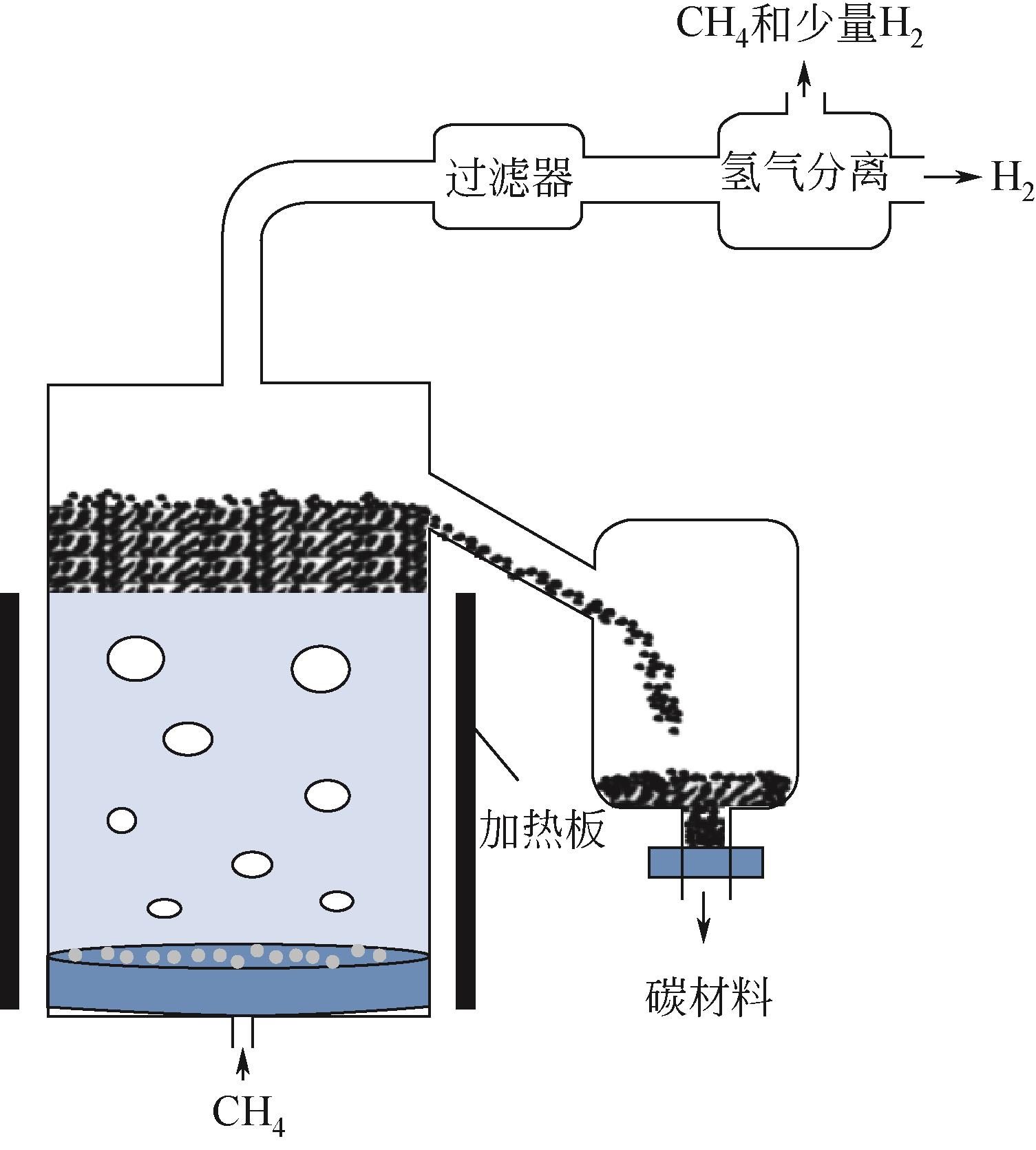
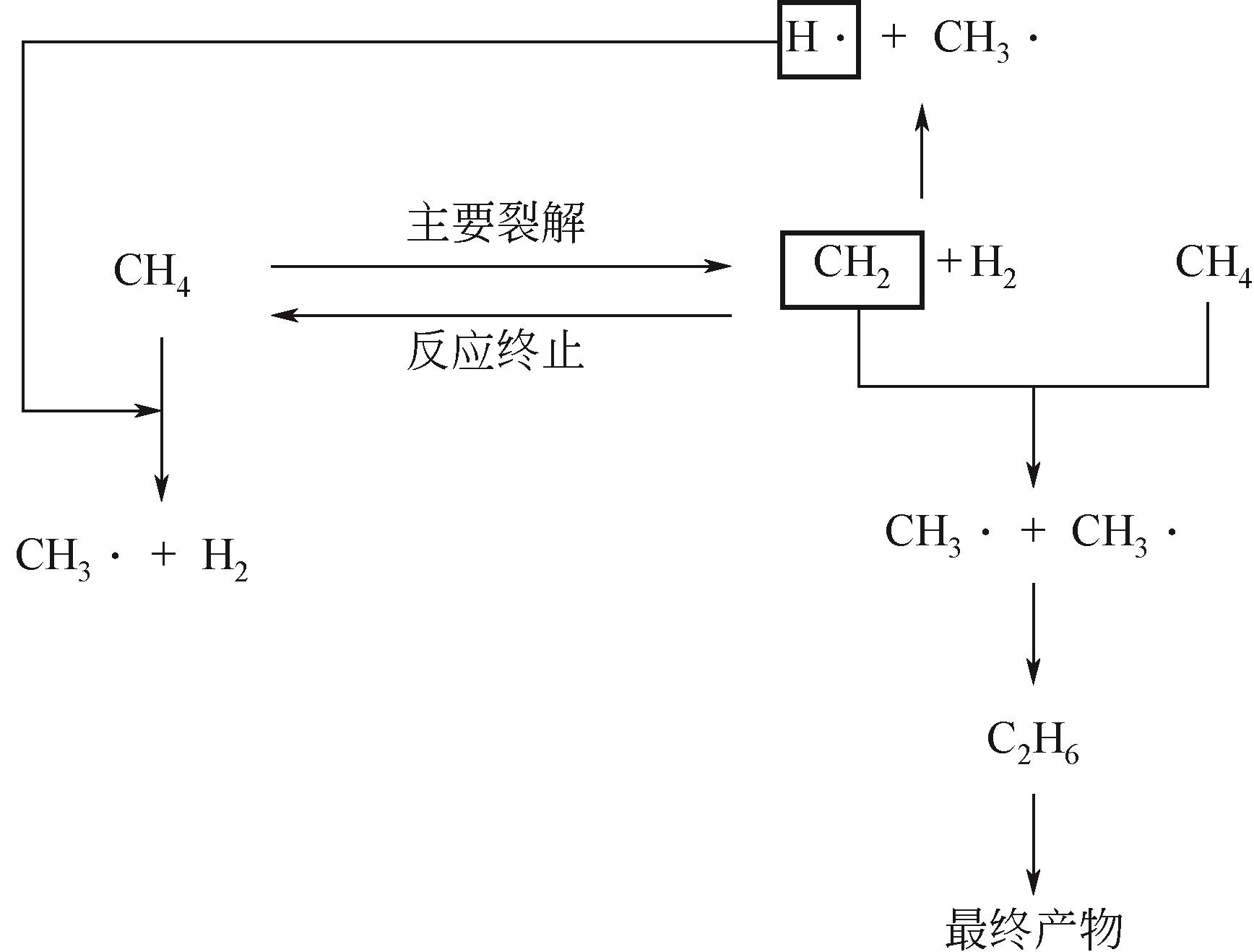
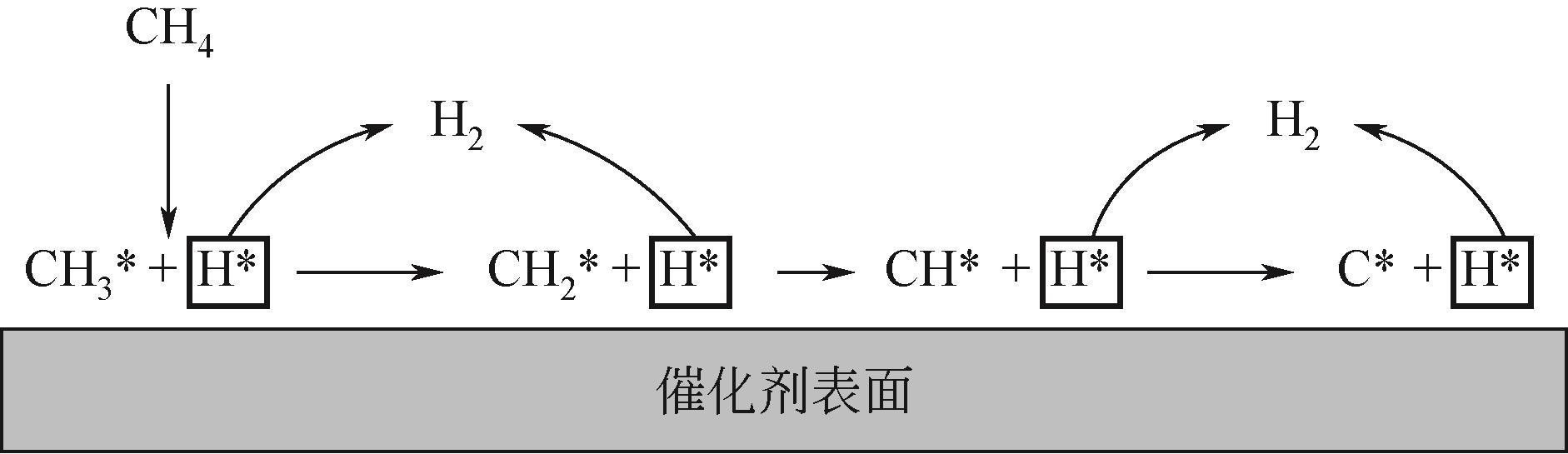
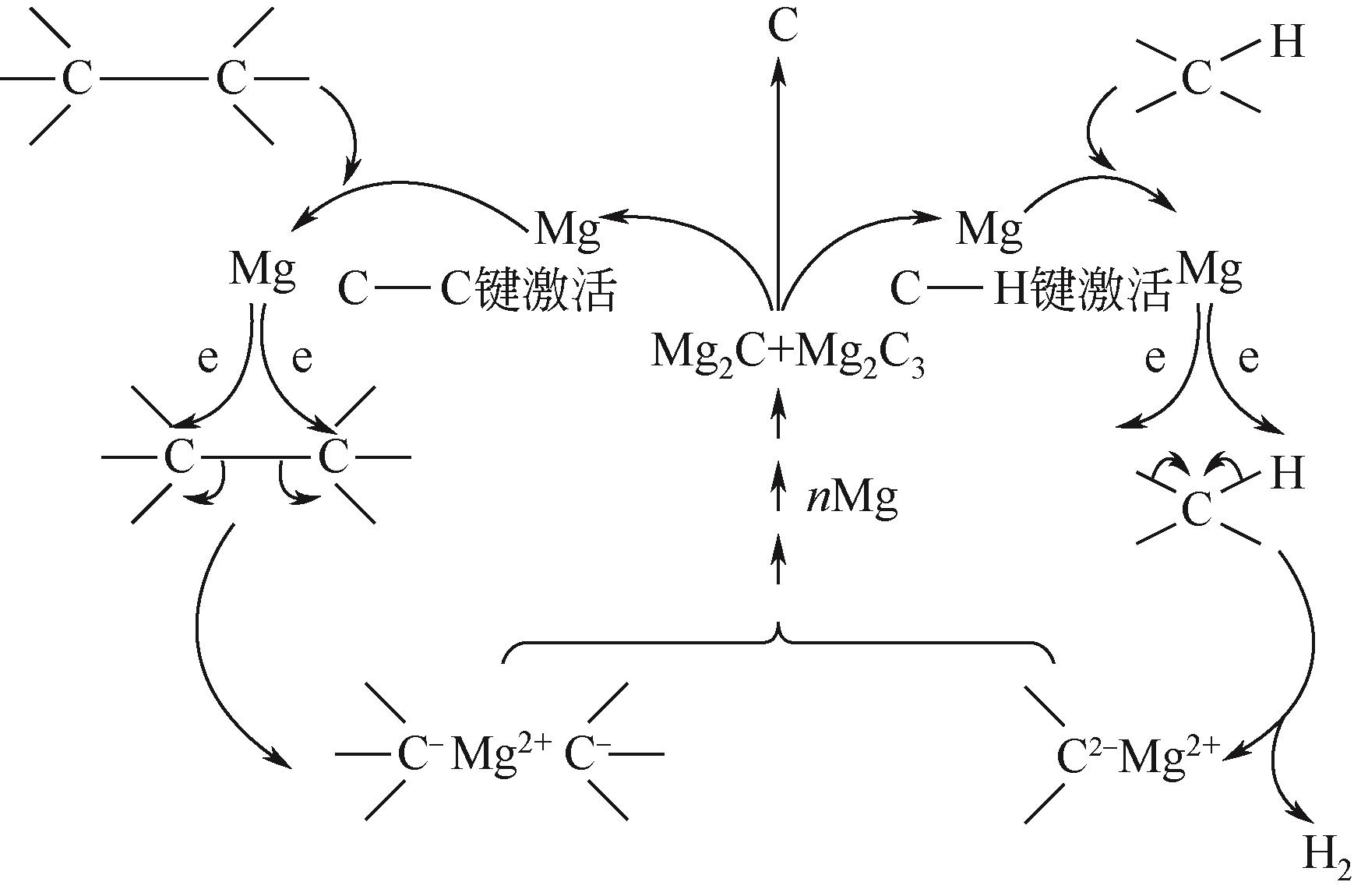
化工进展 ›› 2023, Vol. 42 ›› Issue (3): 1270-1280.DOI: 10.16085/j.issn.1000-6613.2022-0902
熔融金属法甲烷裂解制氢和碳材料研究进展
- 中国石油西南油气田公司天然气研究院,四川 成都 610213
-
收稿日期:2022-05-16修回日期:2022-08-29出版日期:2023-03-15发布日期:2023-04-10 -
通讯作者:何阳东 -
作者简介:何阳东(1992—),男,博士后,研究方向为氢能及碳捕集。E-mail: heyd01@petrochina.com.cn。 -
基金资助:中国石油西南油气田公司博士后基金(20220306-11)
Development of methane pyrolysis based on molten metal technology for coproduction of hydrogen and solid carbon products
HE Yangdong( ), CHANG Honggang, WANG Dan, CHEN Changjie, LI Yaxin
), CHANG Honggang, WANG Dan, CHEN Changjie, LI Yaxin
- Research Institute of Natural Gas Technology, PetroChina Southwest Oil & Gasfield Company, Chengdu 610213, Sichuan, China
-
Received:2022-05-16Revised:2022-08-29Online:2023-03-15Published:2023-04-10 -
Contact:HE Yangdong
摘要:
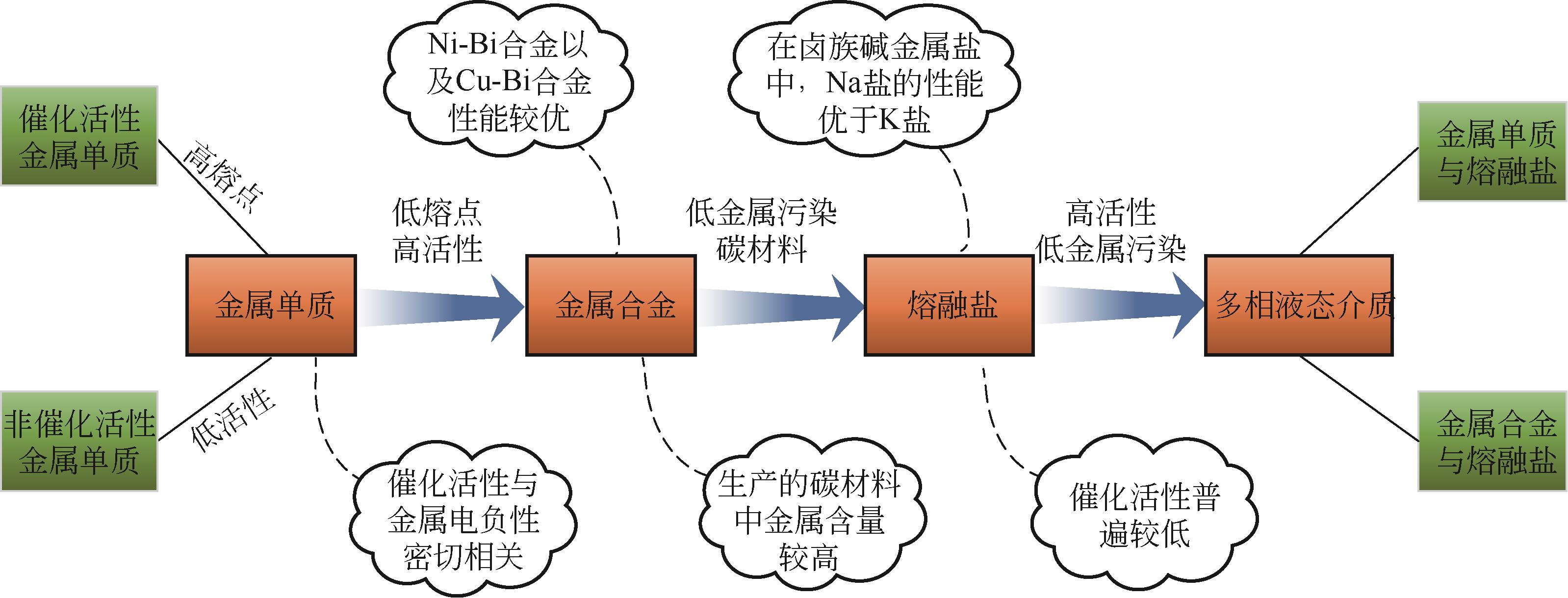
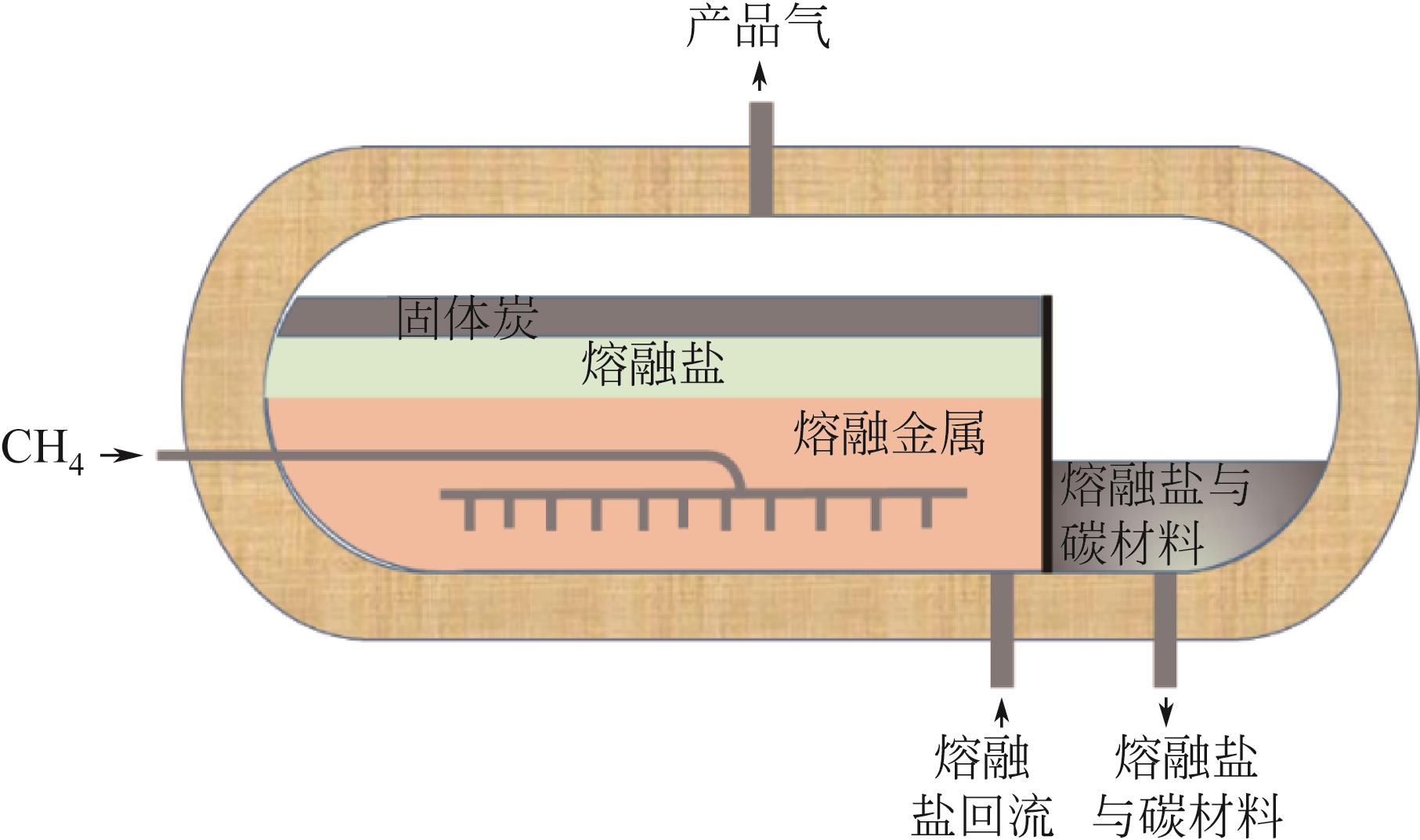
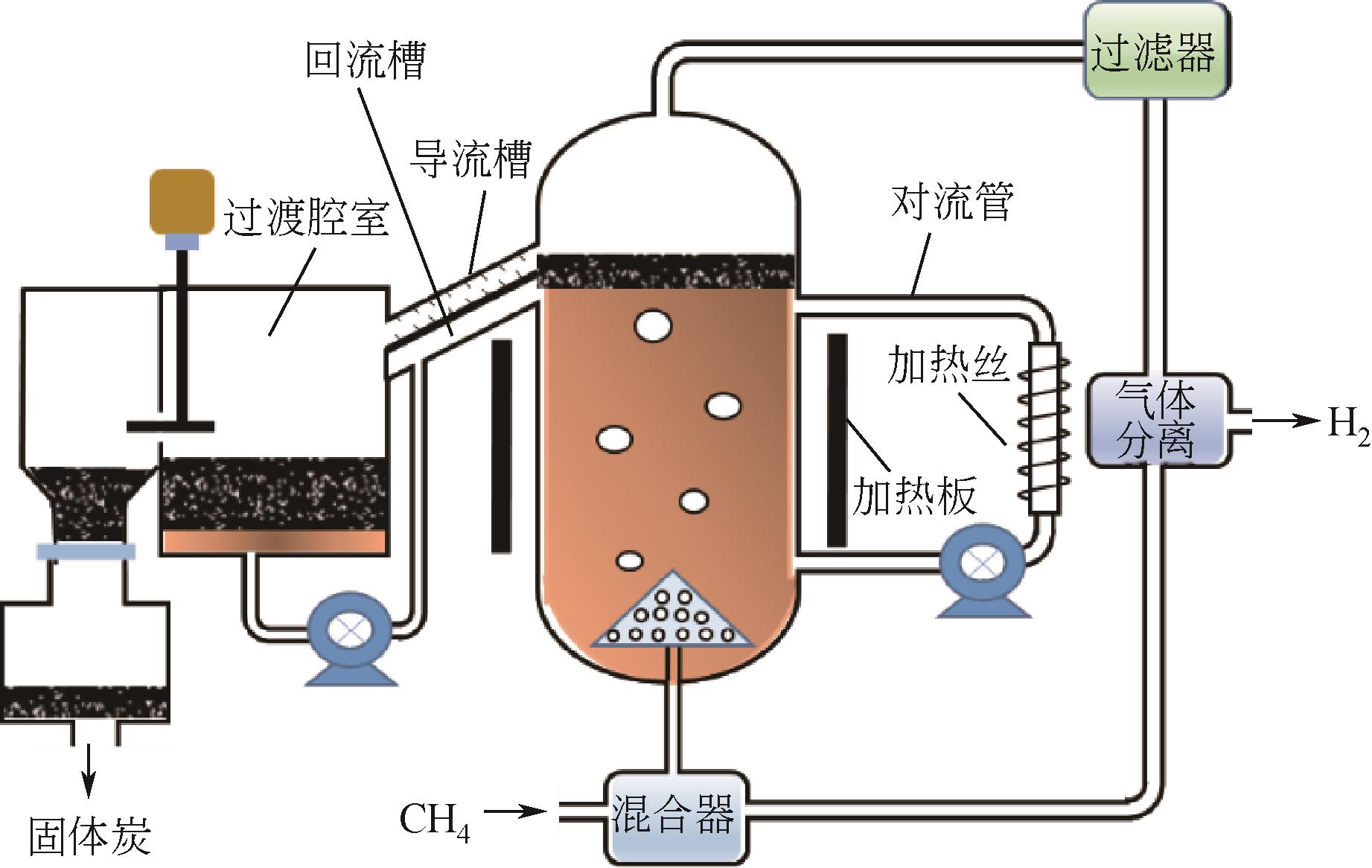
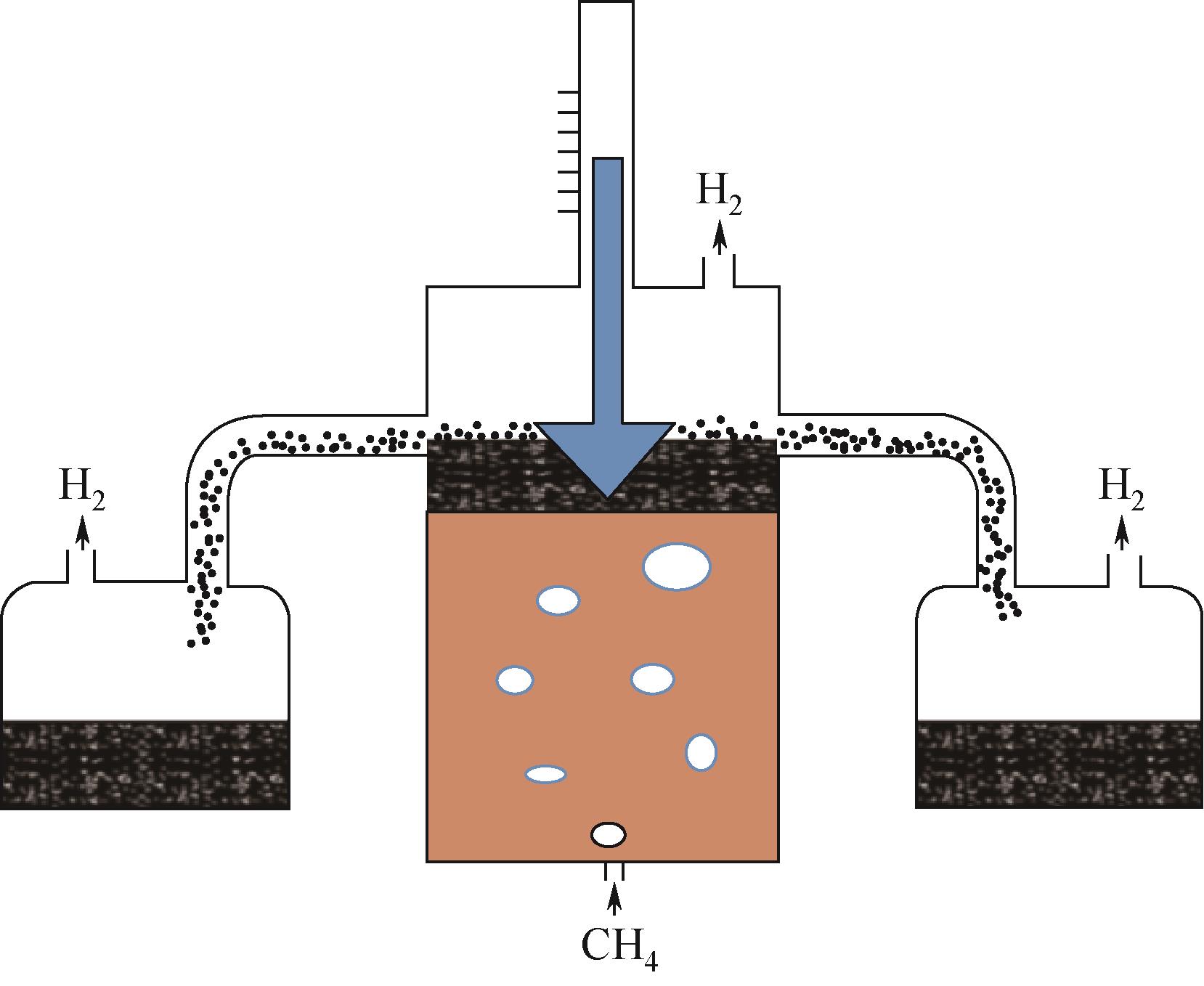
熔融金属法甲烷裂解技术作为近年来新兴的制氢技术,有效地解决了传统甲烷热裂解或催化裂解高能耗、低转化率以及催化剂失活等问题,避免了甲烷蒸汽重整制氢工艺高碳排放。在制氢的同时还能生产出具有附加值的碳产品,因而受到各方广泛关注。本文总结了熔融金属法甲烷裂解技术研究进展,并围绕工艺流程、反应机理、熔融介质的选择以及反应器设计等方面展开,给出了液相介质是否起催化作用的两类甲烷裂解反应机理,并详细阐述了熔融介质选择原则、发展趋势以及不同类型熔融介质的优缺点。再者,技术经济性以及温室气体减排量也在文中详细体现,进一步论证了该工艺的可行性和潜在效益。此外,文中还给出了未来技术发展趋势和建议,指出调控碳材料形貌,使之向高附加值碳材料转变应是未来重点发展方向之一。
中图分类号:
引用本文
何阳东, 常宏岗, 王丹, 陈昌介, 李雅欣. 熔融金属法甲烷裂解制氢和碳材料研究进展[J]. 化工进展, 2023, 42(3): 1270-1280.
HE Yangdong, CHANG Honggang, WANG Dan, CHEN Changjie, LI Yaxin. Development of methane pyrolysis based on molten metal technology for coproduction of hydrogen and solid carbon products[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1270-1280.
| 熔融介质 | 装载高度/mm | 纯化方式① | C/% | Ni/% | Bi/% | Na/% | K/% | Br/% |
|---|---|---|---|---|---|---|---|---|
