5 | 0.0025 | |
| BTO纳米线 | 1.0 | 超声,40kH,100W | RhB染料 | 5 | 0.034 | |
| BTO纳米片 | 1.0 | 超声,40kHz,100W | RhB染料 | 5 | 0.13 | |
| m-Bi2O4 | 1.2.0 | 超声,40kHz,300W | 磺胺甲嘧啶 | 10 | 0.033 | |
| BTO纳米带 | 1.0 | 超声,50kHz,100W | RhB染料 | 10 | 0.015 | |
| 0.7BiFeO3-0.3BaTiO3 | 3.0 | 水力,0.2m/s | RhB染料 | 10 | 0.0018 | |
| 球形PZT | 5.0 | 水力,300r/min | RhB染料 | 10 | 0.010 | |
| BTO纳米线 | 1.0 | 水力,1000r/min | MO染料 | 5 | 0.58min-1·mol-1 |
| 体系 | 化学物质 | 化学物质投加量 /mg·L-1 | 压电材料 | 压电材料投加量 /g·L-1 | 驱动方式 | 污染物 | 污染物浓度 /mg·L-1 | 降解速率 /min-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 压电-Fenton | Fe2+ | 4 | BTO微枝晶 | 9.0 | 超声,40kHz,300W | AO7 | 20 | 0.042 | |
| Fe2+ | 10 | BTO纳米颗粒 | 2.0 | 超声,45kHz,300W | 卡马西平 | 5 | 0.026 | ||
| 压电-过硫酸盐 | PMS | 1000 | MoS2纳米花 | 0.3 | 超声,40kHz,300W | 苯酚 | 10 | 0.020 | |
| PMS | 1000 | MoS2纳米花 | 0.3 | 水力,900r/min | 苯酚 | 10 | — | ||
| PMS | 250 | BaTiO3/MoS2 | 0.1 | 超声,40kHz,100W | 奥硝唑 | 50 | 0.056 | ||
| PMS | 1000 | BTO纳米颗粒 | 0.5 | 超声,40kHz,100W | 苯并噻唑 | 5 | 0.100 | ||
| PMS | 6150 | BTO纳米颗粒 | 10.0 | 水力,1000r/min | 卡马西平 | 2 | 0.0082 | ||
| PMS | 6150 | CNTs/BaTiO3 | 5.0 | 水力,1000r/min | 卡马西平 | 2 | 0.021 | ||
| PDS | 270 | BTO纳米线 | 2.0 | 超声,40kHz,110W | 布洛芬 | 6 | 0.082 | ||
| PDS | 270 | BTO纳米颗粒 | 2.0 | 超声,40kHz,110W | 布洛芬 | 6 | 0.049 | ||
| PDS | 1000 | SrBi2B2O7 | 2.0 | 超声,100W | 磺胺嘧啶 | 10 | 0.052 | ||
| 压电-臭氧 | O3 | 14 | BTO纳米颗粒 | 3.0 | 超声,40kHz,300W | 布洛芬 | 20 | 0.220 | |
| O3 | 14 | BTO纳米颗粒 | 3.0 | 水力,1000r/min | 布洛芬 | 20 | 0.130 | ||
| O3 | 14 | BTO纳米颗粒 | 3.0 | 水力,1000r/min | 硝基苯 | 10 | 0.130 |
表2 压电-化学物质联用降解水中有机污染物
| 体系 | 化学物质 | 化学物质投加量 /mg·L-1 | 压电材料 | 压电材料投加量 /g·L-1 | 驱动方式 | 污染物 | 污染物浓度 /mg·L-1 | 降解速率 /min-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 压电-Fenton | Fe2+ | 4 | BTO微枝晶 | 9.0 | 超声,40kHz,300W | AO7 | 20 | 0.042 | |
| Fe2+ | 10 | BTO纳米颗粒 | 2.0 | 超声,45kHz,300W | 卡马西平 | 5 | 0.026 | ||
| 压电-过硫酸盐 | PMS | 1000 | MoS2纳米花 | 0.3 | 超声,40kHz,300W | 苯酚 | 10 | 0.020 | |
| PMS | 1000 | MoS2纳米花 | 0.3 | 水力,900r/min | 苯酚 | 10 | — | ||
| PMS | 250 | BaTiO3/MoS2 | 0.1 | 超声,40kHz,100W | 奥硝唑 | 50 | 0.056 | ||
| PMS | 1000 | BTO纳米颗粒 | 0.5 | 超声,40kHz,100W | 苯并噻唑 | 5 | 0.100 | ||
| PMS | 6150 | BTO纳米颗粒 | 10.0 | 水力,1000r/min | 卡马西平 | 2 | 0.0082 | ||
| PMS | 6150 | CNTs/BaTiO3 | 5.0 | 水力,1000r/min | 卡马西平 | 2 | 0.021 | ||
| PDS | 270 | BTO纳米线 | 2.0 | 超声,40kHz,110W | 布洛芬 | 6 | 0.082 | ||
