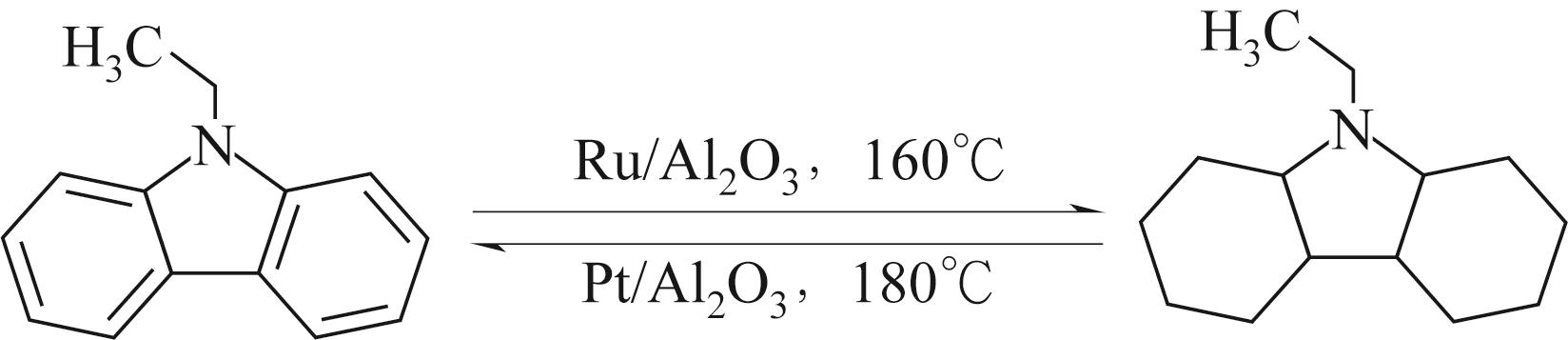化工进展 ›› 2022, Vol. 41 ›› Issue (S1): 108-117.DOI: 10.16085/j.issn.1000-6613.2022-0674
氢能储存技术最新进展
韩利( ), 李琦(
), 李琦( ), 冷国云, 魏雯珍, 李钰颖, 吴玉庭
), 冷国云, 魏雯珍, 李钰颖, 吴玉庭
- 北京工业大学传热强化与过程节能教育部重点实验室,传热与能源应用北京市重点实验室,北京 100124
-
收稿日期:2022-04-15修回日期:2022-06-24出版日期:2022-10-20发布日期:2022-11-10 -
通讯作者:李琦 -
作者简介:韩利(1997—),男,硕士研究生,研究方向为复合相变材料的制备与表征。E-mail:15031364973@163.com。 -
基金资助:北京市自然科学基金(3222026)
Latest research progress of hydrogen energy storage technology
HAN Li( ), LI Qi(
), LI Qi( ), LENG Guoyun, WEI Wenzhen, LI Yuying, WU Yuting
), LENG Guoyun, WEI Wenzhen, LI Yuying, WU Yuting
- Key Laboratory of Heat Transfer Enhancement and Process Energy Saving, Ministry of Education, Beijing University of Technology, Beijing Key Laboratory of Heat Transfer and Energy Application, Beijing 100124, China
