化工进展 ›› 2024, Vol. 43 ›› Issue (1): 60-75.DOI: 10.16085/j.issn.1000-6613.2023-1069
CH4和CO2共转化反应机理研究进展
- 1.天津大学化工学院,天津 300072
2.物质绿色创造与制造海河实验室,天津 300192
-
收稿日期:2023-06-28修回日期:2023-08-15出版日期:2024-01-20发布日期:2024-02-05 -
通讯作者:年瑶,韩优 -
作者简介:成昊霖(2000—),男,硕士研究生,研究方向为理论催化。E-mail:chenghaolin@tju.edu.cn。 -
基金资助:国家自然科学基金(21978210);中国博士后科学基金(2022M722360);天津大学自主创新基金(2023XQM-0012)
Progress in the mechanism of CH4 and CO2co-conversion reactions
CHENG Haolin1( ), NIAN Yao1,2(
), NIAN Yao1,2( ), HAN You1,2(
), HAN You1,2( )
)
- 1.College of Chemical Engineering, Tianjin University, Tianjin 300072, China
2.Haihe Laboratory of Sustainable Chemical Transformations, Tianjin 300192, China
-
Received:2023-06-28Revised:2023-08-15Online:2024-01-20Published:2024-02-05 -
Contact:NIAN Yao, HAN You
摘要:
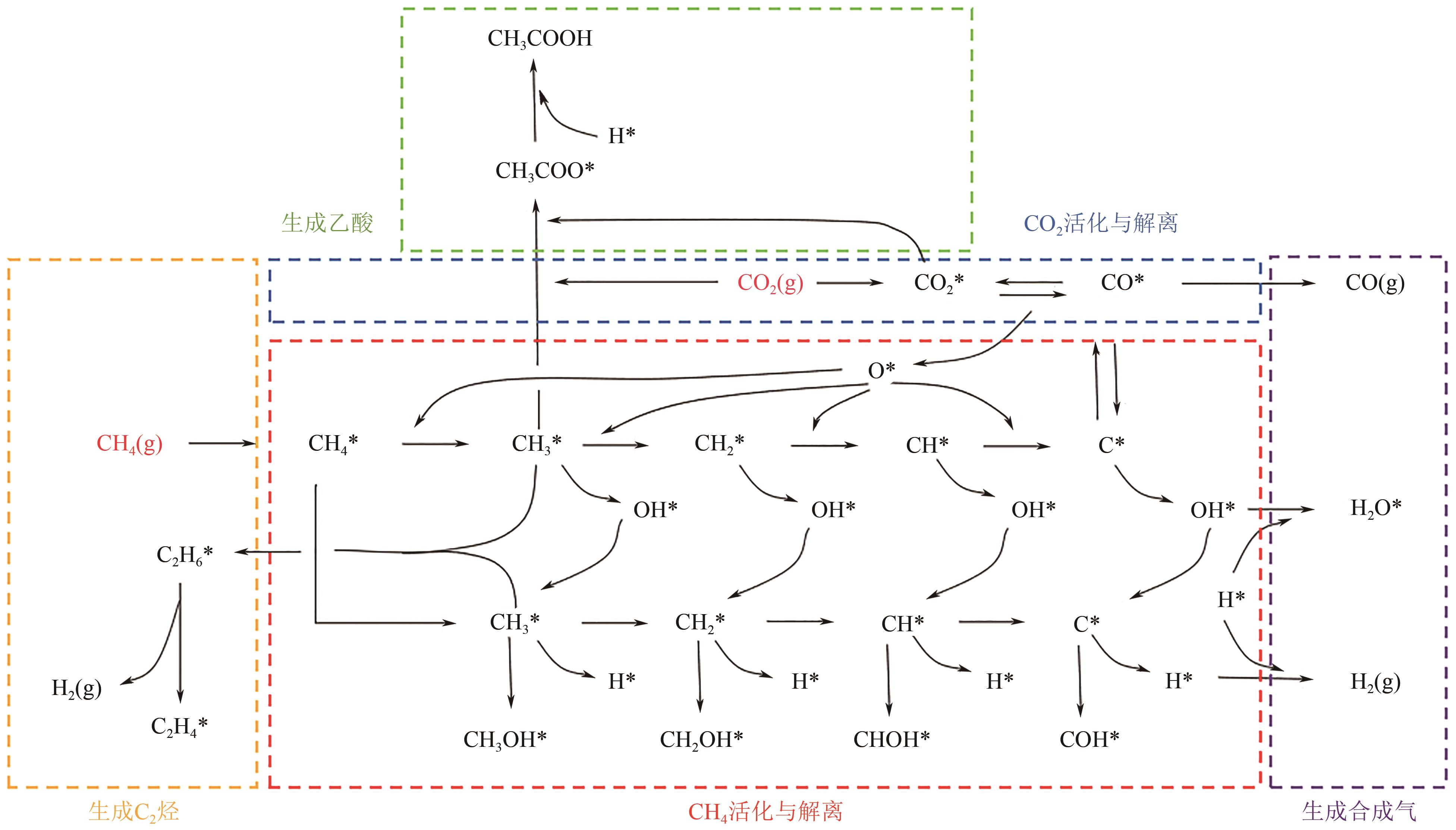
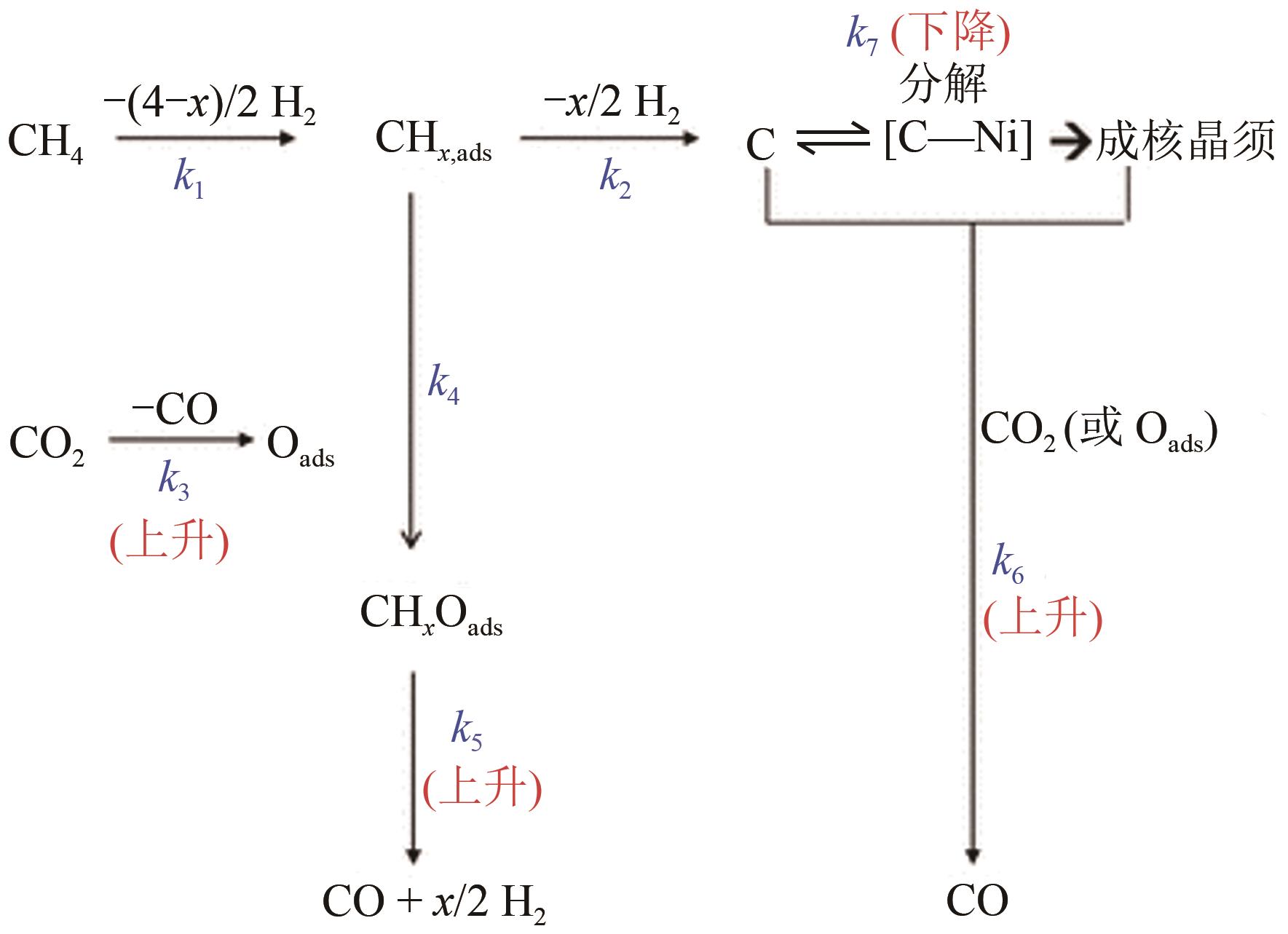
