化工进展 ›› 2024, Vol. 43 ›› Issue (1): 529-540.DOI: 10.16085/j.issn.1000-6613.2023-0258
• 资源与环境化工 • 上一篇
低中放射性废水处理吸附技术及材料
- 1.辽宁省化工资源清洁利用重点实验室,大连理工大学,辽宁 大连 116024
2.一重集团大连工程技术有限公司,辽宁 大连 116600
-
收稿日期:2023-02-24修回日期:2023-05-26出版日期:2024-01-20发布日期:2024-02-05 -
通讯作者:马学虎 -
作者简介:代洪静(1988—),女,博士研究生,高级工程师,研究方向为能源与环保技术。E-mail:daihongjingcfhi@163.com。 -
基金资助:辽宁省“兴辽英才”计划(XLYC1808041);大连市杰出青年项目(2022RJ18);大连市优秀青年项目(2023RY037)
Adsorption technology and materials for the treatment of low and intermediate level radioactive wastewater
DAI Hongjing1,2( ), MA Xuehu1(
), MA Xuehu1( ), WANG Sifang2
), WANG Sifang2
- 1.Liaoning Key Laboratory of Clean Utilization of Chemical Resources, Dalian University of Technology, Dalian 116024, Liaoning, China
2.CFHI Dalian Engineering & Technology Co. , Ltd. , Dalian 116600, Liaoning, China
-
Received:2023-02-24Revised:2023-05-26Online:2024-01-20Published:2024-02-05 -
Contact:MA Xuehu
摘要:
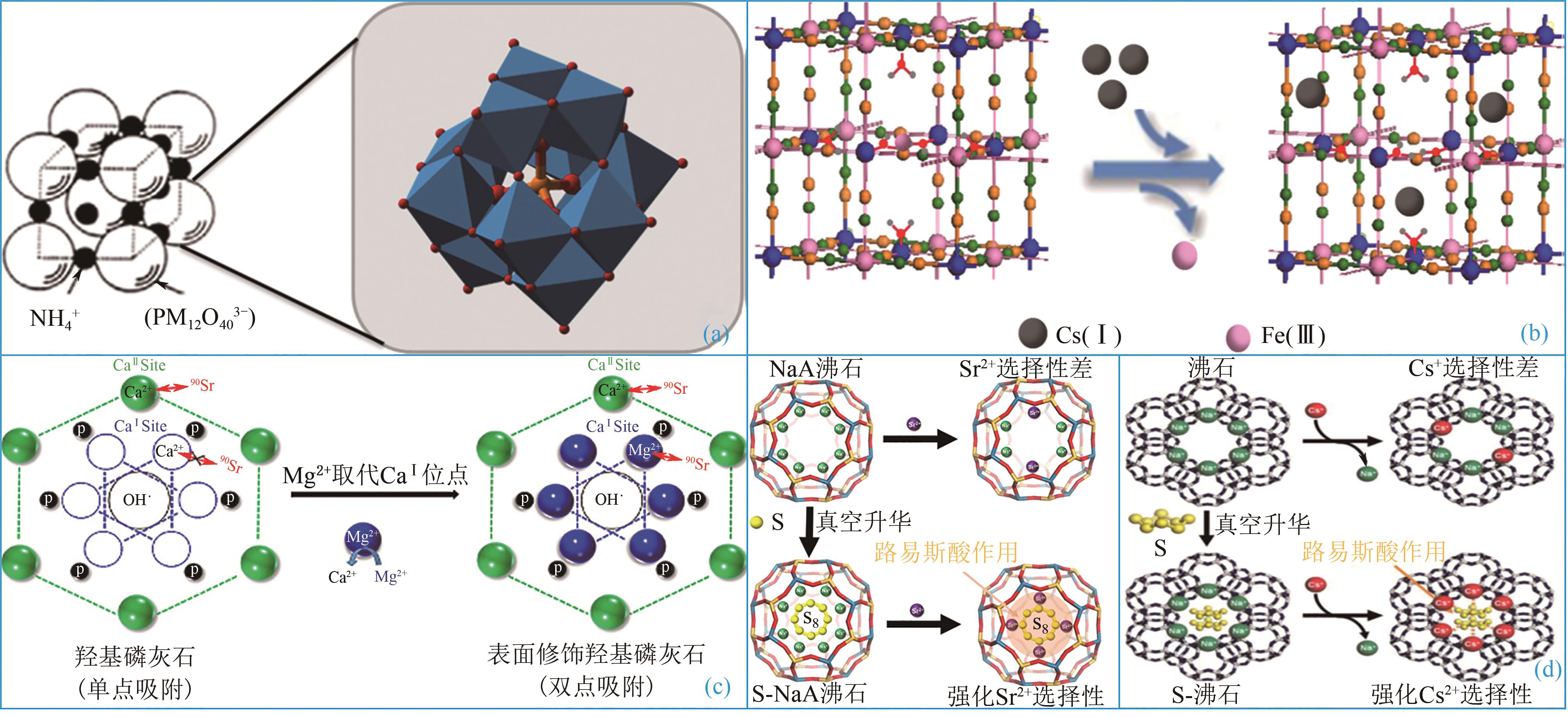
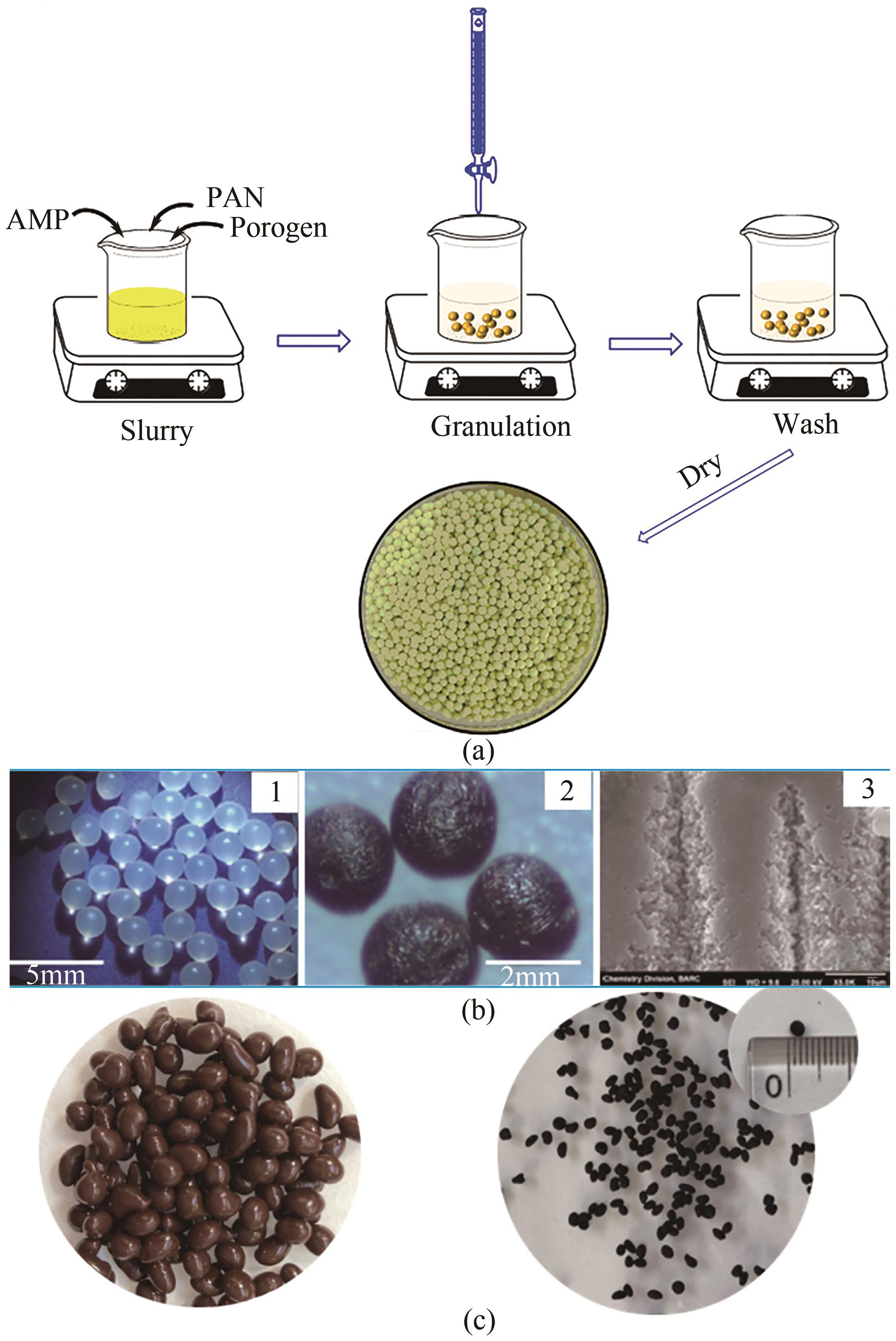
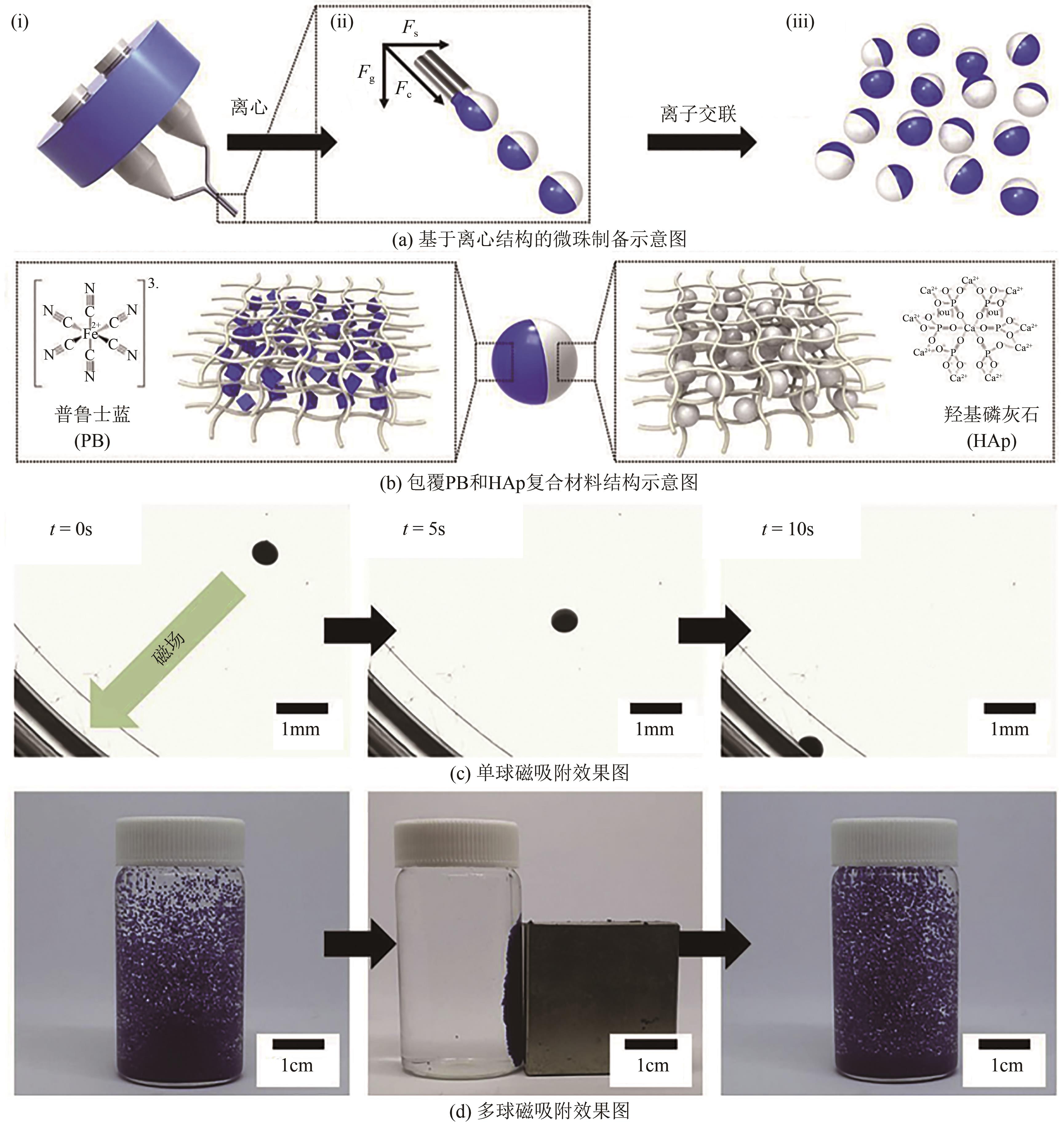
吸附技术是处理核工业产生的低中放射性废水高效、便捷的处理工艺之一。多数纳米吸附材料性能高效,但为适于工程应用需制备为复合吸附剂。本文分析了低中放射性废液的特点及吸附处理技术现状,对适于核工业应用的复合微珠吸附剂的研究进展重点总结,包括外原位固定微珠、聚合物微珠及磁性微珠。从芯材性质、载体特点、制备方法及吸附性能等方面分析了复合吸附剂的优缺点及应用性能提升方法。最后,结合核工业对低中放射性废液的处理需求指出缺乏工程试验及带放射性试验研究为低中放射性废液吸附技术及材料研究的关键问题,提出开发多核素吸附剂、加强低浓度核素吸附的数值模拟及加强复合吸附剂的工程应用考察等方面是未来的重点研究方向。
中图分类号:
引用本文
代洪静, 马学虎, 王四芳. 低中放射性废水处理吸附技术及材料[J]. 化工进展, 2024, 43(1): 529-540.
