化工进展 ›› 2023, Vol. 42 ›› Issue (1): 53-66.DOI: 10.16085/j.issn.1000-6613.2022-1533
电化学二氧化碳还原制甲酸催化剂的研究进展
- 北京化工大学化学学院,化工资源有效利用国家重点实验室,北京 100029
-
收稿日期:2022-08-19修回日期:2022-10-11出版日期:2023-01-25发布日期:2023-02-20 -
通讯作者:杨宇森 -
作者简介:李喆(2000—),女,硕士研究生,研究方向为电催化二氧化碳还原。E-mail:2022200971@mail.buct.edu.cn。 -
基金资助:国家重点研发计划(2021YFC2103500);国家自然科学基金(22172006);北京市自然科学基金(2212012);中央高校基本科研业务费(XK1803-05)
Research progress on catalysts for electrocatalytic reduction of carbon dioxide to formic acid
LI Zhe( ), LI Zeyang, YANG Yusen(
), LI Zeyang, YANG Yusen( ), WEI Min
), WEI Min
- State Key Laboratory of Chemical Resource Engineering, College of Chemistry, Beijing University of Chemical Technology, Beijing 100029, China
-
Received:2022-08-19Revised:2022-10-11Online:2023-01-25Published:2023-02-20 -
Contact:YANG Yusen
摘要:
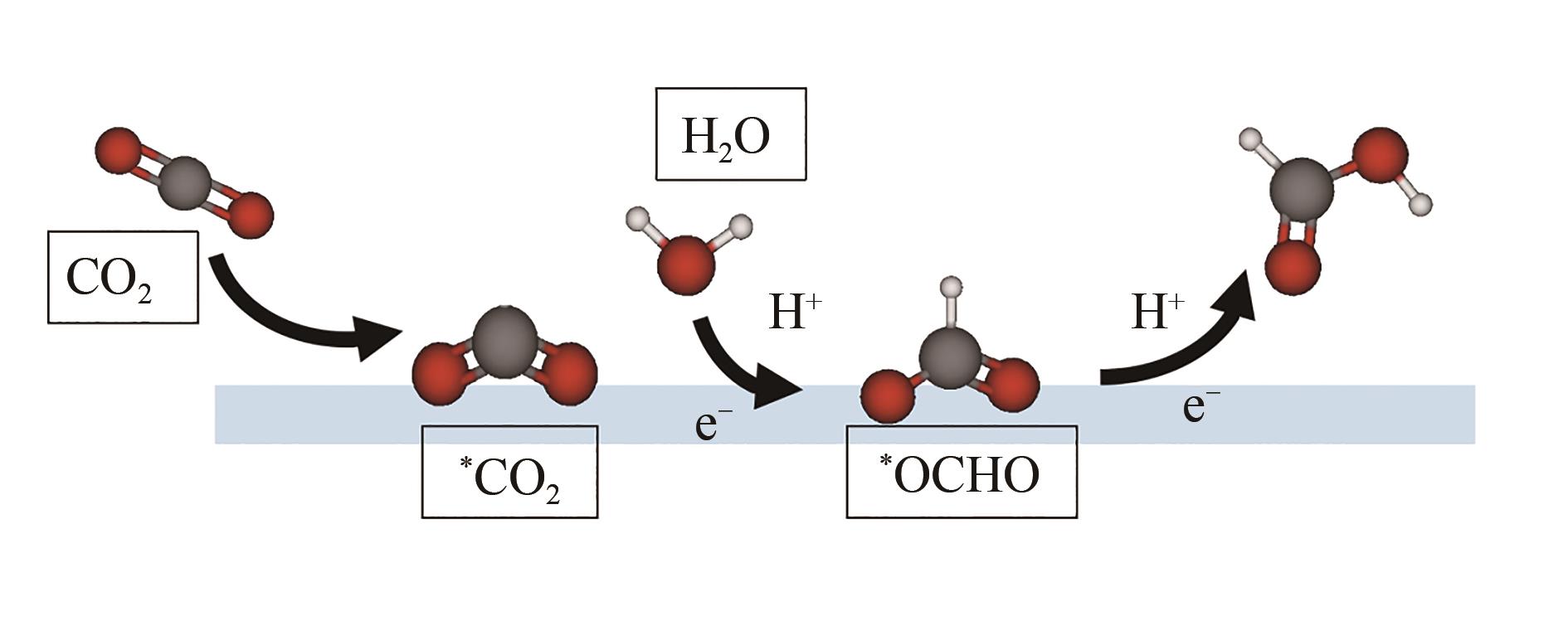
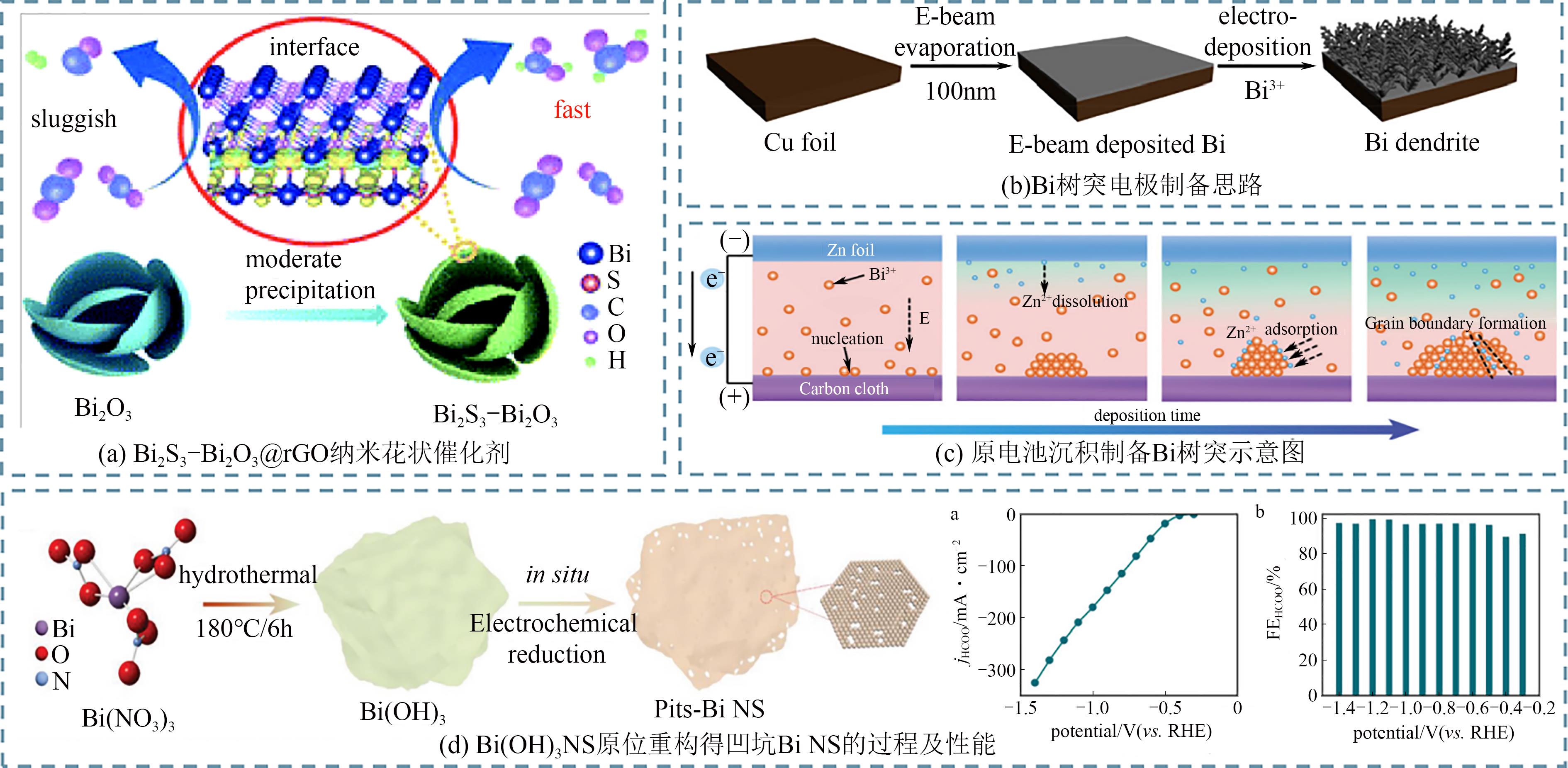
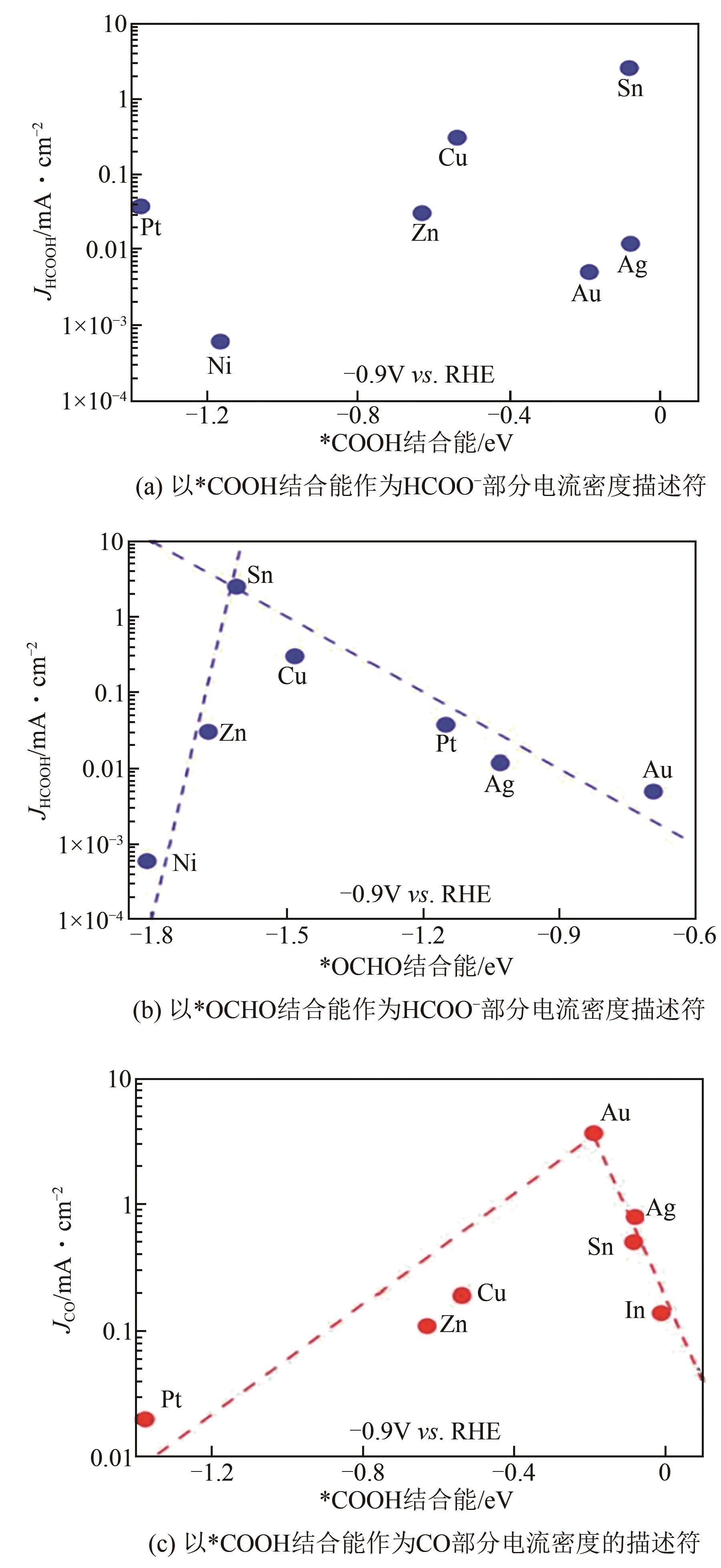
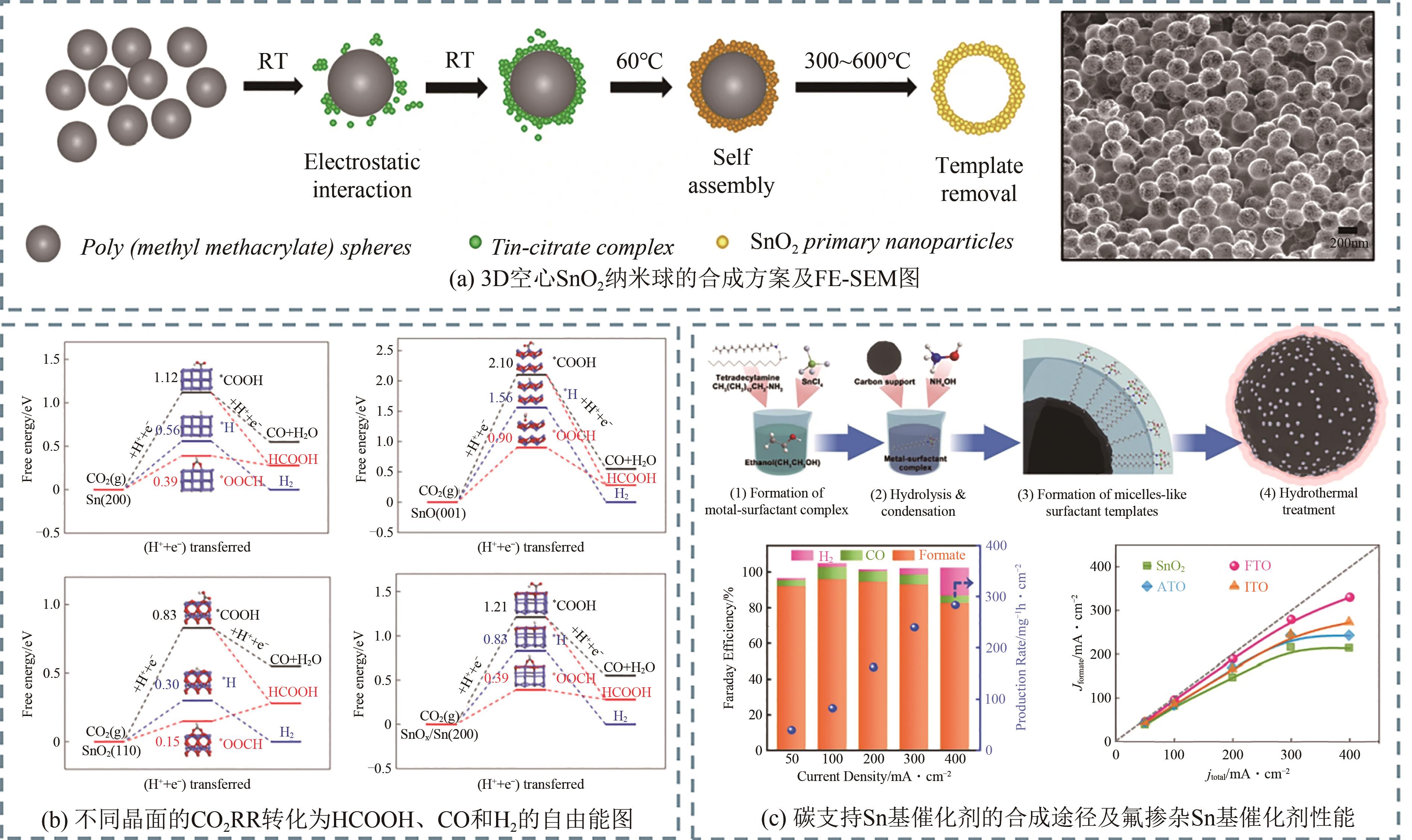
随着日益增长的能源需求,人类社会对于传统碳基化石能源过度依赖,不仅加速了地球上有限能源储备的消耗,还导致大气中二氧化碳(CO2)不断累积。如何对二氧化碳进行可持续的捕获再利用,实现高效的零碳网络循环,已成为人类亟需解决的重大挑战之一。近年来,使用绿色可持续电力的电化学二氧化碳还原反应(CO2RR)生产增值化学品成为研究热点。本文首先介绍了CO2RR的基本电化学反应原理;然后总结了电化学还原CO2制备甲酸/甲酸盐的主要金属基催化剂,着重介绍了Bi、Sn、In三类金属基催化剂的设计调控策略;进一步概括了电化学相关的原位表征手段,分别介绍了原位光谱技术和原位X射线表征技术;最后对电催化二氧化碳还原研究领域的未来发展进行了展望。
中图分类号:
引用本文
李喆, 李泽洋, 杨宇森, 卫敏. 电化学二氧化碳还原制甲酸催化剂的研究进展[J]. 化工进展, 2023, 42(1): 53-66.
LI Zhe, LI Zeyang, YANG Yusen, WEI Min. Research progress on catalysts for electrocatalytic reduction of carbon dioxide to formic acid[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 53-66.
| 物理/化学性质 | 数值 |
|---|---|
| 熔点/℃ | -78.5 |
| 沸点/℃ | -56.6 |
| 溶解度/g·L-1 | 1.45 |
| 第一电离能/eV | 13.79 |
| 电子结合能/eV | 38 |
表1 CO2物理化学性质[6,15]
| 物理/化学性质 | 数值 |
|---|---|
| 熔点/℃ | -78.5 |
| 沸点/℃ | -56.6 |
| 溶解度/g·L-1 | 1.45 |
| 第一电离能/eV | 13.79 |
| 电子结合能/eV | 38 |
| 1 | LAL R. Sequestration of atmospheric CO2 in global carbon pools[J]. Energy & Environmental Science, 2008, 1(1): 86-100. |
| 2 | BUI M, ADJIMAN C S, BARDOW A, et al. Carbon capture and storage (CCS): the way forward[J]. Energy & Environmental Science, 2018, 11(5): 1062-1176. |
| 3 | HO H J, IIZUKA A, SHIBATA E. Carbon capture and utilization technology without carbon dioxide purification and pressurization: a review on its necessity and available technologies[J]. Industrial & Engineering Chemistry Research, 2019, 58(21): 8941-8954. |
| 4 | LI Li, LI Xiaodong, SUN Yongfu, et al. Rational design of electrocatalytic carbon dioxide reduction for a zero-carbon network[J]. Chemical Society Reviews, 2022, 51(4): 1234-1252. |
| 5 | AN Xiaowei, LI Shasha, HAO Xiaoqiong, et al. Common strategies for improving the performances of tin and bismuth-based catalysts in the electrocatalytic reduction of CO2 to formic acid/formate[J]. Renewable and Sustainable Energy Reviews, 2021, 143: 110952. |