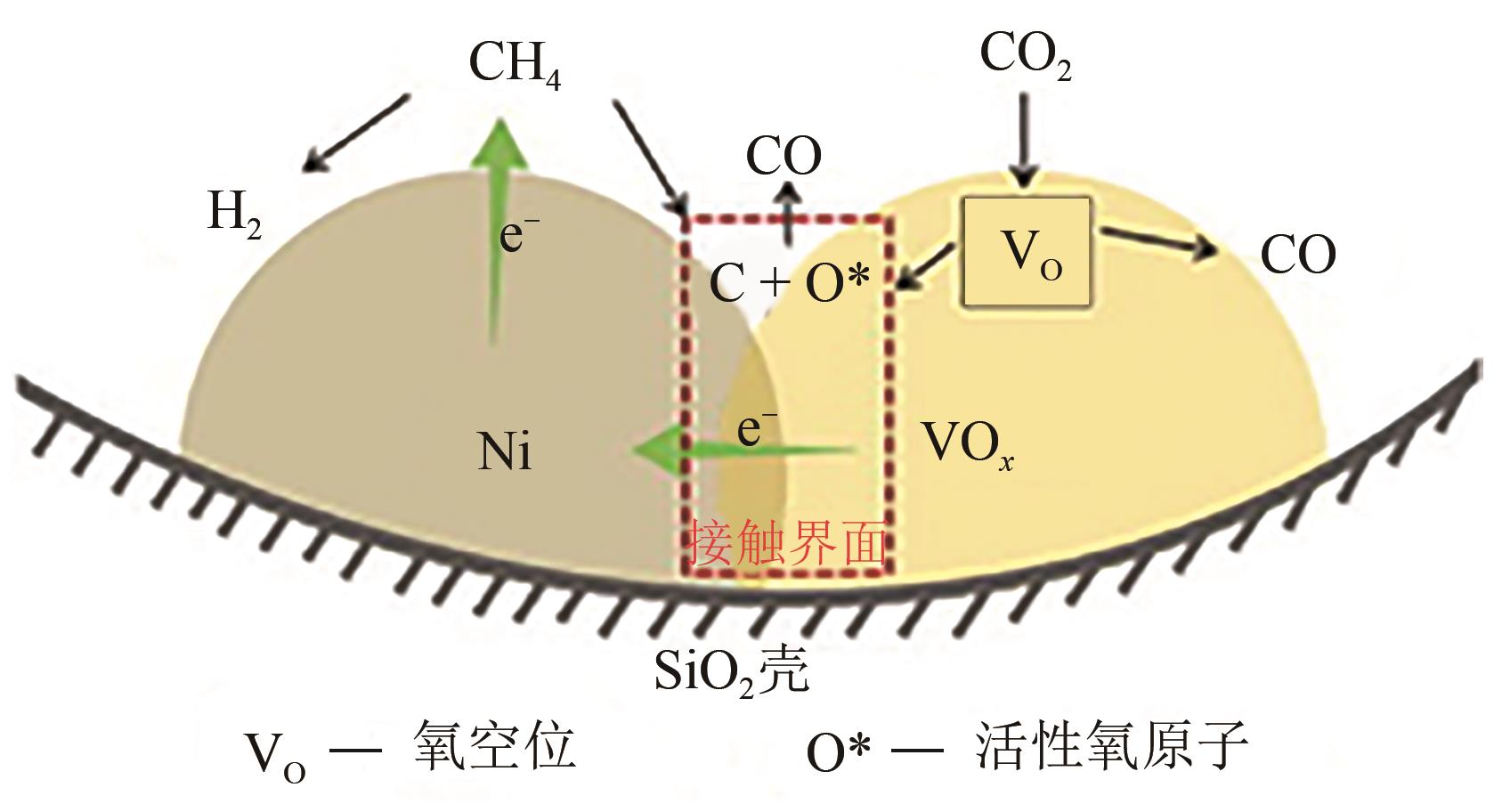
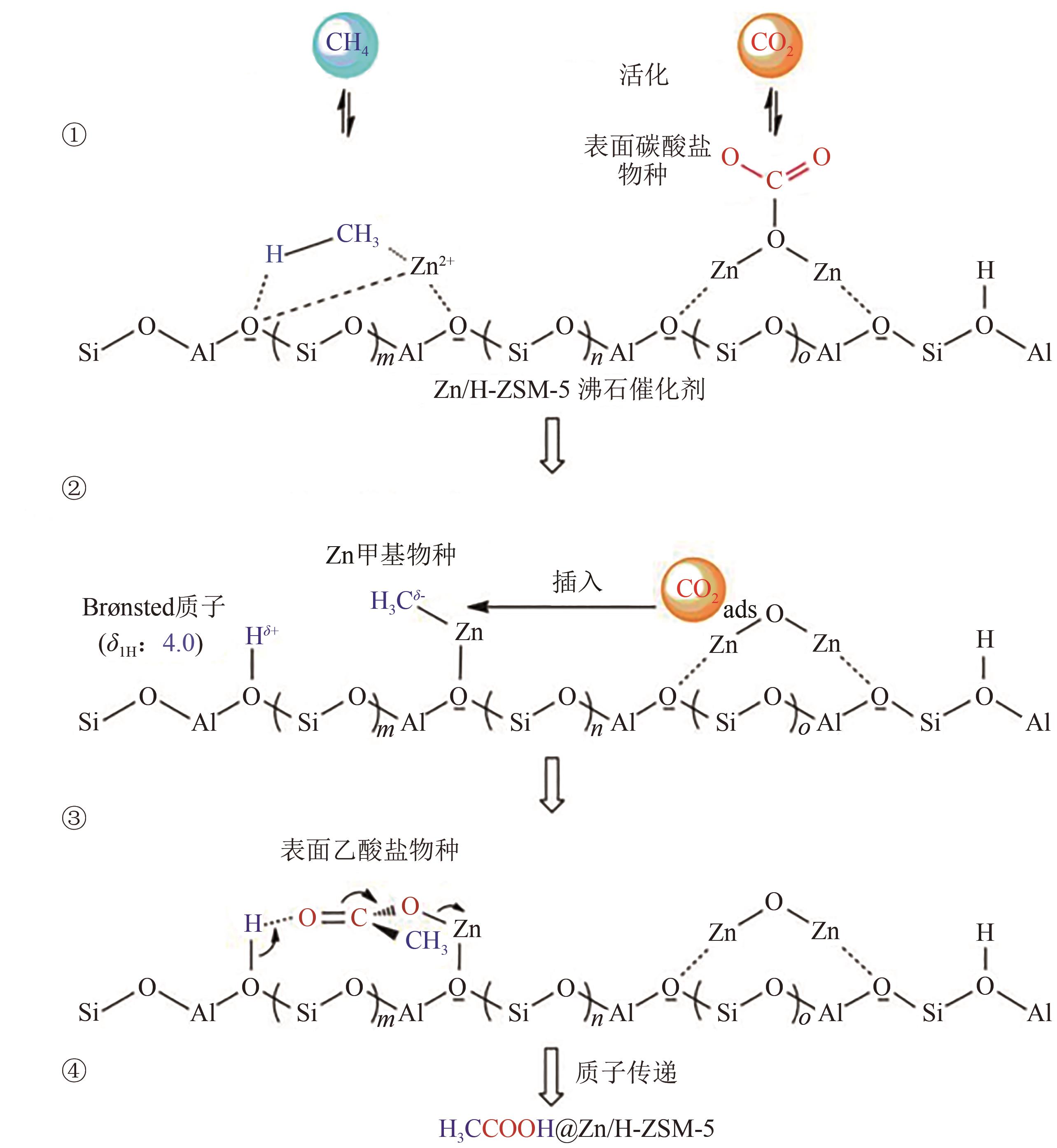
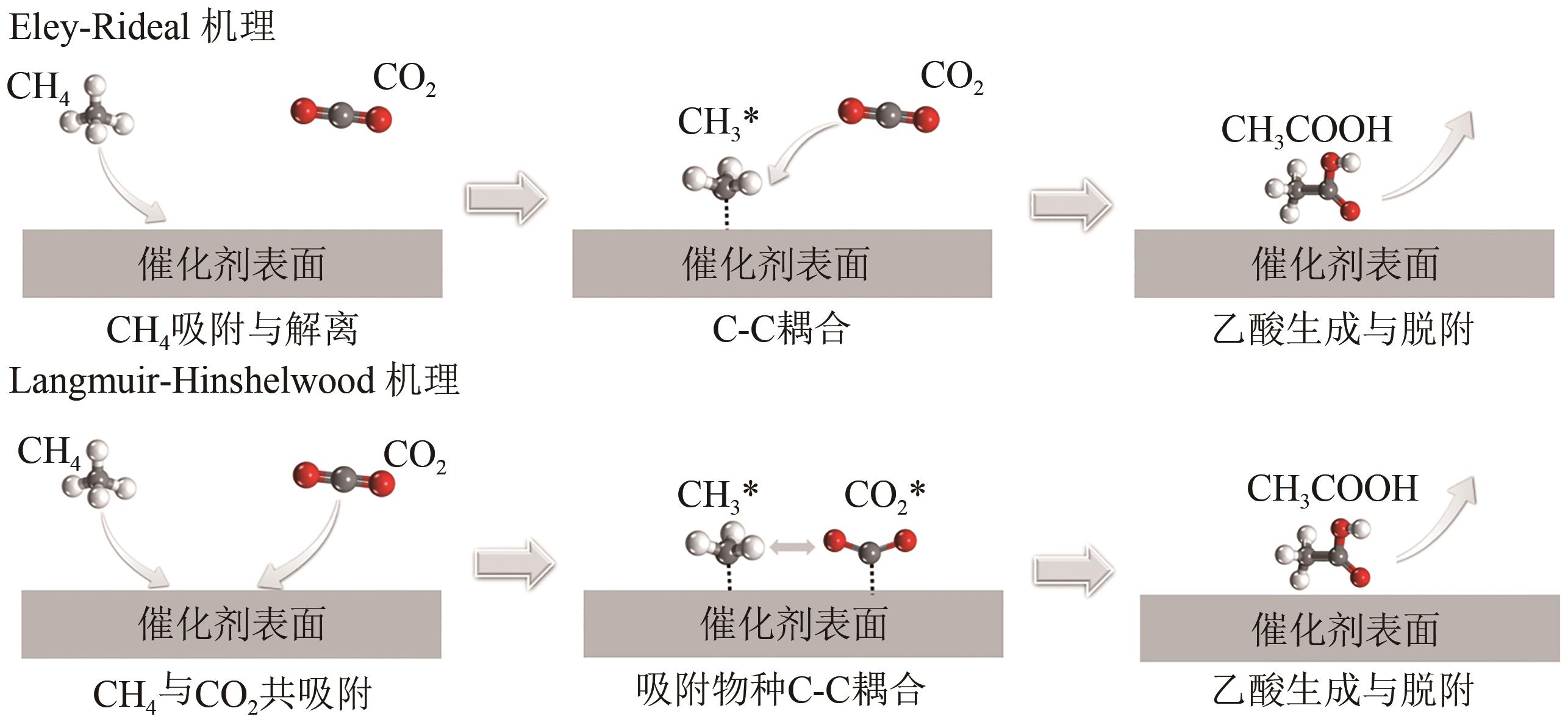
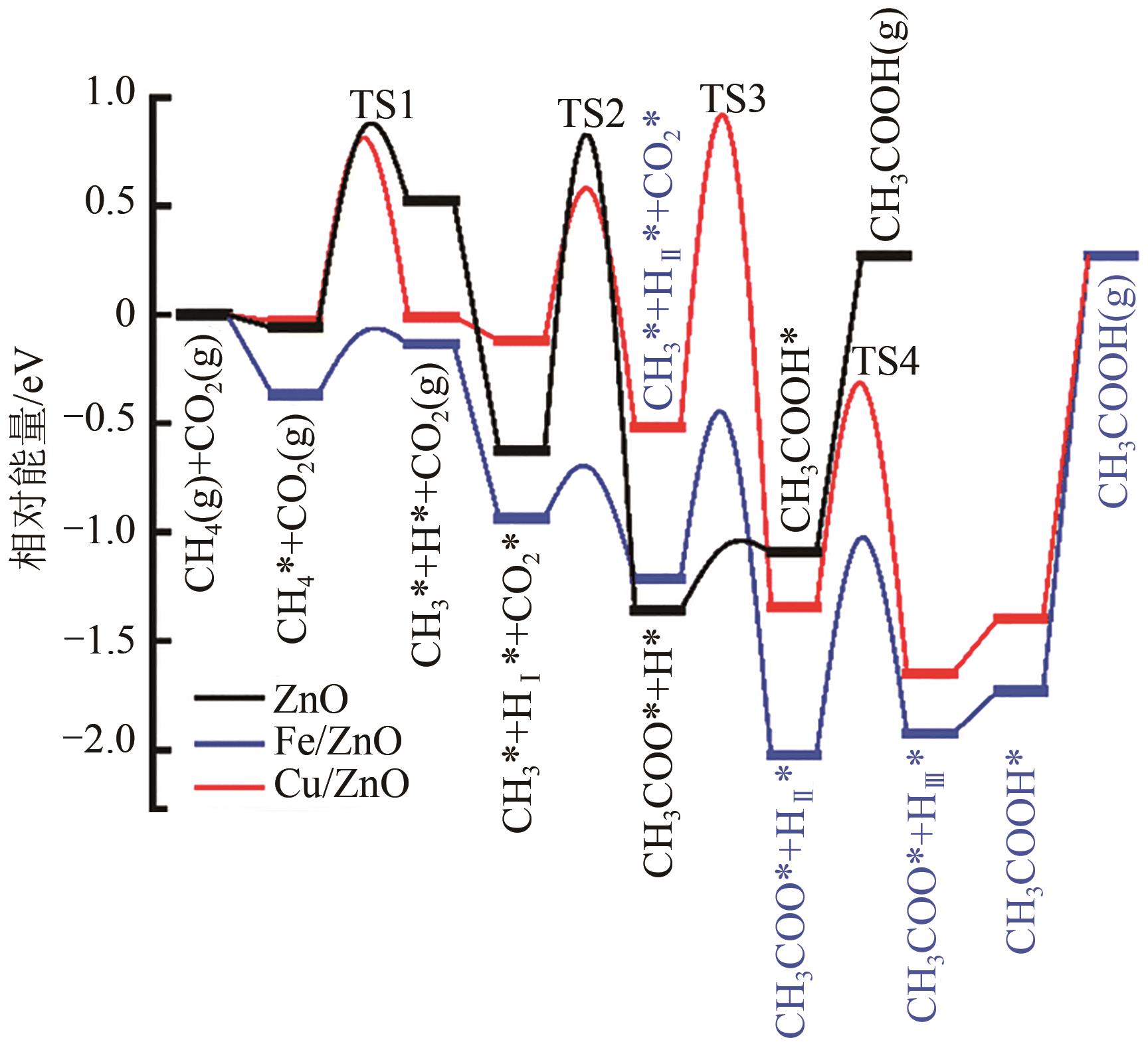
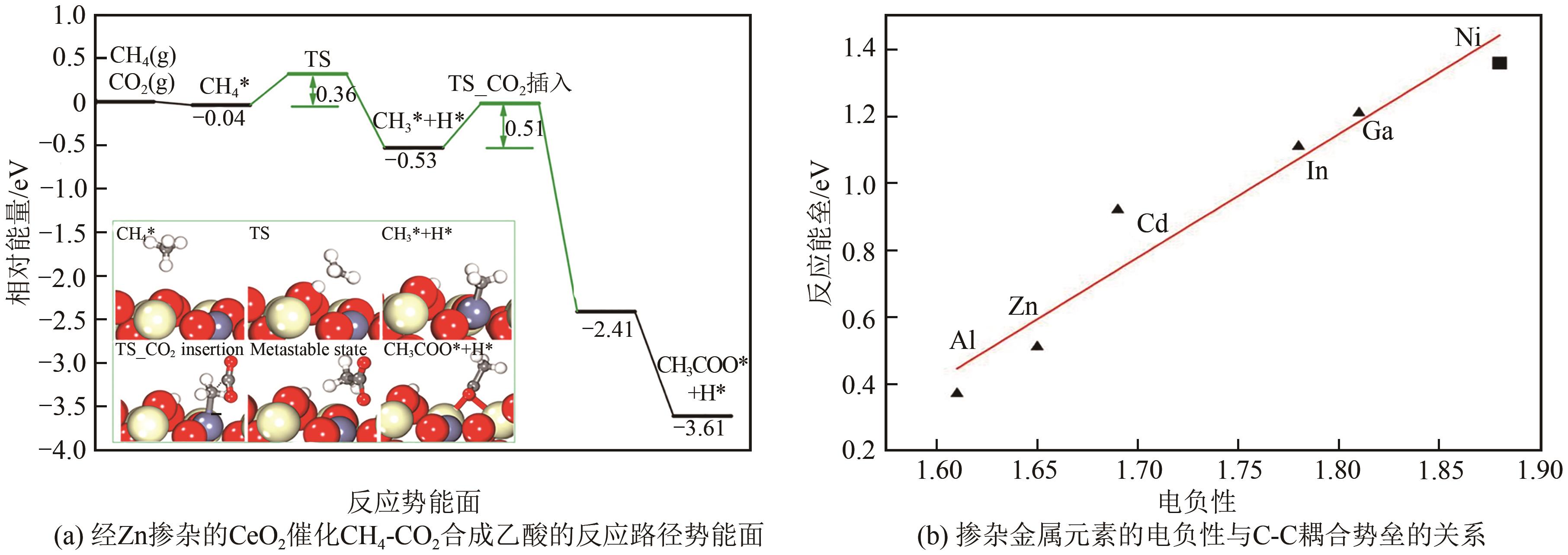
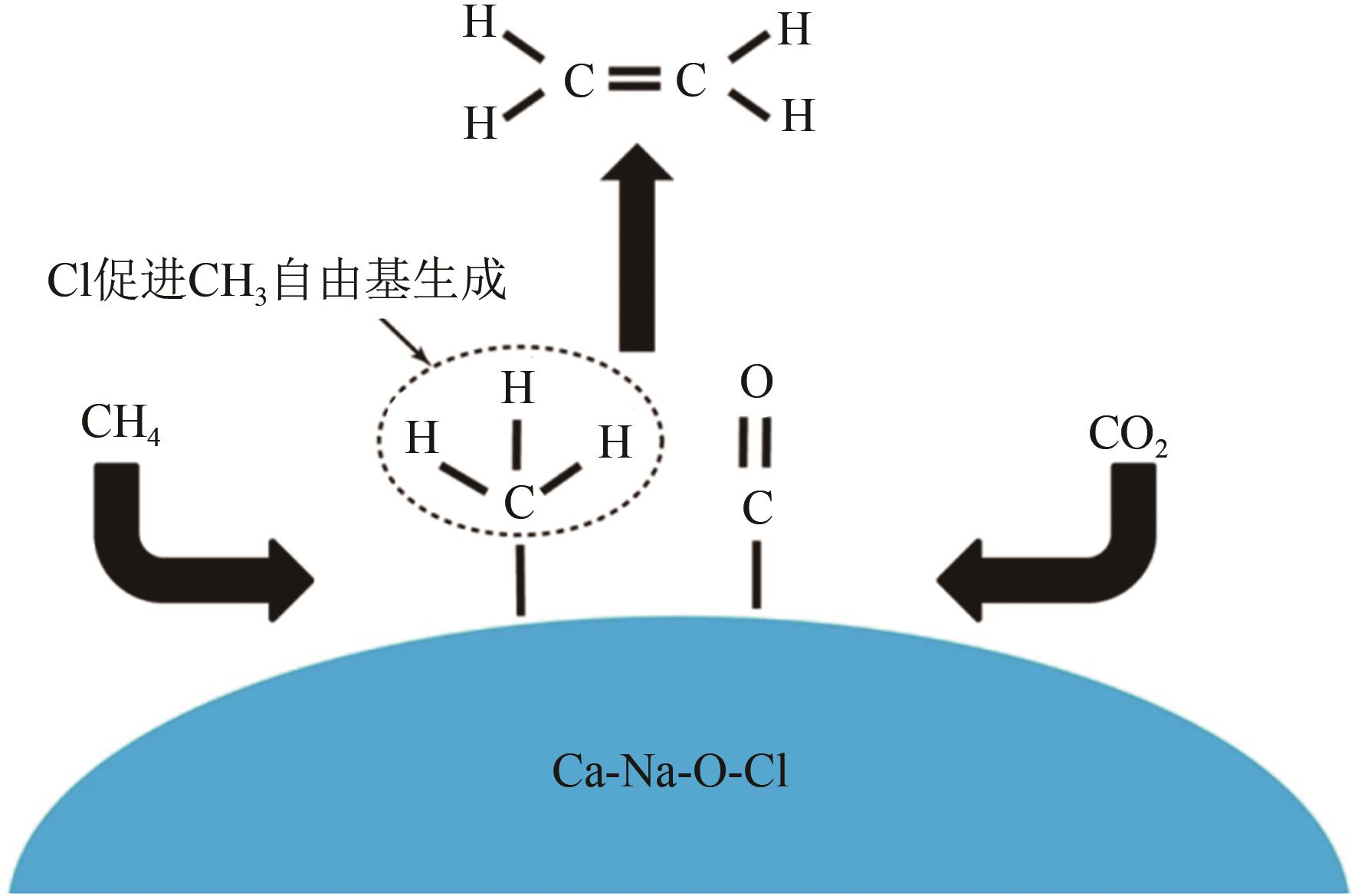
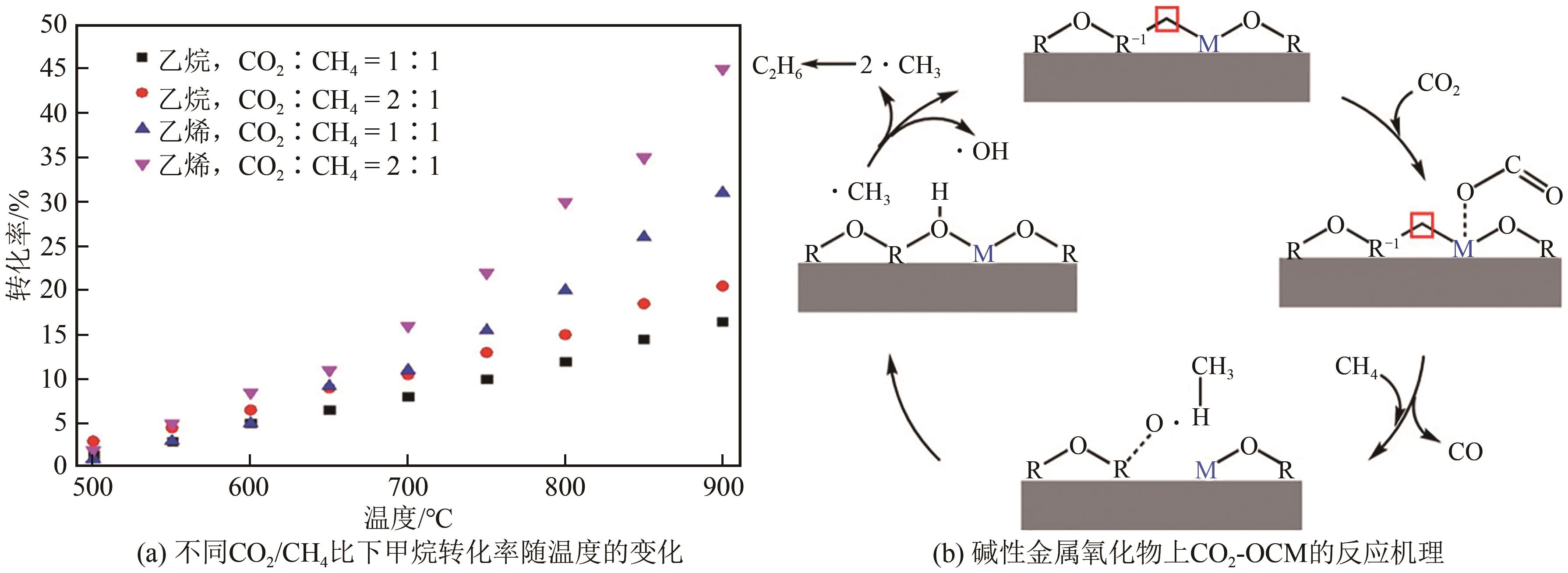
综述了CH4和CO2共转化生成合成气、乙酸和C2烃3种反应路径的反应步骤、关键中间体及反应产物选择性的影响因素。当生成合成气时,CH4与CO2的活化解离是关键步骤,催化剂载体表面为酸性或中性时反应遵循单功能机理,CH4和CO2在同一活性中心被活化,当载体为表面碱性时,CH4和CO2遵循双功能机理,在不同活性中心被活化,通常双功能机理的催化效率更高。当生成乙酸时,C-C耦合过程应被重点关注,该过程中气相CO2可能直接插入M—CH3键(Eley-Rideal机理)或先被吸附活化后再插入(Langmuir-Hinshelwood机理),后者反应能垒更低。当生成C2烃时,活性氧物种被认为是反应过程中的关键中间体,其可能来源于催化剂中的晶格氧或CO2的活化与解离。因此,在催化剂表面构建多个独立活性位点,以分别对CH4和CO2进行多位点协同催化被认为是良好的催化剂改性策略。另外,先进的模拟计算方法和原位表征手段能够深入揭示反应过程中催化剂和反应中间体的动态演变过程及机理,从而为真实CH4和CO2共转化反应过程中催化剂的设计提供理论指导。
中图分类号:
引用本文
成昊霖, 年瑶, 韩优. CH4和CO2共转化反应机理研究进展[J]. 化工进展, 2024, 43(1): 60-75.
