化工进展 ›› 2022, Vol. 41 ›› Issue (9): 4733-4753.DOI: 10.16085/j.issn.1000-6613.2021-2449
丙烷脱氢用高稳定性Pt基催化剂研究进展
张雨宸( ), 张耀远(
), 张耀远( ), 吴芹, 史大昕, 陈康成, 黎汉生(
), 吴芹, 史大昕, 陈康成, 黎汉生( )
)
- 北京理工大学化学与化工学院,北京 100081
-
收稿日期:2021-11-29修回日期:2022-01-13出版日期:2022-09-25发布日期:2022-09-27 -
通讯作者:张耀远,黎汉生 -
作者简介:张雨宸(1997—),男,硕士研究生,研究方向为丙烷脱氢。E-mail:zhangyc912@163.com。 -
基金资助:国家自然科学基金(22108013);中国石油科技创新基金(2020D-5007-0402);北京理工大学青年教师学术启动计划
Advances in high stable Pt based catalysts for propane dehydrogenation
ZHANG Yuchen( ), ZHANG Yaoyuan(
), ZHANG Yaoyuan( ), WU Qin, SHI Daxin, CHEN Kangcheng, LI Hansheng(
), WU Qin, SHI Daxin, CHEN Kangcheng, LI Hansheng( )
)
- School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 100081, China
-
Received:2021-11-29Revised:2022-01-13Online:2022-09-25Published:2022-09-27 -
Contact:ZHANG Yaoyuan, LI Hansheng
摘要:
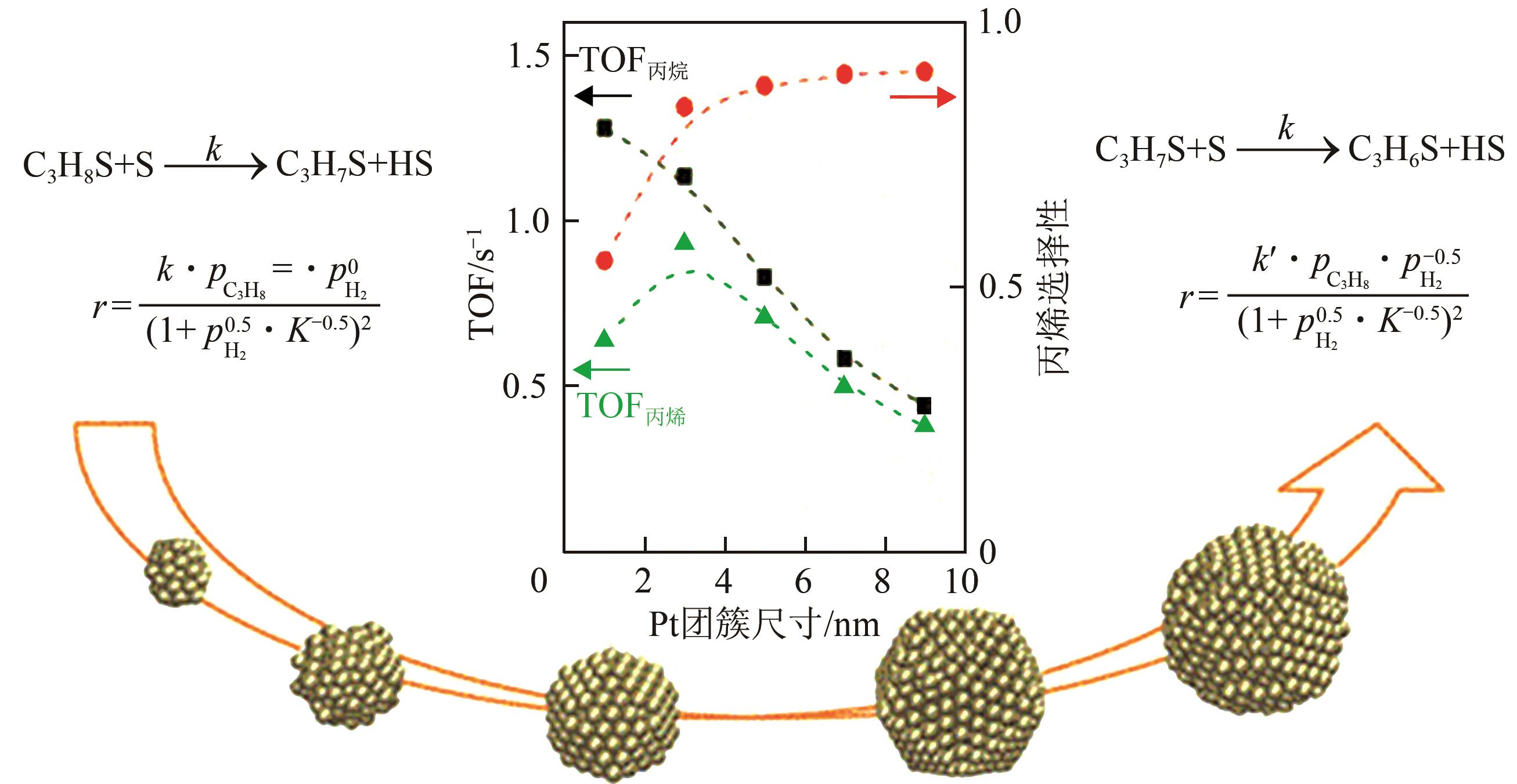
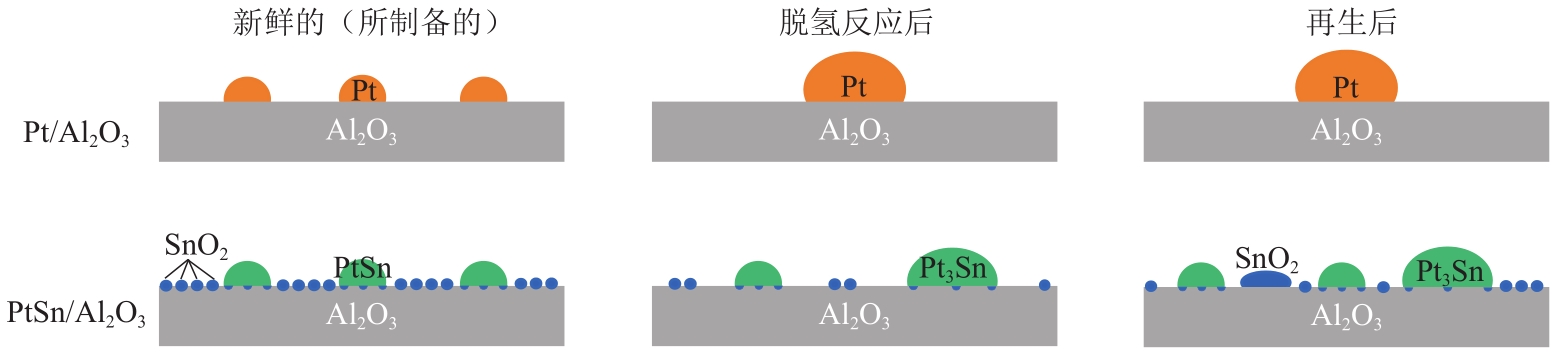
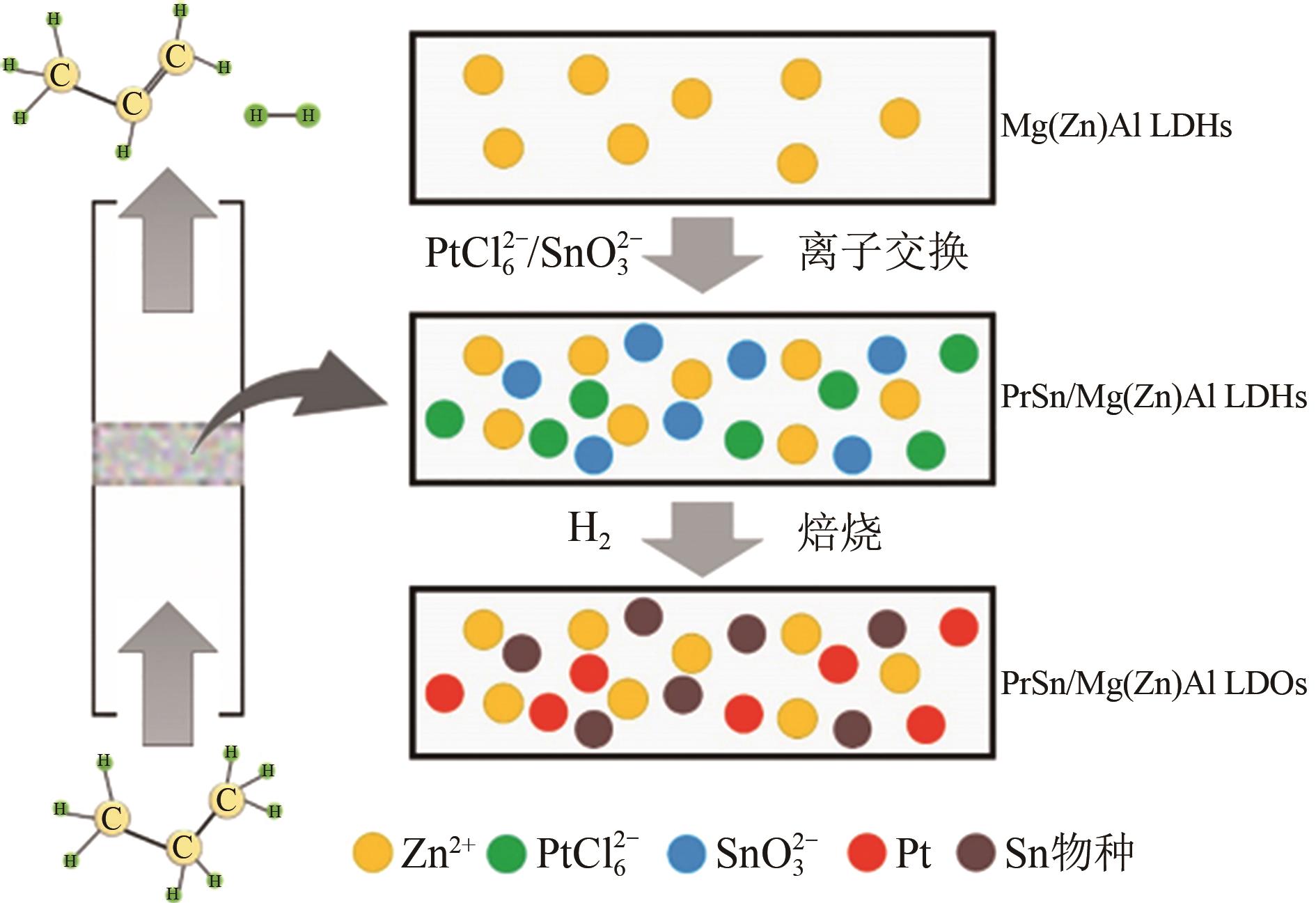
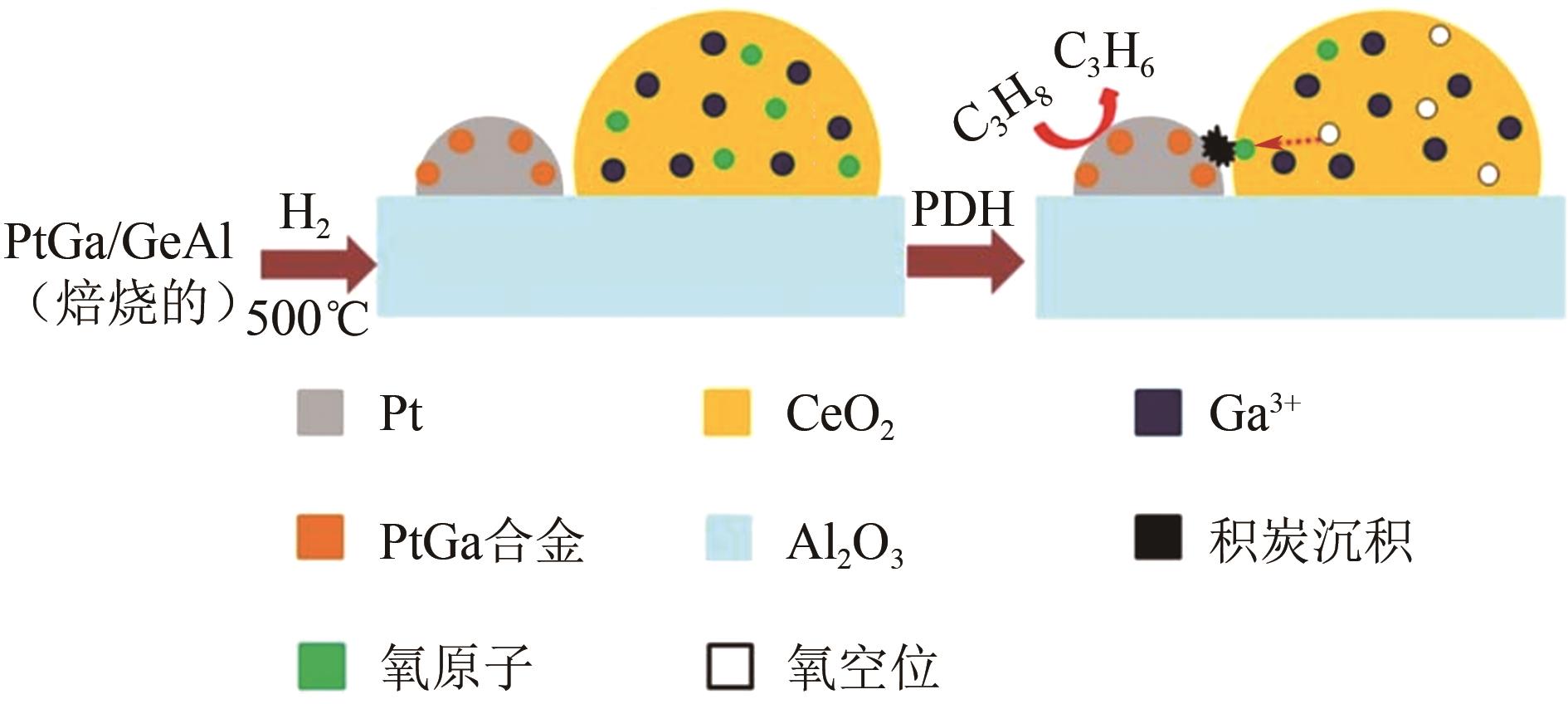
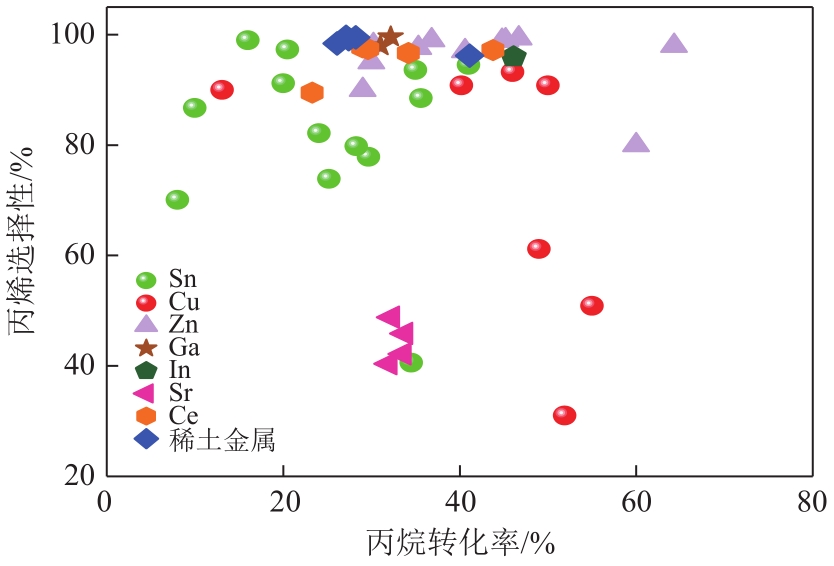
丙烯作为重要的有机化工原料,国内外对其需求量持续增长,造成供不应求。丙烷脱氢(PDH)工艺是以丙烷为原料定向生产丙烯的技术,具有原料来源广泛、丙烯选择性高、产物简单易分离等优势,备受人们关注。本文主要阐述了近十年PDH反应中高稳定Pt基催化剂结构调控和工艺方面的研究进展。总结发现,Pt基催化剂具有最高的反应活性和丙烯选择性,然而,Pt基催化剂在反应中易积炭失活,且在高温下容易烧结团聚,造成稳定性下降。为提高Pt基催化剂的稳定性,研究者主要从催化剂结构设计和操作工艺条件优化两个角度出发。在Pt活性中心的调控方面:①调节Pt位点的结构特性,如分散度、粒径尺寸等;②加入金属助剂,如Sn、Cu、Ga、Zn等;③调控载体的酸性、比表面积、孔结构、金属-载体的相互作用等,能有效改善Pt基催化剂的抗烧结稳定性与抗积炭性能。在操作工艺条件优化方面,通过向丙烷原料中引入CO2、H2、水蒸气或者其他烷烃可增强Pt基催化剂的抗积炭稳定性、提高丙烯的收率。最后,文章指出有效耦合Pt基催化剂结构与操作条件是进一步提高PDH反应中Pt基催化剂稳定性和丙烯收率的关键。
中图分类号:
引用本文