| 6 | SENTHILKUMAR P, MOHAPATRA M, BASU S. The inchoate horizon of electrolyzer designs, membranes and catalysts towards highly efficient electrochemical reduction of CO2 to formic acid[J]. RSC Advances, 2022, 12(3): 1287-1309. |
| 7 | Jörg EPPINGER, HUANG Kuowei. Formic acid as a hydrogen energy carrier[J]. ACS Energy Letters, 2017, 2(1): 188-195. |
| 8 | NIE Renfeng, TAO Yuewen, NIE Yunqing, et al. Recent advances in catalytic transfer hydrogenation with formic acid over heterogeneous transition metal catalysts[J]. ACS Catalysis, 2021, 11(3): 1071-1095. |
| 9 | GAO Y X, JAENICKE S, CHUAH G K. Highly efficient transfer hydrogenation of aldehydes and ketones using potassium formate over AlO(OH)-entrapped ruthenium catalysts[J]. Applied Catalysis A: General, 2014, 484: 51-58. |
| 10 | SZATMÁRI I, PAPP G, JOÓ F, et al. Unexpectedly fast catalytic transfer hydrogenation of aldehydes by formate in 2-propanol-water mixtures under mild conditions[J]. Catalysis Today, 2015, 247: 14-19. |
| 11 | LI Jun, ZHANG Yanmei, HAN Difei, et al. Asymmetric transfer hydrogenation using recoverable ruthenium catalyst immobilized into magnetic mesoporous silica[J]. Journal of Molecular Catalysis A: Chemical, 2009, 298(1/2): 31-35. |
| 12 | JAGADEESH R V, BANERJEE D, AROCKIAM P B, et al. Highly selective transfer hydrogenation of functionalised nitroarenes using cobalt-based nanocatalysts[J]. Green Chemistry, 2015, 17(2): 898-902. |
| 13 | WAGH Y S, ASAO N. Selective transfer semihydrogenation of alkynes with nanoporous gold catalysts[J]. The Journal of Organic Chemistry, 2014, 80(2): 847-851. |
| 14 | LI Jie, CHENG Saisai, DU Tianxing, et al. Pd anchored on C3N4 nanosheets/reduced graphene oxide: an efficient catalyst for the transfer hydrogenation of alkenes[J]. New Journal of Chemistry, 2018, 42(11): 9324-9331. |
| 15 | JIN Song, HAO Zhimeng, ZHANG Kai, et al. Advances and challenges for the electrochemical reduction of CO2 to CO: from fundamentals to industrialization[J]. Angewandte Chemie International Edition, 2021, 60(38): 20627-20648. |
| 16 | JOUNY M, LUC W, JIAO Feng. General techno-economic analysis of CO2 electrolysis systems[J]. Industrial & Engineering Chemistry Research, 2018, 57(6): 2165-2177. |
| 17 | KIBRIA M G, EDWARDS J P, GABARDO C M, et al. Electrochemical CO2 reduction into chemical feedstocks: from mechanistic electrocatalysis models to system design[J]. Advanced Materials, 2019, 31(31): e1807166. |
| 18 | SEIFITOKALDANI A, GABARDO C M, BURDYNY T, et al. Hydronium-induced switching between CO2 electroreduction pathways[J]. Journal of the American Chemical Society, 2018, 140(11): 3833-3837. |
| 19 | FEASTER J T, SHI Chuan, CAVE E R, et al. Understanding selectivity for the electrochemical reduction of carbon dioxide to formic acid and carbon monoxide on metal electrodes[J]. ACS Catalysis, 2017, 7(7): 4822-4827. |
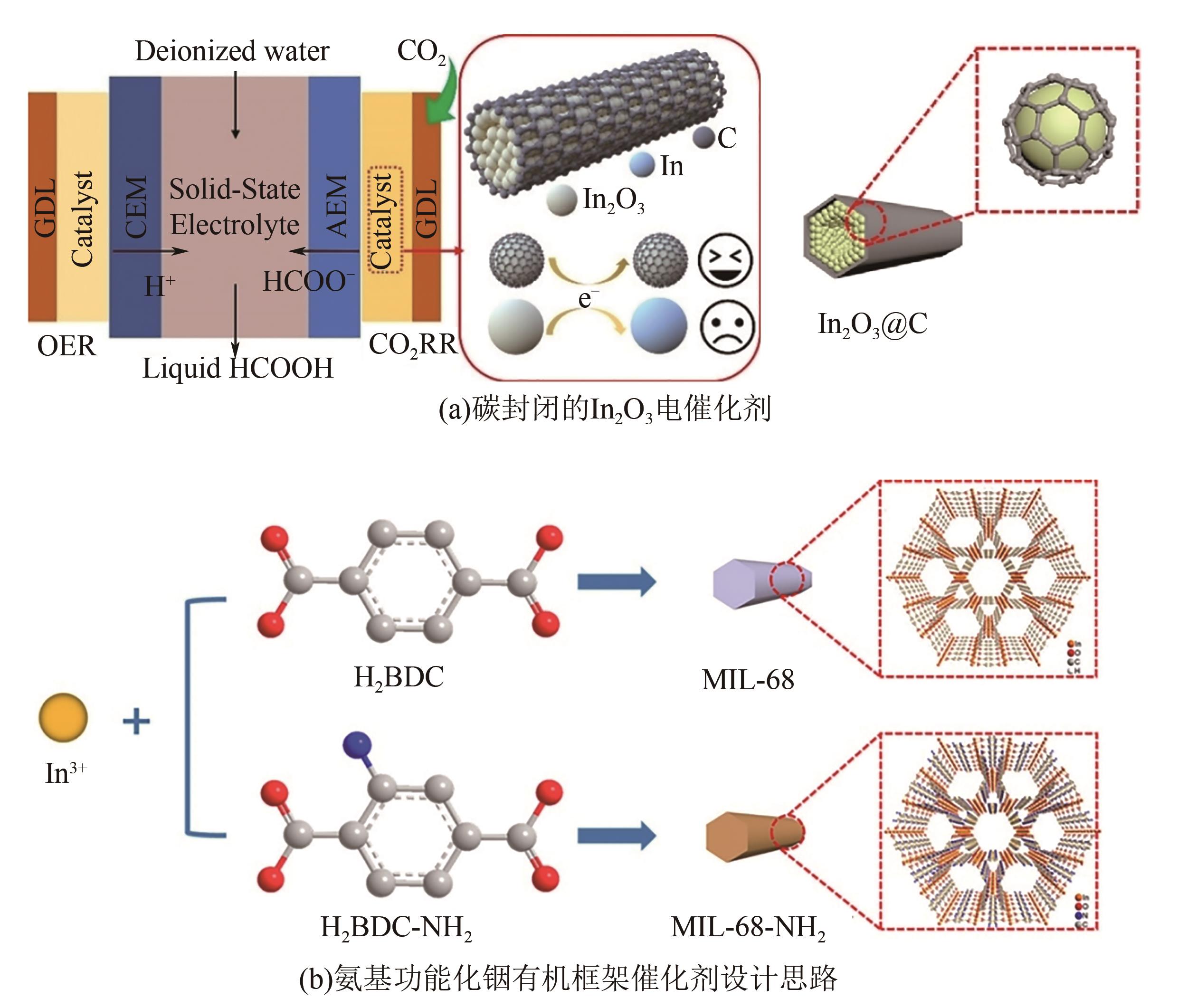
| 20 | 李泽洋, 杨宇森, 卫敏. 二氧化碳还原电催化剂的结构设计及性能研究进展[J]. 化学学报, 2022, 80(2): 199-213. |