DAI Hongjing, MA Xuehu, WANG Sifang. Adsorption technology and materials for the treatment of low and intermediate level radioactive wastewater[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 529-540.
| 吸附剂 | 制备方法 | 材料尺寸/μm | 比表面积/m2·g-1 | 活性成分含量 | 平衡时间/h | 吸附容量/mg·g-1 | 参考文献 | ||
|---|---|---|---|---|---|---|---|---|---|
| 锶 | 铯 | 钴 | |||||||
| 果壳炭-天然沸石 | 逐层黏结 | 400 | 34.93 | 33% | 3.0 | 95.6 | — | — | [ |
| 果壳炭/合成沸石 | 水热 | 400 | 89.17 | 20% | 3.0 | 91.2 | — | — | |
| KNICF/GAC | 沉淀浸渍 | 400 | 694 | — | 5.0 | 13.2 | 49.7 | 0.1 | [ |
| GAC | 400 | 1099 | 100% | 5.0 | 13.2 | 11.1 | 1.0 | ||
| PB/NHPC | 沉淀 | 600 | 1884 | — | 2.0 | — | 100.0 | — | [ |
| 斜发沸石/果壳炭 | 浸渍 | 325 | — | 19% | 2.0 | — | 27.7 | — | [ |
| BC | 热解 | 500 | 6.69 | 100% | 3 | — | 12.9 | 4.4 | [ |
| AC-HAp | 表面合成 | — | — | — | 2.0 | 69.49 | — | — | [ |
| C4BisC6/MMCs-P | 热聚合 | 500 | — | 28.4% | 3.0 | — | 22.7 | — | [ |
| ACC/GO | 真空过滤 | — | 744 | — | — | — | 22.9 | 16.7 | [ |
| MMT-PB | 共沉淀 | — | 259.26 | — | 2.0 | — | 57.47 | — | [ |
| AMP/沸石/SiO2 | 浸渍/冻干 | 100 | 72.2 | 32% | 0.5 | — | 99% | — | [ |
| hf-TiFC | 水热 | 500 | 63.89 | 16% | — | — | 454.54 | — | [ |
| 沸石/PAN | 喷嘴滴加 | 500 | — | 80% | — | 98.1 | 214.1 | — | [ |
| AMP-PAN | 热凝胶 | 1500 | 32.69 | 70% | 2.0 | 16.2 | 81.4 | 9.4 | [ |
| AMP-PAN-N20 | 凝胶制孔 | 1000 | — | 76.1% | 8.0 | — | 55.05 | — | [ |
| KNiFC/PAN | 微震喷射 | 400 | — | 80% | 4.0 | — | 123.0 | — | [ |
| Zr-Mn/PAN | 混合滴加 | 2500 | 215.5 | 16% | 4.0 | — | 21.37 | — | [ |
| ALG/RF | 热凝胶 | 2000 | 568.45 | — | 4.0 | 490.2 | - | — | [ |
| GO-ALG | 凝胶 | 2500 | — | — | 2.0 | — | 144.3 | — | [ |
| 沸石/ALG | 框架凝胶 | — | 1.39 | 10% | 6.0 | 22.0 | — | — | [ |
| zeolite@ALG-Ca | 静电喷射 | 1750 | — | — | 10.0 | 83.3 | — | — | [ |
| AMP/ALG | 凝胶 | — | — | 66% | 5.0 | — | 91.8 | — | [ |
| Co/Mn-CCTS | 溶胶+反相悬浮 | 650 | — | — | 8.0 | — | — | 17.13 | [ |
| NSC@MS-4A | 凝胶 | 100 | 77.07 | 57% | 1.7 | 44.2 | 101.8 | — | [ |
| TiO2/CTS | 水热 | 1750 | — | 50% | 24.0 | 84.6 | — | — | [ |
| HAp-CTS | 静电喷射 | 650 | 37.84 | 4% | 4.0 | 234.2 | — | — | [ |
| CMC/PB-K/PEG | 交联聚合 | 1000 | 33.96 | 10% | 24.0 | — | 149.8 | — | [ |
| CMC/PB-La | 凝胶 | 1100 | — | 73% | 5.0 | — | 35.2 | — | [ |
| mag@silica-CIP | 离子印迹 | — | 158.4 | — | — | — | — | 78.9 | [ |
| PB-HAp-Mas | 微喷射 | 475 | — | — | 2.0 | 29.25 | 24.59 | — | [ |
表1 复合微珠对核素的去除性能
| 吸附剂 | 制备方法 | 材料尺寸/μm | 比表面积/m2·g-1 | 活性成分含量 | 平衡时间/h | 吸附容量/mg·g-1 | 参考文献 | ||
|---|---|---|---|---|---|---|---|---|---|
| 锶 | 铯 | 钴 | |||||||
| 果壳炭-天然沸石 | 逐层黏结 | 400 | 34.93 | 33% | 3.0 | 95.6 | — | — | [ |
| 果壳炭/合成沸石 | 水热 | 400 | 89.17 | 20% | 3.0 | 91.2 | — | — | |
| KNICF/GAC | 沉淀浸渍 | 400 | 694 | — | 5.0 | 13.2 | 49.7 | 0.1 | [ |
| GAC | 400 | 1099 | 100% | 5.0 | 13.2 | 11.1 | 1.0 | ||
| PB/NHPC | 沉淀 | 600 | 1884 | — | 2.0 | — | 100.0 | — | [ |
| 斜发沸石/果壳炭 | 浸渍 | 325 | — | 19% | 2.0 | — | 27.7 | — | [ |
| BC | 热解 | 500 | 6.69 | 100% | 3 | — | 12.9 | 4.4 | [ |
| AC-HAp | 表面合成 | — | — | — | 2.0 | 69.49 | — | — | [ |
| C4BisC6/MMCs-P | 热聚合 | 500 | — | 28.4% | 3.0 | — | 22.7 | — | [ |
| ACC/GO | 真空过滤 | — | 744 | — | — | — | 22.9 | 16.7 | [ |
| MMT-PB | 共沉淀 | — | 259.26 | — | 2.0 | — | 57.47 | — | [ |
| AMP/沸石/SiO2 | 浸渍/冻干 | 100 | 72.2 | 32% | 0.5 | — | 99% | — | [ |
| hf-TiFC | 水热 | 500 | 63.89 | 16% | — | — | 454.54 | — | [ |
| 沸石/PAN | 喷嘴滴加 | 500 | — | 80% | — | 98.1 | 214.1 | — | [ |
| AMP-PAN | 热凝胶 | 1500 | 32.69 | 70% | 2.0 | 16.2 | 81.4 | 9.4 | [ |
| AMP-PAN-N20 | 凝胶制孔 | 1000 | — | 76.1% | 8.0 | — | 55.05 | — | [ |
| KNiFC/PAN | 微震喷射 | 400 | — | 80% | 4.0 | — | 123.0 | — | [ |
| Zr-Mn/PAN | 混合滴加 | 2500 | 215.5 | 16% | 4.0 | — | 21.37 | — | [ |
| ALG/RF | 热凝胶 | 2000 | 568.45 | — | 4.0 | 490.2 | - | — | [ |
| GO-ALG | 凝胶 | 2500 | — | — | 2.0 | — | 144.3 | — | [ |
| 沸石/ALG | 框架凝胶 | — | 1.39 | 10% | 6.0 | 22.0 | — | — | [ |
| zeolite@ALG-Ca | 静电喷射 | 1750 | — | — | 10.0 | 83.3 | — | — | [ |
| AMP/ALG | 凝胶 | — | — | 66% | 5.0 | — | 91.8 | — | [ |
| Co/Mn-CCTS | 溶胶+反相悬浮 | 650 | — | — | 8.0 | — | — | 17.13 | [ |
| NSC@MS-4A | 凝胶 | 100 | 77.07 | 57% | 1.7 | 44.2 | 101.8 | — | [ |
| TiO2/CTS | 水热 | 1750 | — | 50% | 24.0 | 84.6 | — | — | [ |
| HAp-CTS | 静电喷射 | 650 | 37.84 | 4% | 4.0 | 234.2 | — | — | [ |
| CMC/PB-K/PEG | 交联聚合 | 1000 | 33.96 | 10% | 24.0 | — | 149.8 | — | [ |
| CMC/PB-La | 凝胶 | 1100 | — | 73% | 5.0 | — | 35.2 | — | [ |
| mag@silica-CIP | 离子印迹 | — | 158.4 | — | — | — | — | 78.9 | [ |
| PB-HAp-Mas | 微喷射 | 475 | — | — | 2.0 | 29.25 | 24.59 | — | [ |
| 103 | DING Baojun, WANG Ziwei, WANG Xintong, et al. Sr2+ adsorbents produced by microfluidics[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 613: 126072. |
| 104 | KAMBLE Priyanka, SINHAROY Prithwish, PAHAN Sumit, et al. Synthesis and characterization of chitosan-sodium titanate nanocomposite beads for separation of radionuclides from aqueous radioactive waste[J]. Journal of Radioanalytical and Nuclear Chemistry, 2021, 327(2): 691-698. |
| 105 | HU Baiyang, FUGETSU Bunshi, YU Hongwen, et al. Prussian blue caged in spongiform adsorbents using diatomite and carbon nanotubes for elimination of cesium[J]. Journal of Hazardous Materials, 2012, 217/218: 85-91. |
| 106 | TSAI Chengjui, CHANG Yinru, CHEN Manli, et al. Stable poly(vinyl alcohol) and alginate cross-linked granules with immobilized ferric hexacyanoferrate for cesium removal from waters[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 95: 1-10. |
| 107 | FENG Shi, YANG Wenbo, ZHANG Lijing, et al. Casein-hydroxyapatite composite microspheres for strontium-containing wastewater treatment[J]. ACS ES&T Water, 2021, 1(4): 900-909. |