张雨宸, 张耀远, 吴芹, 史大昕, 陈康成, 黎汉生. 丙烷脱氢用高稳定性Pt基催化剂研究进展[J]. 化工进展, 2022, 41(9): 4733-4753.
ZHANG Yuchen, ZHANG Yaoyuan, WU Qin, SHI Daxin, CHEN Kangcheng, LI Hansheng. Advances in high stable Pt based catalysts for propane dehydrogenation[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4733-4753.
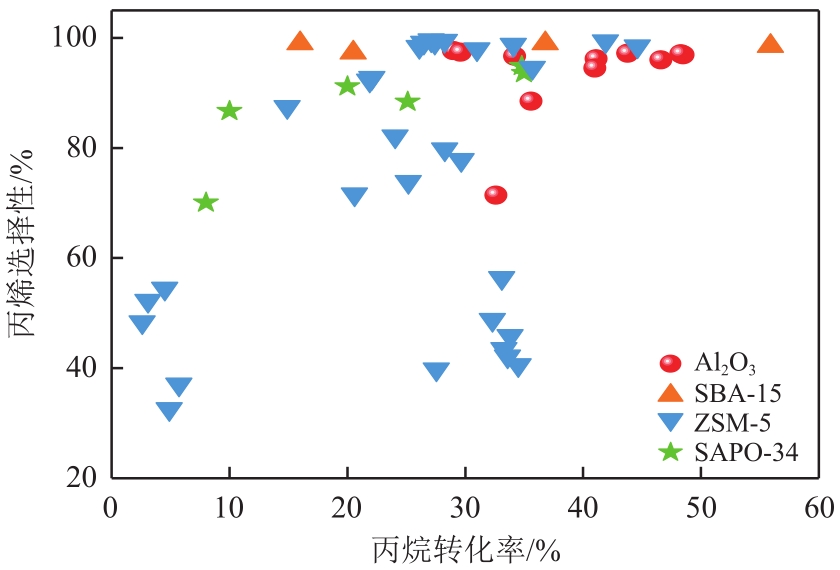
| 催化剂组成 | 反应温度/℃ | 原料及其摩尔分数 | 催化剂质量/g | 丙烷转化率/% | 丙烯选择性/% | 文献 |
|---|---|---|---|---|---|---|
| Pt-Sn/γ-Al2O3 | 600 | 纯丙烷 | 0.3 | 35.6 | 88.5 | [ |
| Pt-Sn/SBA-15-IW | 520 | 8%(体积分数)C3H8+ 13%(体积分数)H2 | 0.03 | 16.0 | 99.0 | [ |
| 0.5Pt-1Sn/SPAO-34 | 600 | 纯丙烷 | — | 35.0 | 93.6 | [ |
| 0.43Pt-1.41Sn/ZSM-5 | 590 | H2/C3H8=0.25 | — | 29.7 | 77.9 | [ |
| Pt(0.7%Sn)Na/AlSBA-15 | 590 | H2/C3H8=0.25 | 1.0 | 20.5 | 97.3 | [ |
| PtSn/HZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 34.5 | 40.6 | [ |
| PtSn/Al2O3 | 600 | H2/C3H8=0.25 | 1.0 | 41.0 | 94.5 | [ |
| Pt3Ga/CeAl | 600 | 26%(体积分数)C3H8+ 26%(体积分数)H2 | 0.15 | 32.2 | 99.6 | [ |
| PtSnNaGa(0.5%)/ZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 31.0 | 98.0 | [ |
| GaPt@S-1 | 600 | C3H8/N2=1∶19 | 0.1 | 45.9 | 92.1 | [ |
| GaPt/SiO2 | 550 | 20%(体积分数)C3H8 | — | 31.9 | 99.0 | [ |
| Pt3Ga/Al2O3 | 620 | 纯丙烷 | 0.15 | 42.0 | 96.7 | [ |
| Pt(0.8%,质量分数)In/Mg(Al)O | 550 | 纯丙烷 | 2.0 | 97.5 | 27.5 | [ |
| PtIn/Mg(Al)O | 620 | H2/C3H8/Ar=7∶8∶35 | 0.3 | 46.1 | 96.0 | [ |
| Pt14In86/SIRAL 10 | 600 | C3H8/N2=1∶9 | 0.2 | 47.0 | 98.0 | [ |
| 0.3%(质量分数)Pt/ Mg(0.6%,质量分数)In(Al)O | 550 | 纯丙烷 | 1.5 | 24.2 | 98.2 | [ |
| 0.3PtSn/1.5In-Al | 580 | H2/C3H8/Ar=7∶8∶35 | 0.3 | 58.41 | 93.0 | [ |