| NiBi | 350 | — | 17.36 | 12.98 | 69.66 | — | — | — |
| NiBi | 350 | 真空加热 | 51.82 | 1.84 | 46.34 | — | — | — |
| NiBi | 350 | 酸洗 | 58.81 | 4.09 | 37.10 | — | — | — |
| NiBi/KBr | 110/110 | 水洗+真空加热 | 85.63 | 0.82 | 1.10 | — | 3.73 | 8.72 |
| NiBi/KBr | 240/110 | 水洗 | 85.68 | 0.22 | 0.66 | — | 4.38 | 9.06 |
| NiBi/KBr | 240/110 | 水洗+真空加热 | 98.22 | 0.23 | 0.32 | — | 0.36 | 0.87 |
| NiBi/KBr | 110/240 | 水洗 | 74.19 | 0.06 | 0.42 | — | 8.07 | 17.26 |
| NiBi/KBr | 110/240 | 水洗+真空加热 | 88.47 | 0.10 | 0.00 | — | 3.60 | 7.83 |
| NiBi/KBr | 110/240 | 水洗+酸洗 | 84.63 | 0 | 0.00 | — | 4.98 | 10.39 |
| NiBi/NaBr | 110/240 | 水洗 | 95.06 | 0.18 | 0.56 | 1.06 | — | 3.14 |
| NiBi/NaBr | 110/240 | 水洗+真空加热 | 97.40 | 0.18 | 0.00 | 0.59 | — | 1.83 |
| NiBi/NaBr | 110/240 | 水洗+酸洗 | 97.34 | 0 | 0.00 | 1.15 | — | 1.51 |
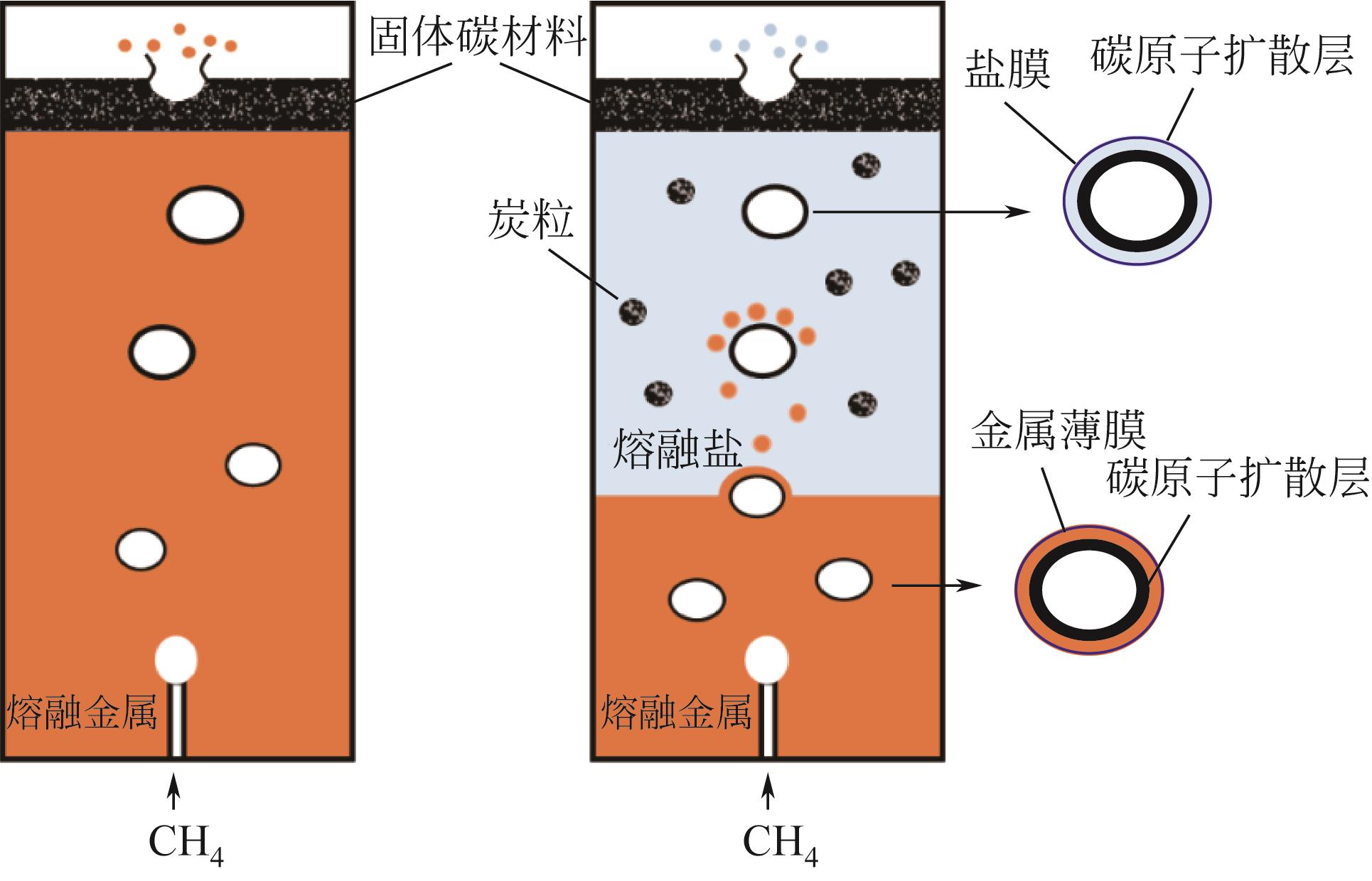
表1 金属熔融与熔融盐两相反应器中甲烷裂解碳材料组分分析结果(质量分数)[29]
| 熔融介质 | 装载高度/mm | 纯化方式① | C/% | Ni/% | Bi/% | Na/% | K/% | Br/% |
|---|---|---|---|---|---|---|---|---|
| NiBi | 350 | — | 17.36 | 12.98 | 69.66 | — | — | — |
| NiBi | 350 | 真空加热 | 51.82 | 1.84 | 46.34 | — | — | — |
| NiBi | 350 | 酸洗 | 58.81 | 4.09 | 37.10 | — | — | — |
| NiBi/KBr | 110/110 | 水洗+真空加热 | 85.63 | 0.82 | 1.10 | — | 3.73 | 8.72 |
| NiBi/KBr | 240/110 | 水洗 | 85.68 | 0.22 | 0.66 | — | 4.38 | 9.06 |
| NiBi/KBr | 240/110 | 水洗+真空加热 | 98.22 | 0.23 | 0.32 | — | 0.36 | 0.87 |
| NiBi/KBr | 110/240 | 水洗 | 74.19 | 0.06 | 0.42 | — | 8.07 | 17.26 |
| NiBi/KBr | 110/240 | 水洗+真空加热 | 88.47 | 0.10 | 0.00 | — | 3.60 | 7.83 |
| NiBi/KBr | 110/240 | 水洗+酸洗 | 84.63 | 0 | 0.00 | — | 4.98 | 10.39 |
| NiBi/NaBr | 110/240 | 水洗 | 95.06 | 0.18 | 0.56 | 1.06 | — | 3.14 |
| NiBi/NaBr | 110/240 | 水洗+真空加热 | 97.40 | 0.18 | 0.00 | 0.59 | — | 1.83 |
| NiBi/NaBr | 110/240 | 水洗+酸洗 | 97.34 | 0 | 0.00 | 1.15 | — | 1.51 |
| 成本 | SMR | 质子交换膜电解水 | 熔融金属法 |
|---|---|---|---|
| 总装置成本/106USD | 42 | 495.8 | 40.8 |
| 总投资成本/106USD | 252.1 | 829.1 | 349.7 |