| 108 | ZONG Youli, ZHANG Yongde, LIN Xiaoyan, et al. Preparation of a novel microsphere adsorbent of prussian blue capsulated in carboxymethyl cellulose sodium for Cs(Ⅰ) removal from contaminated water[J]. Journal of Radioanalytical and Nuclear Chemistry, 2017, 311(3): 1577-1591. |
| 109 | HU Jiayin, CHEN Shangqing, ZHANG Ningluo, et al. Porous composite CMC-KCuFC-PEG spheres for efficient cesium removal from wastewater[J]. New Journal of Chemistry, 2019, 43(24): 9658-9665. |
| 110 | Joanna BOK-BADURA, Alicja KAZEK-KĘSIK, Krzysztof KAROŃ, et al. Highly efficient copper hexacyanoferrate-embedded pectin sorbent for radioactive cesium ions removal[J]. Water Resources and Industry, 2022, 28: 100190. |
| 111 | 顾恩熙, 付凌霄, 王焕. 废水中去除银胶体的负载Fe3+树脂氧化法性能研究[J]. 核技术, 2019, 42(7):29-35. |
| GU Enxi, FU Lingxiao, WANG Huan. Fe3+ loaded resin for removal of Ag colloid by oxidation method from waste water[J]. Nuclear Techniques, 2019, 42(07): 29-35. | |
| 112 | 杜明阳, 邹京, 豆俊峰, 等. 钾改性蒙脱石磁性微球对铯的吸附性能[J]. 环境化学, 2021, 40(3): 779-789. |
| DU Mingyang, ZOU Jing, DOU Junfeng, et al. Adsorption properties of potassium modified montmorillonite magnetic microspheres for cesium[J]. Environmental Chemistry, 2021, 40(3): 779-789. | |
| 113 | CHOI J W, LEE H K, CHOI S J. Magnetite double-network composite using hydroxyapatite-manganese dioxide for Sr2+ removal from aqueous solutions[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105360. |
| 114 | LEE H K, CHOI J W, CHOI S J. Magnetic ion-imprinted polymer based on mesoporous silica for selective removal of Co(Ⅱ) from radioactive wastewater[J]. Separation Science and Technology, 2021, 56(11): 1842-1852. |
| 115 | MAJIDNIA Zohreh, IDRIS Ani. Evaluation of cesium removal from radioactive waste water using maghemite PVA-alginate beads[J]. Chemical Engineering Journal, 2015, 262: 372-382. |
| 116 | BOUKHALFA N, DARDER M, BOUTAHALA M, et al. Composite nanoarchitectonics: Alginate beads encapsulating sepiolite/magnetite/prussian blue for removal of cesium ions from water[J]. Bulletin of the Chemical Society of Japan, 2021, 94(1): 122-132. |
| 117 | PARK B, GHOREISHIAN S M, KIM Y, et al. Dual-functional micro-adsorbents: Application for simultaneous adsorption of cesium and strontium[J]. Chemosphere, 2021, 263: 128266. |
| 118 | ZHANG Kun, LI Hailong, LI Zhanguo, et al. Molecular dynamics and density functional theory simulations of cesium and strontium adsorption on illite/smectite[J]. Journal of Radioanalytical and Nuclear Chemistry, 2022, 331(7): 2983-2992. |
| 119 | ZENG Jianping, ZHANG Yan, CHEN Yuhang, et al. Molecular dynamics simulation of the adsorption properties of graphene oxide/graphene composite for alkali metal ions[J]. Journal of Molecular Graphics and Modelling, 2022, 114: 108184. |
| 120 | KHANMOHAMMADI Hossein, BAYATI Behrouz, Javad RAHBAR-SHAHROUZI, et al. Molecular simulation of the ion exchange behavior of Cu2+, Cd2+ and Pb2+ ions on different zeolites exchanged with sodium[J]. Journal of Environmental Chemical Engineering, 2019, 7(3): 103040. |
| 1 | MURDOCK C E. Public health in a radioactive age: Environmental pollution, popular therapies, and narratives of danger in the federal republic of Germany, 1949—1970[J]. Central European History, 2019, 52(1): 45-64. |
| 2 | CAO Yiyao, ZHOU Lei, REN Hong, et al. Determination, separation and application of 137Cs: A review[J]. International Journal of Environmental Research and Public Health, 2022, 19(16): 10183. |
| 3 | OTHMAN Zaki. Performance improvement of a radioactive forced circulation evaporator system[J]. Arab Journal of Nuclear Sciences and Applications, 2016, 49(1): 76-86. |
| 4 | BENGIAT Ravell, BOGOSLAVSKY Benny, MANDLER Daniel, et al. Selective binding and precipitation of cesium ions from aqueous solutions: A size-driven supramolecular reaction[J]. Chemistry—A European Journal, 2018, 24(13): 3161-3164. |
| 5 | COMBERNOUX Nicolas, SCHRIVE Luc, LABED Véronique, et al. Treatment of radioactive liquid effluents by reverse osmosis membranes: From lab-scale to pilot-scale[J]. Water Research, 2017, 123: 311-320. |
| 6 | BANERJEE D, SANDHYA U, SUMIT P, et al. Removal of 137Cs and 90Sr from low-level radioactive effluents by hexacyanoferrate loaded synthetic 4A type zeolite[J]. Journal of Radioanalytical and Nuclear Chemistry, 2017, 311(1): 893-902. |
| 7 | FISKUM S K, PEASE L F, PETERSON R A. Review of ion exchange technologies for cesium removal from caustic tank waste[J]. Solvent Extraction and Ion Exchange, 2020, 38(6): 573-611. |
| 8 | HIMANSHU Patel. Fixed-bed column adsorption study: A comprehensive review[J]. Applied Water Science, 2019, 9(3): 45. |
| 9 | LEHTO Jukka, KOIVULA Risto, LEINONEN Heikki, et al. Removal of radionuclides from fukushima daiichi waste effluents[J]. Separation & Purification Reviews, 2019, 48(2): 122-142. |
| 10 | LI Xindai, XU Guangming, XIA Meng, et al. Research on the remediation of cesium pollution by adsorption: Insights from bibliometric analysis[J]. Chemosphere, 2022, 308: 136445. |
| 11 | ZHANG Xiaoyuan, LIU Yu. Nanomaterials for radioactive wastewater decontamination[J]. Environmental Science: Nano, 2020, 7(4): 1008-1040. |
| 12 | 张丽莹, 李晓静, 曾进忠, 等. 华龙一号活化腐蚀产物沉积源项评估[J]. 辐射防护, 2019, 39(3): 192-197. |
| ZHANG Liying, LI Xiaojing, ZENG Jinzhong, et al. Assessment of deposit source term of activated corrosion products for HPR1000 nuclear power plants[J]. Radiation Protection, 2019, 39(3): 192-197. | |