| PDS | 270 | BTO纳米颗粒 | 2.0 | 超声,40kHz,110W | 布洛芬 | 6 | 0.049 | ||
| PDS | 1000 | SrBi2B2O7 | 2.0 | 超声,100W | 磺胺嘧啶 | 10 | 0.052 | ||
| 压电-臭氧 | O3 | 14 | BTO纳米颗粒 | 3.0 | 超声,40kHz,300W | 布洛芬 | 20 | 0.220 | |
| O3 | 14 | BTO纳米颗粒 | 3.0 | 水力,1000r/min | 布洛芬 | 20 | 0.130 | ||
| O3 | 14 | BTO纳米颗粒 | 3.0 | 水力,1000r/min | 硝基苯 | 10 | 0.130 |
| 1 | 胡徐腾. 我国化石能源清洁利用前景展望[J]. 化工进展, 2017, 36(9): 3145-3151. |
| HU Xuteng. Outlook of clean utilization of fossil energy in China[J]. Chemical Industry and Engineering Progress, 2017, 36(9): 3145-3151. | |
| 2 | 陈菁泉, 连欣燕, 马晓君, 等. 中国全要素能源效率测算及其驱动因素[J]. 中国环境科学, 2022, 42(5): 2453-2463. |
| CHEN Jingquan, LIAN Xinyan, MA Xiaojun, et al. Total factor energy efficiency measurement and drivers in China[J]. China Environmental Science, 2022, 42(5): 2453-2463. | |
| 3 | 张羽就, 席佳锐, 陈玲, 等. 中国城镇污水处理厂能耗统计与基准分析[J]. 中国给水排水, 2021, 37(8): 8-17. |
| ZHANG Yujiu, XI Jiarui, CHEN Ling, et al. Energy consumption statistics and benchmarking analysis of urban wastewater treatment plants(WWTPs) in China[J]. China Water & Wastewater, 2021, 37(8): 8-17. | |
| 4 | 宋鹏, 张慧敏, 毛显强. 面向碳达峰目标的重庆市碳减排路径[J]. 中国环境科学, 2022, 42(3): 1446-1455. |
| SONG Peng, ZHANG Huimin, MAO Xianqiang. Research on Chongqing’s carbon emission reduction path towards the goal of carbon peak[J]. China Environmental Science, 2022, 42(3): 1446-1455. | |
| 5 | 黄晟, 王静宇, 郭沛, 等. 碳中和目标下能源结构优化的近期策略与远期展望[J]. 化工进展, 2022, 41(11): 5695-5708. |
| HUANG Sheng, WANG Jingyu, GUO Pei, et al. Short-term strategy and long-term prospect of energy structure optimization under carbon neutrality target[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5695-5708. | |
| 6 | ZHENG Ying, ZHUANG Wei, ZHAO Mengshang, et al. Role of driven approach on the piezoelectric ozonation processes: Comparing ultrasound with hydro-energy as driving forces[J]. Journal of Hazardous Materials, 2021, 418: 126392. |
| 7 | ZHENG Ying, YANG Jing, GONG Bingrou, et al. Hydraulic-driven piezo-activation of peroxymonosulfate for carbamazepine degradation with ultralow energy consumption[J]. Chemical Engineering Journal, 2022, 441: 136116. |
| 8 | ZHANG An, LIU Zhiyong, GENG Xinhui, et al. Ultrasonic vibration driven piezocatalytic activity of lead-free K0.5Na0.5NbO3 materials[J]. Ceramics International, 2019, 45(17): 22486-22492. |
| 9 | YUAN Baowei, WU Jiang, QIN Ni, et al. Enhanced piezocatalytic performance of (Ba, Sr)TiO3 nanowires to degrade organic pollutants[J]. ACS Applied Nano Materials, 2018, 1(9): 5119-5127. |