| LI Zeyang, YANG Yusen, WEI Min. Structural design and performance of electrocatalysts for carbon dioxide reduction: a review[J]. Acta Chimica Sinica, 2022, 80(2): 199-213. | |
| 21 | ZHANG Baohua, JIANG Yinzhu, GAO Mingxia, et al. Recent progress on hybrid electrocatalysts for efficient electrochemical CO2 reduction[J]. Nano Energy, 2021, 80: 105504. |
| 22 | KOMATSU S, YANAGIHARA T, HIRAGA Y, et al. Electrochemical reduction of CO2 at Sb and Bi electrodes in KHCO3 solution[J]. Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 1995, 63(3): 217-224. |
| 23 | ZHANG Xiaolong, SUN Xinghuan, GUO Sixuan, et al. Formation of lattice-dislocated bismuth nanowires on copper foam for enhanced electrocatalytic CO2 reduction at low overpotential[J]. Energy & Environmental Science, 2019, 12(4): 1334-1340. |
| 24 | ZHENG Hongzhi, WU Guanglei, GAO Guanhui, et al. The bismuth architecture assembled by nanotubes used as highly efficient electrocatalyst for CO2 reduction to formate[J]. Chemical Engineering Journal, 2021, 421: 129606. |
| 25 | ZHANG Xia, LEI Tao, LIU Yuyu, et al. Enhancing CO2 electrolysis to formate on facilely synthesized Bi catalysts at low overpotential[J]. Applied Catalysis B: Environmental, 2017, 218: 46-50. |
| 26 | ZHAO Meiming, GU Yaliu, GAO Weicheng, et al. Atom vacancies induced electron-rich surface of ultrathin Bi nanosheet for efficient electrochemical CO2 reduction[J]. Applied Catalysis B: Environmental, 2020, 266: 118625. |
| 27 | WANG Haiying, WEI Dun, HE Yingjie, et al. Carbon nanoarchitectonics with Bi nanoparticle encapsulation for improved electrochemical deionization performance[J]. ACS Applied Materials & Interfaces, 2022, 14(11): 13177-13185. |
| 28 | KOH J H, WON DA HYE, EOM T, et al. Facile CO2 electro-reduction to formate via oxygen bidentate intermediate stabilized by high-index Planes of Bi dendrite catalyst[J]. ACS Catalysis, 2017, 7(8): 5071-5077. |
| 29 | 郑元波, 张前, 石坚, 等. 电催化还原CO2生成多种产物催化剂研究进展[J]. 化工进展, 2022, 41(3): 1209-1223. |
| ZHENG Yuanbo, ZHANG Qian, SHI Jian, et al. Research progress of catalysts for electrocatalytic reduction of CO2 to various products[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1209-1223. | |
| 30 | YANG Xuxiao, DENG Peilin, LIU Dongyu, et al. Partial sulfuration-induced defect and interface tailoring on bismuth oxide for promoting electrocatalytic CO2 reduction[J]. Journal of Materials Chemistry A, 2020, 8(5): 2472-2480. |
| 31 | CHEN Jialei, CHEN Shan, LI Youzeng, et al. Galvanic-cell deposition enables the exposure of bismuth grain boundary for efficient electroreduction of carbon dioxide[J]. Small, 2022, 18(22): 2201633. |
| 32 | YUAN Yuliang, WANG Qiyou, QIAO Yan, et al. In situ structural reconstruction to generate the active sites for CO2 electroreduction on bismuth ultrathin nanosheets[J]. Advanced Energy Materials, 2022, 12(29): 2200970. |