| PtSnSr(1.2%)/HZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 33.6 | 42.2 | [ |
| PtZn-Sn/SBA-15 | 576 | H2/C3H8/He=1∶1∶3 | 0.3 | 36.8 | 99.0 | [ |
| PtSn-Mg(3%Zn)AlO | 550 | N2/C3H8=0.25 | 0.05 | 45.2 | 99.0 | [ |
| 10Zn0.1Pt/HZ | 525 | 5%(体积分数)C3H8 | 0.24 | 60.0 | 80.0 | [ |
| Pt0Zn d+/SiO2 | 550 | C3H8/Ar=1∶4 | — | 35.3 | 97.6 | [ |
| Zn(4%,质量分数)/TiZrO x | 550 | C3H8/N2=2∶3 | 0.05 | 30.0 | 95.0 | [ |
| Pt-Zn/Na-Beta | 555 | 纯丙烷 | 0.04 | 29.0 | 90.0 | [ |
| PtZn@S-1 | 600 | C3H8/H2/N2=1∶1∶2 | 0.2 | 46.7 | 99.3 | [ |
| 0.1Pt2Zn/Si-Beta | 550 | 10%(体积分数)C3H8 | 0.1 | 64.3 | 98.0 | [ |
| PtNa/Zn(1.0%)-ZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 40.6 | 97.0 | [ |
| PtZn4@S-1-H | 550 | C3H8/N2=10∶30 | 0.3 | 44.8 | 98.9 | [ |
| Pt/1.2Ce-Al2O3 | 580 | H2/C3H8=1∶2 | 2.0 | 23.3 | 89.5 | [ |
| PtSnNa/Ce(0.6)-MA | 590 | H2/C3H8=0.25 | 2.0 | 34.2 | 96.7 | [ |
| Pt-Sn/1.1Ce-Al | 576 | H2/C3H8/Ar=1∶1∶5 | 0.3 | 43.78 | 97.2 | [ |
| PtSnNa/1.0La-Al | 590 | H2/C3H8=0.25 | 2.0 | 41.1 | 96.2 | [ |
| PtSnNa/ZSM-5(稀土金属质量分数3%) | 590 | H2/C3H8=0.25 | 2.0 | 28.2 | 99.5 | [ |
| 0.1Pt10Cu/Al2O3 | 600 | C3H8/H2/N2=8∶8∶34 | 0.25 | 13.1 | 90.0 | [ |
| Pt-0.5%(质量分数)Cu/θ-Al2O3 | 600 | N2/C3H8/H2=1∶1∶1 | 0.1 | 55.0 | 50.9 | [ |
| Cu0.7Pt0.1/S-1 | 610 | 纯丙烷 | 1.0 | 50.0 | 90.8 | [ |
| Cu1.0Pt0.1@ZSM-5(Si/Al=150) | 610 | 纯丙烷 | 1.0 | 51.9 | 31.0 | [ |
| Pt-(0.5Cu)/Al2O3 | 600 | H2/C3H8=1∶1 | — | 40.2 | 90.8 | [ |
表1 不同助剂在PDH反应中的丙烯选择性和转化率
| 催化剂组成 | 反应温度/℃ | 原料及其摩尔分数 | 催化剂质量/g | 丙烷转化率/% | 丙烯选择性/% | 文献 |
