化工进展 ›› 2023, Vol. 42 ›› Issue (4): 1832-1846.DOI: 10.16085/j.issn.1000-6613.2022-1109
甲烷催化部分氧化制合成气催化剂的研究进展
- 1.广东佛燃科技有限公司,广东 佛山 528000
2.中国科学院广州能源研究所,广东 广州 510640
-
收稿日期:2022-06-13修回日期:2022-08-22出版日期:2023-04-25发布日期:2023-05-08 -
通讯作者:杨润农 -
作者简介:阮鹏(1980—),男,高级工程师,研究方向为新型燃气技术的应用。E-mail:ruanpeng@fsgas.com。 -
基金资助:佛山市社会领域科技攻关专项(2120001008444)
Advances in catalysts for catalytic partial oxidation of methane to syngas
RUAN Peng1( ), YANG Runnong1,2(
), YANG Runnong1,2( ), LIN Zirong1, SUN Yongming2
), LIN Zirong1, SUN Yongming2
- 1.Guangdong Foran Technology Company Limited, Foshan 528000, Guangdong, China
2.Guangzhou Institute of Energy Conversion, Chinese Academy of Science, Guangzhou 510640, Guangdong, China
-
Received:2022-06-13Revised:2022-08-22Online:2023-04-25Published:2023-05-08 -
Contact:YANG Runnong
摘要:
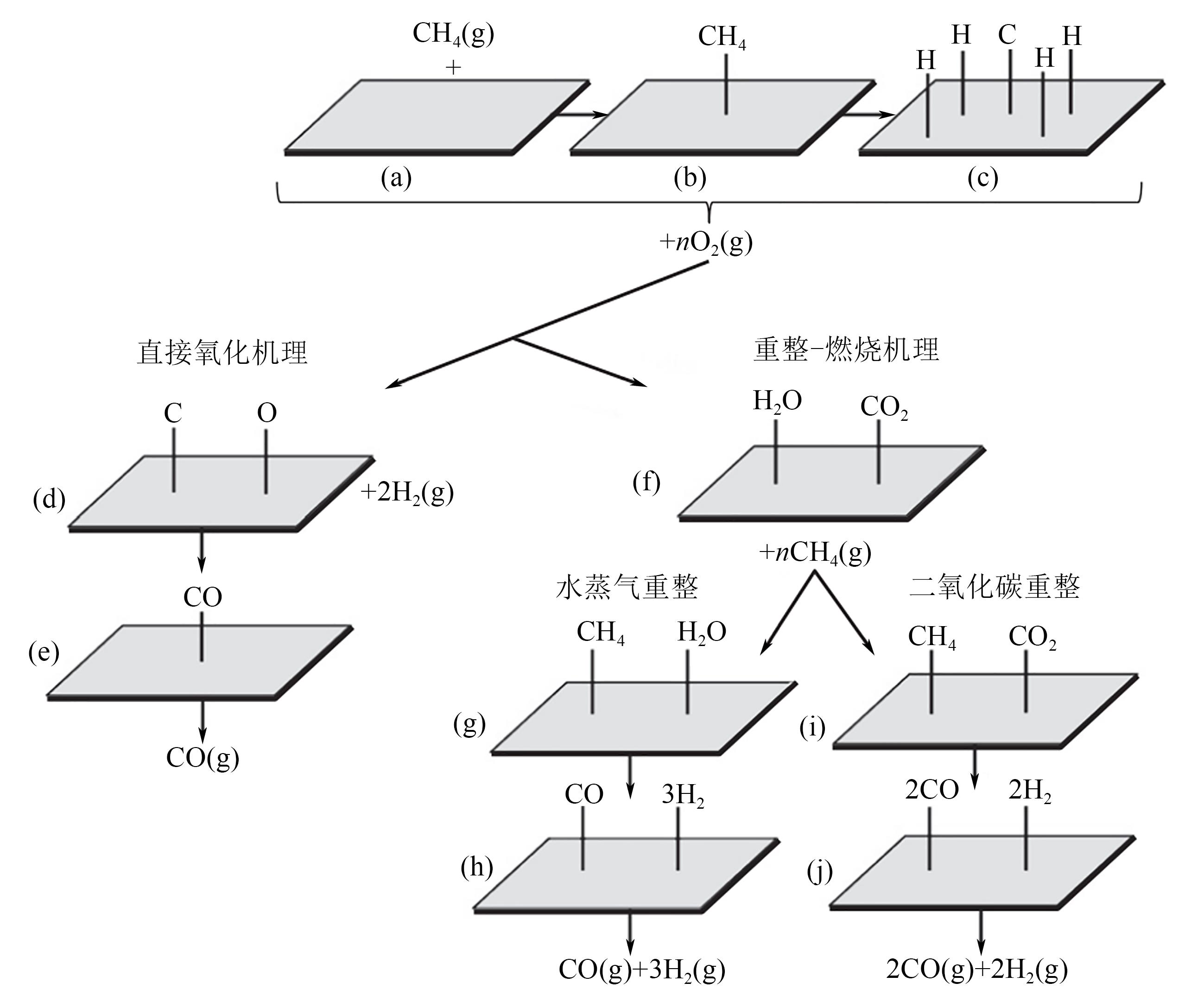
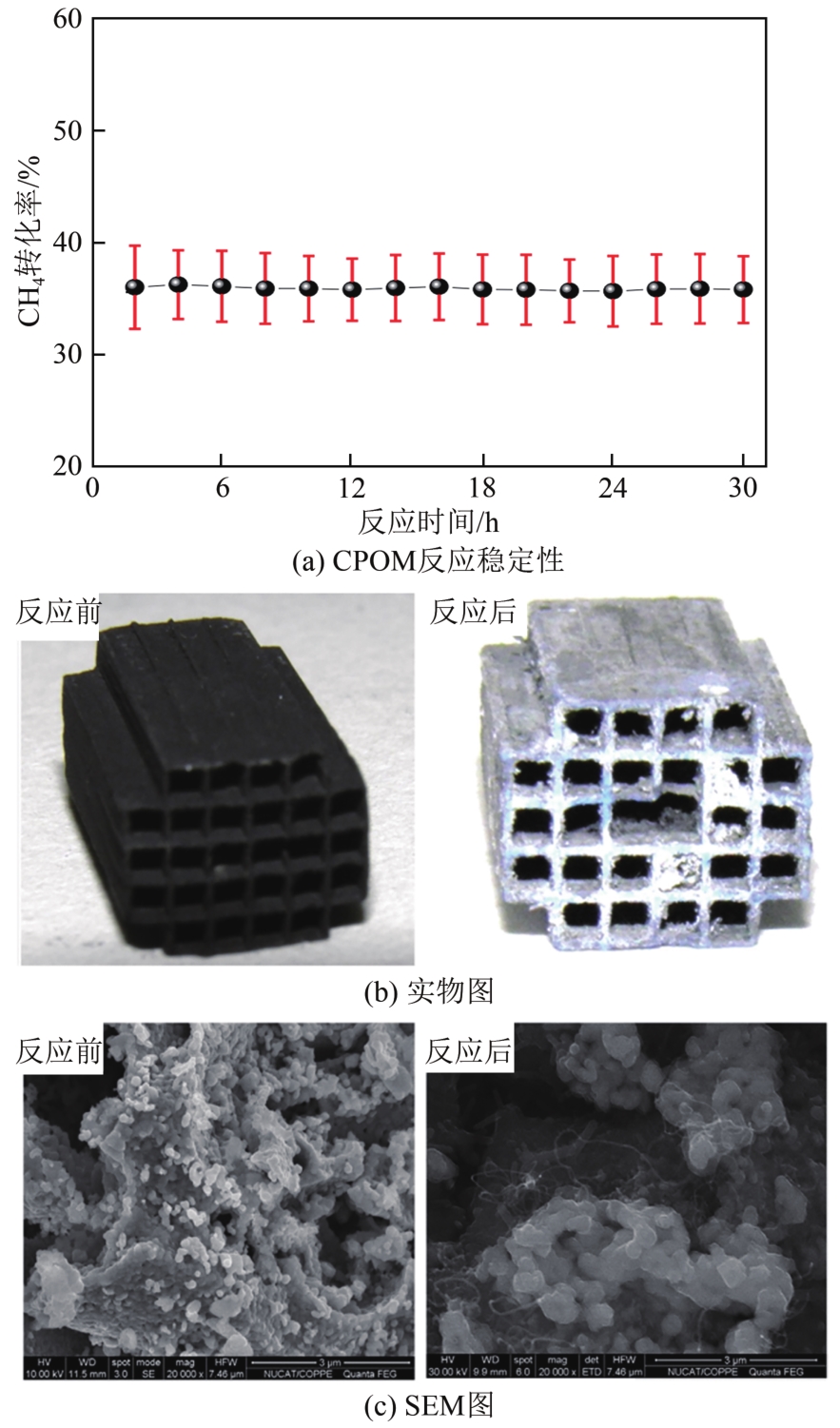
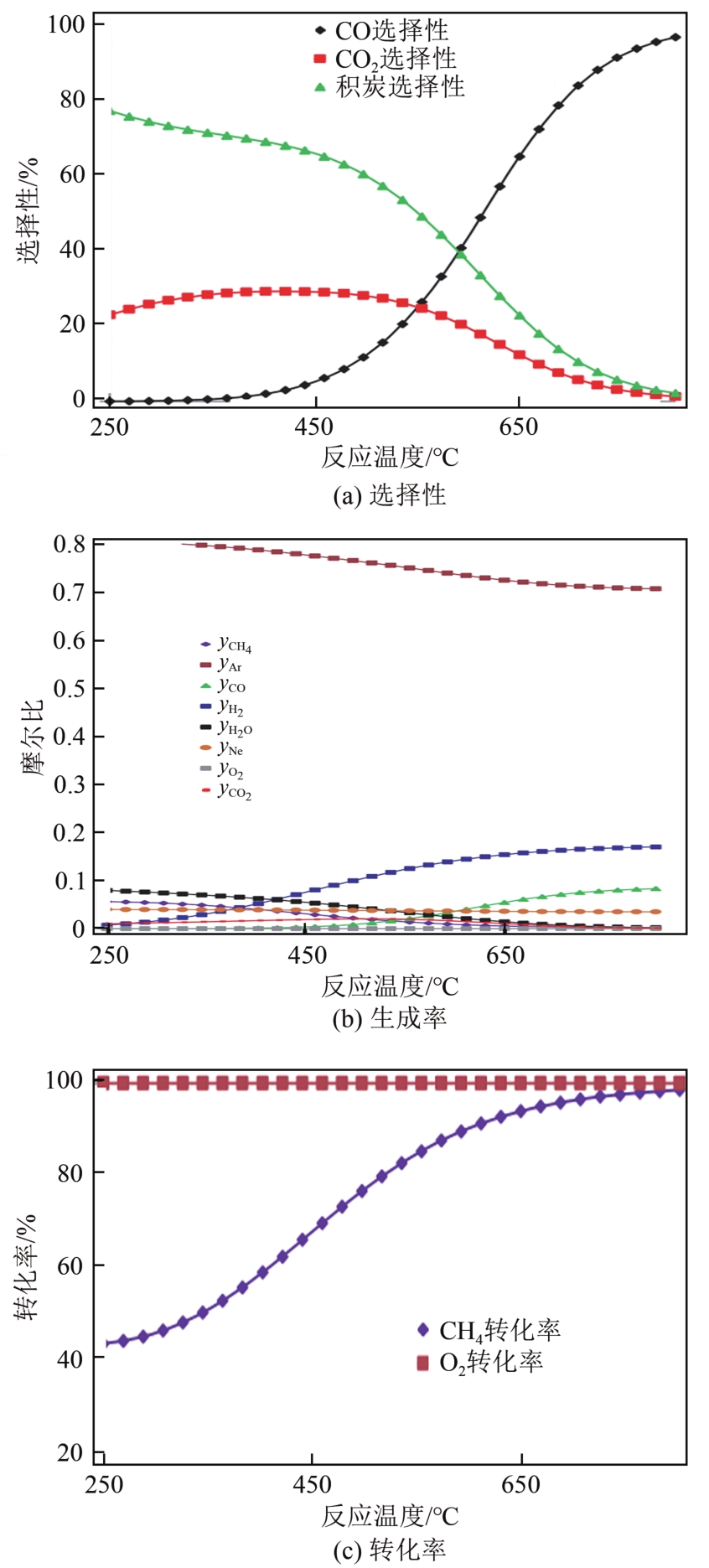
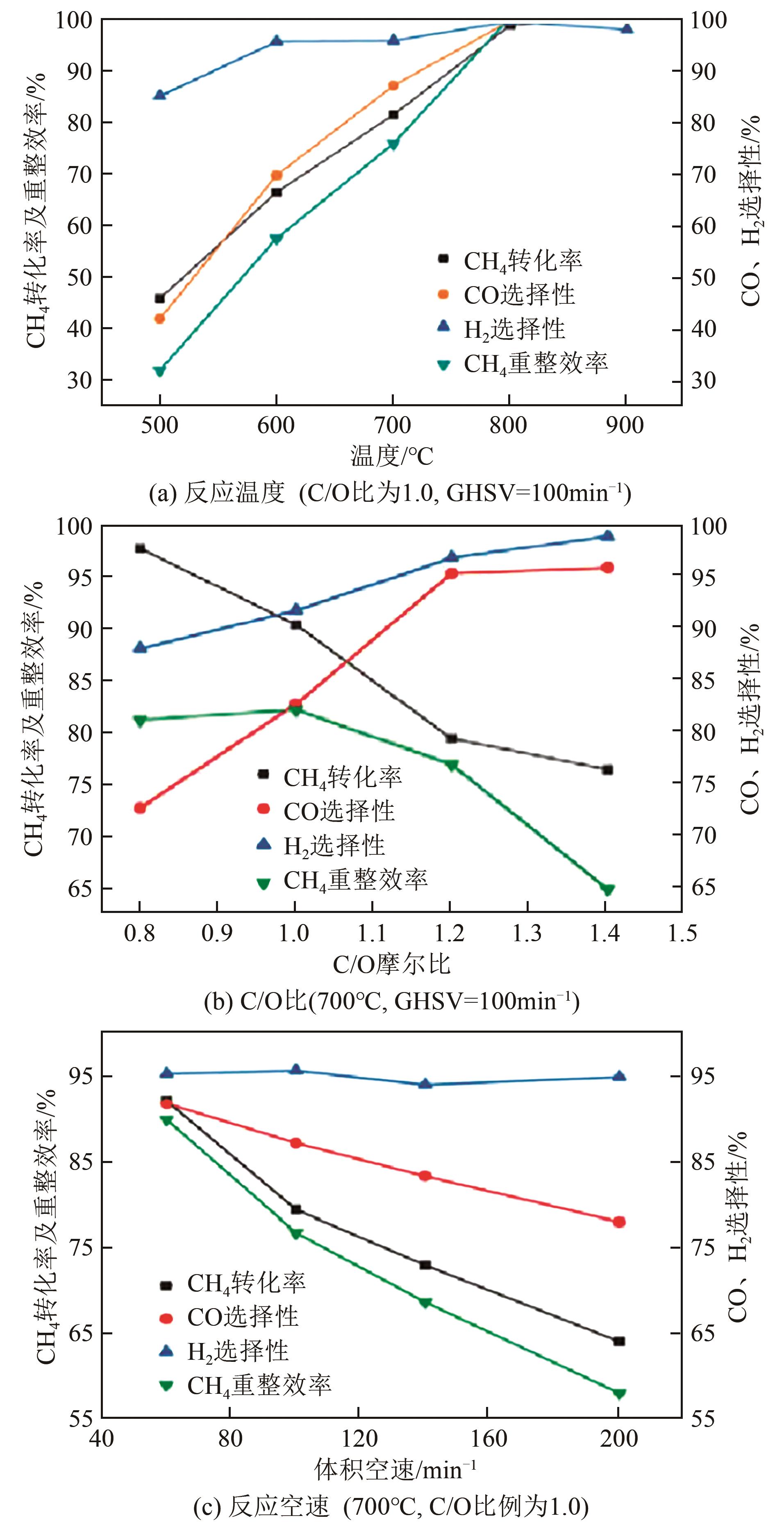
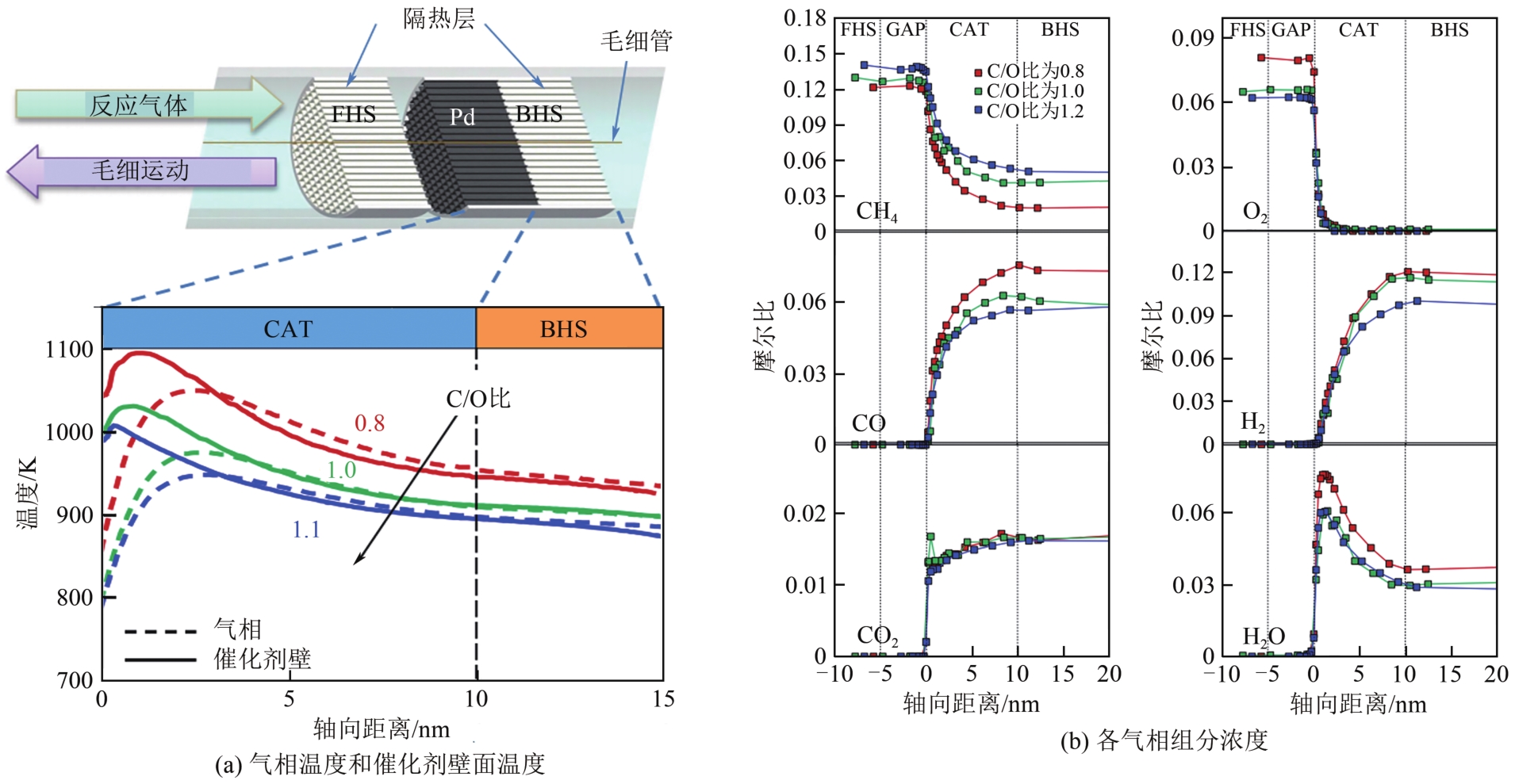
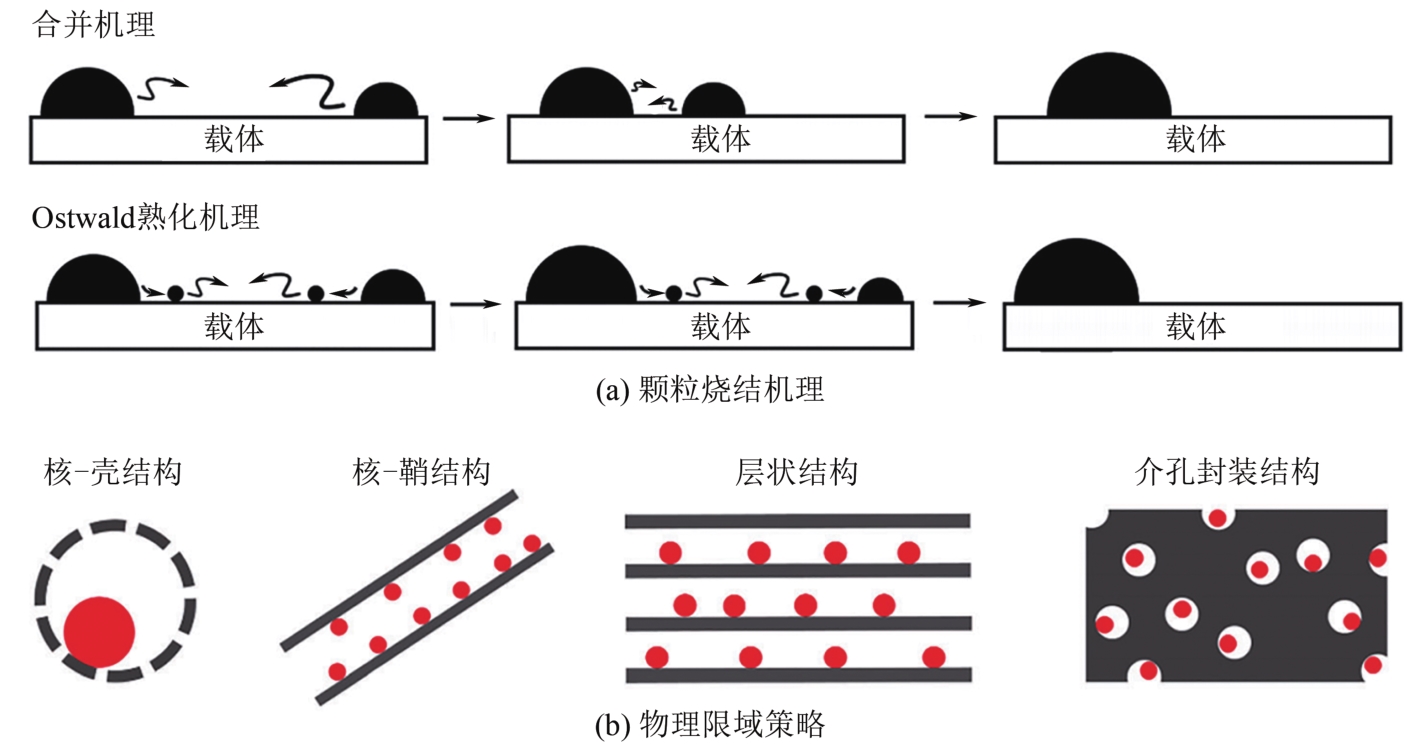
天然气是一种前景广阔的清洁燃料,甲烷作为天然气的主要成分,其高效利用具有重要的现实意义。在众多甲烷转化途径中,甲烷催化部分氧化(CPOM)具有能耗低、合成气组分适宜、反应迅速等优势。本文简要介绍了CPOM反应机理,即直接氧化机理和燃烧-重整机理;重点综述了过渡金属、贵金属、双金属和钙钛矿这四类CPOM催化剂的研究现状;分析了反应温度、反应气体碳氧比和反应空速对CPOM反应特性的影响;阐述了积炭和烧结这两种催化剂失活的主要原因及应对措施。根据研究结果可知,通过选取合适的催化剂组分、采用优化的制备方法、精确控制催化剂活性组分分布和微观结构等措施,可以保证更多的有效活性位更稳定地暴露在催化剂表面,以此提高催化性能(包括甲烷转化率、合成气选择性、合成气生成率、反应稳定性等)。最后指出了对CPOM催化剂微观结构的合理设计与可控制备以及对CPOM反应机理的深入研究仍将是今后关注的重点。
中图分类号:
引用本文
阮鹏, 杨润农, 林梓荣, 孙永明. 甲烷催化部分氧化制合成气催化剂的研究进展[J]. 化工进展, 2023, 42(4): 1832-1846.
RUAN Peng, YANG Runnong, LIN Zirong, SUN Yongming. Advances in catalysts for catalytic partial oxidation of methane to syngas[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1832-1846.