CHENG Haolin, NIAN Yao, HAN You. Progress in the mechanism of CH4 and CO2co-conversion reactions[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 60-75.
| 反应式 | ||
|---|---|---|
| CH4(g)+3CO2(g) | +235.1 | +209.2 |
| CH4(g)+CO2(g) | +247.5 | +170.8 |
| CH4(g)+CO2(g) | +36.4 | +71.1 |
| 2CH4(g)+CO2(g) | +58.8 | +88.0 |
| 2CH4(g)+2CO2(g) | +189.7 | +208.3 |
| 2CH4(g)+CO2(g) | +8.5 | +115.0 |
表1 CH4和CO2共转化相关反应的焓变和自由能变[6]
| 反应式 | ||
|---|---|---|
| CH4(g)+3CO2(g) | +235.1 | +209.2 |
| CH4(g)+CO2(g) | +247.5 | +170.8 |
| CH4(g)+CO2(g) | +36.4 | +71.1 |
| 2CH4(g)+CO2(g) | +58.8 | +88.0 |
| 2CH4(g)+2CO2(g) | +189.7 | +208.3 |
| 2CH4(g)+CO2(g) | +8.5 | +115.0 |
| 解离物种 | Ru | Rh | Pd |
|---|---|---|---|
| CH3 | -2.70 | -2.58 | -2.20 |
| CH2 | -4.07 | -3.77 | -2.93 |
| CH | -5.64 | -5.45 | -4.46 |
表2 CH x 在Ru、Rh和Pd上的吸附能[41] (单位:eV)
| 解离物种 | Ru | Rh | Pd |
|---|---|---|---|
| CH3 | -2.70 | -2.58 | -2.20 |
| CH2 | -4.07 | -3.77 | -2.93 |
| CH | -5.64 | -5.45 | -4.46 |
| 催化剂 | 温度/K | V(CO2)/V(CH4) | C2烃选择性/% | C2烃产率/% |
|---|---|---|---|---|
| CaO-ZnO | 1123 | 2.3 | 80.0 | 4.3 |
| La2O3-ZnO | 1123 | 2.0 | 90.6 | 2.8 |
| Cr2O3-CaO | 850 | 2.3 | 63.0 | 4.0 |
| CeO2-CaO | 850 | 1.0 | 62.0 | 3.2 |
| MnO2-SrCO3 | 875 | 2.3 | 51.0 | 4.5 |
| Na2WO4-Mn/SiO2 | 1093.15 | 2.0 | 94.0 | 4.5 |
表3 CO2和CH4生成C2烃反应中二元金属氧化物催化剂的催化性能
| 催化剂 | 温度/K | V(CO2)/V(CH4) | C2烃选择性/% | C2烃产率/% |
|---|---|---|---|---|
| CaO-ZnO | 1123 | 2.3 | 80.0 | 4.3 |
| La2O3-ZnO | 1123 | 2.0 | 90.6 | 2.8 |
| Cr2O3-CaO | 850 | 2.3 | 63.0 | 4.0 |
| CeO2-CaO | 850 | 1.0 | 62.0 | 3.2 |
| MnO2-SrCO3 | 875 | 2.3 | 51.0 | 4.5 |
| Na2WO4-Mn/SiO2 | 1093.15 | 2.0 | 94.0 | 4.5 |
| 1 | JIANG Yao, TAN Peng, QI Shichao, et al. Metal-organic frameworks with target-specific active sites switched by photoresponsive motifs: Efficient adsorbents for tailorable CO2 capture[J]. Angewandte Chemie International Edition, 2019, 58(20): 6600-6604. |
| 2 | XIE L, DING J, KONG X, et al. Microwave-assisted synthesis of Mg-gallate for efficient CO2 capture[J]. Materials Today Sustainability, 2023, 22: 100356. |
| 3 | 杨海霞, 吴聃. 国际能源署发布《2022年全球二氧化碳排放》报告[J]. 世界石油工业, 2023, 30(2): 80-81. |
| YANG Haixia, WU Dan. The International Energy Agency releases the report “Global CO2 emissions by 2022”[J]. World Petroleum Industry, 2023, 30(2): 80-81. | |
| 4 | 张彦 著. 谋划甲烷控排双多边国际合作——基于G20成员国甲烷排放结构的差异性[J]. 可持续发展经济导刊, 2023(4): 16-19. |
| ZHANG Yanzhu. Thought of bilateral and multilateral cooperation on methane emission control based on differences in methane emission portfolios among G20 members[J]. China Sustainability Tribune, 2023(4): 16-19. | |
| 5 | 李志勤, 李侨, 黄伟, 等. 酸改性CoPd/TiO2催化CH4-CO2直接合成C2含氧化合物[J]. 化工进展, 2020, 39(3): 1035-1042. |
| LI Zhiqin, LI Qiao, HUANG Wei, et al. Direct synthesis of C2-oxygenates from CH4 and CO2 over acid-modified CoPd/TiO2 catalyst[J]. Chemical Industry and Engineering Progress, 2020, 39(3): 1035-1042. | |
| 6 | 赵云涛. CH4与CO2或其他含氧化合物在金属氧化物表面基于C-C直接耦合的密度泛函理论研究[D]. 天津: 天津大学, 2020. |
| ZHAO Yuntao. Theoretical study of conversion of CH4 with CO2 or other oxygenates through direct C-C coupling on metal oxide surface[D]. Tianjin: Tianjin University, 2020. | |
| 7 | CAI Xiaojiao, HU Yunhang. Advances in catalytic conversion of methane and carbon dioxide to highly valuable products[J]. Energy Science & Engineering, 2019, 7(1): 4-29. |
| 8 | WANG Ye, YAO Lu, WANG Yannan, et al. Low-temperature catalytic CO2 dry reforming of methane on Ni-Si/ZrO2 catalyst[J]. ACS Catalysis, 2018, 8(7): 6495-6506. |
| 9 | YAN Xiaoliang, HU Tong, LIU Peng, et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2 [J]. Applied Catalysis B: Environmental, 2019, 246: 221-231. |
| 10 | BODROV N, APELBAUM L, TEMKIN M J K K. Kinetics of the reaction of methane with steam on the surface of nickel[J]. Kinetics and Catalysis, 1964, 5: 696. |
| 11 | ROSTRUPNIELSEN J R, HANSEN J H B. CO2-reforming of methane over transition metals[J]. Journal of Catalysis, 1993, 144(1): 38-49. |
| 12 | 张云飞, 张晓娣, 王影, 等. 镍基CH4-CO2重整催化剂研究综述[J]. 洁净煤技术, 2022, 28(5): 29-50. |
| ZHANG Yunfei, ZHANG Xiaodi, WANG Ying, et al. Review of nickel-based CH4-CO2 reforming catalysts[J]. Clean Coal Technology, 2022, 28(5): 29-50. | |
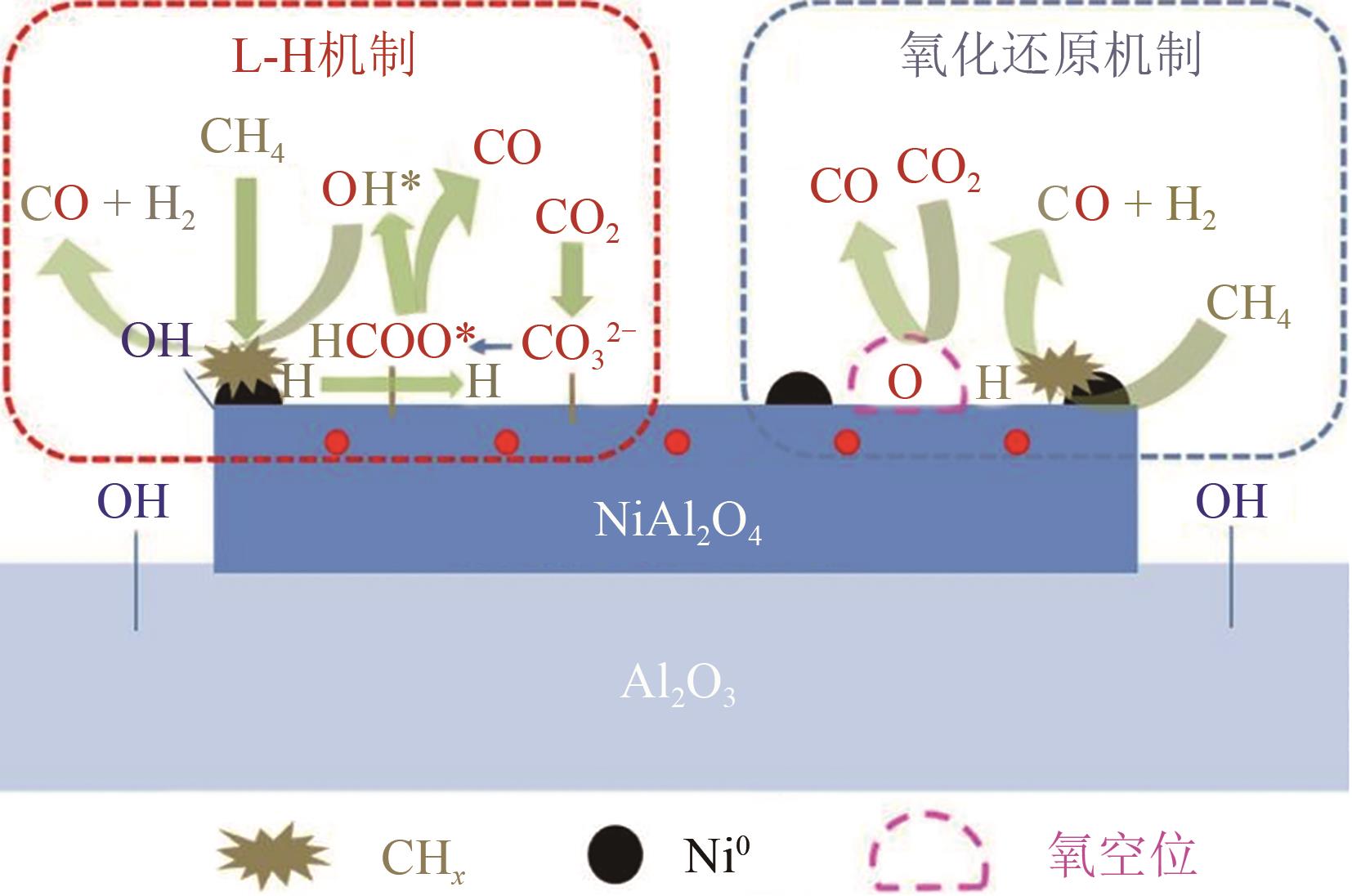
| 13 | ZHANG Shuangshuang, YING Ming, YU Jun, et al. Ni x Al1O2- δ mesoporous catalysts for dry reforming of methane: The special role of NiAl2O4 spinel phase and its reaction mechanism[J]. Applied Catalysis B: Environmental, 2021, 291: 120074. |
| 14 | DAS S, ASHOK J, BIAN Z F, et al. Silica-ceria sandwiched Ni core-shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights[J]. Applied Catalysis B: Environmental, 2018, 230: 220-236. |