| 10 | FENG Yawei, LING Lili, WANG Yanxu, et al. Engineering spherical lead zirconate titanate to explore the essence of piezo-catalysis[J]. Nano Energy, 2017, 40: 481-486. |
| 11 | LIANG Zhang, YAN Changfeng, RTIMI Sami, et al. Piezoelectric materials for catalytic/photocatalytic removal of pollutants: Recent advances and outlook[J]. Applied Catalysis B: Environmental, 2019, 241: 256-269. |
| 12 | TU Shuchen, GUO Yuxi, ZHANG Yihe, et al. Piezocatalysis and piezo-photocatalysis: Catalysts classification and modification strategy, reaction mechanism, and practical application[J]. Advanced Functional Materials, 2020, 30(48): 2005158. |
| 13 | WANG Mengye, WANG Biao, HUANG Feng, et al. Enabling PIEZOpotential in PIEZOelectric semiconductors for enhanced catalytic activities[J]. Angewandte Chemie International Edition, 2019, 58(23): 7526-7536. |
| 14 | 孙奇薇, 薛国梁, 周学凡, 等. 压电催化降解有机污染物的研究进展[J]. 中国有色金属学报, 2021, 31(8): 1997-2013. |
| SUN Qiwei, XUE Guoliang, ZHOU Xuefan, et al. Research progress in piezoelectric degradation of organic pollutants[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(8): 1997-2013. | |
| 15 | HONG Kuangsheng, XU Huifang, KONISHI Hiromi, et al. Piezoelectrochemical effect: A new mechanism for azo dye decolorization in aqueous solution through vibrating piezoelectric microfibers[J]. The Journal of Physical Chemistry C, 2012, 116(24): 13045-13051. |
| 16 | JIN Chengchao, LIU Daiming, HU Jing, et al. The role of microstructure in piezocatalytic degradation of organic dye pollutants in wastewater[J]. Nano Energy, 2019, 59: 372-379. |
| 17 | MASEKELA Daniel, HINTSHO-MBITA Nomso C, Simanye SAM, et al. Application of BaTiO3-based catalysts for piezocatalytic, photocatalytic and piezo-photocatalytic degradation of organic pollutants and bacterial disinfection in wastewater: A comprehensive review[J]. Arabian Journal of Chemistry, 2023, 16(2): 104473. |
| 18 | LIN He, WU Zheng, JIA Yanmin, et al. Piezoelectrically induced mechano-catalytic effect for degradation of dye wastewater through vibrating Pb(Zr0.52Ti0.48)O3 fibers[J]. Applied Physics Letters, 2014, 104(16): 162907. |
| 19 | WU Jyh Ming, CHANG Wei’en, CHANG Yuting, et al. Piezo-catalytic effect on the enhancement of the ultra-high degradation activity in the dark by single-and few-layers MoS2 nanoflowers[J]. Advanced Materials, 2016, 28(19): 3718-3725. |