| 甲烷转化途径 | 目标产物 | 优势 | 劣势 |
|---|---|---|---|
| 直接转化 | 烯烃、烷烃、醇类、醛类、 芳香烃等 | 工艺简单,运输成本低 | 高温、高压条件下反应,反应转化率低,产物选择性低 |
| 间接转化 | 合成气(CO+H2) | 工艺相对成熟,能源利用率高 | 工艺较复杂、生产成本较高 |
| 水蒸气重整 | 工艺成熟 | 强吸热反应、高能耗,对催化剂和反应设备要求高、投资成本高,产物氢碳比较高 | |
| 二氧化碳重整 | 以常见的温室气体CO2为原料、反应绿色环保 | 强吸热反应、高能耗,产物氢碳比较低,催化剂易积炭 | |
催化部分氧化 (CPOM) | 弱放热反应、能耗低,产物氢碳比理想,反应迅速 | 工艺尚不成熟,存在安全隐患 | |
| 自热重整 | 自供热、能耗低 | 控制复杂,对反应设备要求高 |
表1 不同甲烷转化途径的反应特点
| 甲烷转化途径 | 目标产物 | 优势 | 劣势 |
|---|---|---|---|
| 直接转化 | 烯烃、烷烃、醇类、醛类、 芳香烃等 | 工艺简单,运输成本低 | 高温、高压条件下反应,反应转化率低,产物选择性低 |
| 间接转化 | 合成气(CO+H2) | 工艺相对成熟,能源利用率高 | 工艺较复杂、生产成本较高 |
| 水蒸气重整 | 工艺成熟 | 强吸热反应、高能耗,对催化剂和反应设备要求高、投资成本高,产物氢碳比较高 | |
| 二氧化碳重整 | 以常见的温室气体CO2为原料、反应绿色环保 | 强吸热反应、高能耗,产物氢碳比较低,催化剂易积炭 | |
催化部分氧化 (CPOM) | 弱放热反应、能耗低,产物氢碳比理想,反应迅速 | 工艺尚不成熟,存在安全隐患 | |
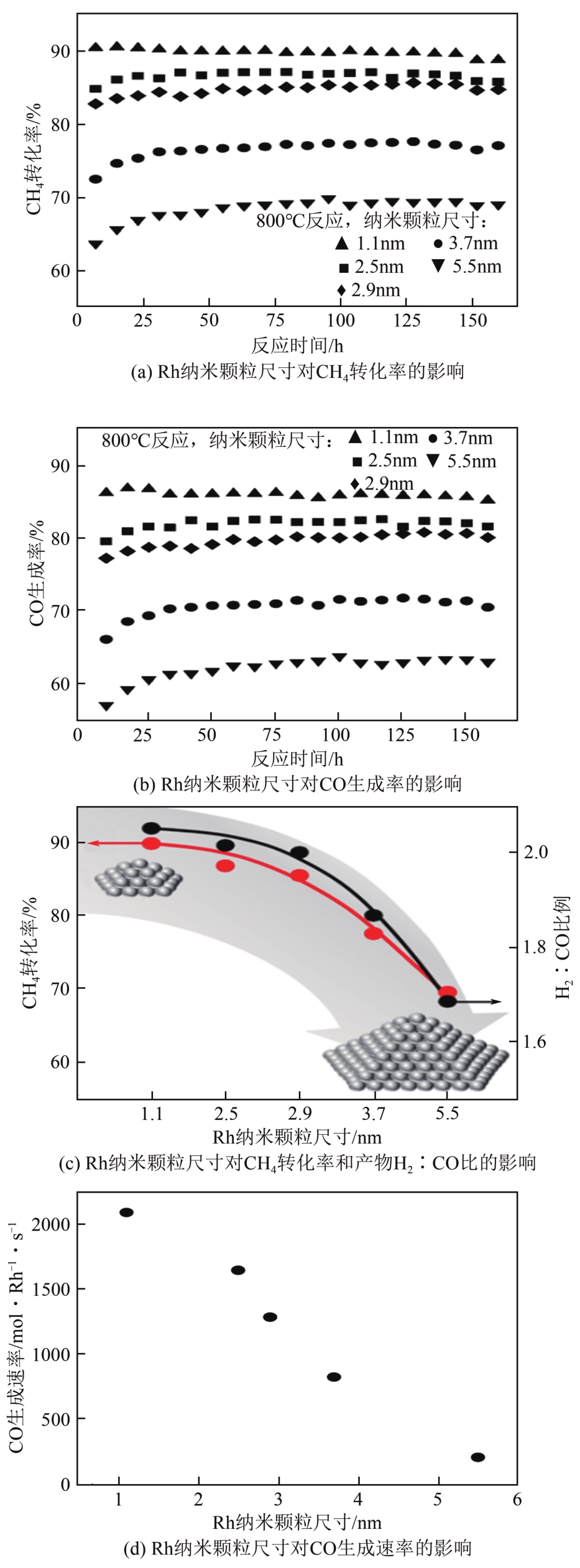
| 自热重整 | 自供热、能耗低 | 控制复杂,对反应设备要求高 |
| 催化剂组成 | 反应条件 | 不同CH4转化率下的温度/℃ | 不同CO选择性下的温度/℃ | 不同H2选择性下的温度/℃ | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|
| T50 | T90 | T50 | T90 | T50 | T90 | |||
| Ni(7.7)/Al2O3 | C/O比例为1.0 WHSV=157500mL/(g·h) | 600 | — | 600 | 800 | <600 | 780 | [ |
| Ni(2.8)Co(2.6)/Al2O3 | 755 | — | 763 | — | 765 | — | ||
| Co(6.8)/Al2O3 | — | — | — | — | — | — | ||
| Ni(2.5)/La-CeOx | C/O比例为1.0 | <500 | 625 | <550 | 700 | * | * | [ |
| Ni(5)/La-CeOx | <500 | 625 | <550 | — | * | * | ||
| Ni(10)/La-CeOx | 618 | 645 | 630 | — | * | * | ||
| Ni/MgO | C/O比例为1.0 WHSV=520000mL/(g·h) | 503 | 625 | 503 | 510 | * | * | [ |
| La-Ni@SiO2 | C/O比例为1.0 WHSV=72000mL/(g·h) | <650 | 738 | <650 | 712 | <650 | 730 | [ |
| Rh(0.5)/Al2O3 | C/O比例为1.0 GHSV=6000h-1 | 525 | 765 | 535 | 730 | <500 | 560 | [ |
| Rh(1)/γ-Al2O3 | C/O比例为1.0 WHSV=48000mL/(g·h) | 478 | 765 | 588 | 800 | — | — | [ |
| Rh(1)/γ-Al2O3-W | 470 | 765 | 580 | 800 | — | — | ||
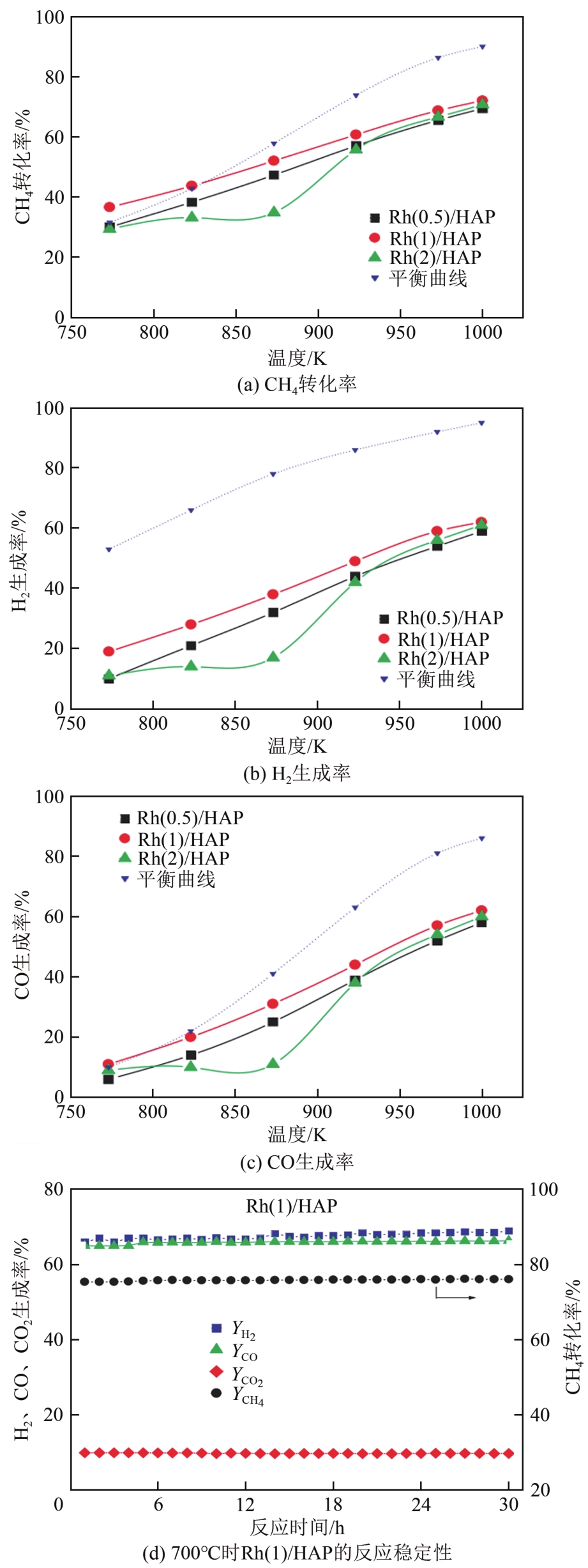
| Rh(0.5)/HAP | C/O比例为1.0 GHSV=192000h-1 | 613 | — | 685 | — | 680 | — | [ |
| Rh(1)/HAP | 587 | — | 667 | — | 662 | — | ||
| Rh(2)/HAP | 637 | — | 677 | — | 677 | — | ||
| Pt(0.5)-Ru(0.5)/CeZrO x -Al2O3 | C/O比例为1.0 GHSV=53000h-1 | 565 | 785 | 670 | — | 550 | 610 | [ |
| Pt(0.5)-Ru(0.5)/CeZrO x -Al2O3 | C/O比例为0.75 GHSV=26000h-1 | 570 | 780 | 640 | — | 578 | 595 | [ |
| La(0.5)Ca(0.5)Co(1)O3-δ | C/O比例为1.0 GHSV=12200h-1 | 800 | 860 | 765 | 853 | 760 | 850 | [ |
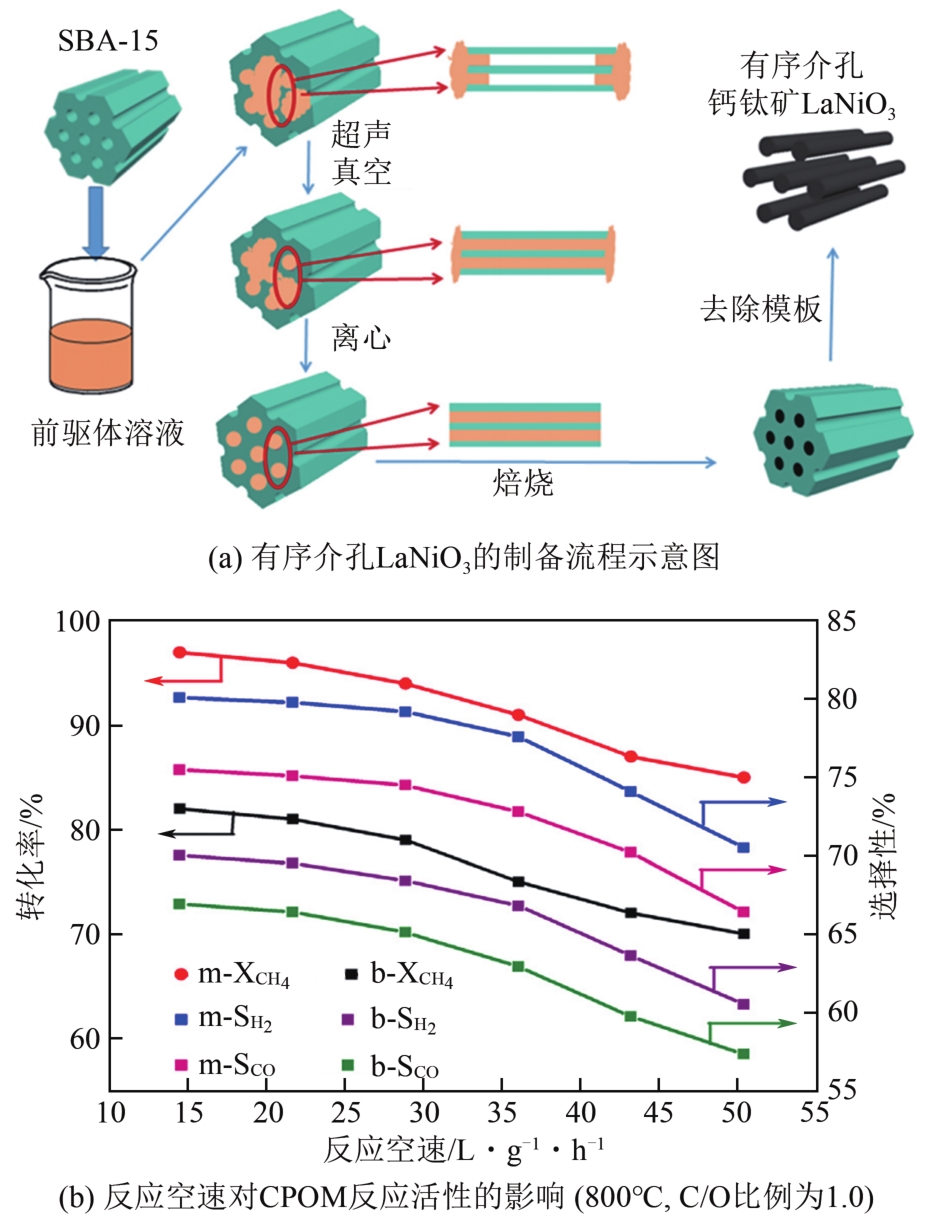
| LaNiO3 | C/O比例为1.0 WHSV=216000 mL/(g·h) | 350 | — | 525 | — | 430 | — | [ |
| 有序介孔LaNiO3 | <300 | 740 | 490 | — | <300 | — | ||
表2 不同催化剂的CPOM反应活性
| 催化剂组成 | 反应条件 | 不同CH4转化率下的温度/℃ | 不同CO选择性下的温度/℃ | 不同H2选择性下的温度/℃ | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|
| T50 | T90 | T50 | T90 | T50 | T90 | |||
| Ni(7.7)/Al2O3 | C/O比例为1.0 WHSV=157500mL/(g·h) | 600 | — | 600 | 800 | <600 | 780 | [ |
| Ni(2.8)Co(2.6)/Al2O3 | 755 | — | 763 | — | 765 | — | ||
| Co(6.8)/Al2O3 | — | — | — | — | — | — | ||
| Ni(2.5)/La-CeOx | C/O比例为1.0 | <500 | 625 | <550 | 700 | * | * | [ |
| Ni(5)/La-CeOx | <500 | 625 | <550 | — | * | * | ||
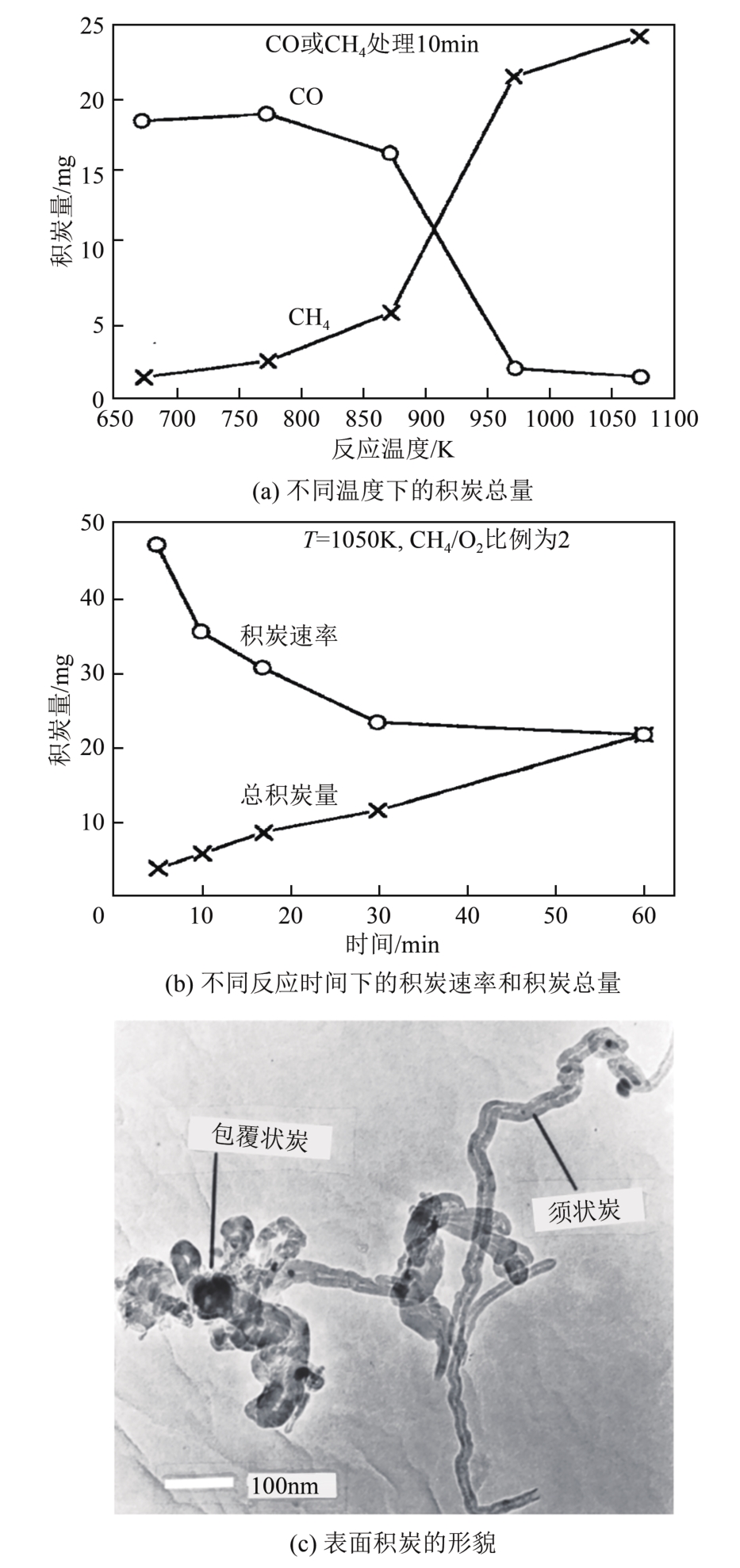
| Ni(10)/La-CeOx | 618 | 645 | 630 | — | * | * | ||
| Ni/MgO | C/O比例为1.0 WHSV=520000mL/(g·h) | 503 | 625 | 503 | 510 | * | * | [ |
| La-Ni@SiO2 | C/O比例为1.0 WHSV=72000mL/(g·h) | <650 | 738 | <650 | 712 | <650 | 730 | [ |
| Rh(0.5)/Al2O3 | C/O比例为1.0 GHSV=6000h-1 | 525 | 765 | 535 | 730 | <500 | 560 | [ |
| Rh(1)/γ-Al2O3 | C/O比例为1.0 WHSV=48000mL/(g·h) | 478 | 765 | 588 | 800 | — | — | [ |
| Rh(1)/γ-Al2O3-W | 470 | 765 | 580 | 800 | — | — | ||
| Rh(0.5)/HAP | C/O比例为1.0 GHSV=192000h-1 | 613 | — | 685 | — | 680 | — | [ |
| Rh(1)/HAP | 587 | — | 667 | — | 662 | — | ||
| Rh(2)/HAP | 637 | — | 677 | — | 677 | — | ||
| Pt(0.5)-Ru(0.5)/CeZrO x -Al2O3 | C/O比例为1.0 GHSV=53000h-1 | 565 | 785 | 670 | — | 550 | 610 | [ |
| Pt(0.5)-Ru(0.5)/CeZrO x -Al2O3 | C/O比例为0.75 GHSV=26000h-1 | 570 | 780 | 640 | — | 578 | 595 | [ |
| La(0.5)Ca(0.5)Co(1)O3-δ | C/O比例为1.0 GHSV=12200h-1 | 800 | 860 | 765 | 853 | 760 | 850 | [ |
| LaNiO3 | C/O比例为1.0 WHSV=216000 mL/(g·h) | 350 | — | 525 | — | 430 | — | [ |
| 有序介孔LaNiO3 | <300 | 740 | 490 | — | <300 | — | ||
| 1 | 吴玉玺, 韩婷婷, 解子恒, 等. 直接碳固体氧化物燃料电池研究进展: 碳燃料和逆向Boudouard反应催化剂[J]. 储能科学与技术, 2021, 10(6): 1977-1986. |
| WU Yuxi, HAN Tingting, XIE Ziheng, et al. Recent progress in direct carbon solid oxide fuel cells: carbon fuels and reverse Boudouard reaction catalysts[J]. Energy Storage Science and Technology, 2021, 10(6): 1977-1986. | |