| 运行成本/106USD·a-1 | 94.5 | 582.4 | 122 |
| 原料成本/106USD·a-1 | 63.2 | 543.8 | 92.8 |
| 净电输出/MWe | 12.6① | — | 5.4① |
| 1kg H2的碳排放/kg | 9.3 | 0 | 2.5 |
| 氢气售价(IRR=10%)/USD·kg-1 | 1.26 | 7.13② | 1.39③ |
| 与SMR工艺IRR收益为10%相等时,碳税价格/USD·t-1 | — | 585 | 18 |
表2 不同制氢工艺年产10万吨氢气投资成本
| 成本 | SMR | 质子交换膜电解水 | 熔融金属法 |
|---|---|---|---|
| 总装置成本/106USD | 42 | 495.8 | 40.8 |
| 总投资成本/106USD | 252.1 | 829.1 | 349.7 |
| 运行成本/106USD·a-1 | 94.5 | 582.4 | 122 |
| 原料成本/106USD·a-1 | 63.2 | 543.8 | 92.8 |
| 净电输出/MWe | 12.6① | — | 5.4① |
| 1kg H2的碳排放/kg | 9.3 | 0 | 2.5 |
| 氢气售价(IRR=10%)/USD·kg-1 | 1.26 | 7.13② | 1.39③ |
| 与SMR工艺IRR收益为10%相等时,碳税价格/USD·t-1 | — | 585 | 18 |
| CO2捕集条件 | SMR | 熔融金属法 (碳供能) | 熔融金属法 (氢供能) | 熔融金属法 (天然气供能) | 熔融金属法 (电供能) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| — | MDEA脱碳 | — | MEA脱碳 | — | MEA脱碳 | ||||||
| 反应温度/℃ | 890 | 890 | 1200 | 1200 | 1200 | 1200 | 1200 | 1200 | |||
| 反应压力/MPa | 3.2 | 3.2 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| NG流率/kg·s-1 | 2.62 | 2.81 | 3.86 | 3.86 | 7.31 | 5.33 | 5.33 | 3.86 | |||
| H2流率/kg·s-1 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | |||
| 1mol CH4的H2产率/mol | 2.49 | 2.48 | 1.65 | 1.63 | 0.93 | 1.25 | 1.23 | 1.71 | |||
| 1kg H2的CO2排放量/kg | 9.18 | 1.57 | 5.26 | 0.45 | 1.46 | 6.16 | 0.56 | 1.01 | |||
| CO2减排率/% | — | 83 | 43 | 95 | 84 | 33 | 94 | 89 | |||
表3 不同供能方式对碳排放的影响
| CO2捕集条件 | SMR | 熔融金属法 (碳供能) | 熔融金属法 (氢供能) | 熔融金属法 (天然气供能) | 熔融金属法 (电供能) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| — | MDEA脱碳 | — | MEA脱碳 | — | MEA脱碳 | ||||||
| 反应温度/℃ | 890 | 890 | 1200 | 1200 | 1200 | 1200 | 1200 | 1200 | |||
| 反应压力/MPa | 3.2 | 3.2 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| NG流率/kg·s-1 | 2.62 | 2.81 | 3.86 | 3.86 | 7.31 | 5.33 | 5.33 | 3.86 | |||
| H2流率/kg·s-1 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | |||
| 1mol CH4的H2产率/mol | 2.49 | 2.48 | 1.65 | 1.63 | 0.93 | 1.25 | 1.23 | 1.71 | |||