| 15 | YENTEKAKIS I V, GOULA G, HATZISYMEON M, et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane[J]. Applied Catalysis B: Environmental, 2019, 243: 490-501. |
| 16 | ZHOU Wei, WANG Binghao, TANG Long, et al. Photocatalytic dry reforming of methane enhanced by “dual-path” strategy with excellent low-temperature catalytic performance[J]. Advanced Functional Materials, 2023, 33(27): 2214068. |
| 17 | AZANCOT L, BOBADILLA L F, CENTENO M A, et al. IR spectroscopic insights into the coking-resistance effect of potassium on nickel-based catalyst during dry reforming of methane[J]. Applied Catalysis B: Environmental, 2021, 285: 119822. |
| 18 | BARAŃSKI A. On the usefulness of Campbell’s concept of the rate-determining step[J]. Solid State Ionics, 1999, 117(1/2): 123-128. |
| 19 | CHEN Shuyue, ZAFFRAN J, YANG Bo. Descriptor design in the computational screening of Ni-based catalysts with balanced activity and stability for dry reforming of methane reaction[J]. ACS Catalysis, 2020, 10(5): 3074-3083. |
| 20 | WEI Junmei, IGLESIA E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts[J]. Journal of Catalysis, 2004, 224(2): 370-383. |
| 21 | BRADFORD M C J, VANNICE M A. Catalytic reforming of methane with carbon dioxide over nickel catalysts Ⅱ. Reaction kinetics[J]. Applied Catalysis A: General, 1996, 142(1): 97-122. |
| 22 | OSAKI T. Effect of nickel diameter on the rates of elementary steps involved in CO2 reforming of CH4 over Ni/Al2O3 catalysts[J]. Catalysis Letters, 2015, 145(11): 1931-1940. |
| 23 | 余长春, 李然家, 王伟, 等. CO2/CH4干重整转化催化剂的积炭控制研究[J]. 石油化工, 2020, 49(10): 925-930. |
| YU Changchun, LI Ranjia, WANG Wei, et al. Study of carbon deposition controlling over CO2/CH4 dry reforming catalyst[J]. Petrochemical Technology, 2020, 49(10): 925-930. | |
| 24 | WANG Baochuan, CHEN Shuyue, ZHANG Jiaming, et al. Propagating DFT uncertainty to mechanism determination, degree of rate control, and coverage analysis: The kinetics of dry reforming of methane[J]. The Journal of Physical Chemistry C, 2019, 123(50): 30389-30397. |
| 25 | BIAN Zhoufeng, DAS S, Minghui WAI, et al. A review on bimetallic nickel-based catalysts for CO2 reforming of methane[J]. ChemPhysChem, 2017, 18(22): 3117-3134. |
| 26 | KAWI S, KATHIRASER Y, NI Jun, et al. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane[J]. ChemSusChem, 2015, 8(21): 3556-3575. |
| 27 | MORTENSEN P M, DYBKJÆR I. Industrial scale experience on steam reforming of CO2-rich gas[J]. Applied Catalysis A: General, 2015, 495: 141-151. |
| 28 | ÁLVAREZ MORENO A, RAMIREZ-REINA T, IVANOVA S, et al. Bimetallic Ni-Ru and Ni-Re catalysts for dry reforming of methane: Understanding the synergies of the selected promoters[J]. Frontiers in Chemistry, 2021, 9: 694976. |
| 29 | LU Yao, GUO Dan, ZHAO Yifan, et al. Enhanced catalytic performance of Ni x -V@HSS catalysts for the DRM reaction: The study of interfacial effects on Ni-VO x structure with a unique yolk-shell structure[J]. Journal of Catalysis, 2021, 396: 65-80. |
| 30 | LOVELL E C, FULLER A, SCOTT J, et al. Enhancing Ni-SiO2 catalysts for the carbon dioxide reforming of methane: Reduction-oxidation-reduction pre-treatment[J]. Applied Catalysis B: Environmental, 2016, 199: 155-165. |
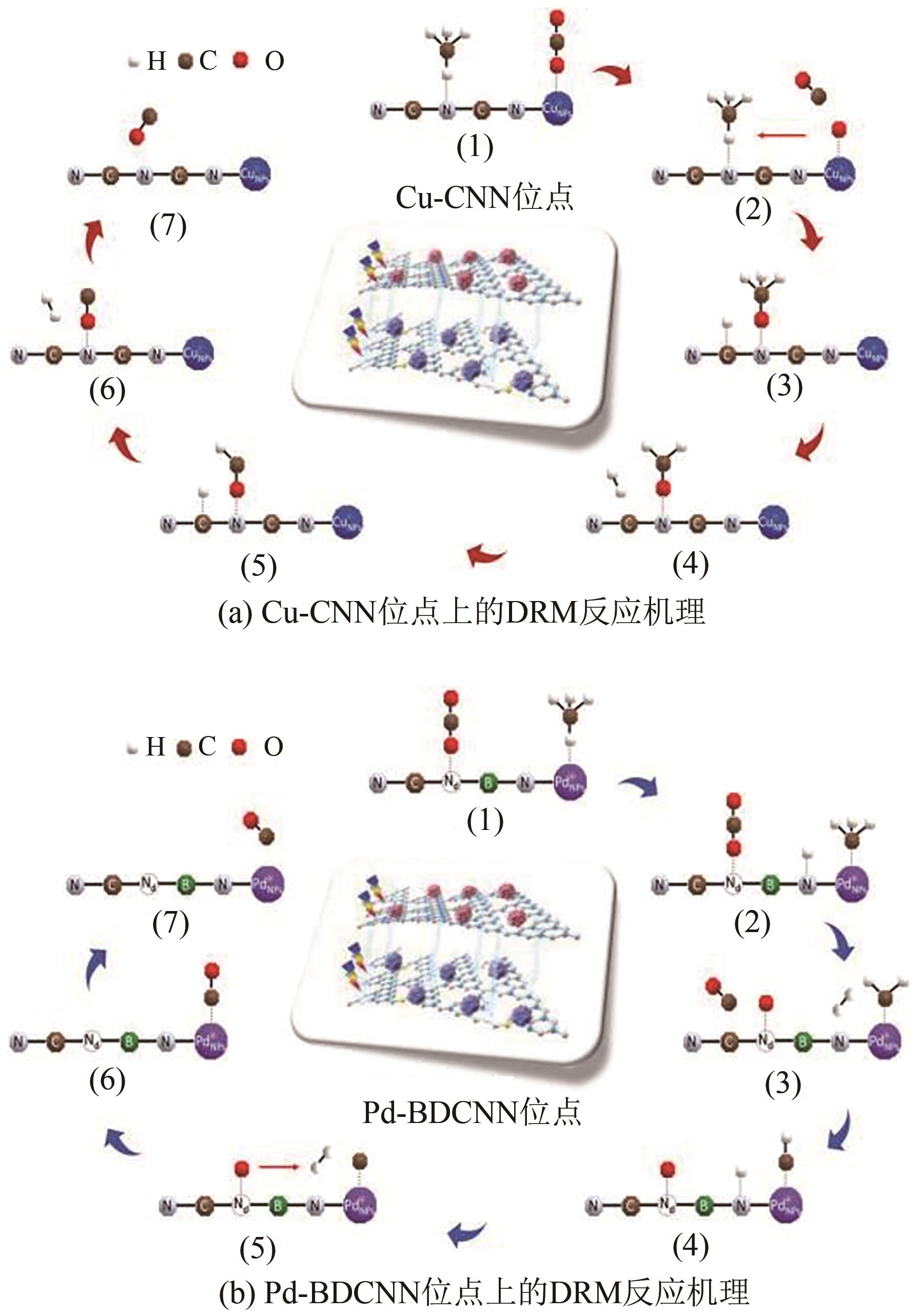
| 31 | BAN Tao, YU Xiyang, KANG Haozhe, et al. Design of single-atom and Frustrated-Lewis-Pair dual active sites for direct conversion of CH4 and CO2 to acetic acid[J]. Journal of Catalysis, 2022, 408: 206-215. |
| 32 | KURIOKA M, NAKATA K, JINTOKU T, et al. Palladium-catalyzed acetic acid synthesis from methane and carbon monoxide or dioxide[J]. Chemistry Letters, 1995, 24(3): 244. |
| 33 | TANIGUCHI Y, HAYASHIDA T, KITAMURA T, et al. Vanadium-catalyzed acetic acid synthesis from methane and carbon dioxide[M]// Studies in Surface Science and Catalysis. Amsterdam: Elsevier, 1998: 439-442. |
| 34 | ZERELLA M, MUKHOPADHYAY S, BELL A T. Synthesis of mixed acid anhydrides from methane and carbon dioxide in acid solvents[J]. Organic Letters, 2003, 5(18): 3193-3196. |
| 35 | WU Jianfeng, YU Simin, WANG Wei D, et al. Mechanistic insight into the formation of acetic acid from the direct conversion of methane and carbon dioxide on zinc-modified H-ZSM-5 zeolite[J]. Journal of the American Chemical Society, 2013, 135(36): 13567-13573. |
| 36 | WANG Sen, GUO Shujia, LUO Yaoya, et al. Direct synthesis of acetic acid from carbon dioxide and methane over Cu-modulated BEA, MFI, MOR and TON zeolites: A density functional theory study[J]. Catalysis Science & Technology, 2019, 9(23): 6613-6626. |