| 20 | LAN Shenyu, FENG Jinxi, XIONG Ya, et al. Performance and mechanism of piezo-catalytic degradation of 4-chlorophenol: Finding of effective piezo-dechlorination[J]. Environmental Science & Technology, 2017, 51(11): 6560-6569. |
| 21 | YOU Huilin, JIA Yanmin, WU Zheng, et al. Strong piezo-electrochemical effect of multiferroic BiFeO3 square micro-sheets for mechanocatalysis[J]. Electrochemistry Communications, 2017, 79: 55-58. |
| 22 | TU Shuchen, HUANG Hongwei, ZHANG Tierui, et al. Controllable synthesis of multi-responsive ferroelectric layered perovskite-like Bi4Ti3O12: Photocatalysis and piezoelectric-catalysis and mechanism insight[J]. Applied Catalysis B: Environmental, 2017, 219: 550-562. |
| 23 | XU Xiaoli, WU Zheng, XIAO Lingbo, et al. Strong piezo-electro-chemical effect of piezoelectric BaTiO3 nanofibers for vibration-catalysis[J]. Journal of Alloys and Compounds, 2018, 762: 915-921. |
| 24 | XU Xiaoli, JIA Yanmin, XIAO Lingbo, et al. Strong vibration-catalysis of ZnO nanorods for dye wastewater decolorization via piezo-electro-chemical coupling[J]. Chemosphere, 2018, 193: 1143-1148. |
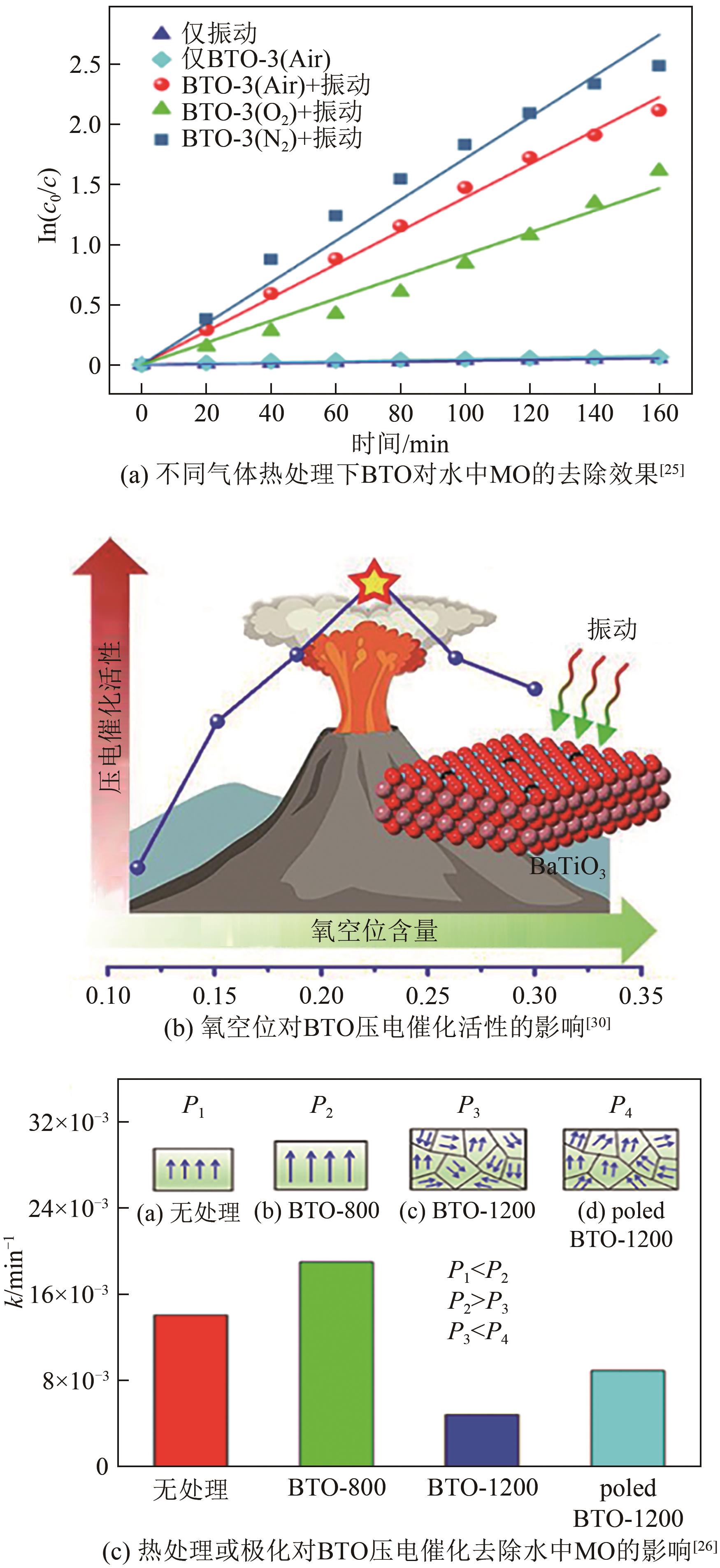
| 25 | WU Jiang, QIN Ni, BAO Dinghua. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration[J]. Nano Energy, 2018, 45: 44-51. |
| 26 | WU Jiang, XU Qi, LIN Enzhu, et al. Insights into the role of ferroelectric polarization in piezocatalysis of nanocrystalline BaTiO3 [J]. ACS Applied Materials & Interfaces, 2018, 10(21): 17842-17849. |
| 27 | WANG Shensong, WU Zheng, CHEN Jie, et al. Lead-free sodium niobate nanowires with strong piezo-catalysis for dye wastewater degradation[J]. Ceramics International, 2019, 45(9): 11703-11708. |
| 28 | YU Chengye, TAN Mengxi, LI Yang, et al. Ultrahigh piezocatalytic capability in eco-friendly BaTiO3 nanosheets promoted by 2D morphology engineering[J]. Journal of Colloid and Interface Science, 2021, 596: 288-296. |
| 29 | LIU Fengling, CHEN Haoxuan, XU Chenmin, et al. Monoclinic dibismuth tetraoxide (m-Bi2O4) for piezocatalysis: New use for neglected materials[J]. Chemical Communications, 2021, 57(22): 2740-2743. |
| 30 | WANG Penglei, LI Xinyong, FAN Shiying, et al. Impact of oxygen vacancy occupancy on piezo-catalytic activity of BaTiO3 nanobelt[J]. Applied Catalysis B: Environmental, 2020, 279: 119340. |