| 13 | SIROUX Brice, WISSOCQ Aubéry, BEAUCAIRE Catherine, et al. Adsorption of strontium and caesium onto an Na-illite and Na-illite/Na-smectite mixtures: Implementation and application of a multi-site ion-exchange model[J]. Applied Geochemistry, 2018, 99: 65-74. |
| 14 | PARK S M, ALESSI D S, BAEK K. Selective adsorption and irreversible fixation behavior of cesium onto 2∶1 layered clay mineral: A mini review[J]. Journal of Hazardous Materials, 2019, 369: 569-576. |
| 15 | 马鸿宾, 魏新渝, 熊小伟, 等. 离子交换技术去除核电厂放射性废液中痕量核素研究进展[J]. 水处理技术, 2016, 42(1): 7-11, 19. |
| MA Hongbin, WEI Xinyu, XIONG Xiaowei, et al. Research progress of application of ion exchange technology on the removal of trace radionuclides from liquid radioactive waste innuclear power plant[J]. Technology of Water Treatment, 2016, 42(1): 7-11, 19. | |
| 16 | 李富海, 梁维江, 方军, 等. 模拟Co胶体在压水堆停堆氧化运行期间的溶解行为研究[J]. 原子能科学技术, 2022, 56(10): 1996-2003. |
| LI Fuhai, LIANG Weijiang, FANG Jun, et al. Dissolution behavior of simulated Co colloid in oxidation operation process during shutdown of PWRs[J]. Atomic Energy Science and Technology, 2022, 56(10): 1996-2003. | |
| 17 | SONWALKAR V M, MOHANTA S, PAL S K, et al. Identification of the contributors (Ag-110m) for higher radiation field on primary heat transport system of Tarapur Atomic Power Station-3 and its impact on collective dose[J]. Radiation Protection and Environment, 2018, 41(2): 66. |
| 18 | 刘昱, 刘佩, 张明乾. 压水堆核电站废液处理系统的比较[J]. 辐射防护, 2010, 30(1): 42-47. |
| LIU Yu, LIU Pei, ZHANG Mingqian. Comparison of liquid radwaste treatment systems at pressurized water reactor nuclear power plant[J]. Radiation Protection, 2010, 30(1): 42-47. | |
| 19 | 魏新渝, 马鸿宾, 熊小伟, 等. 反渗透技术去除核电厂放射性废液中痕量核素的研究进展[J]. 水处理技术, 2015, 41(12): 10-14, 19. |
| WEI Xinyu, MA Hongbin, XIONG Xiaowei, et al. Research progress of application of RO technology in the removal of trace radionuclides from radioactive liquid waste of nuclear power plant[J]. Technology of Water Treatment, 2015, 41(12): 10-14, 19. | |
| 20 | CHEN Zongyuan, WANG Siyuan, HOU Huijuan, et al. China's progress in radionuclide migration study over the past decade (2010—2021): Sorption, transport and radioactive colloid[J]. Chinese Chemical Letters, 2022, 33(7): 3405-3412. |
| 21 | KIM K W, BAEK Y J, LEE K Y, et al. Treatment of radioactive waste seawater by coagulation-flocculation method using ferric hydroxide and poly acrylamide[J]. Journal of Nuclear Science and Technology, 2016, 53(3): 439-450. |
| 22 | KIM K W, SHON W J, OH M K, et al. Evaluation of dynamic behavior of coagulation-flocculation using hydrous ferric oxide for removal of radioactive nuclides in wastewater[J]. Nuclear Engineering and Technology, 2019, 51(3): 738-745. |
| 23 | XU Yao, GU Ping, ZHANG Guanghui, et al. Investigation of coagulation as a pretreatment for microfiltration in cesium removal by copper ferrocyanide adsorption[J]. Journal of Radioanalytical and Nuclear Chemistry, 2017, 313(2): 435-444. |
| 24 | ZHANG Mingdong, GU Ping, YAN Su, et al. Effective removal of radioactive cobalt from aqueous solution by a layered metal sulfide adsorbent: Mechanism, adsorption performance, and practical application[J]. Separation and Purification Technology, 2021, 256: 117775. |
| 25 | 李元, 林建中, 汤东升, 等. 沉淀絮凝—吸附处理放射性废水的模拟实验[J]. 南方能源建设, 2015, 2(4): 81-87. |
| LI Yuan, LIN Jianzhong, TANG Dongsheng, et al. Flocculation and adsorption experiment for treatment of simulated radioactive wastewater[J]. Southern Energy Construction, 2015, 2(4): 81-87. | |
| 26 | 李俊雄, 顾健, 王晓伟, 等. 内陆AP1000核电厂放射性废液处理系统设计改进[J]. 电力建设, 2014, 35(4): 96-100. |
| LI Junxiong, GU Jian, WANG Xiaowei, et al. Design improvement of radioactive waste liquid processing system in inland AP1000 nuclear power plant[J]. Electric Power Construction, 2014, 35(4): 96-100. | |
| 27 | CHUA Siewfen, NOURI Alireza, Weilun ANG, et al. The emergence of multifunctional adsorbents and their role in environmental remediation[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104793. |
| 28 | AHMAD W A, SHAHADAT M, WAZED A S, et al. Recent advances and future perspectives of polymer-based magnetic nanomaterials for detection and removal of radionuclides: A review [J]. Journal of Molecular Liquids, 2022, 365: 119976. |
| 29 | 陈思璠, 尉继英, 赵璇. 离子交换树脂去除模拟放射性废液中的铯[J]. 应用化学, 2019, 36(1): 41-50. |
| CHEN Sifan, WEI Jiying, ZHAO Xuan. Removal of cesium by ion exchange resins in simulated radioactive wastewater[J]. Chinese Journal of Applied Chemistry, 2019, 36(1): 41-50. | |
| 30 | WANG Jianlong, ZHUANG Shuting. Removal of cesium ions from aqueous solutions using various separation technologies[J]. Reviews in Environmental Science and Bio/Technology, 2019, 18(2): 231-269. |
| 31 | PUTRA D I, OCHIAI S, TOMIHARA S, et al. Determination of low level 137Cs in environmental water sample using AMP method and a review comparing with other adsorbents[J]. Journal of Hunan University Natural Sciences, 2021, 48(6). |
| 32 | LI Juexuan, ZAN Yongxi, ZHANG Zhengping, et al. Prussian blue nanocubes decorated on nitrogen-doped hierarchically porous carbon network for efficient sorption of radioactive cesium[J]. Journal of Hazardous Materials, 2020, 385: 121568. |
| 33 | JIN Wanqin, TOUTIANOUSH Ali, PYRASCH Mario, et al. Self-assembled films of Prussian blue and analogues: Structure and morphology, elemental composition, film growth, and nanosieving of ions[J]. The Journal of Physical Chemistry B, 2003, 107(44): 12062-12070. |
| 34 | WU Xinyue, RU Yue, BAI Yang, et al. PBA composites and their derivatives in energy and environmental applications[J]. Coordination Chemistry Reviews, 2022, 451: 214260. |