-
Received:2022-04-15Revised:2022-06-24Online:2022-10-20Published:2022-11-10 -
Contact:LI Qi
摘要:
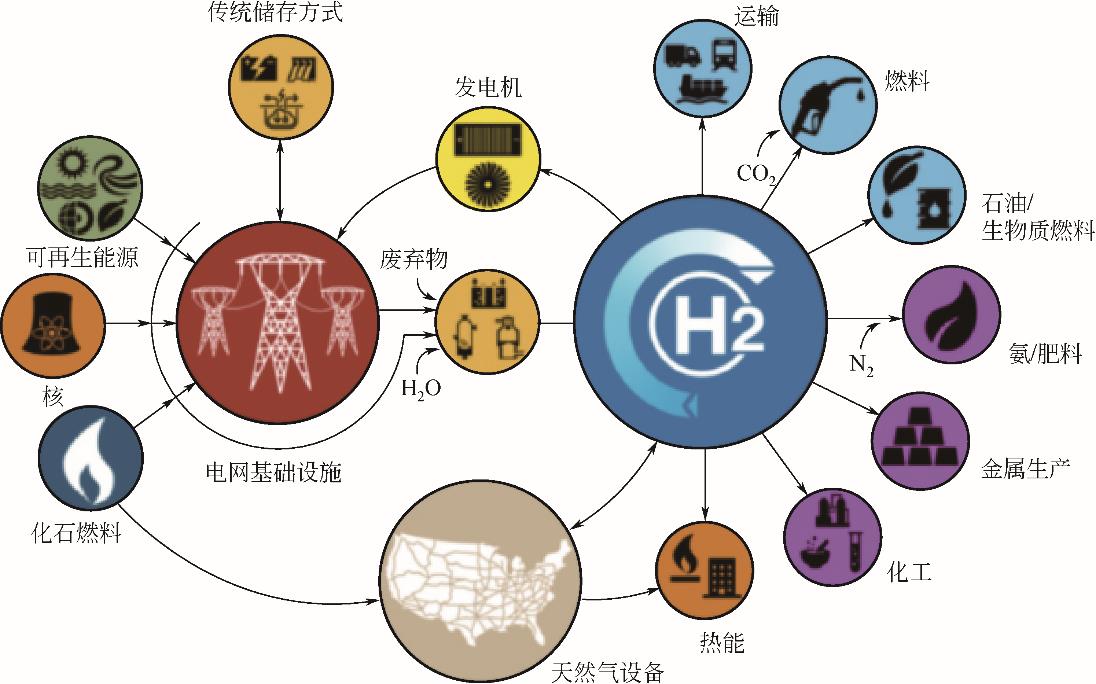
氢能是全球能源技术革命的重要发展方向,在氢能产业发展过程中,开发高效、安全和低成本的氢能储存技术是实现大规模用氢的必要保障和关键。本文综述了当前主流的四种氢能储存技术,即高压气态储氢、低温液态储氢、有机液态储氢、固体材料储氢的原理和技术特点,分析整理了这几种储氢技术的优缺点,讨论了各类储氢方式的最新研究现状和面临的关键挑战,并对未来储氢技术的优化和发展趋势进行了展望。可以发现,为了提高储氢量,研究人员都将重心放在开发具有成本效益、提高能量密度的储氢技术上。其中,高压气态储氢应着力开发低成本、高性能的碳纤维复合材料,降低Ⅳ型瓶的成本;低温液态储氢应把研究重点放在降低液压成本以及寻求廉价易得的保温材料上;对于有机液态储氢来说,寻求高效催化剂可以大幅度提高其储氢能力;固体材料储氢应着力研发高效催化剂,寻求可以提高氢气与材料相互作用力的途径。政府、企业及科研院应大力推进储氢技术的研究,加速氢能产业发展,早日实现碳中和目标。
中图分类号:
引用本文
韩利, 李琦, 冷国云, 魏雯珍, 李钰颖, 吴玉庭. 氢能储存技术最新进展[J]. 化工进展, 2022, 41(S1): 108-117.