|---|---|---|---|---|---|---|
| Pt-Sn/γ-Al2O3 | 600 | 纯丙烷 | 0.3 | 35.6 | 88.5 | [ |
| Pt-Sn/SBA-15-IW | 520 | 8%(体积分数)C3H8+ 13%(体积分数)H2 | 0.03 | 16.0 | 99.0 | [ |
| 0.5Pt-1Sn/SPAO-34 | 600 | 纯丙烷 | — | 35.0 | 93.6 | [ |
| 0.43Pt-1.41Sn/ZSM-5 | 590 | H2/C3H8=0.25 | — | 29.7 | 77.9 | [ |
| Pt(0.7%Sn)Na/AlSBA-15 | 590 | H2/C3H8=0.25 | 1.0 | 20.5 | 97.3 | [ |
| PtSn/HZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 34.5 | 40.6 | [ |
| PtSn/Al2O3 | 600 | H2/C3H8=0.25 | 1.0 | 41.0 | 94.5 | [ |
| Pt3Ga/CeAl | 600 | 26%(体积分数)C3H8+ 26%(体积分数)H2 | 0.15 | 32.2 | 99.6 | [ |
| PtSnNaGa(0.5%)/ZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 31.0 | 98.0 | [ |
| GaPt@S-1 | 600 | C3H8/N2=1∶19 | 0.1 | 45.9 | 92.1 | [ |
| GaPt/SiO2 | 550 | 20%(体积分数)C3H8 | — | 31.9 | 99.0 | [ |
| Pt3Ga/Al2O3 | 620 | 纯丙烷 | 0.15 | 42.0 | 96.7 | [ |
| Pt(0.8%,质量分数)In/Mg(Al)O | 550 | 纯丙烷 | 2.0 | 97.5 | 27.5 | [ |
| PtIn/Mg(Al)O | 620 | H2/C3H8/Ar=7∶8∶35 | 0.3 | 46.1 | 96.0 | [ |
| Pt14In86/SIRAL 10 | 600 | C3H8/N2=1∶9 | 0.2 | 47.0 | 98.0 | [ |
| 0.3%(质量分数)Pt/ Mg(0.6%,质量分数)In(Al)O | 550 | 纯丙烷 | 1.5 | 24.2 | 98.2 | [ |
| 0.3PtSn/1.5In-Al | 580 | H2/C3H8/Ar=7∶8∶35 | 0.3 | 58.41 | 93.0 | [ |
| PtSnSr(1.2%)/HZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 33.6 | 42.2 | [ |
| PtZn-Sn/SBA-15 | 576 | H2/C3H8/He=1∶1∶3 | 0.3 | 36.8 | 99.0 | [ |
| PtSn-Mg(3%Zn)AlO | 550 | N2/C3H8=0.25 | 0.05 | 45.2 | 99.0 | [ |
| 10Zn0.1Pt/HZ | 525 | 5%(体积分数)C3H8 | 0.24 | 60.0 | 80.0 | [ |
| Pt0Zn d+/SiO2 | 550 | C3H8/Ar=1∶4 | — | 35.3 | 97.6 | [ |