| 35 | YAO Chuqing, DAI Yaodong, CHANG Shuquan, et al. Removal of cesium and strontium for radioactive wastewater by Prussian blue nanorods[J]. Environmental Science and Pollution Research, 2023, 30(13): 36807-36823. |
| 36 | BALASOORIYA I L, CHEN J, KORALE G S, et al. Applications of nano hydroxyapatite as adsorbents: A review [J]. Nanomaterials, 2022, 12(14): 2324. |
| 37 | METWALLY S S, AHMED I M, RIZK H E. Modification of hydroxyapatite for removal of cesium and strontium ions from aqueous solution[J]. Journal of Alloys and Compounds, 2017, 709: 438-444. |
| 38 | SIHN Youngho, YANG Hee-Man, PARK Chan Woo, et al. Post-substitution of magnesium at CaI of nano-hydroxyapatite surface for highly efficient and selective removal of radioactive 90Sr from groundwater[J]. Chemosphere, 2022, 295: 133874. |
| 39 | HANDLEY-SIDHU S, MULLAN T K, GRAIL Q, et al. Influence of pH, competing ions and salinity on the sorption of strontium and cobalt onto biogenic hydroxyapatite[J]. Scientific Reports, 2016, 6: 23361. |
| 40 | TAN Liqiang, WANG Song, DU Weigang, et al. Effect of water chemistries on adsorption of Cs(I) onto graphene oxide investigated by batch and modeling techniques[J]. Chemical Engineering Journal, 2016, 292: 92-97. |
| 41 | SIROUX Brice, BEAUCAIRE Catherine, TABARANT Michel, et al. Adsorption of strontium and caesium onto an Na-MX80 bentonite: Experiments and building of a coherent thermodynamic modelling[J]. Applied Geochemistry, 2017, 87: 167-175. |
| 42 | CLAVERIE Marie, GARCIA Just, PREVOST Thierry, et al. Inorganic and hybrid (organic-inorganic) lamellar materials for heavy metals and radionuclides capture in energy wastes management—A review[J]. Materials, 2019, 12(9): 1399. |
| 43 | YANG Shubin, OKADA Naoya, NAGATSU Masaaki. The highly effective removal of Cs+ by low turbidity chitosan-grafted magnetic bentonite[J]. Journal of Hazardous Materials, 2016, 301: 8-16. |
| 44 | OKUMURA Masahiko, NAKAMURA Hiroki, MACHIDA Masahiko. Mechanism of strong affinity of clay minerals to radioactive cesium: First-principles calculation study for adsorption of cesium at frayed edge sites in muscovite[J]. Journal of the Physical Society of Japan, 2013, 82(3): 0338002. |
| 45 | PARK S M, LEE J, JEON E K, et al. Adsorption characteristics of cesium on the clay minerals: Structural change under wetting and drying condition[J]. Geoderma, 2019, 340: 49-54. |
| 46 | FANG Xianghong, FANG Fang, LU Chunhai, et al. Removal of Cs+, Sr2+, and Co2+ ions from the mixture of organics and suspended solids aqueous solutions by zeolites[J]. Nuclear Engineering and Technology, 2017, 49(3): 556-561. |
| 47 | OSMANLIOGLU A E. Treatment of radioactive liquid waste by sorption on natural zeolite in Turkey[J]. Journal of Hazardous Materials, 2006, 137(1): 332-335. |
| 48 | ALBY Delhia, CHARNAY Clarence, HERAN Marc, et al. Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: Synthesis and shaping, sorption capacity, mechanisms, and selectivity—A review[J]. Journal of Hazardous Materials, 2018, 344: 511-530. |
| 49 | YANG H M, JEON H, LEE Y, et al. Sulfur-modified zeolite A as a low-cost strontium remover with improved selectivity for radioactive strontium[J]. Chemosphere, 2022, 299: 134309. |
| 50 | ZHENG Wei, FENG Sheng, FENG Shanshan, et al. A novel S-doped PB/GO nanocomposite for efficient adsorption and removal of cesium ions[J]. Journal of Radioanalytical and Nuclear Chemistry, 2020, 326(2): 879-891. |
| 51 | XING Min, ZHUANG Shuting, WANG Jianlong. Adsorptive removal of strontium ions from aqueous solution by graphene oxide[J]. Environmental Science and Pollution Research, 2019, 26(29): 29669-29678. |
| 52 | FANG Fang, KONG Lingtao, HUANG Jiarui, et al. Removal of cobalt ions from aqueous solution by an amination graphene oxide nanocomposite[J]. Journal of Hazardous Materials, 2014, 270: 1-10. |
| 53 | 曹林园, 王辉, 张鹏, 等. 功能化石墨烯吸附模拟反应堆冷却剂中银的研究[J]. 原子能科学技术, 2020, 54(4): 663-670. |
| CAO Linyuan, WANG Hui, ZHANG Peng, et al. Adsorption behavior of silver on functional graphene in simulated reactor coolant[J]. Atomic Energy Science and Technology, 2020, 54(4): 663-670. | |
| 54 | XING Min, WANG Jianlong. Nanoscaled zero valent iron/graphene composite as an efficient adsorbent for Co(Ⅱ) removal from aqueous solution[J]. Journal of Colloid and Interface Science, 2016, 474: 119-128. |
| 55 | WEN Tao, WU Xilin, LIU Mancheng, et al. Efficient capture of strontium from aqueous solutions using graphene oxide-hydroxyapatite nanocomposites[J]. Dalton Transactions, 2014, 43(20): 7464-7472. |
| 56 | BOLISETTY Sreenath, MEZZENGA Raffaele. Amyloid-carbon hybrid membranes for universal water purification[J]. Nature Nanotechnology, 2016, 11(4): 365-371. |
| 57 | KWAK C H, LIM C, KIM S, et al. Surface modification of carbon materials and its application as adsorbents[J]. Journal of Industrial and Engineering Chemistry, 2022, 116: 21-31. |
| 58 | 李琦, 苟全录, 余小东. 海阳核电厂(AP1000机组)放射性废物管理系统建设探讨[J]. 辐射防护, 2018, 38(1): 80-87. |
| LI Qi, GOU Quanlu, YU Xiaodong. Discussion on construction of a radwaste management system in Haiyang Nuclear Power Plant (AP1000)[J]. Radiation Protection, 2018, 38(1): 80-87. | |
| 59 | HO K, PARK D, PARK M K, et al. Adsorption mechanism of methyl iodide by triethylenediamine and quinuclidine-impregnated activated carbons at extremely low pressures[J]. Chemical Engineering Journal, 2020, 396: 125215. |
| 60 | ALEXEY Makarov, ALEXEY Safonov, ANASTASIIA Sitanskaia, et al. Clay and carbon materials-based engineered barriers for technetium immobilization[J]. Progress in Nuclear Energy, 2022, 152: 104398. |