| 33 | CHEN Chubai, LI Yifan, YANG Peidong. Address the “alkalinity problem” in CO2 electrolysis with catalyst design and translation[J]. Joule, 2021, 5(4): 737-742. |
| 34 | KIBRIA NABIL S, MCCOY S, KIBRIA M G. Comparative life cycle assessment of electrochemical upgrading of CO2 to fuels and feedstocks[J]. Green Chemistry, 2021, 23(2): 867-880. |
| 35 | XIE Yi, Pengfei OU, WANG Xue, et al. High carbon utilization in CO2 reduction to multi-carbon products in acidic media[J]. Nature Catalysis, 2022, 5(6): 564-570. |
| 36 | MONTEIRO M C O, PHILIPS M F, SCHOUTEN K J P, et al. Efficiency and selectivity of CO2 reduction to CO on gold gas diffusion electrodes in acidic media[J]. Nature Communications, 2021, 12: 4943. |
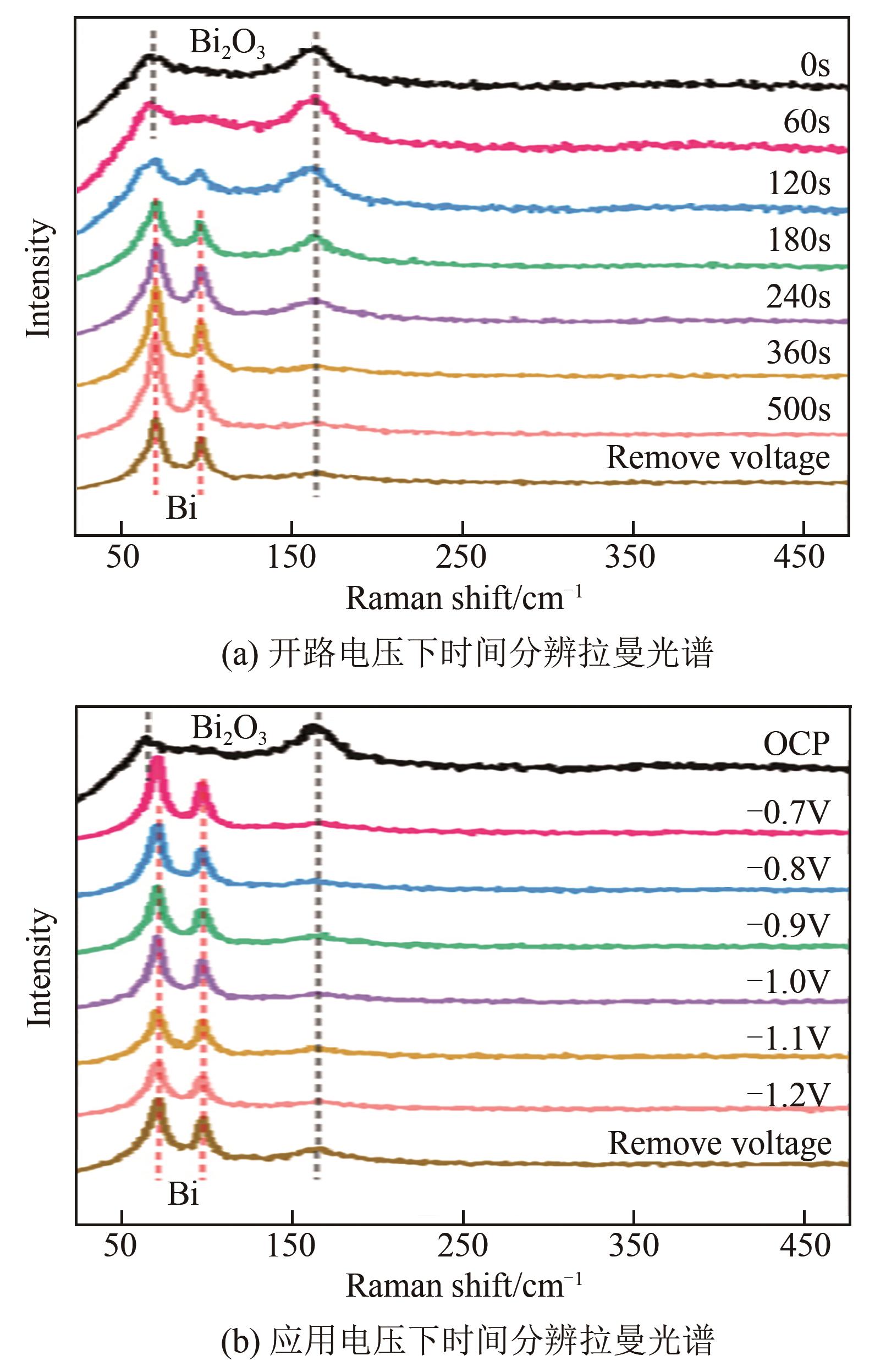
| 37 | BONDUE C J, GRAF M, GOYAL A, et al. Suppression of hydrogen evolution in acidic electrolytes by electrochemical CO2 reduction[J]. Journal of the American Chemical Society, 2021, 143(1): 279-285. |
| 38 | HUANG J E, LI Fengwang, OZDEN A, et al. CO2 electrolysis to multicarbon products in strong acid[J]. Science, 2021, 372(6546): 1074-1078. |
| 39 | QIAO Yan, LAI Wenchuan, HUANG Kai, et al. Engineering the local microenvironment over Bi nanosheets for highly selective electrocatalytic conversion of CO2 to HCOOH in strong acid[J]. ACS Catalysis, 2022, 12(4): 2357-2364. |
| 40 | VESBORG P C K, JARAMILLO T F. Addressing the terawatt challenge: scalability in the supply of chemical elements for renewable energy[J]. RSC Advances, 2012, 2(21): 7933. |
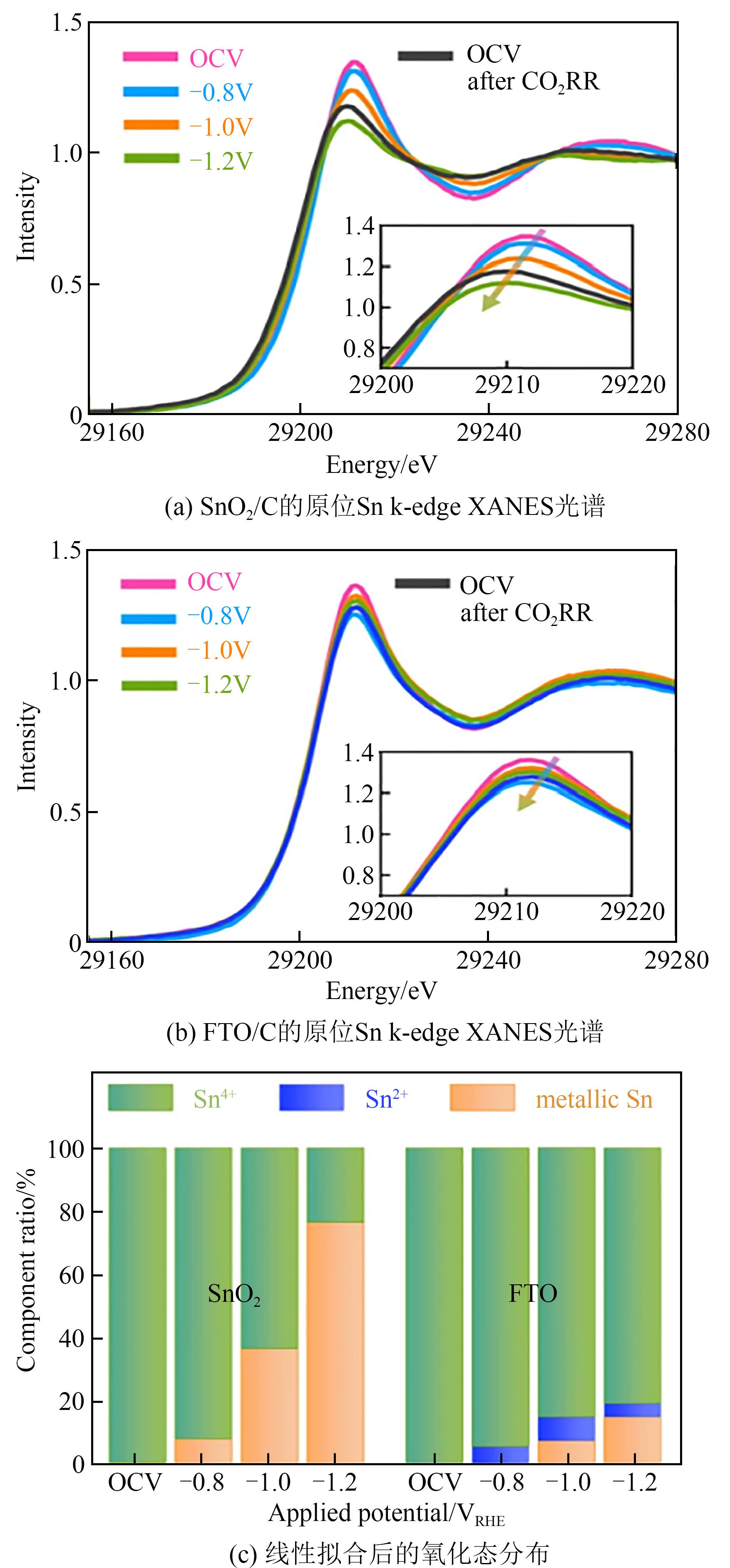
| 41 | ZHAO Shulin, LI Sheng, GUO Tao, et al. Advances in Sn-based catalysts for electrochemical CO2 reduction[J]. Nano-Micro Letters, 2019, 11(1): 62. |
| 42 | DUTTA A, KUZUME A, RAHAMAN M, et al. Monitoring the chemical state of catalysts for CO2 electroreduction: an in operando study[J]. ACS Catalysis, 2015, 5(12): 7498-7502. |
| 43 | NGUYEN-PHAN T D, HU L M, HOWARD B H, et al. High current density electroreduction of CO2 into formate with tin oxide nanospheres[J]. Scientific Reports, 2022, 12: 8420. |
| 44 | CUI Chaonan, HAN Jinyu, ZHU Xinli, et al. Promotional effect of surface hydroxyls on electrochemical reduction of CO2 over SnO x /Sn electrode[J]. Journal of Catalysis, 2016, 343: 257-265. |
| 45 | CHEN Y H, KANAN M W. Tin oxide dependence of the CO2 reduction efficiency on tin electrodes and enhanced activity for tin/tin oxide thin-film catalysts[J]. Journal of the American Chemical Society, 2012, 134(4): 1986-1989. |
| 46 | AN Xiaowei, LI Shasha, YOSHIDA Akihiro, et al. Electrodeposition of tin-based electrocatalysts with different surface tin species distributions for electrochemical reduction of CO2 to HCOOH[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(10): 9360-9368. |
| 47 | KO Y J, KIM J Y, LEE W H, et al. Exploring dopant effects in stannic oxide nanoparticles for CO2 electro-reduction to formate[J]. Nature Communications, 2022, 13(1): 2205. |