| Zn(4%,质量分数)/TiZrO x | 550 | C3H8/N2=2∶3 | 0.05 | 30.0 | 95.0 | [ |
| Pt-Zn/Na-Beta | 555 | 纯丙烷 | 0.04 | 29.0 | 90.0 | [ |
| PtZn@S-1 | 600 | C3H8/H2/N2=1∶1∶2 | 0.2 | 46.7 | 99.3 | [ |
| 0.1Pt2Zn/Si-Beta | 550 | 10%(体积分数)C3H8 | 0.1 | 64.3 | 98.0 | [ |
| PtNa/Zn(1.0%)-ZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 40.6 | 97.0 | [ |
| PtZn4@S-1-H | 550 | C3H8/N2=10∶30 | 0.3 | 44.8 | 98.9 | [ |
| Pt/1.2Ce-Al2O3 | 580 | H2/C3H8=1∶2 | 2.0 | 23.3 | 89.5 | [ |
| PtSnNa/Ce(0.6)-MA | 590 | H2/C3H8=0.25 | 2.0 | 34.2 | 96.7 | [ |
| Pt-Sn/1.1Ce-Al | 576 | H2/C3H8/Ar=1∶1∶5 | 0.3 | 43.78 | 97.2 | [ |
| PtSnNa/1.0La-Al | 590 | H2/C3H8=0.25 | 2.0 | 41.1 | 96.2 | [ |
| PtSnNa/ZSM-5(稀土金属质量分数3%) | 590 | H2/C3H8=0.25 | 2.0 | 28.2 | 99.5 | [ |
| 0.1Pt10Cu/Al2O3 | 600 | C3H8/H2/N2=8∶8∶34 | 0.25 | 13.1 | 90.0 | [ |
| Pt-0.5%(质量分数)Cu/θ-Al2O3 | 600 | N2/C3H8/H2=1∶1∶1 | 0.1 | 55.0 | 50.9 | [ |
| Cu0.7Pt0.1/S-1 | 610 | 纯丙烷 | 1.0 | 50.0 | 90.8 | [ |
| Cu1.0Pt0.1@ZSM-5(Si/Al=150) | 610 | 纯丙烷 | 1.0 | 51.9 | 31.0 | [ |
| Pt-(0.5Cu)/Al2O3 | 600 | H2/C3H8=1∶1 | — | 40.2 | 90.8 | [ |
| 催化剂组成 | 反应温度/℃ | 原料及其摩尔分数 | 催化剂质量/g | 丙烷转化率/% | 丙烯选择性/% | 文献 |
|---|---|---|---|---|---|---|
| Pt-Sn/γ-Al2O3 | 600 | 纯丙烷 | 0.3 | 35.6 | 88.5 | [ |
| PtSnNa/Ce(0.6)-介孔Al2O3 | 590 | H2/C3H8=0.25 | 2.0 | 34.2 | 96.7 | [ |
| Pt-Sn/1.1Ce-Al | 576 | H2/C3H8/Ar=1∶1∶5 | 0.3 | 43.8 | 97.2 | [ |
| PtSnNa/1.0La-Al | 590 | H2/C3H8=0.25 | 2.0 | 41.1 | 96.2 | [ |
| PtSn/Al2O3 | 600 | H2/C3H8=0.25 | 1.0 | 41.0 | 94.5 | [ |
| PtSn/Al2O3 | 590 | C3H8∶H2∶He=1∶1.25∶4 | 0.05 | 48.5 | 96.9 | [ |