HAN Li, LI Qi, LENG Guoyun, WEI Wenzhen, LI Yuying, WU Yuting. Latest research progress of hydrogen energy storage technology[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 108-117.
| 储氢方式 | 优点 | 缺点 |
|---|---|---|
| 高压气态储氢 | 技术成熟、结构简单 | 储氢密度低、安全性较差 |
| 低温液态储氢 | 储氢密度大、安全性好 | 氢液化能耗大、储氢容器要求高 |
| 有机液态储氢 | 纯度高、储氢密度大 | 成本高、能耗大、操作空间苛刻 |
| 固体材料储氢 | 易携带、安全性好 | 单位质量储氢密度低、充放氢 效率低 |
表1 四种储氢方式优缺点
| 储氢方式 | 优点 | 缺点 |
|---|---|---|
| 高压气态储氢 | 技术成熟、结构简单 | 储氢密度低、安全性较差 |
| 低温液态储氢 | 储氢密度大、安全性好 | 氢液化能耗大、储氢容器要求高 |
| 有机液态储氢 | 纯度高、储氢密度大 | 成本高、能耗大、操作空间苛刻 |
| 固体材料储氢 | 易携带、安全性好 | 单位质量储氢密度低、充放氢 效率低 |
| 类型 | 材质 | 工作压力 /MPa | 质量储氢 密度/% | 使用寿命 /a |
|---|---|---|---|---|
| Ⅰ型 | 纯钢制金属瓶 | 17.5~20 | 约1 | 15 |
| Ⅱ型 | 钢制内胆纤维缠绕瓶 | 26.3~30 | 约1.5 | 15 |
| Ⅲ型 | 铝内胆纤维缠绕瓶 | 30~70 | 2.4~4.1 | 15~20 |
| Ⅳ型 | 塑料内胆纤维缠绕瓶 | >70 | 2.5~5.7 | 15~20 |
表2 不同类型储氢罐性能对比
| 类型 | 材质 | 工作压力 /MPa | 质量储氢 密度/% | 使用寿命 /a |
|---|---|---|---|---|
| Ⅰ型 | 纯钢制金属瓶 | 17.5~20 | 约1 | 15 |
| Ⅱ型 | 钢制内胆纤维缠绕瓶 | 26.3~30 | 约1.5 | 15 |
| Ⅲ型 | 铝内胆纤维缠绕瓶 | 30~70 | 2.4~4.1 | 15~20 |
| Ⅳ型 | 塑料内胆纤维缠绕瓶 | >70 | 2.5~5.7 | 15~20 |
| 配位体 | 示例 |
|---|---|
| [AlH4]- | NaAlH4,Ca(AlH4)2,Ti(AlH4)4 |
| [BH4]- | LiBH4,NaBH4,Al(BH4)3 |
| VIIIB 族元素 | Mg2NiH4,Mg2FeH6 |
表3 配位氢化物分类
| 配位体 | 示例 |
|---|---|
| [AlH4]- | NaAlH4,Ca(AlH4)2,Ti(AlH4)4 |
| [BH4]- | LiBH4,NaBH4,Al(BH4)3 |
| VIIIB 族元素 | Mg2NiH4,Mg2FeH6 |
| 1 | PIVOVAR B, RUSTAGI N, SATYAPAL S. Hydrogen at scale (H2@Scale) key to a clean, economic, and sustainable energy system[J]. The Electrochemical Society, 2018, 27(1): 47-52. |
| 2 | 中国氢能联盟. 中国氢能源及燃料电池产业白皮书2019[R]. 2019. |
| China Hydrogen Alliance. White paper of hydrogen energy and fuel cell industryon in China in 2019[R]. 2019. | |
| 3 | 中国氢能联盟. 中国氢能源及燃料电池产业白皮书2020[R]. 2020. |
| China Hydrogen Alliance. White paper of hydrogen energy and fuel cell industryon in China in 2020[R]. 2019. | |
| 4 | 李建勋. 加氢站氢气充装和放散过程分析[J]. 煤气与热力, 2020, 40(5): 15-20, 45. |
| LI Jianxun. Analysis of hydrogen filling and venting process in hydrogen refueling station[J]. Gas&Heat, 2020, 40(5): 15-20, 45. | |
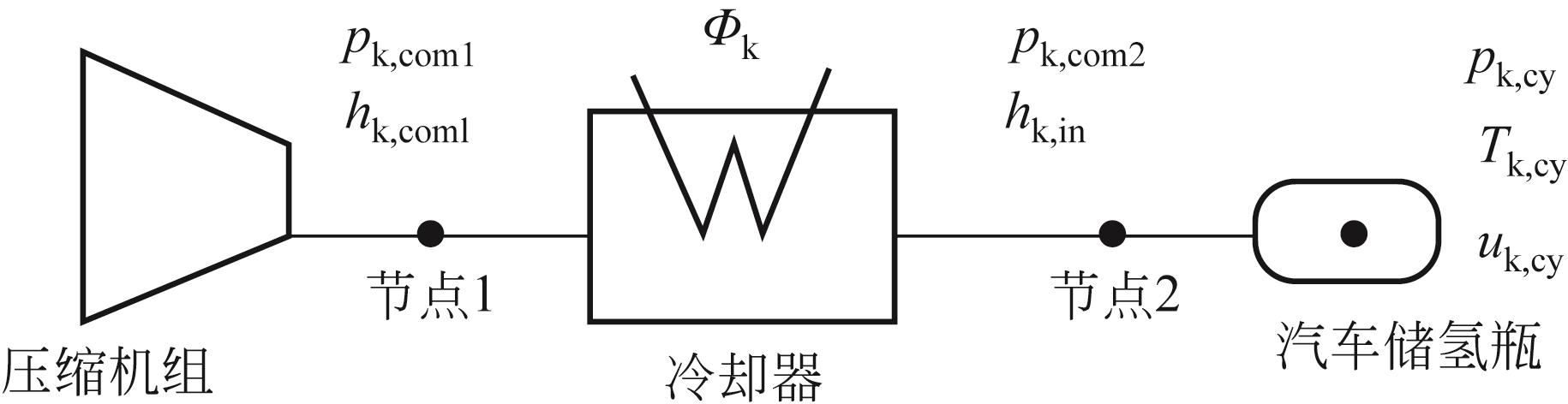
| 5 | 李建, 张立新, 李瑞懿, 等. 高压储氢容器研究进展[J]. 储能科学与技术, 2021, 10(5): 1835-1844. |
| LI Jian, ZHANG Lixin, LI Ruiyi, et al. High-pressure gaseous hydrogen storage vessels: Currentstatus and prospects[J]. Energy Storage Science and Technology, 2021, 10(5): 1835-1844. | |
| 6 | QIN Y Q, GONG Y, YUAN Y W, et al. Failure analysis on leakage of hydrogen storage tank for vehicles occurring in oil circulation fatigue test[J]. Engineering Failure Analysis, 2020, 117(2): 104830. |