| 61 | HARO-DEL R D A, AL-JOUBORI S, KONTOGIANNIS O, et al. The removal of caesium ions using supported clinoptilolite[J]. Journal of Hazardous Materials, 2015, 289: 1-8. |
| 62 | DONG Zhimin, LI Zifan, ZENG Dongling, et al. Highly selective adsorption of radioactive cesium by novel calix[4] biscrown-6 functionalized millimetre-sized hierarchically porous carbon spheres[J]. Separation and Purification Technology, 2023, 304: 122255. |
| 63 | BALLOVA S, PIPÍŠKA M, FRIŠTÁK V, et al. Pyrogenic carbon for decontamination of low-level radioactive effluents: Simultaneous separation of 137Cs and 60Co[J]. Progress in Nuclear Energy, 2020, 129: 103484. |
| 64 | DASHTINEJAD Maryam, SAMADFAM Mohammad, FASIHI Javad, et al. Synthesis, characterization, and cesium sorption performance of potassium nickel hexacyanoferrate-loaded granular activated carbon[J]. Particulate Science and Technology, 2014, 32(4): 348-354. |
| 65 | VANDERHEYDEN S R, YPERMAN J, CARLEER R, et al. Enhanced cesium removal from real matrices by nickel-hexacyanoferrate modified activated carbons[J]. Chemosphere, 2018, 202: 569-575. |
| 66 | Daemin OH, KIM Bokseong, KANG Sungwon, et al. Enhanced immobilization of Prussian blue through hydrogel formation by polymerization of acrylic acid for radioactive cesium adsorption[J]. Scientific Reports, 2019, 9: 16334. |
| 67 | SEO Younggyo, HWANG Yuhoon. Prussian blue immobilized on covalent organic polymer-grafted granular activated carbon for cesium adsorption from water[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 105950. |
| 68 | AL-JUBOURI S M, CURRY N A, HOLMES S M. Hierarchical porous structured zeolite composite for removal of ionic contaminants from waste streams and effective encapsulation of hazardous waste[J]. Journal of Hazardous Materials, 2016, 320: 241-251. |
| 69 | HUANG Tao, CAO Zhenxing, JIN Junxun, et al. Hydroxyapatite nanoparticle functionalized activated carbon particle electrode that removes strontium from spiked soils in a unipolar three-dimensional electrokinetic system[J]. Journal of Environmental Management, 2021, 280: 111697. |
| 70 | LIU Xiaojing, WANG Jianlong. Electro-assisted adsorption of Cs(Ⅰ) and Co(Ⅱ) from aqueous solution by capacitive deionization with activated carbon cloth/graphene oxide composite electrode[J]. Science of The Total Environment, 2020, 749: 141524. |
| 71 | ALAMUDY H A, CHO K. Selective adsorption of cesium from an aqueous solution by a montmorillonite-prussian blue hybrid[J]. Chemical Engineering Journal, 2018, 349: 595-602. |
| 72 | MARTIN Pipíška, Florková EVA, PETER Nemeček, et al. Evaluation of Co and Zn competitive sorption by zeolitic material synthesized from fly ash using 60Co and 65Zn as radioindicators[J]. Journal of Radioanalytical and Nuclear Chemistry, 2019, 319(3): 855-867. |
| 73 | NAYL A A, AHMED I M, ABD-ELHAMID A I, et al. Selective sorption of 134Cs and 60Co radioisotopes using synthetic nanocopper ferrocyanide-SiO2 materials[J]. Separation and Purification Technology, 2020, 234: 116060. |
| 74 | WU Yan, ZHANG Xiaoxia, WEI Yuezhou, et al. Development of adsorption and solidification process for decontamination of Cs-contaminated radioactive water in Fukushima through silica-based AMP hybrid adsorbent[J]. Separation and Purification Technology, 2017, 181: 76-84. |
| 75 | INGALE S V, RAMU R, SASTRY P U, et al. Synthesis and characterization of ammonium molybdophosphate-silica nano-composite (AMP-SiO2) as a prospective sorbent for the separation of 137Cs from nuclear waste[J]. Journal of Radioanalytical and Nuclear Chemistry, 2014, 301(2): 409-415. |
| 76 | YOSHIDA Seiichiro, IWAMURA Shinichiroh, OGINO Isao, et al. Continuous-flow separation of cesium ion by ammonium molybdophosphate immobilized in a silica microhoneycomb (AMP-SMH)[J]. Adsorption, 2019, 25(6): 1089-1098. |
| 77 | SOLANGE S, ADRIANA C G, MÉLODIE T, et al. Nanoporous ammonium molybdophosphate-silica hybrids as regenerable ultra-selective extraction agents for radiocesium monitoring[J]. New Journal of Chemistry, 2013, 37(12): 3877-3880. |
| 78 | HUNT R D, COLLINS J L, ADU-WUSU K, et al. Monosodium titanate in hydrous titanium oxide spheres for the removal of strontium and key actinides from salt solutions at the savannah river site[J]. Separation Science and Technology, 2005, 40(14): 2933-2946. |
| 79 | YANG Dongjiang, LIU Hongwei, ZHENG Zhanfeng, et al. Titanate-based adsorbents for radioactive ions entrapment from water[J]. Nanoscale, 2013, 5(6): 2232-2242. |
| 80 | LIN Zhi, FERDOV Stanislav. Temperature and time controlled crystallization in Na2O-SiO2-TiO2-H2O system[J]. Microporous and Mesoporous Materials, 2022, 335: 111835. |
| 81 | YANG H M, PARK C W, KIM I, et al. Hollow flower-like titanium ferrocyanide structure for the highly efficient removal of radioactive cesium from water[J]. Chemical Engineering Journal, 2020, 392: 123713. |
| 82 | YUSAN Sabriye, ERENTURK Sema. Adsorption characterization of strontium on PAN/zeolite composite adsorbent[J]. World Journal of Nuclear Science and Technology, 2011, 1(1): 6-12. |
| 83 | FAGHIHIAN Hossein, IRAVANI Mozhgan, MOAYED Mohammad, et al. Preparation of a novel PAN-zeolite nanocomposite for removal of Cs+ and Sr2+ from aqueous solutions: Kinetic, equilibrium, and thermodynamic studies[J]. Chemical Engineering Journal, 2013, 222: 41-48. |
| 84 | 游新锋, 李腾, 牟凌, 等. 球形亚铁氰化镍钾聚丙烯腈吸附剂的制备及应用[J]. 核化学与放射化学, 2021, 43(1): 91-98. |
| YOU Xinfeng, LI Teng, MOU Ling, et al. Preparation and application of spherical ferrocyanide nickel potassium polyacrylonitrile composite adsorbent[J].Journal of Nuclear and Radiochemistry, 2021, 43(1): 91-98. | |