| 1kg H2的CO2排放量/kg | 9.18 | 1.57 | 5.26 | 0.45 | 1.46 | 6.16 | 0.56 | 1.01 | |||
| CO2减排率/% | — | 83 | 43 | 95 | 84 | 33 | 94 | 89 | |||
| 1 | ABÁNADES A, RUBBIA C, SALMIERI D. Thermal cracking of methane into Hydrogen for a CO2-free utilization of natural gas[J]. International Journal of Hydrogen Energy, 2013, 38(20): 8491-8496. |
| 2 | HE Yangdong, ZHU Lin, FAN Junming, et al. Life cycle assessment of CO2 emission reduction potential of carbon capture and utilization for liquid fuel and power cogeneration[J]. Fuel Processing Technology, 2021, 221: 106924. |
| 3 | HE Yangdong, ZHU Lin, LI Luling, et al. Hydrogen and power cogeneration based on chemical looping combustion: is it capable of reducing carbon emissions and the cost of production?[J]. Energy & Fuels, 2020, 34(3): 3501-3512. |
| 4 | NOH Y G, LEE Y J, KIM J, et al. Enhanced efficiency in CO2-free hydrogen production from methane in a molten liquid alloy bubble column reactor with zirconia beads[J]. Chemical Engineering Journal, 2022, 428: 131095. |
| 5 | ABÁNADES A, RUIZ E, FERRUELO E M, et al. Experimental analysis of direct thermal methane cracking[J]. International Journal of Hydrogen Energy, 2011, 36(20): 12877-12886. |
| 6 | SERBAN M, LEWIS M A, MARSHALL C L, et al. Hydrogen production by direct contact pyrolysis of natural gas[J]. Energy & Fuels, 2003, 17(3): 705-713. |
| 7 | AO Dongyi, TANG Yongliang, XU Xiaofeng, et al. Highly conductive PDMS composite mechanically enhanced with 3D-graphene network for high-performance EMI shielding application[J]. Nanomaterials (Basel, Switzerland), 2020, 10(4): 768. |
| 8 | GEIßLER T, ABÁNADES A, HEINZEL A,et al. Hydrogen production via methane pyrolysis in a liquid metal bubble column reactor with a packed bed[J]. Chemical Engineering Journal, 2016, 299: 192-200. |