| PtSn/Al2O3 | 680 | — | 0.1 | 32.6 | 71.4 | [ |
| Pt-Sn/SBA-15-IW | 520 | 8%(体积分数)C3H8+ 13%(体积分数)H2 | 0.03 | 16.0 | 99.0 | [ |
| Pt(0.7%Sn)Na/AlSBA-15 | 590 | H2/C3H8=0.25 | 1.0 | 20.5 | 97.3 | [ |
| PtSn(0.2Al)/SBA-15 | 590 | C3H8/Ar=1∶5 | 0.2 | 55.9 | 98.5 | [ |
| PtZn-Sn/SBA-15 | 576 | H2/C3H8/He=1∶1∶3 | 0.3 | 36.8 | 99.0 | [ |
| PtSn/NaZSM-5(Si/Al=108) | 590 | H2/C3H8=0.25 | 2.0 | 26.5 | 99.2 | [ |
| PtSnNa/ZSM-5 | 590 | H2/C3H8=0.25 | 1.5 | 34.1 | 98.8 | [ |
| PtSnNa/ZFS | 590 | H2/C3H8=0.25 | 1.5 | 41.9 | 99.4 | [ |
| PtSnNa/ZSM-5 | 590 | H2/C3H8=0.25 | 1.5 | 35.7 | 94.6 | [ |
| PtSnNa/ZQ | 590 | H2/C3H8=0.25 | 1.5 | 44.6 | 98.5 | [ |
| 0.5Pt-1Sn/SPAO-34 | 600 | 纯丙烷 | — | 35.0 | 93.6 | [ |
| 0.5%(质量分数)Pt-1%(质量分数) Sn/SAPO-34 | 600 | 纯丙烷 | — | 34.8 | 94.9 | [ |
| 0.41Pt-2.36Sn/ZSM-5 | 590 | H2/C3H8=0.25 | — | 28.3 | 79.8 | [ |
| PtSnNaGa(0.5%)/ZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 31.0 | 98.0 | [ |
| PtSn/HZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 34.5 | 40.6 | [ |
| PtSnSr(1.8%)/HZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 33.8 | 45.9 | [ |
| PtSnNa/ZSM-5(稀土金属质量分数3%) | 590 | H2/C3H8=0.25 | 2.0 | 28.2 | 99.5 | [ |
表2 不同载体在PDH反应中的丙烯选择性和转化率
| 催化剂组成 | 反应温度/℃ | 原料及其摩尔分数 | 催化剂质量/g | 丙烷转化率/% | 丙烯选择性/% | 文献 |
|---|---|---|---|---|---|---|
| Pt-Sn/γ-Al2O3 | 600 | 纯丙烷 | 0.3 | 35.6 | 88.5 | [ |
| PtSnNa/Ce(0.6)-介孔Al2O3 | 590 | H2/C3H8=0.25 | 2.0 | 34.2 | 96.7 | [ |
| Pt-Sn/1.1Ce-Al | 576 | H2/C3H8/Ar=1∶1∶5 | 0.3 | 43.8 | 97.2 | [ |
| PtSnNa/1.0La-Al | 590 | H2/C3H8=0.25 | 2.0 | 41.1 | 96.2 | [ |