| 7 | YU S, HONG L, WEI Z, et al. Research on hydrogen permeability of polyamide 6 as the liner material for type Ⅳ hydrogen storage tank[J]. International Journal of Hydrogen Energy, 2020, 45(46): 24980-24990. |
| 8 | YERSAK T A, BAKER D R, YANAGISAWA Y, et al. Predictive model for depressurization-induced blistering of type Ⅳ tank liners for hydrogen storage[J]. International Journal of Hydrogen Energy, 2017, 42(48): 28910-28917. |
| 9 | CORGNALE C, HARDY B, CHAHINE R, et al. Hydrogen storage in a two-liter adsorbent prototype tank for fuel cell driven vehicles[J]. Applied Energy, 2019, 250: 333-343. |
| 10 | ZU L, KOUSSIOS S, BEUKERS A, et al. A novel design solution for improving the performance of composite toroidal hydrogen storage tanks[J]. International Journal of Hydrogen Energy, 2012, 37(19): 14343-14350. |
| 11 | ZUO Z, JIANG W B, QIN X, et al. Numerical investigation on full thermodynamic venting process of liquid hydrogen in an on-orbit storage tank[J]. International Journal of Hydrogen Energy, 2020, 45(51): 27792-27805. |
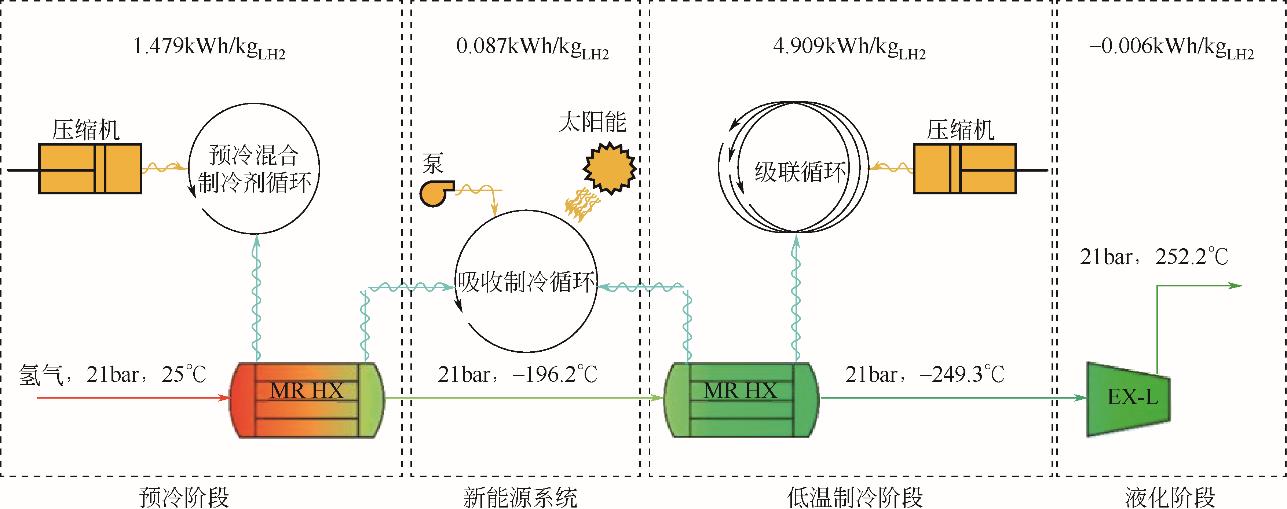
| 12 | AASADNIAA M, MEHRPOOYA M. Conceptual design and analysis of a novel process for hydrogen liquefaction assisted by absorption precooling system[J]. Journal of Cleaner Production, 2018, 205: 565-588. |
| 13 | JIANG W, SUN P, LI P, et al. Transient thermal behavior of multi-layer insulation coupled with vapor cooled shield used for liquid hydrogen storage tank[J]. Energy, 2021, 231: 120859. |
| 14 | 陈国邦, 张鹏. 低温绝热与传热技术[M]. 北京: 科学出版社, 2004. |
| CHEN Guobang, ZHANG Peng. Low temperature insulation and heat transfer technology[M]. Beijing: Science Press, 2004. | |
| 15 | 胡伟峰, 申麟, 彭小波, 等. 低温推进剂长时间在轨的蒸发量控制关键技术分析[J]. 低温工程, 2011(3): 8. |
| HU Weifeng, SHEN Lin, PENG Xiaobo, et al. Key technology analysis of boil-off control study on cryogenic propellant long-term application on orbit[J]. Cryogenic Engineering, 2011(3): 8. | |
| 16 | XU X, XU H, YANG B, et al. A novel composite insulation system of hollow glass microspheres and multilayer insulation with self-evaporating vapor cooled shield for liquid hydrogen storage[J]. Energy Technology, 2020, 8(9): 2000591. |
| 17 | NOTARDONATO W U, SWANGER A M, FESMIRE J E, et al. Zero boil-off methods for large-scale liquid hydrogen tanks using integrated refrigeration and storage[J]. Materials Science and Engineering, 2017, 278: doi:10.1088/1757-899X/278/1/D12012. |