| 48 | LIU Xue, HUANG Jiaqi, ZHANG Qiang, et al. Nanostructured metal oxides and sulfides for lithium-sulfur batteries[J]. Advanced Materials, 2017, 29(20): 1601759. |
| 49 | ZHENG Xueli, DE LUNA Phil, GARCÍA DE ARQUER F Pelayo, et al. Sulfur-modulated tin sites enable highly selective electrochemical reduction of CO2 to formate[J]. Joule, 2017, 1(4): 794-805. |
| 50 | CHEN Tao, LIU Tong, DING Tao, et al. Surface oxygen injection in tin disulfide nanosheets for efficient CO2 electroreduction to formate and syngas[J]. Nano-Micro Letters, 2021, 13(1): 1-11. |
| 51 | ZHANG An, HE Rong, LI Huiping, et al. Nickel doping in atomically thin tin disulfide nanosheets enables highly efficient CO2 reduction[J]. Angewandte Chemie International Edition, 2018, 57(34): 10954-10958. |
| 52 | TUREKIAN K K, WEDEPOHL K H. Distribution of the elements in some major units of the earth’s crust[J]. Geological Society of America Bulletin, 1961, 72(2): 175. |
| 53 | HAN Na, DING Pan, HE Le, et al. CO2 reduction: promises of main group metal-based nanostructured materials for electrochemical CO2 reduction to formate[J]. Advanced Energy Materials, 2020, 10(11): 2070046. |
| 54 | DETWEILER Z M, WHITE J L, BERNASEK S L, et al. Anodized indium metal electrodes for enhanced carbon dioxide reduction in aqueous electrolyte[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2014, 30(25): 7593-7600. |
| 55 | ZU Xiaolong, LI Xiaodong, LIU Wei, et al. Efficient and robust carbon dioxide electroreduction enabled by atomically dispersed Sn δ + sites[J]. Advanced Materials, 2019, 31(15): 1808135. |
| 56 | WANG Zhitong, ZHOU Yansong, LIU Dongyu, et al. Carbon-confined indium oxides for efficient carbon dioxide reduction in a solid-state electrolyte flow cell[J]. Angewandte Chemie International Edition, 2022, 61(21): e202200552. |
| 57 | WIJAYA D T, LEE C W. Metal-Organic framework catalysts: a versatile platform for bioinspired electrochemical conversion of carbon dioxide[J]. Chemical Engineering Journal, 2022, 446: 137311. |
| 58 | WANG Zhitong, ZHOU Yansong, XIA Chenfeng, et al. Efficient electroconversion of carbon dioxide to formate by a reconstructed amino-functionalized indium-organic framework electrocatalyst[J]. Angewandte Chemie International Edition, 2021, 60(35): 19107-19112. |
| 59 | SHANG Huishan, WANG Tao, PEI Jiajing, et al. Design of a single-atom indium δ +-N4 interface for efficient electroreduction of CO2 to formate[J]. Angewandte Chemie International Edition, 2020, 59(50): 22465-22469. |
| 60 | GAO Dunfeng, ZHOU Hu, CAI Fan, et al. Pd-containing nanostructures for electrochemical CO2 reduction reaction[J]. ACS Catalysis, 2018, 8(2): 1510-1519. |
| 61 | KLINKOVA A, DE LUNA P, DINH C T, et al. Rational design of efficient palladium catalysts for electroreduction of carbon dioxide to formate[J]. ACS Catalysis, 2016, 6(12): 8115-8120. |
| 62 | HAN Na, SUN Mingzi, ZHOU Yuan, et al. Alloyed palladium-silver nanowires enabling ultrastable carbon dioxide reduction to formate[J]. Advanced Materials, 2021, 33(4): e2005821. |