| 37 | ZHANG Riguang, SONG Luzhi, LIU Hongyan, et al. The interaction mechanism of CO2 with CH3 and H on Cu (111) surface in synthesis of acetic acid from CH4/CO2: A DFT study[J]. Applied Catalysis A: General, 2012, 443/444: 50-58. |
| 38 | ZHOU Lei, LI Wei, HU Tongliang. Computational study of Zn single-atom catalysts on In2O3 nanomaterials for direct synthesis of acetic acid from CH4 and CO2 [J]. ACS Applied Nano Materials, 2022, 5(7): 10015-10025. |
| 39 | MAHYUDDIN M H, TANAKA S, SHIOTA Y, et al. Room-temperature activation of methane and direct formations of acetic acid and methanol on Zn-ZSM-5 zeolite: A mechanistic DFT study[J]. Bulletin of the Chemical Society of Japan, 2020, 93(3): 345-354. |
| 40 | WU Bo, LIN Tiejun, LU Zhengxing, et al. Fe binuclear sites convert methane to acetic acid with ultrahigh selectivity[J]. Chem, 2022, 8(6): 1658-1672. |
| 41 | DING Yihui, HUANG Wei, WANG Yonggang. Direct synthesis of acetic acid from CH4 and CO2 by a step-wise route over Pd/SiO2 and Rh/SiO2 catalysts[J]. Fuel Processing Technology, 2007, 88(4): 319-324. |
| 42 | SHAVI R, KO J, CHO A, et al. Mechanistic insight into the quantitative synthesis of acetic acid by direct conversion of CH4 and CO2: An experimental and theoretical approach[J]. Applied Catalysis B: Environmental, 2018, 229: 237-248. |
| 43 | 章日光, 黄伟, 王宝俊. Co-Pd催化剂上CH4/CO2合成乙酸反应中CO2与表面金属物种作用的密度泛函理论研究[J]. 高等学校化学学报, 2009, 30(11): 2252-2257. |
| ZHANG Riguang, HUANG Wei, WANG Baojun. Density functional theory study on interaction of CO2 with metal surface carbon species in synthesis of acetic acid from CH4/CO2 on Co-Pd catalysts[J]. Chemical Journal of Chinese Universities, 2009, 30(11): 2252-2257. | |
| 44 | NIE Xiaowa, REN Xianxuan, TU Chunyan, et al. Computational and experimental identification of strong synergy of the Fe/ZnO catalyst in promoting acetic acid synthesis from CH4 and CO2 [J]. Chemical Communications, 2020, 56(28): 3983-3986. |
| 45 | ZHAO Yuntao, CUI Chaonan, HAN Jinyu, et al. Direct C-C coupling of CO2 and the methyl group from CH4 activation through facile insertion of CO2 into Zn-CH3 σ-bond[J]. Journal of the American Chemical Society, 2016, 138(32): 10191-10198. |
| 46 | ORTIZ-BRAVO C A, CHAGAS C A, TONIOLO F S. Oxidative coupling of methane (OCM): An overview of the challenges and opportunities for developing new technologies[J]. Journal of Natural Gas Science and Engineering, 2021, 96: 104254. |
| 47 | ZOU Shihui, LI Zhinian, ZHOU Qiuyue, et al. Surface coupling of methyl radicals for efficient low-temperature oxidative coupling of methane[J]. Chinese Journal of Catalysis, 2021, 42(7): 1117-1125. |
| 48 | KIANI D, SOURAV S, BALTRUSAITIS J, et al. Oxidative coupling of methane (OCM) by SiO2-supported tungsten oxide catalysts promoted with Mn and Na[J]. ACS Catalysis, 2019, 9(7): 5912-5928. |
| 49 | NGUYEN T N, NHAT T T P, TAKIMOTO K, et al. High-throughput experimentation and catalyst informatics for oxidative coupling of methane[J]. ACS Catalysis, 2020, 10(2): 921-932. |
| 50 | LIU Wenchi, RALSTON W T, MELAET G, et al. Oxidative coupling of methane (OCM): Effect of noble metal (M=Pt, Ir, Rh) doping on the performance of mesoporous silica MCF-17 supported Mn x O y -Na2WO4 catalysts[J]. Applied Catalysis A: General, 2017, 545: 17-23. |
| 51 | LIU Zichen, XU Shuman, HAO Jing, et al. Bifunctional catalysts composed of low silicon-content SAPO-34 nanosheets and In2O3/ZrO2 with improved performance for CO2 hydrogenation[J]. Greenhouse Gases: Science and Technology, 2022, 12(2): 305-320. |
| 52 | ASAMI K, FUJITA T, KUSAKABE K, et al. Conversion of methane with carbon dioxide into C2 hydrocarbons over metal oxides[J]. Applied Catalysis A: General, 1995, 126(2): 245-255. |
| 53 | LIU Jingwei, HUO Minfeng, ZHU Yan. Surface basic sites and lattice oxygen sensitive Mn-Na2WO4/SiO2 catalysts for oxidative coupling of methane[J]. Journal of Nanoscience and Nanotechnology, 2017, 17(12): 8818-8826. |
| 54 | ASAMI K, KUSAKABE K, ASHI N, et al. Synthesis of ethane and ethylene from methane and carbon dioxide over praseodymium oxide catalysts[J]. Applied Catalysis A: General, 1997, 156(1): 43-56. |
| 55 | 蔡迎春, 丑凌军, 张兵, 等. Mn-CaO催化剂上甲烷-二氧化碳共活化制C2烃研究II.催化剂表征及反应机理研究[J]. 分子催化, 2005, 19(5): 327-331. |
| CAI Yingchun, CHOU Lingjun, ZHANG Bing, et al. Selective conversion of CH4 and CO2 to C2 hydrocarbons over Mn-CaO catalysts Ⅱ. Catalysts characterization and mechanism deduce[J]. Journal of Molecular Catalysis, 2005, 19(5): 327-331. | |
| 56 | ZHANG Xianhua, PEI Chunlei, CHANG Xin, et al. FeO6 octahedral distortion activates lattice oxygen in perovskite ferrite for methane partial oxidation coupled with CO2 splitting[J]. Journal of the American Chemical Society, 2020, 142(26): 11540-11549. |