| 18 | 曹军文, 覃祥富, 耿嘎, 等. 氢气储运技术的发展现状与展望[J]. 石油学报(石油加工), 2021, 37(6): 1461-1478. |
| CAO Junwen, Tan Xiangfu, GENG Ga, et al. Current status and projects of hydrogen storage and transportation technology[J]. Chinese Journal of Petroleum (Petroleum Processing), 2021, 37(6): 1461-1478. | |
| 19 | KIM T W, KIM M, KIM S K, et al. Remarkably fast low-temperature hydrogen storage into aromatic benzyltoluenes over MgO-supported Ru nanoparticles with homolytic and heterolytic H2 adsorption[J]. Applied Catalysis B: Environmental, 2021, 286: 119889. |
| 20 | CRABTREE, ROBERT H. Hydrogen storage in liquid organi cheterocycles[J]. Energy & Environmental Science, 2008, 1(1): 134-138. |
| 21 | EBLAGON K M, TAM K, TSANG S C. Comparison of catalytic performance of supported ruthenium and rhodium for hydrogenation of 9-ethylcarbazole for hydrogen storage applications[J]. Energy&Environmental Science, 2012, 5(9): 8621-8630. |
| 22 | YU H, YANG X, JIANG X, et al. LaNi5.5 particles for reversible hydrogen storage in N-ethylcarbazole[J]. Nano Energy, 2021, 80: 105476. |
| 23 | YANG X, WU Y M, YU H G, et al. A YH3 promoted palladium catalyst for reversible hydrogen storage of N-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2020, 45(58): 33657-33662. |
| 24 | WANG S D, HUANG H Y, BRUNEAN C, et al. Iridium-catalyzed hydrogenation and dehydrogenation of N-heterocycles in water under mild conditions[J]. ChemSusChem, 2019, 12(11): 2350-2354. |
| 25 | WANG Z H, BELLI J, JENSEN C M, et al. Catalysed low temperature H2 release from nitrogen heterocycles[J]. Faraday Discussions, 2011, 151: 297-305. |
| 26 | SOGAARD A, SCHEUERMEYER M, BÖSMANN A, et al. Homogeneously-catalysed hydrogen release/storage using the 2-methylindole/2-methylindoline LOHC system in molten salt-organic biphasic reaction systems[J]. Chemical Communications, 2019, 55(14): 2046-2049. |
| 27 | VEREVKIN S P, KONNOVA M E, ZHERIKOVA K V, et al. Sustainable hydrogen storage: Thermochemistry of amino-alcohols as seminal liquid organic hydrogen carriers[J]. The Journal of Chemical Thermodynamics, 2021, 163: 106610. |
| 28 | ZOU Y Q, WOLFF N V, ANABY A, et al. Ethylene glycol as an efficient and reversible liquid-organic hydrogen carrier[J]. Nature Catalysis, 2019, 2(5): 415-422. |
| 29 | SHAO Z, LI Y, LIU C, et al. Reversible interconversion between methanol-diamine and diamide for hydrogen storage based on manganese catalyzed (de)hydrogenation[J]. Nature Communications, 2020, 11(1): doi: 10.1038/S41467-020-14380-3. |