| 2 | 宋乃建, 郭明媛, 南皓雄, 等. 过渡金属基催化剂用于氧析出反应的研究进展[J]. 储能科学与技术, 2021, 10(6): 1906-1917. |
| SONG Naijian, GUO Mingyuan, Haoxiong NAN, et al. Recent advances in transition metal-based catalysts for oxygen evolution reaction[J]. Energy Storage Science and Technology, 2021, 10(6): 1906-1917. | |
| 3 | HE Feng, LI Fanxing. Perovskite promoted iron oxide for hybrid water-splitting and syngas generation with exceptional conversion[J]. Energy & Environmental Science, 2015, 8(2): 535-539. |
| 4 | SZIMA S, CORMOS A M, CORMOS C C. Flexible hydrogen and power co-generation based on dry methane reforming with carbon capture[C]//28th European Symposium on Computer Aided Proless Engineering, Part B, Amsterdam: Elsevier, 2018: 1281-1286. |
| 5 | 刘蕊. 双金属氧化物载氧体在化学链甲烷部分氧化中的反应机理研究[D]. 天津: 天津大学, 2020. |
| LIU Rui. Reaction mechanism of bimetal oxide oxygen carriers for chemical looping partial oxidation of methane[D]. Tianjin: Tianjin University, 2020. | |
| 6 | 张翔宇, 李振花. 甲烷部分氧化制合成气催化剂的研究进展[J]. 化工进展, 2002, 21(12): 903-907. |
| ZHANG Xiangyu, LI Zhenhua. Progress in catalyst of partial oxidation of methane to syngas[J]. Chemical Industry and Engineering Progress, 2002, 21(12): 903-907. | |
| 7 | 余长林, 周晓春. 甲烷催化部分氧化制合成气研究新进展[J]. 天然气化工, 2011, 36(5): 67-72. |
| YU Changlin, ZHOU Xiaochun. Research progress in preparation of syngas by catalytic partial oxidation of methane[J]. Natural Gas Chemical Industry, 2011, 36(5): 67-72. | |
| 8 | DIPU A L, OHBUCHI S, NISHIKAWA Y, et al. Direct nonoxidative conversion of methane to higher hydrocarbons over silica-supported nickel phosphide catalyst[J]. ACS Catalysis, 2020, 10(1): 375-379. |
| 9 | ABDELSAYED V, SHEKHAWAT D, SMITH M, et al. Microwave-assisted conversion of low rank coal under methane environment[J]. Energy & Fuels, 2019, 33(2): 905-915. |
| 10 | LI Duanxing, BASLYMAN W S, SIRITANARATKUL B, et al. Oxidative-coupling-assisted methane aromatization: A simulation study[J]. Industrial & Engineering Chemistry Research, 2019, 58(51): 22884-22892. |
| 11 | 徐锋, 李凡, 朱丽华, 等. 甲烷直接催化氧化制甲醇催化剂及反应机理[J]. 化工进展, 2019, 38(10): 4564-4573. |
| XU Feng, LI Fan, ZHU Lihua, et al. Catalyst and reaction mechanism for direct catalytic oxidation of methane to methanol[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4564-4573. | |
| 12 | BAI Shuxing, LIU Fangfang, HUANG Bolong, et al. High-efficiency direct methane conversion to oxygenates on a cerium dioxide nanowires supported rhodium single-atom catalyst[J]. Nature Communications, 2020, 11: 954. |
| 13 | 于彦存. 甲烷部分氧化制合成气Ni-Ce x Zr1- x O2催化剂的研究[D]. 天津: 天津大学, 2007. |
| YU Yancun. Study on partial oxidation of methane to syngas over Ni-Ce x Zr1- x O2 catalyst[D]. Tianjin: Tianjin University, 2007. | |
| 14 | LIU Bing, LI Wenping, XU Yuebing, et al. Insight into the intrinsic active site for selective production of light olefins in cobalt-catalyzed Fischer-Tropsch synthesis[J]. ACS Catalysis, 2019, 9(8): 7073-7089. |
| 15 | 田丰源, 周明扬, 汪维, 等. 基于固体氧化物燃料电池的甲烷电化学部分氧化[J]. 陶瓷学报, 2021, 42(3): 406-413. |
| TIAN Fengyuan, ZHOU Mingyang, WANG Wei, et al. Electrochemical partial oxidation of methane through solid oxide fuel cell[J]. Journal of Ceramics, 2021, 42(3): 406-413. | |
| 16 | ZHANG Haotian, SUN Zhuxing, HU Yun hang. Steam reforming of methane: Current states of catalyst design and process upgrading[J]. Renewable and Sustainable Energy Reviews, 2021, 149: 111330. |
| 17 | FAN L Y, MOKHOV A, SAADABADI S A, et al. Methane steam reforming reaction in solid oxide fuel cells: Influence of electrochemical reaction and anode thickness[J]. Journal of Power Sources, 2021, 507: 230276. |
| 18 | 杨杰, 常辉, 隋志军, 等. 化学链催化甲烷氧化反应研究进展[J]. 化工进展, 2021, 40(4): 1928-1947. |
| YANG Jie, CHANG Hui, SUI Zhijun, et al. Advances in chemical looping methane oxidation[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 1928-1947. | |
| 19 | NIU Juntian, GUO Fan, RAN Jingyu, et al. Methane dry (CO2) reforming to syngas (H2/CO) in catalytic process: From experimental study and DFT calculations[J]. International Journal of Hydrogen Energy, 2020, 45(55): 30267-30287. |