| PtSn/Al2O3 | 600 | H2/C3H8=0.25 | 1.0 | 41.0 | 94.5 | [ |
| PtSn/Al2O3 | 590 | C3H8∶H2∶He=1∶1.25∶4 | 0.05 | 48.5 | 96.9 | [ |
| PtSn/Al2O3 | 680 | — | 0.1 | 32.6 | 71.4 | [ |
| Pt-Sn/SBA-15-IW | 520 | 8%(体积分数)C3H8+ 13%(体积分数)H2 | 0.03 | 16.0 | 99.0 | [ |
| Pt(0.7%Sn)Na/AlSBA-15 | 590 | H2/C3H8=0.25 | 1.0 | 20.5 | 97.3 | [ |
| PtSn(0.2Al)/SBA-15 | 590 | C3H8/Ar=1∶5 | 0.2 | 55.9 | 98.5 | [ |
| PtZn-Sn/SBA-15 | 576 | H2/C3H8/He=1∶1∶3 | 0.3 | 36.8 | 99.0 | [ |
| PtSn/NaZSM-5(Si/Al=108) | 590 | H2/C3H8=0.25 | 2.0 | 26.5 | 99.2 | [ |
| PtSnNa/ZSM-5 | 590 | H2/C3H8=0.25 | 1.5 | 34.1 | 98.8 | [ |
| PtSnNa/ZFS | 590 | H2/C3H8=0.25 | 1.5 | 41.9 | 99.4 | [ |
| PtSnNa/ZSM-5 | 590 | H2/C3H8=0.25 | 1.5 | 35.7 | 94.6 | [ |
| PtSnNa/ZQ | 590 | H2/C3H8=0.25 | 1.5 | 44.6 | 98.5 | [ |
| 0.5Pt-1Sn/SPAO-34 | 600 | 纯丙烷 | — | 35.0 | 93.6 | [ |
| 0.5%(质量分数)Pt-1%(质量分数) Sn/SAPO-34 | 600 | 纯丙烷 | — | 34.8 | 94.9 | [ |
| 0.41Pt-2.36Sn/ZSM-5 | 590 | H2/C3H8=0.25 | — | 28.3 | 79.8 | [ |
| PtSnNaGa(0.5%)/ZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 31.0 | 98.0 | [ |
| PtSn/HZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 34.5 | 40.6 | [ |
| PtSnSr(1.8%)/HZSM-5 | 590 | H2/C3H8=0.25 | 2.0 | 33.8 | 45.9 | [ |
| PtSnNa/ZSM-5(稀土金属质量分数3%) | 590 | H2/C3H8=0.25 | 2.0 | 28.2 | 99.5 | [ |
| 1 | 杜凯敏, 范杰. 丙烷氧化脱氢制丙烯研究进展[J]. 化工进展, 2019, 38(6): 2697-2706. |
| DU Kaimin, FAN Jie. Research progress on oxidative dehydrogenation of propane to propene[J]. Chemical Industry and Engineering Progress, 2019, 38(6): 2697-2706. | |