| 31 | SUN Yanhua, LI Xiaoning, VIJAYAKUMAR Amruthalakshmi, et al. Hydrogen generation and degradation of organic dyes by new piezocatalytic 0.7BiFeO3-0.3BaTiO3 nanoparticles with proper band alignment[J]. ACS Applied Materials & Interfaces, 2021, 13(9): 11050-11057. |
| 32 | WANG Chunyang, HU Cheng, CHEN Fang, et al. Design strategies and effect comparisons toward efficient piezocatalytic system[J]. Nano Energy, 2023, 107: 108093. |
| 33 | Yanping HA, CHEN Yinglong, SHEN Minghu, et al. Hierarchical MoS2 hollow microspheres with high piezoelectric catalytic performance prepared by the soft-template method[J]. Inorganic Chemistry Communications, 2023, 148: 110349. |
| 34 | LI Hongjing, XIONG Yi, WANG Yumin, et al. High piezocatalytic capability in CuS/MoS2 nanocomposites using mechanical energy for degrading pollutants[J]. Journal of Colloid and Interface Science, 2022, 609: 657-666. |
| 35 | HE Qingshen, YI Yuyan, SHI Wenjun, et al. Determination of the key role to affect the piezocatalytic activity of graphitic carbon nitride for tetracycline hydrochloride degradation in water[J]. Chemosphere, 2023, 317: 137828. |
| 36 | HE Dongcai, WANG Weijie, FENG Nan, et al. Defect-modified nano-BaTiO3 as a sonosensitizer for rapid and high-efficiency sonodynamic sterilization[J]. ACS Applied Materials & Interfaces, 2023, 15(12): 15140-15151. |
| 37 | WANG Chunyang, CHEN Fang, HU Cheng, et al. Efficient piezocatalytic H2O2 production of atomic-level thickness Bi4Ti3O12 nanosheets with surface oxygen vacancy[J]. Chemical Engineering Journal, 2022, 431: 133930. |
| 38 | ZHENG Ying, ZHUANG Wei, ZHANG Xiaohui, et al. Grape-like CNTs/BaTiO3 nanocatalyst boosted hydraulic-driven piezo-activation of peroxymonosulfate for carbamazepine removal[J]. Chemical Engineering Journal, 2022, 449: 137826. |
| 39 | XIA Dehua, TANG Zhuoyun, WANG Yunchen, et al. Piezo-catalytic persulfate activation system for water advanced disinfection: Process efficiency and inactivation mechanisms[J]. Chemical Engineering Journal, 2020, 400: 125894. |
| 40 | YANG Guodong, CHEN Qin, WANG Weijun, et al. Cocatalyst engineering in piezocatalysis: A promising strategy for boosting hydrogen evolution[J]. ACS Applied Materials & Interfaces, 2021, 13(13): 15305-15314. |