| 30 | NAZIR G, REHMAN A, HUSSAIN S A, et al. Heteroatoms-doped hierarchical porous carbons: Multifunctional materials for effective methylene blue removal and cryogenic hydrogen storage[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 630. |
| 31 | 张集. 活性炭纤维改性及储氢性能研究[D]. 大连: 大连理工大学, 2020. |
| ZHANG Ji. Study on activated carbon fiber modification and hydrogen storage[D]. Dalian: Dalian University of Technology, 2020. | |
| 32 | GEORGE J K, YADAV A, VERMA N. Electrochemical hydrogen storage behavior of Ni-Ceria impregnated carbon micro-nanofibers[J]. International Journal of Hydrogen Energy, 2021, 46(2): 2491-2502. |
| 33 | BARBARA, PANELLA, et al. Hydrogen adsorption in different carbon nanostructures[J]. Carbon, 2005. 43(10): 2209-2214. |
| 34 | JOKAR F, NGUYEN D D, POURKHALIL M, et al. Effect of single-and multiwall carbon nanotubes with activated carbon on hydrogen storage[J]. Chemical Engineering & Technology, 2021, 44(3): 387-394. |
| 35 | EDGAR M V, ROCÍO T, MAURICIO M, et al. Hydrogen storage in purified multi-walled carbon nanotubes: gas hydrogenation cycles effect on the adsorption kinetics and their performance[J]. Heliyon, 2021, 7(12): e08494. |
| 36 | BADER N, OUEDERNI A. Optimization of biomass-based carbon materials for hydrogen storage[J]. Journal of Energy Storage, 2016, 5: 77-84. |
| 37 | RAHIMI M, ABBASPOUR-FARD M H, ROHANI A. Machine learning approaches to rediscovery and optimization of hydrogen storage on porous bio-derived carbon[J]. Cleaner Production, 329: 129714. |
| 38 | ARIHARAN A, RAMESH K, VINAYAGAMOORTHI R, et al. Biomass derived phosphorous containing porous carbon material for hydrogen storage and high-performance supercapacitor applications[J]. The Journal of Energy Storage, 2021, 35(7): 102185. |
| 39 | LI Y, XIAO Y, DONG H, et al. Polyacrylonitrile-based highly porous carbon materials for exceptional hydrogen storage[J]. International journal of hydrogen energy, 2019, 44(41): 23210-23215. |
| 40 | GAO P, LI J W, ZHANG J, et al. Computational exploration of magnesium-decorated carbon nitride (g-C3N4) monolayer as advanced energy storage materials[J]. International Journal of Hydrogen Energy, 2021, 46(42): 21739-21747. |
| 41 | HUO Y, ZHANG Y, WANG C, et al. Boron-doping effect on the enhanced hydrogen storage of titanium-decorated porous graphene: A first-principles study[J]. International Journal of Hydrogen Energy, 2021, 46 (80): 40301-40311. |