| 85 | Süleyman İNAN, Yüksel ALTAŞ. Preparation of zirconium-manganese oxide/polyacrylonitrile (Zr-Mn oxide/PAN) composite spheres and the investigation of Sr(Ⅱ) sorption by experimental design[J]. Chemical Engineering Journal, 2011, 168(3): 1263-1271. |
| 86 | PARK Y, LEE Y C, SHIN W S, et al. Removal of cobalt, strontium and cesium from radioactive laundry wastewater by ammonium molybdophosphate-polyacrylonitrile (AMP-PAN)[J]. Chemical Engineering Journal, 2010, 162(2): 685-695. |
| 87 | DING Dahu, ZHANG Zhenya, CHEN Rongzhi, et al. Selective removal of cesium by ammonium molybdophosphate-polyacrylonitrile bead and membrane[J]. Journal of Hazardous Materials, 2017, 324: 753-761. |
| 88 | LIU Qi, GE Haojie, LIU Can, et al. Highly selective and easily regenerated novel porous polyacrylonitrile-ammonium phosphomolybdate beads for cesium removal from geothermal water[J]. Journal of Water Process Engineering, 2022: 103339. |
| 89 | TRANTER T J, HERBST R S, TODD T A, et al. Evaluation of ammonium molybdophosphate-polyacrylonitrile (AMP-PAN) as a cesium selective sorbent for the removal of 137Cs from acidic nuclear waste solutions[J]. Advances in Environmental Research, 2002, 6(2): 107-121. |
| 90 | 王晓伟, 梁成强, 杜志辉, 等. 聚丙烯腈基钛硅酸钠复合材料对废水中低浓度Cs+选择性吸附性能研究[J]. 海军工程大学学报, 2020, 32(4): 1-5, 21. |
| WANG Xiaowei, LIANG Chengqiang, DU Zhihui, et al. Selective adsorption of low concentration Cs+ by polyacrylonitrile-based titanium silicate sodium composites[J]. Journal of Naval University of Engineering, 2020, 32(4): 1-5, 21. | |
| 91 | EL-NAGGAR M R, EL-SHERIF E A, MAREE R M, et al. Batch and fixed bed column investigations of the sorptive removal of cesium ions from aqueous solutions using modified graphene-alginate nanocompositebeads[J]. Journal of Radiation Research and Applied Sciences, 2021, 14(1): 146-158. |
| 92 | XIA Meng, ZHENG Xianming, DU Mingyang, et al. The adsorption of Cs+ from wastewater using lithium-modified montmorillonite caged in calcium alginate beads[J]. Chemosphere, 2018, 203: 271-280. |
| 93 | YE Xiushen, WU Zhijian, LI Wu, et al. Rubidium and cesium ion adsorption by an ammonium molybdophosphate-calcium alginate composite adsorbent[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2009, 342(1/2/3): 76-83. |
| 94 | MIMURA Hitoshi, ONODERA Yoshio. Selective uptake and recovery of cesium ions by composite columns of ammonium molybdophosphate (AMP)-calcium alginate[J]. Journal of Nuclear Science and Technology, 2002, 39(3): 282-285. |
| 95 | ZHANG Yahui, LIN Xiaoyan, HU Shuhong, et al. Core-shell zeolite@Alg-Ca particles for removal of strontium from aqueous solutions[J]. RSC Advances, 2016, 6(78): 73959-73973. |
| 96 | LAI Yuchen, CHANG Yinru, CHEN Manli, et al. Poly(vinyl alcohol) and alginate cross-linked matrix with immobilized Prussian blue and ion exchange resin for cesium removal from waters[J]. Bioresource Technology, 2016, 214: 192-198. |
| 97 | DWIVEDI C, KUMAR A, JUBY K A, et al. Preparation and evaluation of alginate-assisted spherical resorcinol-formaldehyde resin beads for removal of cesium from alkaline waste[J]. Chemical Engineering Journal, 2012, 200/201/202: 491-498. |
| 98 | HONG H J, KIM B G, RYU J, et al. Preparation of highly stable zeolite-alginate foam composite for strontium(90Sr) removal from seawater and evaluation of Sr adsorption performance[J]. Journal of Environmental Management, 2018, 205: 192-200. |
| 99 | LI Qiang, SU Haijia, TAN Tianwei. Synthesis of ion-imprinted chitosan-TiO2 adsorbent and its multi-functional performances[J]. Biochemical Engineering Journal, 2008, 38(2): 212-218. |
| 100 | CHEN Yuwei, WANG Jianlong. Removal of cesium from radioactive wastewater using magnetic chitosan beads cross-linked with glutaraldehyde[J]. Nuclear Science and Techniques, 2016, 27(2): 43. |
| 101 | 刘法邦, 贾铭椿, 门金凤, 等. 钴/锰印迹半胱氨酸-壳聚糖对低浓度Mn2+和Co2+的选择性吸附[J]. 原子能科学技术, 2015, 49(6): 984-991. |
| LIU Fabang, JIA Mingchun, Jinfeng MEN, et al. Selective adsorption of Mn2+ and Co2+ in dilute solution by cobalt/manganese imprinted cysteine-chitosan[J]. Atomic Energy Science and Technology, 2015, 49(6): 984-991. | |
| 102 | GOYAL Nitin, GAO Peng, WANG Zhe, et al. Nanostructured chitosan/molecular sieve-4A an emergent material for the synergistic adsorption of radioactive major pollutants cesium and strontium[J]. Journal of Hazardous Materials, 2020, 392: 122494. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [4] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| [5] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [6] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [7] | 于志庆, 黄文斌, 王晓晗, 邓开鑫, 魏强, 周亚松, 姜鹏. B掺杂Al2O3@C负载CoMo型加氢脱硫催化剂性能[J]. 化工进展, 2023, 42(7): 3550-3560. |
| [8] | 单雪影, 张濛, 张家傅, 李玲玉, 宋艳, 李锦春. 阻燃型环氧树脂的燃烧数值模拟[J]. 化工进展, 2023, 42(7): 3413-3419. |
| [9] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [10] | 朱雅静, 徐岩, 简美鹏, 李海燕, 王崇臣. 金属有机框架材料用于海水提铀的研究进展[J]. 化工进展, 2023, 42(6): 3029-3048. |
| [11] | 许春树, 姚庆达, 梁永贤, 周华龙. 氧化石墨烯/碳纳米管对几种典型高分子材料的性能影响[J]. 化工进展, 2023, 42(6): 3012-3028. |
| [12] | 杨竞莹, 施万胜, 黄振兴, 谢利娟, 赵明星, 阮文权. 改性纳米零价铁材料制备的研究进展[J]. 化工进展, 2023, 42(6): 2975-2986. |
| [13] | 张宁, 吴海滨, 李钰, 李剑锋, 程芳琴. 漂浮型光催化材料的制备及其在水处理领域的应用研究进展[J]. 化工进展, 2023, 42(5): 2475-2485. |
| [14] | 陈飞, 刘成宝, 陈丰, 钱君超, 邱永斌, 孟宪荣, 陈志刚. g-C3N4基超级电容器用电极材料的研究进展[J]. 化工进展, 2023, 42(5): 2566-2576. |
| [15] | 刘念, 陈葵, 武斌, 纪利俊, 吴艳阳, 韩金玲. 蛋黄-壳介孔磁性炭微球的制备及其对红霉素的高效吸附[J]. 化工进展, 2023, 42(5): 2724-2732. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||