| 41 | WEI Yan, ZHANG Yiwen, GENG Wei, et al. Efficient bifunctional piezocatalysis of Au/BiVO4 for simultaneous removal of 4-chlorophenol and Cr (Ⅵ) in water[J]. Applied Catalysis B: Environmental, 2019, 259: 118084. |
| 42 | PAN Meilan, ZHANG Chen, WANG Jiong, et al. Multifunctional piezoelectric heterostructure of BaTiO3@Graphene: De complexation of Cu-EDTA and recovery of Cu[J]. Environmental Science & Technology, 2019, 53(14): 8342-8351. |
| 43 | PAN Meilan, LIU Subiao, CHEW Jiawei. Unlocking the high redox activity of MoS2 on dual-doped graphene as a superior piezocatalyst[J]. Nano Energy, 2020, 68: 104366. |
| 44 | KUMAR Manish, SINGH Gurpreet, VAISH Rahul. A reduced graphene oxide/bismuth vanadate composite as an efficient piezocatalyst for degradation of organic dye[J]. Materials Advances, 2021, 2(12): 4093-4101. |
| 45 | LI Shengjuan, ZHANG Mei, GAO Yulai, et al. ZnO-Zn/CNT hybrid film as light-free nanocatalyst for degradation reaction[J]. Nano Energy, 2013, 2(6): 1329-1336. |
| 46 | Weibiao LYU, KONG L, LAN Shenyu, et al. Enhancement effect in the piezoelectric degradation of organic pollutants by piezo-Fenton process[J]. Journal of Chemical Technology & Biotechnology, 2017, 92: 152-156. |
| 47 | 张森, 郑莹, 韩仕强, 等. 低频超声驱动BaTiO3压电-芬顿体系降解水中卡马西平[J]. 净水技术, 2020, 39(7): 130-138. |
| ZHANG Sen, ZHENG Ying, HAN Shiqiang, et al. Degradation of carbamazepine in water by piezo-Fenton process based on BaTiO3 driven with low frequency ultrasound[J]. Water Purification Technology, 2020, 39(7): 130-138. | |
| 48 | LIU Shuhui, JING Binghua, NIE Chunyang, et al. Piezoelectric activation of peroxymonosulfate by MoS2 nanoflowers for the enhanced degradation of aqueous organic pollutants[J]. Environmental Science: Nano, 2021, 8(3): 784-794. |
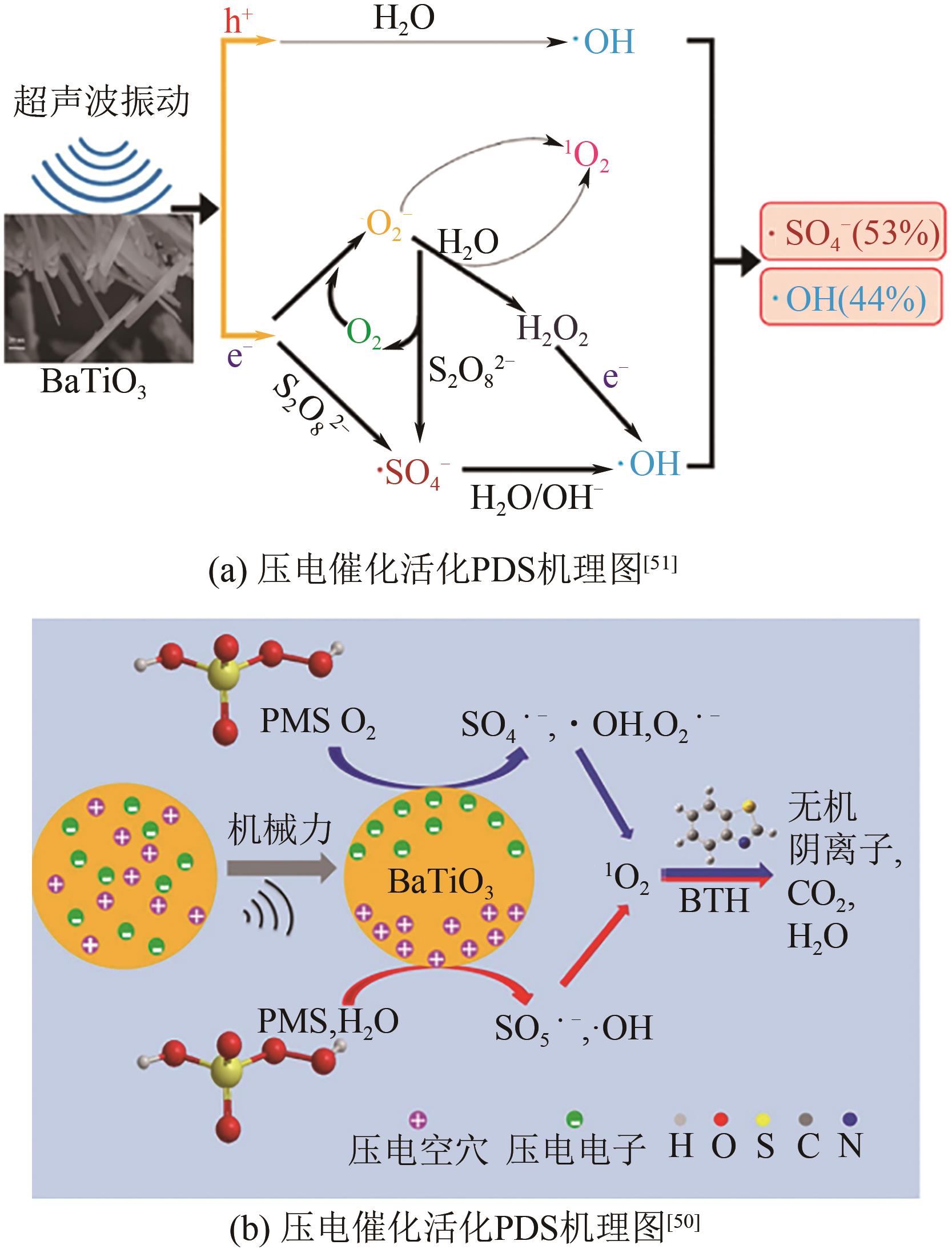
| 49 | CHEN Yanxi, LAN Shenyu, ZHU Mingshan. Construction of piezoelectric BaTiO3/MoS2 heterojunction for boosting piezo-activation of peroxymonosulfate[J]. Chinese Chemical Letters, 2021, 32(6): 2052-2056. |
| 50 | LAN Shenyu, CHEN Yanxi, ZENG Lixi, et al. Piezo-activation of peroxymonosulfate for benzothiazole removal in water[J]. Journal of Hazardous Materials, 2020, 393: 122448. |
| 51 | PENG Fei, YIN Ran, LIAO Yuhong, et al. Kinetics and mechanisms of enhanced degradation of ibuprofen by piezo-catalytic activation of persulfate[J]. Chemical Engineering Journal, 2020, 392: 123818. |