| 42 | FARHA O K, ERYAZICI I, JEONG N C, et al.Metal-organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit?[J]. Journal of the American Chemical Society,2012, 134(36). |
| 43 | LANGMI H W, REN J, NORTH B, et al. Hydrogen Storage in Metal-Organic Frameworks: A Review[J]. Electrochimica Acta, 2014, 128: 368-392. |
| 44 | KASSAOUI M, LAKHAL M, BENYOUSSEF A, et al. Enhancement of hydrogen storage properties of metal-organic framework-5 by substitution (Zn, Cd and Mg) and decoration (Li, Be and Na)[J]. International Journal of Hydrogen Energy, 2021, 45(52): 26426-26436. |
| 45 | RAHALI S, BELHOCINE Y, SEYDOU M, et al. Multiscale study of the structure and hydrogen storage capacity of an aluminum metal-organic framework[J]. International Journal of Hydrogen Energy, 2017, 42(22): 15271-15282. |
| 46 | LEE S Y, PARK S J. Effect of platinum doping of activated carbon on hydrogen storage behaviors of metal-organic frameworks-5[J]. International Journal of Hydrogen Energy, 2011, 36(14): 8381-8387. |
| 47 | SAMUEL A, YANG H W, ANDREW J. Rapid solvothermal synthesis of an isoreticular metal-organic framework with permanent porosity for hydrogen storage[J]. Microporous and Mesoporous Materials, 2012, 153: 88-93. |
| 48 | PUTTIMATE T, PALMARIN D, SOPHIDA T, et al. Reversible hydrogen sorption and kinetics of hydrogen storage tank based on MgH2 modified by TiF4 and activated carbon[J]. International Journal of Hydrogen Energy, 2018, 43(27): 12260-12270. |
| 49 | ZHANG J, YAN S, XIA J L, et al. Stabilization of low-valence transition metal towards advanced catalytic effects on the hydrogen storage performance of magnesium hydride[J]. Journal of Magnesium and Alloys, 2021, 9(02): 647-657. |
| 50 | FU H, NONG J W, WEN X B, et al. Facile and low-cost synthesis of carbon-supported manganese monoxide nanocomposites and evaluation of their superior catalytic effect toward magnesium hydride[J]. Journal of Alloys and Compounds, 2021, 887: 161380. |
| 51 | YE Y, LU J, DING J, et al. Numerical simulation on the storage performance of a phase change materials based metal hydride hydrogen storage tank[J]. Applied Energy, 2020, 278: 115682. |
| 52 | YANG Y A, JING D A, WW A, et al. The storage performance of metal hydride hydrogen storage tanks with reaction heat recovery by phase change materials[J]. Applied Energy, 299: 117255. |