| 9 | CHEN C J, BACK M H, BACK R A. The thermal decomposition of methane. Ⅱ. Secondary reactions, autocatalysis and carbon formation; non-Arrhenius behaviour in the reaction of CH3 with ethane[J]. Canadian Journal of Chemistry, 1976, 54(20): 3175-3184. |
| 10 | CHEN C J, BACK M H, BACK R A. Mechanism of the thermal decomposition of methane[J]. ACS Symposium Series, 1976(32): 1-16. |
| 11 | ROSCOE J M, THOMPSON M J. Thermal decomposition of methane: Autocatalysis[J]. International Journal of Chemical Kinetics, 1985, 17(9): 967-990. |
| 12 | KHAN M S, CRYNES B L. Survey of recent methane pyrolysis literature[J]. Industrial & Engineering Chemistry, 1970, 62(10): 54-59. |
| 13 | KEVORKIAN V, HEATH C E, BOUDART M. The decomposition of methane in shock waves[J]. The Journal of Physical Chemistry, 1960, 64(8): 964-968. |
| 14 | KOZLOV G I, KNORRE V G. Single-pulse shock tube studies on the kinetics of the thermal decomposition of methane[J]. Combustion and Flame, 1962, 6: 253-263. |
| 15 | CHEN Q Q, LUA A C. Kinetic reaction and deactivation studies on thermocatalytic decomposition of methane by electroless nickel plating catalyst[J]. Chemical Engineering Journal, 2020, 389: 124366. |
| 16 | WANG Jiaofei, LI Xiaoming, ZHOU Yang, et al. Mechanism of methane decomposition with hydrogen addition over activated carbon via in-situ pyrolysis-electron impact ionization time-of-flight mass spectrometry[J]. Fuel, 2020, 263: 116734. |
| 17 | YADAV M D, DASGUPTA K, PATWARDHAN A W, et al. Kinetic study of single-walled carbon nanotube synthesis by thermocatalytic decomposition of methane using floating catalyst chemical vapour deposition[J]. Chemical Engineering Science, 2019, 196: 91-103. |
| 18 | WANG K, LI W S, ZHOU X P. Hydrogen generation by direct decomposition of hydrocarbons over molten magnesium[J]. Journal of Molecular Catalysis A: Chemical, 2008, 283(1/2): 153-157. |
| 19 | ZHOU Lu, ENAKONDA L R, HARB M, et al. Fe catalysts for methane decomposition to produce hydrogen and carbon nano materials[J]. Applied Catalysis B: Environmental, 2017, 208: 44-59. |