| 20 | HAMBALI H U, JALIL A A, ABDULRASHEED A A, et al. Zeolite and clay based catalysts for CO2 reforming of methane to syngas: A review[J]. International Journal of Hydrogen Energy, 2022, 47(72): 30759-30787. |
| 21 | 阮勇哲, 卢遥, 王胜平. 甲烷干重整Ni基催化剂失活及抑制失活研究进展[J]. 化工进展, 2018, 37(10): 3850-3857. |
| RUAN Yongzhe, LU Yao, WANG Shengping. Progress in deactivation and anti-deactivation of nickel-based catalysts for methane dry reforming[J]. Chemical Industry and Engineering Progress, 2018, 37(10): 3850-3857. | |
| 22 | 王奕然, 曾令志, 娄舒洁, 等. 天然气制氢技术研究进展[J]. 石化技术与应用, 2019, 37(5): 361-366. |
| WANG Yiran, ZENG Lingzhi, LOU Shujie, et al. Review of hydrogen production from natural gas[J]. Petrochemical Technology & Application, 2019, 37(5): 361-366. | |
| 23 | 王芬. 甲烷合成与转化的抗积碳、抗烧结催化剂研究[D]. 厦门: 厦门大学, 2019. |
| WANG Fen. Study on coke and sintering resistant catalysts for methane synthesis and conversion[D]. Xiamen: Xiamen University, 2019. | |
| 24 | 龚思琦. 催化部分氧化-固体氧化物燃料电池系统特性研究[D]. 北京: 清华大学, 2019. |
| GONG Siqi. Research on characteristics of catalytic partial oxidation-solid oxide fuel cell systems[D]. Beijing: Tsinghua University, 2019. | |
| 25 | FAN Dongjie, GAO Yi, LIU Fangsheng, et al. Autothermal reforming of methane over an integrated solid oxide fuel cell reactor for power and syngas co-generation[J]. Journal of Power Sources, 2021, 513: 230536. |
| 26 | CHERIF A, NEBBALI R, SHEFFIELD J W, et al. Numerical investigation of hydrogen production via autothermal reforming of steam and methane over Ni/Al2O3 and Pt/Al2O3 patterned catalytic layers[J]. International Journal of Hydrogen Energy, 2021, 46(75): 37521-37532. |
| 27 | LIANDER H. The utilisation of natural gases for the ammonia process[J]. Transactions of the Faraday Society, 1929, 25: 462-472. |
| 28 | PRETTRE M, EICHNER C, PERRIN M. The catalytic oxidation of methane to carbon monoxide and hydrogen[J]. Transactions of the Faraday Society, 1946, 42: 335-339. |
| 29 | 罗春容. SiO2和Al2O3负载的Rh、Ru、Ir催化剂上甲烷部分氧化制合成气反应机理研究[D]. 厦门: 厦门大学, 2008. |
| LUO Chunrong. Mechanistic study of the partial oxidation of methane to synthesis gas over SiO2- and Al2O3-supported Rh, Ru and Ir catalysts[D]. Xiamen: Xiamen University, 2008. | |
| 30 | SMITH M W, SHEKHAWAT D. Fuel Cells: Technologies for fuel processing. Chapters 5—Catalytic partial oxidation[M]. Amsterdam: Elsevier, 2011: 73-128. |
| 31 | HU Y H, RUCKENSTEIN E. Transient kinetic studies of partial oxidation of CH4 [J]. Journal of Catalysis, 1996, 158(1): 260-266. |
| 32 | HICKMAN D A, SCHMIDT L D. Production of syngas by direct catalytic oxidation of methane[J]. Science, 1993, 259(5093): 343-346. |
| 33 | HICKMAN D A, SCHMIDT L D. Synthesis gas formation by direct oxidation of methane over Pt monoliths[J]. Journal of Catalysis, 1992, 138(1): 267-282. |
| 34 | VERMEIREN W J M, BLOMSMA E, JACOBS P A. Catalytic and thermodynamic approach of the oxyreforming reaction of methane[J]. Catalysis Today, 1992, 13(2/3): 427-436. |
| 35 | WENG Weizheng, CHEN Mingshu, YAN Qiangu, et al. Mechanistic study of partial oxidation of methane to synthesis gas over supported rhodium and ruthenium catalysts using in situ time-resolved FTIR spectroscopy[J]. Catalysis Today, 2000, 63(2/3/4): 317-326. |
| 36 | HOU Zhaoyin, GAO Jing, GUO Jianzhong, et al. Deactivation of Ni catalysts during methane autothermal reforming with CO2 and O2 in a fluidized-bed reactor[J]. Journal of Catalysis, 2007, 250(2): 331-341. |
| 37 | JALALI R, NEMATOLLAHI B, REZAEI M, et al. Mesoporous nanostructured Ni/MgAl2O4 catalysts: Highly active and stable catalysts for syngas production in combined dry reforming and partial oxidation[J]. International Journal of Hydrogen Energy, 2019, 44(21): 10427-10442. |
| 38 | 余林, 袁书华, 田久英, 等. 甲烷部分氧化制合成气载体及助剂对Ni系催化剂活性的影响[J]. 催化学报, 2001, 22(4): 383-386. |
| YU Lin, YUAN Shuhua, TIAN Jiuying, et al. Partial oxidation of methane to syngas: Effects of support and promoter on catalytic performance of Ni/Al2O3 catalyst[J]. Chinese Journal of Catalysis, 2001, 22(4): 383-386. | |
| 39 | OSMAN A I, MEUDAL J, LAFFIR F, et al. Enhanced catalytic activity of Ni on η-Al2O3 and ZSM-5 on addition of ceria zirconia for the partial oxidation of methane[J]. Applied Catalysis B: Environmental, 2017, 212: 68-79. |