| 52 | ZHU Mude, CHEN Xueqin, TANG Yi, et al. Piezo-promoted persulfate activation by SrBi2B2O7 for efficient sulfadiazine degradation from water[J]. Journal of Hazardous Materials, 2022, 437: 129359. |
| 53 | 庄玮, 杨婧, 龚冰柔, 等. 钛酸钡压电臭氧化降解水中的硝基苯[J]. 中国环境科学, 2021, 41(10): 4654-4661. |
| ZHUANG Wei, YANG Jing, GONG Bingrou, et al. Degradation of nitrobenzene from water by piezoelectric ozonation of Barium titanate[J]. China Environmental Science, 2021, 41(10): 4654-4661. | |
| 54 | SHAO Dengkui, ZHANG Ling, SUN Songmei, et al. Oxygen reduction reaction for generating H2O2 through a piezo-catalytic process over bismuth oxychloride[J]. ChemSusChem, 2018, 11(3): 527-531. |
| 55 | RAN Maoxi, XU Hai, BAO Yan, et al. Selective production of CO from organic pollutants by coupling piezocatalysis and advanced oxidation processes[J]. Angewandte Chemie International Edition, 2023, 62(22): e202303728. |
| 56 | LIU Wenyuan, FU Pengbo, ZHANG Yayun, et al. Efficient hydrogen production from wastewater remediation bypiezoelectricity coupling advanced oxidation processes[J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(7): e2218813120. |
| 57 | YOU Huilin, WU Zheng, ZHANG Luohong, et al. Harvesting the vibration energy of BiFeO3 nanosheets for hydrogen evolution[J]. Angewandte Chemie International Edition, 2019, 58(34): 11779-11784. |
| 58 | MENG Fanqing, MA Wei, DUAN Chunying, et al. High efficient degradation of levofloxacin by edge-selectively Fe@3D-WS2: Self-renewing behavior and degradation mechanism study[J]. Applied Catalysis B: Environmental, 2019, 252: 187-197. |
| 59 | ZHAO Xiaona, LEI Yuanchao, FANG Pengfei, et al. Piezotronic effect of single/few-layers MoS2 nanosheets composite with TiO2 nanorod heterojunction[J]. Nano Energy, 2019, 66: 104168. |
| 60 | FENG Yawei, LI Hao, LING Lili, et al. Enhanced photocatalytic degradation performance by fluid-induced piezoelectric field[J]. Environmental Science & Technology, 2018, 52(14): 7842-7848. |
| 61 | ZHUANG Wei, ZHENG Ying, SHUAI Yi, et al. Correction: Hydraulic-driven piezoelectric ozonation process for nitrobenzene degradation: Synergy, energy consumption, impact factors, mechanism, and application potential[J]. Environmental Science: Water Research & Technology, 2023, 9(2): 654. |
| 62 | 王勇, 王颖. 中国实现碳减排双控目标的可行性及最优路径——能源结构优化的视角[J]. 中国环境科学, 2019, 39(10): 4444-4455. |