| 20 | PLEVAN M, GEIßLER T, ABÁNADES A, et al. Thermal cracking of methane in a liquid metal bubble column reactor: Experiments and kinetic analysis[J]. International Journal of Hydrogen Energy, 2015, 40(25): 8020-8033. |
| 21 | TANG Yongliang, PENG Peng, WANG Shuangyue, et al. Continuous production of graphite nanosheets by bubbling chemical vapor deposition using molten copper[J]. Chemistry of Materials, 2017, 29(19): 8404-8411. |
| 22 | ZENG J R, TARAZKAR M, PENNEBAKER T, et al. Catalytic methane pyrolysis with liquid and vapor phase tellurium[J]. ACS Catalysis, 2020, 10(15): 8223-8230. |
| 23 | PÉREZ B J L, MEDRANO JIMÉNEZ J A, BHARDWAJ R, et al. Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment[J]. International Journal of Hydrogen Energy, 2020, 46(7): 4917-4935. |
| 24 | UPHAM D C, AGARWAL V, KHECHFE A, et al. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon[J]. Science, 2017, 358(6365): 917-921. |
| 25 | PALMER C, BUNYAN E, GELINAS J, et al. CO2-Free hydrogen production by catalytic pyrolysis of hydrocarbon feedstocks in molten Ni-Bi[J]. Energy & Fuels, 2020, 34(12): 16073-16080. |
| 26 | 敖东羿. 多层高品质石墨烯的大量制备及其应用研究[D]. 成都: 电子科技大学, 2020. |
| AO Dongyi. Study on massive production of multilayer high-quality graphene and its applications[D]. Chengdu: University of Electronic Science and Technology of China, 2020. | |
| 27 | PALMER C, TARAZKAR M, KRISTOFFERSEN H H, et al. Methane pyrolysis with a molten Cu-Bi alloy catalyst[J]. ACS Catalysis, 2019, 9(9): 8337-8345. |
| 28 | RIEDEWALD F, SOUSA-GALLAGHER M. Novel waste printed circuit board recycling process with molten salt[J]. Methods X, 2015, 2: 100-106. |
| 29 | RAHIMI N, KANG D, GELINAS J, et al. Solid carbon production and recovery from high temperature methane pyrolysis in bubble columns containing molten metals and molten salts[J]. Carbon, 2019, 151: 181-191. |