| 40 | KONDRATENKO V A, BERGER-KARIN C, KONDRATENKO E V. Partial oxidation of methane to syngas over γ-Al2O3-supported Rh nanoparticles: Kinetic and mechanistic origins of size effect on selectivity and activity[J]. ACS Catalysis, 2014, 4(9): 3136-3144. |
| 41 | LANZA R, CANU P, JÄRÅS S G. Methane partial oxidation over Pt-Ru catalyst: An investigation on the mechanism[J]. Applied Catalysis A: General, 2010, 375(1): 92-100. |
| 42 | SCARABELLO A, NOGARE D D, CANU P, et al. Partial oxidation of methane on Rh/ZrO2 and Rh/Ce-ZrO2 on monoliths: Catalyst restructuring at reaction conditions[J]. Applied Catalysis B: Environmental, 2015, 174/175: 308-322. |
| 43 | LANZA R, CANU P, JÄRÅS S G. Partial oxidation of methane over Pt-Ru bimetallic catalyst for syngas production[J]. Applied Catalysis A: General, 2008, 348(2): 221-228. |
| 44 | XU Xia, LANE A M. Water-treated Rh/γ-Al2O3 catalyst for methane partial oxidation[J]. Applied Petrochemical Research, 2016, 6(2): 163-166. |
| 45 | BOUKHA Z, GIL-CALVO M, DE RIVAS B, et al. Behaviour of Rh supported on hydroxyapatite catalysts in partial oxidation and steam reforming of methane: On the role of the speciation of the Rh particles[J]. Applied Catalysis A: General, 2018, 556: 191-203. |
| 46 | RABE S, TRUONG T B, VOGEL F. Low temperature catalytic partial oxidation of methane for gas-to-liquids applications[J]. Applied Catalysis A: General, 2005, 292: 177-188. |
| 47 | JAVED A H, SHAHZAD N, BUTT F A, et al. Synthesis of bimetallic Co-Ni/ZnO nanoprisms (ZnO-NPr) for hydrogen-rich syngas production via partial oxidation of methane[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106887. |
| 48 | CINAR T, ALTINCEKIC T G. Synthesis and investigation of bimetallic Ni-Co/Al2O3 nanocatalysts using the polyol process[J]. Particulate Science and Technology, 2016, 34(6): 725-735. |
| 49 | FAKEEHA A H, ARAFAT Y, IBRAHIM A A, et al. Highly selective syngas/H2 production via partial oxidation of CH4 using (Ni, Co and Ni-Co)/ZrO2-Al2O3 catalysts: Influence of calcination temperature[J]. Processes, 2019, 7(3): 141. |
| 50 | ZAGAYNOV I V, LOKTEV A S, MUKHIN I E, et al. Influence of the Ni/Co ratio in bimetallic NiCo catalysts on methane conversion into synthesis gas[J]. Mendeleev Communications, 2017, 27(5): 509-511. |
| 51 | CHEEPHAT C, DAORATTANACHAI P, LAOSIRIPOJANA N. Effects of Re additional and Co-fed water reactants on bimetallic Ni-Fe based catalysts for catalytic partial oxidation of methane[J]. Journal of Sustainable Energy & Environment, 2017, 8: 59-63. |
| 52 | ZHU Huaiyu, WANG Wei, RAN Ran, et al. Iron incorporated Ni-ZrO2 catalysts for electric power generation from methane[J]. International Journal of Hydrogen Energy, 2012, 37(12): 9801-9808. |
| 53 | BAWORNRUTTANABOONYA K, LAOSIRIPOJANA N, MUJUMDAR A S, et al. Catalytic partial oxidation of CH4 over bimetallic Ni-Re/Al2O3: Kinetic determination for application in microreactor [J]. AIChE Journal, 2018, 64(5): 1691-1701. |
| 54 | CIMINO S, LISI L, RUSSO G, et al. Effect of partial substitution of Rh catalysts with Pt or Pd during the partial oxidation of methane in the presence of sulphur[J]. Catalysis Today, 2010, 154(3/4): 283-292. |
| 55 | TOMISHIGE K, KANAZAWA S, SATO M, et al. Catalyst design of Pt-modified Ni/Al2O3 catalyst with flat temperature profile in methane reforming with CO2 and O2 [J]. Catalysis Letters, 2002, 84: 69-74. |
| 56 | PAULETTO G, LIBRETTO N, BOFFITO D C, et al. Ni/CeO2 promoted Ru and Pt supported on FeCrAl gauze for cycling methane catalytic partial oxidation-CPOX[J]. Applied Catalysis B: Environmental, 2021, 286: 119849. |
| 57 | LI L C, DOSTAGIR N H, SHROTRI A, et al. Partial oxidation of methane to syngas via formate intermediate found for a ruthenium-rhenium bimetallic catalyst[J]. ACS Catalysis, 2021, 11(7): 3782-3789. |
| 58 | BRACKMANN R, PEREZ C A, SCHMAL M. LaCoO3 perovskite on ceramic monoliths-Pre and post reaction analyzes of the partial oxidation of methane[J]. International Journal of Hydrogen Energy, 2014, 39(26): 13991-14007. |
| 59 | BASHAN V, UST Y. Perovskite catalysts for methane combustion: Applications, design, effects for reactivity and partial oxidation[J]. International Journal of Energy Research, 2019, 43(14): 7755-7789. |