| 63 | ZHANG Shengli, YAN Zhong, LI Yafei, et al. Atomically thin arsenene and antimonene: semimetal-semiconductor and indirect-direct band-gap transitions[J]. Angewandte Chemie International Edition, 2015, 54(10): 3112-3115. |
| 64 | LI Fengwang, XUE Mianqi, LI Jiezhen, et al. Unlocking the electrocatalytic activity of antimony for CO2 reduction by two-dimensional engineering of the bulk material[J]. Angewandte Chemie International Edition, 2017, 56(46): 14718-14722. |
| 65 | SHUI Ziqing, WANG Yu, LI Yafei. Activity origin of antimony nanosheets toward selective electroreduction of CO2 to formic acid[J]. The Journal of Physical Chemistry C, 2022, 126(8): 4015-4023. |
| 66 | HANDOKO A D, WEI Fengxia, JENNDY, et al. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques[J]. Nature Catalysis, 2018, 1(12): 922-934. |
| 67 | LI Xiaodong, WANG Shumin, LI Li, et al. Progress and perspective for in situ studies of CO2 reduction[J]. Journal of the American Chemical Society, 2020, 142(21): 9567-9581. |
| 68 | YANG Chengwu, Christof WÖLL. IR spectroscopy applied to metal oxide surfaces: adsorbate vibrations and beyond[J]. Advances in Physics: X, 2017, 2(2): 373-408. |
| 69 | WEI Xing, YIN Zhenglei, Kangjie LYU, et al. Highly selective reduction of CO2 to C2+ hydrocarbons at copper/polyaniline interfaces[J]. ACS Catalysis, 2020, 10(7): 4103-4111. |
| 70 | SUI Pengfei, GAO Minrui, LIU Subiao, et al. Carbon dioxide valorization via formate electrosynthesis in a wide potential window[J]. Advanced Functional Materials, 2022, 32(32): 2203794. |
| 71 | KIM Y, PARK S, SHIN S J, et al. Time-resolved observation of C—C coupling intermediates on Cu electrodes for selective electrochemical CO2 reduction[J]. Energy & Environmental Science, 2020, 13(11): 4301-4311. |
| 72 | DUNWELL M, YANG Xuan, SETZLER B P, et al. Examination of near-electrode concentration gradients and kinetic impacts on the electrochemical reduction of CO2 using surface-enhanced infrared spectroscopy[J]. ACS Catalysis, 2018, 8(5): 3999-4008. |
| 73 | BANERJEE S, ZHANG Zhuoqun, HALL A S, et al. Surfactant perturbation of cation interactions at the electrode-electrolyte interface in carbon dioxide reduction[J]. ACS Catalysis, 2020, 10(17): 9907-9914. |
| 74 | ZOU Yuqin, WANG Shuangyin. An investigation of active sites for electrochemical CO2 reduction reactions: from in situ characterization to rational design[J]. Advanced Science, 2021, 8(9): 2003579. |
| 75 | WANG Hongbo, TANG Chongyang, SUN Bo, et al. In-situ structural evolution of Bi2O3 nanoparticle catalysts for CO2 electroreduction[J]. International Journal of Extreme Manufacturing, 2022, 4(3): 035002. |
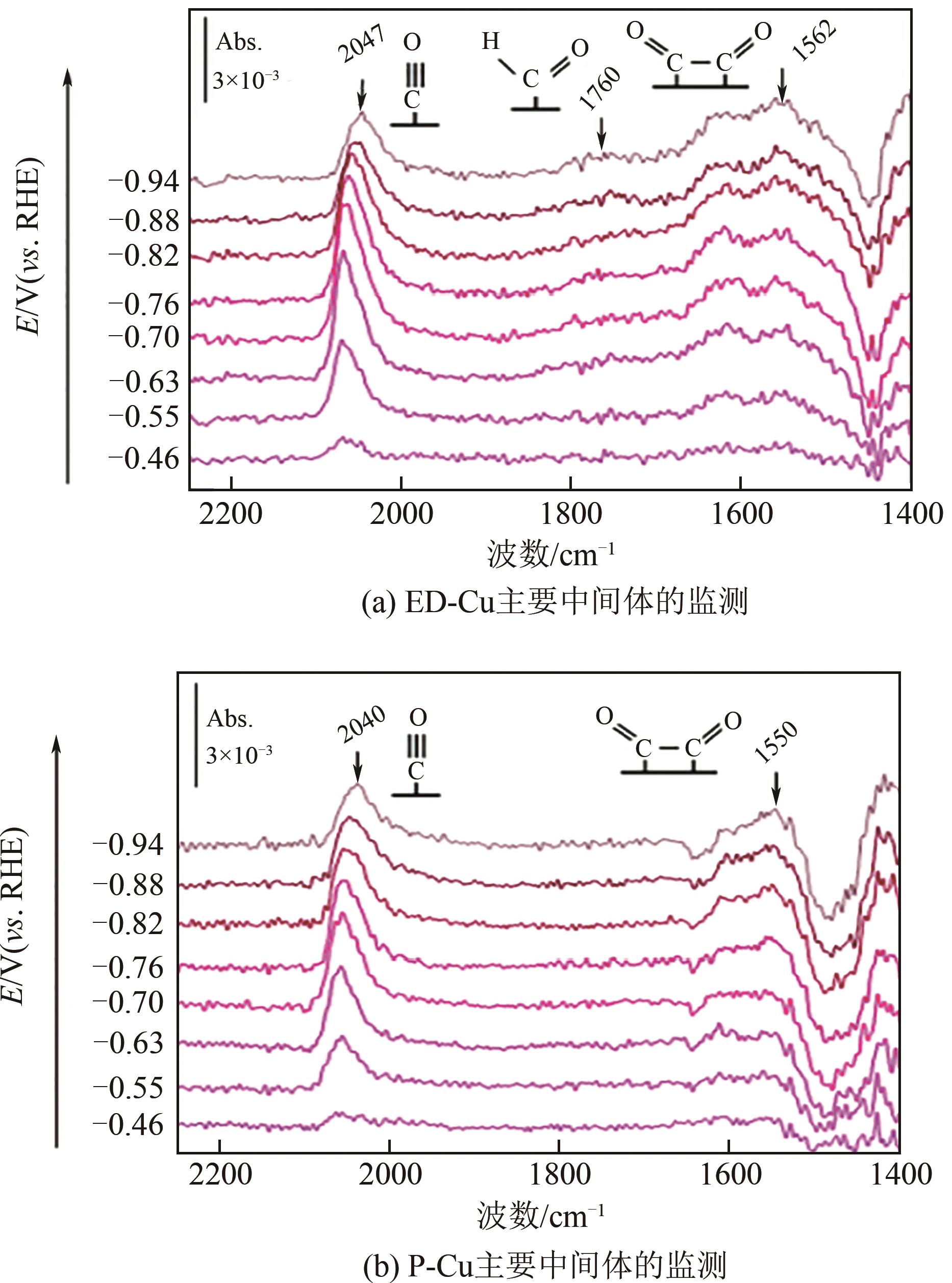
| 76 | HE Ming, CHANG Xiaoxia, CHAO Tzu-Hsuan, et al. Selective enhancement of methane formation in electrochemical CO2 reduction enabled by a Raman-inactive oxygen-containing species on Cu[J]. ACS Catalysis, 2022, 12(10): 6036-6046. |