| WANG Yong, WANG Ying. Feasibility and optimal pathway of China’s double targets for carbon reduction—The perspective of energy structure optimization[J]. China Environmental Science, 2019, 39(10): 4444-4455. | |
| 63 | 黄晟, 王静宇, 李振宇. 碳中和目标下石油与化学工业绿色低碳发展路径分析[J]. 化工进展, 2022, 41(4): 1689-1703. |
| HUANG Sheng, WANG Jingyu, LI Zhenyu. Analysis of green and low-carbon development path of petroleum and chemical industry under the goal of carbon neutrality[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1689-1703. | |
| 64 | WANG Zichen, XIANG Manqi, HUO Bingjie, et al. A novel ZnO/CQDs/PVDF piezoelectric system for efficiently degradation of antibiotics by using water flow energy in pipeline: Performance and mechanism[J]. Nano Energy, 2023, 107: 108162. |
| 65 | WANG Jingxue, LIANG Yanting, WANG Zichen, et al. High efficiently degradation of organic pollutants via low-speed water flow activation of Cu2O@MoS2/PVDF modified pipeline with piezocatalysis performance[J]. Chemical Engineering Journal, 2023, 458: 141409. |
| [1] | 张茜, 李皓芯, 张天阳, 李子富, 孙文俊, 敖秀玮. 基于紫外线的高级氧化或高级还原技术降解水中全氟或多氟烷基化合物[J]. 化工进展, 2024, 43(8): 4587-4600. |
| [2] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [3] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [4] | 章萍萍, 丁书海, 高晶晶, 赵敏, 俞海祥, 刘玥宏, 谷麟. 碳量子点修饰半导体复合光催化剂降解水中有机污染物[J]. 化工进展, 2023, 42(10): 5487-5500. |
| [5] | 刘怡璇, 林跃朝, 马伟芳. 可见光催化降解水中卤代有机污染物的研究进展[J]. 化工进展, 2022, 41(S1): 571-579. |
| [6] | 段毅, 邹烨, 周书葵, 杨柳. 过渡金属单原子催化剂活化H2O2/PMS/PDS降解有机污染物的研究进展[J]. 化工进展, 2022, 41(8): 4147-4158. |
| [7] | 徐虎, 郭泓凯, 柴昌盛, 郝相忠, 杨子元, 徐卫军. 碳纤维类材料用于电芬顿体系电极的研究现状[J]. 化工进展, 2022, 41(7): 3707-3718. |
| [8] | 寇悦, 陈宇, 叶黄凡, 王庆宏, 陈春茂. 炼化含盐污水处理全过程有机污染物降解特征[J]. 化工进展, 2022, 41(4): 2202-2208. |
| [9] | 许泽涛, 曹怡婷, 王俏, 王志红. 固相钴基催化剂活化过一硫酸盐在水处理中的研究进展[J]. 化工进展, 2022, 41(2): 730-739. |
| [10] | 刘肖贝, 张西华, 熊梅, 赵赫. 退役锂电池放电废水特征有机污染物解析[J]. 化工进展, 2022, 41(10): 5619-5629. |
| [11] | 王文霞, 刘小丰, 陈浠, 许艳虹, 蒙振邦, 郑俊霞, 安太成. 多孔g-C3N4基光催化材料的制备及应用研究进展[J]. 化工进展, 2022, 41(1): 300-309. |
| [12] | 孙刘鑫, 王培茗, 杨俊浩, 刘清, 崔咪芬, 乔旭. 离子强度对吸附有机污染物影响的研究进展[J]. 化工进展, 2021, 40(6): 3239-3257. |
| [13] | 罗艳红, 岳秀萍, 姜悦如, 赵博玮, 高艳娟, 段燕青. 高级氧化技术降解吲哚的研究进展[J]. 化工进展, 2021, 40(2): 1025-1034. |
| [14] | 孟宪政, 庄瑞杰, 于庆君, 唐晓龙, 易红宏, 冯勇超, 隗晶慧, 陈超祺. 制药行业有机废气催化燃烧研究进展[J]. 化工进展, 2021, 40(2): 789-799. |
| [15] | 陈惠超, 李雪, 梁潇, 王梦. 机械化学方法在环境污染控制领域的应用研究进展[J]. 化工进展, 2021, 40(11): 6332-6346. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||