| 53 | ARDAHAIE S S, HOSSEINI M J, EISAPOUR M, et al. A novel porous metal hydride tank for hydrogen energy storage and consumption assisted by PCM jackets and spiral tubes[J]. Journal of Cleaner Production, 2021, 311: 127674. |
| 54 | 张静, 白晨光, 潘复生, 等. 配位氢化物储氢材料的研究进展[J]. 兵器材料科学与工程, 2008, 31(6): 4. |
| ZHANG Jing, BAI Chenguang, PAN Fusheng, et al. Progress in complex hydrides for hydrogen storage[J]. Weapon Materials Science and Engineering, 2008, 31(6): 4. | |
| 55 | 龚金明, 刘道平, 谢应明. 储氢材料的研究概况与发展方向[J]. 天然气化工(C1化学与化工), 2010, 35(5): 71-78. |
| GONG Jinming, LIU Daoping, XIE Yingming. Progress on hydrogen storage materials[J].Natural Gas Chemical Industry, 2010, 35(5): 71-78. | |
| 56 | CAO Z, FELDERHOFF M. Mechanochemical synthesis and dehydrogenation properties of Yb(AlH4)3 [J]. International Journal of Hydrogen Energy, 2021, 46(52): 26437-26444. |
| 57 | XIAO X, QIN T, JIANG Y, et al. Significantly enhanced hydrogen desorption properties of Mg(AlH4)2 nanoparticles synthesized using solvent free strategy[J], Progress in Natural Science-materials International, 2017, 27(1): 112-120. |
| 58 | YUAN J, CHEN J, HUANG H, et al. Enhanced hydrogen storage properties of NaBH4-Mg(BH4)2 composites by NdF3 addition[J]. Progress in Natural Science-materials International 2021, 31(4): 521-526. |
| 59 | WU D F, OUYANG L Z, HUANG J M, et al. Synthesis and hydrogen storage property tuning of Zr(BH4)4·8NH3 via physical vapour deposition and composite formation[J]. International Journal of Hydrogen Energy, 2018, 43(41): 19182-19188. |
| [1] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [2] | 张瑞杰, 刘志林, 王俊文, 张玮, 韩德求, 李婷, 邹雄. 水冷式复叠制冷系统的在线动态模拟与优化[J]. 化工进展, 2023, 42(S1): 124-132. |
| [3] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [4] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [5] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [6] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [7] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [8] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [9] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [10] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [11] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [12] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [13] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [14] | 李春利, 韩晓光, 刘加朋, 王亚涛, 王晨希, 王洪海, 彭胜. 填料塔液体分布器的研究进展[J]. 化工进展, 2023, 42(9): 4479-4495. |
| [15] | 刘炫麟, 王驿凯, 戴苏洲, 殷勇高. 热泵中氨基甲酸铵分解反应特性及反应器结构优化[J]. 化工进展, 2023, 42(9): 4522-4530. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||