| 77 | ZHAO Yang, LIU Xunlin, LIU Zhixiao, et al. Spontaneously Sn-doped Bi/BiO x core-shell nanowires toward high-performance CO2 electroreduction to liquid fuel[J]. Nano Letters, 2021, 21(16): 6907-6913. |
| 78 | HOLLANDER J M, JOLLY W L. X-ray photoelectron spectroscopy[J]. Accounts of Chemical Research, 1970, 3(6): 193-200. |
| 79 | CHOI Y W, SCHOLTEN F, SINEV I, et al. Enhanced stability and CO/formate selectivity of plasma-treated SnO x /AgO x catalysts during CO2 electroreduction[J]. Journal of the American Chemical Society, 2019, 141(13): 5261-5266. |
| 80 | LI Jing, WU Donghuan, MALKANI A S, et al. Hydroxide is not a promoter of C2+ product formation in the electrochemical reduction of CO on copper[J]. Angewandte Chemie International Edition, 2020, 59(11): 4464-4469. |
| 81 | GABARDO C M, SEIFITOKALDANI A, EDWARDS J P, et al. Combined high alkalinity and pressurization enable efficient CO2 electroreduction to CO[J]. Energy & Environmental Science, 2018, 11(9): 2531-2539. |
| 82 | THORSON M R, SIIL K I, KENIS P J A. Effect of cations on the electrochemical conversion of CO2 to CO[J]. Journal of the Electrochemical Society, 2012, 160(1): F69-F74. |
| 83 | GAO Feiyue, BAO Ruicheng, GAO Minrui, et al. Electrochemical CO2-to-CO conversion: electrocatalysts, electrolytes, and electrolyzers[J]. Journal of Materials Chemistry A, 2020, 8(31): 15458-15478. |
| 84 | KIM D, CHOI W, LEE H W, et al. Electrocatalytic reduction of low concentrations of CO2 gas in a membrane electrode assembly electrolyzer[J]. ACS Energy Letters, 2021, 6(10): 3488-3495. |
| 85 | GE Lei, RABIEE H, LI Mengran, et al. Electrochemical CO2 reduction in membrane-electrode assemblies[J]. Chem., 2022, 8(3): 663-692. |
| 86 | 李冰玉, 毛庆, 赵健, 等. 二氧化碳电化学还原反应器的研究进展[J]. 化工进展, 2019, 38(11): 4901-4910. |
| LI Bingyu, MAO Qing, ZHAO Jian, et al. Research progress in CO2 electroreduction reactor[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 4901-4910. | |
| 87 | WEI Xinfa, LI Yan, CHEN Lisong, et al. Formic acid electro-synthesis by concurrent cathodic CO2 reduction and anodic CH3 OH oxidation[J]. Angewandte Chemie International Edition, 2021, 60(6): 3148-3155. |
| 88 | LI Yu, HUO Cuizhu, WANG Hongjuan, et al. Coupling CO2 reduction with CH3OH oxidation for efficient electrosynthesis of formate on hierarchical bifunctional CuSn alloy[J]. Nano Energy, 2022, 98: 107277. |
| 89 | GUO Shengyuan, ASSET T, ATANASSOV P. Catalytic hybrid electrocatalytic/biocatalytic cascades for carbon dioxide reduction and valorization[J]. ACS Catalysis, 2021, 11(9): 5172-5188. |
| 90 | NAHA S, JOSHI C, CHANDRASHEKHAR B, et al. Bioelectrosynthesis of organic and inorganic chemicals in bioelectrochemical system[J]. Journal of Hazardous, Toxic, and Radioactive Waste, 2020, 24(2): 03120001. |