| 60 | CIHLAR J J, VRBA R, CASTKOVA K, et al. Effect of transition metal on stability and activity of La-Ca-M-(Al)-O (M = Co, Cr, Fe and Mn) perovskite oxides during partial oxidation of methane[J]. International Journal of Hydrogen Energy, 2017, 42(31): 19920-19934. |
| 61 | SANTOS M, NETO R C, NORONHA F B, et al. Perovskite as catalyst precursors in the partial oxidation of methane: the effect of cobalt, nickel and pretreatment[J]. Catalysis Today, 2018, 299: 229-241. |
| 62 | ZHU T L, FLYTZANI-STEPHANOPOULOS M. Catalytic partial oxidation of methane to synthesis gas over Ni-CeO2 [J]. Applied Catalysis A: General, 2001, 208(1/2): 403-417. |
| 63 | CHOUDHARY V R, MAMMAN A S, SANSARE S D. Selective oxidation of methane to CO and H2 over Ni/MgO at low temperatures[J]. Angewandte Chemie International Edition in English, 1992, 31(9): 1189-1190. |
| 64 | LI Lei, YAO Yao, SUN Bo, et al. Highly active and stable lanthanum-doped core-shell-structured Ni@SiO2 catalysts for the partial oxidation of methane to syngas[J]. ChemCatChem, 2013, 5(12): 3781-3787. |
| 65 | 龚思琦, 曾洪瑜, 史翊翔, 等. 基于甲烷催化部分氧化的SOFC性能研究[J]. 燃烧科学与技术, 2019, 25(1): 60-65. |
| GONG Siqi, ZENG Hongyu, SHI Yixiang, et al. Study of performance of SOFC based on catalytic partial oxidation of methane[J]. Journal of Combustion Science and Technology, 2019, 25(1): 60-65. | |
| 66 | DUAN Qianlin, WANG Junwen, DING Chuanmin, et al. Partial oxidation of methane over Ni based catalyst derived from order mesoporous LaNiO3 perovskite prepared by modified nanocasting method[J]. Fuel, 2017, 193: 112-118. |
| 67 | OSMAN A I. Catalytic hydrogen production from methane partial oxidation: Mechanism and kinetic study[J]. Chemical Engineering & Technology, 2020, 43(4): 641-648. |
| 68 | GUO Songsong, WANG Junwen, DING Chuanmin, et al. Confining Ni nanoparticles in honeycomb-like silica for coking and sintering resistant partial oxidation of methane[J]. International Journal of Hydrogen Energy, 2018, 43(13): 6603-6613. |
| 69 | STOTZ H, MAIER L, DEUTSCHMANN O. Methane oxidation over palladium: On the mechanism in fuel-rich mixtures at high temperatures[J]. Topics in Catalysis, 2017, 60(1/2): 83-109. |
| 70 | JAWORSKI Z, ZAKRZEWSKA B, PIANKO-OPRYCH P. On thermodynamic equilibrium of carbon deposition from gaseous C—H—O mixtures: Updating for nanotubes[J]. Reviews in Chemical Engineering, 2017, 33(3): 217-235. |
| 71 | JAWORSKI Z, PIANKO-OPRYCH P. On nanotube carbon deposition at equilibrium in catalytic partial oxidation of selected hydrocarbon fuels[J]. International Journal of Hydrogen Energy, 2017, 42(27): 16920-16931. |
| 72 | CLARIDGE J B, GREEN M L H, TSANG S C, et al. A study of carbon deposition on catalysts during the partial oxidation of methane to synthesis gas[J]. Catalysis Letters, 1993, 22(4): 299-305. |
| 73 | TANG S, LIN J, TAN K L. Partial oxidation of methane to syngas over Ni/MgO, Ni/CaO and Ni/CeO2 [J]. Catalysis Letters, 1998, 51: 169-175. |
| 74 | GAO Jing, HOU Zhaoyin, GUO Jianzhong, et al. Catalytic conversion of methane and CO2 to synthesis gas over a La2O3-modified SiO2 supported Ni catalyst in fluidized-bed reactor[J]. Catalysis Today, 2008, 131(1/2/3/4): 278-284. |
| 75 | NICHELE V, SIGNORETTO M, PINNA F, et al. Ni/ZrO2 catalysts in ethanol steam reforming: Inhibition of coke formation by CaO-doping[J]. Applied Catalysis B: Environmental, 2014, 150/151: 12-20. |
| 76 | DAI Yunqian, LU Ping, CAO Zhenming, et al. The physical chemistry and materials science behind sinter-resistant catalysts[J]. Chemical Society Reviews, 2018, 47(12): 4314-4331. |
| 77 | 薛亚楠. 高度分散Pt催化剂的制备及用于POM催化性能的研究[D]. 太原: 太原理工大学, 2019. |
| XUE Yanan. Study on the preparation of highly dispersed Pt catalysts and performance for partial oxidation of methane[D]. Taiyuan: Taiyuan University of Technology, 2019. | |
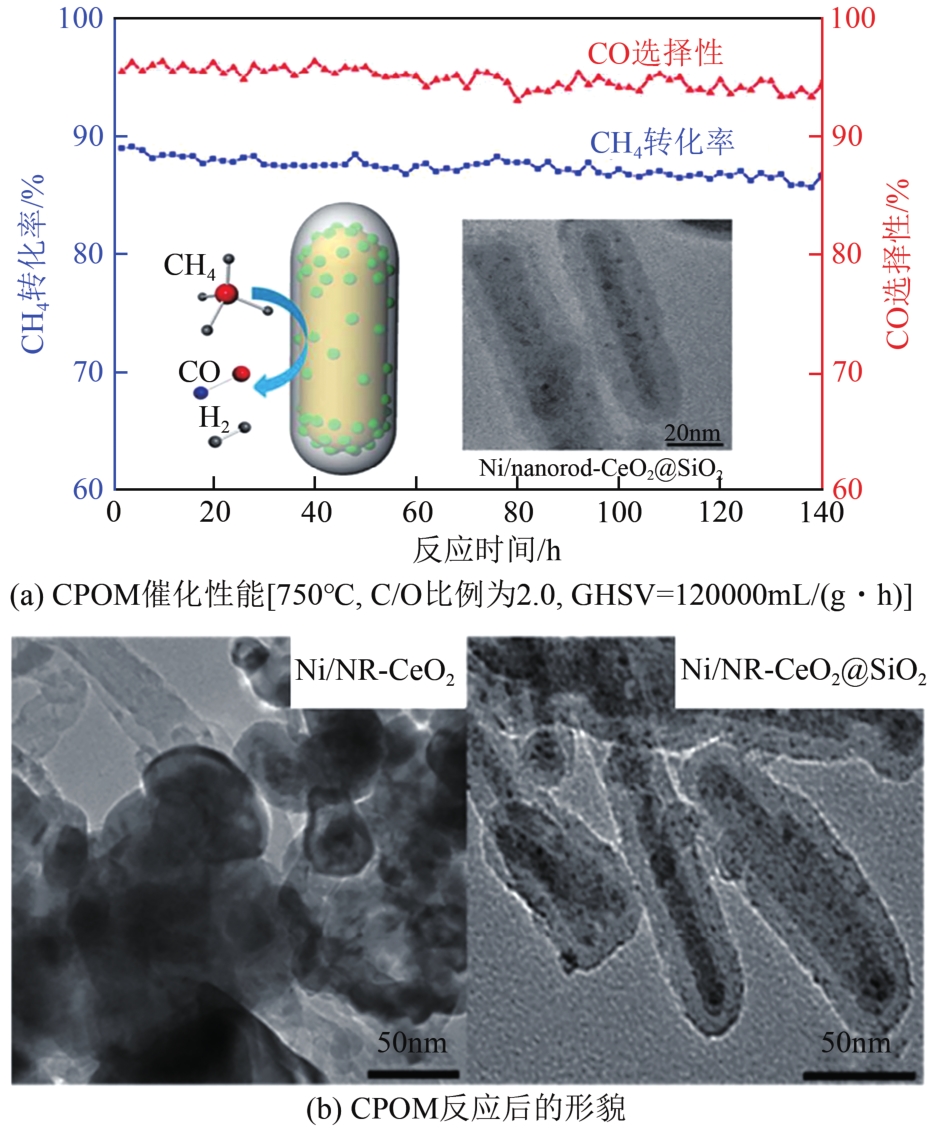
| 78 | ZHU Shaohong, LIAN Xinyi, FAN Tingting, et al. Thermally stable core-shell Ni/nanorod-CeO2@SiO2 catalyst for partial oxidation of methane at high temperatures[J]. Nanoscale, 2018, 10(29): 14031-14038. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [14] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [15] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||