| 30 | WICHTERLE K. Breakup of gas bubbles rising in molten metals[J]. Steel Research International, 2010, 81(5): 356-361. |
| 31 | KANG D, RAHIMI N, GORDON M J, et al. Catalytic methane pyrolysis in molten MnCl2-KCl[J]. Applied Catalysis B: Environmental, 2019, 254: 659-666. |
| 32 | KANG D, PALMER C, MANNINI D, et al. Catalytic methane pyrolysis in molten alkali chloride salts containing iron[J]. ACS Catalysis, 2020, 10(13): 7032-7042. |
| 33 | PARKINSON B, PATZSCHKE C F, NIKOLIS D, et al. Methane pyrolysis in monovalent alkali halide salts: Kinetics and pyrolytic carbon properties[J]. International Journal of Hydrogen Energy, 2021, 46(9): 6225-6238. |
| 34 | PARKINSON B, TABATABAEI M, UPHAM D C, et al. Hydrogen production using methane: Techno-economics of decarbonizing fuels and chemicals[J]. International Journal of Hydrogen Energy, 2018, 43(5): 2540-2555. |
| 35 | 芶富均, 陈建军, 叶宗标, 等. 一种催化辅助甲烷裂解制氢的设备: CN113213423A[P]. 2021-08-06. |
| GOU Fujun, CHEN Jianjun, YE Zongbiao,et al. Catalysis-assisted methane cracking hydrogen production equipment: CN113213423A[P]. 2021-08-06. | |
| 36 | KUDINOV I V, PIMENOV A A, KRYUKOV Y A, et al. A theoretical and experimental study on hydrodynamics, heat exchange and diffusion during methane pyrolysis in a layer of molten tin[J]. International Journal of Hydrogen Energy, 2021, 46(17): 10183-10190. |
| 37 | PARKINSON B, MATTHEWS J W, MCCONNAUGHY T B, et al. Techno-economic analysis of methane pyrolysis in molten metals: Decarbonizing natural gas[J]. Chemical Engineering & Technology, 2017, 40(6): 1022-1030. |
| 38 | TIMMERBERG S, KALTSCHMITT M, FINKBEINER M. Hydrogen and hydrogen-derived fuels through methane decomposition of natural gas-GHG emissions and costs[J]. Energy Conversion and Management: X, 2020, 7: 100043. |