| 91 | CASTAÑEDA-LOSADA L, ADAM D, PACZIA N, et al. Bioelectrocatalytic cofactor regeneration coupled to CO2 fixation in a redox-active hydrogel for stereoselective C—C bond formation[J]. Angewandte Chemie International Edition, 2021, 60(38): 21056-21061. |
| 92 | WU Yun, LI Weichao, WANG Lutian, et al. Enhancing the selective synthesis of butyrate in microbial electrosynthesis system by gas diffusion membrane composite biocathode[J]. Chemosphere, 2022, 308: 136088. |
| 93 | LIANG Qinjun, GAO Yu, LI Zhigang, et al. Electricity-driven ammonia oxidation and acetate production in microbial electrosynthesis systems[J]. Frontiers of Environmental Science & Engineering, 2021, 16(4): 1-10. |
| 94 | ZHENG Tingting, ZHANG Menglu, WU Lianghuan, et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering[J]. Nature Catalysis, 2022, 5(5): 388-396. |
| 95 | ZHANG Zhen, WEN Guobin, LUO Dan, et al. “Two ships in a bottle” design for Zn-Ag-O catalyst enabling selective and long-lasting CO2 electroreduction[J]. Journal of the American Chemical Society, 2021, 143(18): 6855-6864. |
| 96 | ZHAO Qing, MARTIREZ J M P, CARTER E A. Revisiting understanding of electrochemical CO2 reduction on Cu(111): competing proton-coupled electron transfer reaction mechanisms revealed by embedded correlated wavefunction theory[J]. Journal of the American Chemical Society, 2021, 143(16): 6152-6164. |
| 97 | CHEN Baotong, LI Boran, TIAN Ziqi, et al. Enhancement of mass transfer for facilitating industrial-level CO2 electroreduction on atomic Ni-N4 sites[J]. Advanced Energy Materials, 2021, 11(40): 2102152. |
| 98 | ZHANG Ningqiang, ZHANG Xinxin, KANG Yikun, et al. A supported Pd2 dual-atom site catalyst for efficient electrochemical CO2 reduction[J]. Angewandte Chemie International Edition, 2021, 60(24): 13388-13393. |
| 99 | NØRSKOV J K, BLIGAARD T, ROSSMEISL J, et al. Towards the computational design of solid catalysts[J]. Nature Chemistry, 2009, 1(1): 37-46. |
| 100 | SUN Zhehao, YIN Hang, LIU Kaili, et al. Machine learning accelerated calculation and design of electrocatalysts for CO2 reduction[J]. SmartMat, 2022, 3(1): 68-83. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [3] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [4] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [5] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [6] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [7] | 刘炫麟, 王驿凯, 戴苏洲, 殷勇高. 热泵中氨基甲酸铵分解反应特性及反应器结构优化[J]. 化工进展, 2023, 42(9): 4522-4530. |
| [8] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [9] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [10] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [11] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [12] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [13] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [14] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [15] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||