| 57 | VAN ALPHEN S, SLAETS J, CEULEMANS S, et al. Effect of N2 on CO2-CH4 conversion in a gliding arc plasmatron: Can this major component in industrial emissions improve the energy efficiency?[J]. Journal of CO2 Utilization, 2021, 54: 101767. |
| 58 | SAVINOV S Y, LEE H, SONG H K, et al. Decomposition of methane and carbon dioxide in a radio-frequency discharge[J]. Industrial & Engineering Chemistry Research, 1999, 38(7): 2540-2547. |
| 59 | CHEN Changlin, XU Yide, LI Guangjin, et al. Oxidative coupling of methane by carbon dioxide: A highly C2 selective La2O3/ZnO catalyst[J]. Catalysis Letters, 1996, 42(3): 149-153. |
| 60 | ZHANG Yongzheng, CHO Yohei, YAMAGUCHI A, et al. CO2 oxidative coupling of methane using an earth-abundant CaO-based catalyst[J]. Scientific Reports, 2019, 9(1): 15454. |
| 61 | WANG Ye, TAKAHASHI Y, OHTSUKA Y. Carbon dioxide-induced selective conversion of methane to C2 hydrocarbons on CeO2 modified with CaO[J]. Applied Catalysis A: General, 1998, 172(2): L203-L206. |
| 62 | ARINAGA A M, ZIEGELSKI M C, MARKS T J. Alternative oxidants for the catalytic oxidative coupling of methane[J]. Angewandte Chemie International Edition, 2021, 60(19): 10502-10515. |
| 63 | YOON Suji, Seoyeon LIM, CHOI Jae-Wook, et al. Study on the unsteady state oxidative coupling of methane: Effects of oxygen species from O2, surface lattice oxygen, and CO2 on the C2+ selectivity[J]. RSC Advances, 2020, 10(59): 35889-35897. |
| 64 | SHI Jia, YAO Lu, HU Changwei. Effect of CO2 on the structural variation of Na2WO4/Mn/SiO2 catalyst for oxidative coupling of methane to ethylene[J]. Journal of Energy Chemistry, 2015, 24(4): 394-400. |
| 65 | WANG Ye, OHTSUKA Y. CaO-ZnO catalyst for selective conversion of methane to C2 hydrocarbons using carbon dioxide as the oxidant[J]. Journal of Catalysis, 2000, 192(1): 252-255. |
| 66 | 翟林燕, 于淼, 周倩, 等. 常压等离子体作用下CO2氧化CH4转化反应的光谱分析[J]. 光谱学与光谱分析, 2012, 32(3): 734-738. |
| ZHAI Linyan, YU Miao, ZHOU Qian, et al. Spectral analysis of the reaction of CH4 with CO2 as oxidant under plasma at atmospheric pressure[J]. Spectroscopy and Spectral Analysis, 2012, 32(3): 734-738. | |
| 67 | CAI Yingchun, CHOU Lingjun, LI Shuben, et al. Selective conversion of methane to C2 hydrocarbons using carbon dioxide over Mn-SrCO3 catalysts[J]. Catalysis Letters, 2003, 86(4): 191-195. |
| 68 | FILARDI L R, YANG Feipeng, GUO Jinghua, et al. Surface basicity controls C-C coupling rates during carbon dioxide-assisted methane coupling over bifunctional Ca/ZnO catalysts[J]. Physical Chemistry Chemical Physics, 2023, 25(14): 9859-9867. |
| 69 | LIU Yu, HOU Ruiling, LIU Xuxia, et al. Performance of Na2WO4-Mn/SiO2 catalyst for conversion of CH4 with CO2 into C2 hydrocarbons and its mechanism[M]// Natural Gas Conversion V, Proceedings of the 5th International Natural Gas Conversion Symposium, Amsterdam: Elsevier, 1998: 307-311. |
| 70 | KIANI D, SOURAV S, WACHS I E, et al. Synthesis and molecular structure of model silica-supported tungsten oxide catalysts for oxidative coupling of methane (OCM)[J]. Catalysis Science & Technology, 2020, 10(10): 3334-3345. |
| 71 | WANG Jiyang, FU Yu, KONG Wenbo, et al. Investigation of atom-level reaction kinetics of carbon-resistant bimetallic NiCo-reforming catalysts: Combining microkinetic modeling and density functional theory[J]. ACS Catalysis, 2022, 12(8): 4382-4393. |
| 72 | 中国工程科技知识中心. CO2与甲烷重整转化制备合成气关键技术与工程示范[EB/OL]. (2015-11-24)[2023-07-26]. . |
| China Knowledge Center for Engineering Science and Technology. Key technology and engineering demonstration of CO2 and methane reforming conversion for syngas preparation[EB/OL]. (2015-11-24)[2023-07-26]. . |
| [1] | 于笑笑, 巢艳红, 刘海燕, 朱文帅, 刘植昌. D-A共轭聚合强化光电性能及光催化CO2转化[J]. 化工进展, 2024, 43(1): 292-301. |
| [2] | 杨梦茹, 彭琴, 常玉龙, 邱淑兴, 张溅波, 江霞. 生物炭替代煤粉/焦炭高炉炼铁碳减排技术研究进展[J]. 化工进展, 2024, 43(1): 490-500. |
| [3] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [4] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [5] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [6] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [7] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [8] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [9] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [10] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [11] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [12] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [13] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [14] | 奚永兰, 王成成, 叶小梅, 刘洋, 贾昭炎, 曹春晖, 韩挺, 张应鹏, 田雨. 微纳米气泡在厌氧消化中的应用研究进展[J]. 化工进展, 2023, 42(8): 4414-4423. |
| [15] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||