| 39 | ABÁNADES A, RATHNAM R K, GEIßLER T, et al. Development of methane decarbonisation based on liquid metal technology for CO2-free production of hydrogen[J]. International Journal of Hydrogen Energy, 2016, 41(19): 8159-8167. |
| 40 | STEINBERG M. Fossil fuel decarbonization technology for mitigating global warming[J]. International Journal of Hydrogen Energy, 1999, 24(8): 771-777. |
| 41 | RODAT S, ABÁNADS S, FLAMANT G. Co-production of hydrogen and carbon black from solar thermal methane splitting in a tubular reactor prototype[J]. Solar Energy, 2011, 85(4): 645-652. |
| 42 | DUFOUR J, GÁLVEZ J L, SERRANO D P, et al. Life cycle assessment of hydrogen production by methane decomposition using carbonaceous catalysts[J]. International Journal of Hydrogen Energy, 2010, 35(3): 1205-1212. |
| 43 | POSTELS S, ABÁNADES A, VON DER ASSEN N, et al. Life cycle assessment of hydrogen production by thermal cracking of methane based on liquid-metal technology[J]. International Journal of Hydrogen Energy, 2016, 41(48): 23204-23212. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [3] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [4] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [5] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [6] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [7] | 史柯柯, 刘木子, 赵强, 李晋平, 刘光. 镁基储氢材料的性能及研究进展[J]. 化工进展, 2023, 42(9): 4731-4745. |
| [8] | 刘木子, 史柯柯, 赵强, 李晋平, 刘光. 固体储氢材料的研究进展[J]. 化工进展, 2023, 42(9): 4746-4769. |
| [9] | 邵志国, 任雯, 许世佩, 聂凡, 许毓, 刘龙杰, 谢水祥, 李兴春, 王庆吉, 谢加才. 终温对油基钻屑热解产物分布和特性影响[J]. 化工进展, 2023, 42(9): 4929-4938. |
| [10] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [11] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [12] | 王兰江, 梁瑜, 汤琼, 唐明兴, 李学宽, 刘雷, 董晋湘. 快速热解铂前体合成高分散的Pt/HY催化剂及其萘深度加氢性能[J]. 化工进展, 2023, 42(8): 4159-4166. |
| [13] | 郭晋, 张耕, 陈国华, 朱鸣, 谭粤, 李蔚, 夏莉, 胡昆. 车载液氢气瓶设计技术的研究进展[J]. 化工进展, 2023, 42(8): 4221-4229. |
| [14] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [15] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||