| 2 | SATTLER J J H B, RUIZ-MARTINEZ J, SANTILLAN-JIMENEZ E, et al. Catalytic dehydrogenation of light Alkanes on metals and metal oxides[J]. Chemical Reviews, 2014, 114(20): 10613-10653. |
| 3 | 余长林, 葛庆杰, 徐恒泳, 等. 丙烷脱氢制丙烯研究新进展[J]. 化工进展, 2006, 25(9): 977-982. |
| YU Changlin, GE Qingjie, XU Hengyong, et al. New development of dehydrogenation of propane to propylene[J]. Chemical Industry and Engineering Progress, 2006, 25(9): 977-982. | |
| 4 | 黄燕青, 陈辉. 丙烷脱氢工艺对比[J]. 山东化工, 2020, 49(15): 89-92. |
| HUANG Yanqing, CHEN Hui. Comparisonof various propane dehydrogenation processes[J]. Shandong Chemical Industry, 2020, 49(15): 89-92. | |
| 5 | 朱刚. 烷烃脱氢FBD工艺技术分析[J]. 技术与市场, 2015, 22(10): 42-43. |
| ZHU Gang. Technical analysis of alkane dehydrogenation FBD process [J]. Technology and Market, 2015, 22(10): 42-43. | |
| 6 | 张彩凤, 付辉, 周大鹏, 等. 丙烷脱氢工艺及其市场分析[J]. 精细石油化工进展, 2018, 19(5): 39-42. |
| ZHANG Caifeng, FU Hui, ZHOU Dapeng, et al. Technology and market analysis of propane dehydrogenation[J]. Advances in Fine Petrochemicals, 2018, 19(5): 39-42. | |
| 7 | 李春义, 王国玮. 丙烷/异丁烷脱氢铂系催化剂研究进展Ⅰ.烷烃脱氢反应的热力学、动力学及反应机理[J]. 石化技术与应用, 2017, 35(1):1-5. |
| LI Chunyi, WANG Guowei. Progress in Pt-based catalysts for propane/isobutane dehydrogenation Ⅰ.Thermodynamics, kinetics and reaction mechanism[J]. Petrochemical Technology & Application, 2017, 35(1): 1-5. | |
| 8 | KUMAR M S, CHEN D, WALMSLEY J C, et al. Dehydrogenation of propane over Pt-SBA-15: effect of Pt particle size[J]. Catalysis Communications, 2008, 9(5): 747-750. |
| 9 | YANG Minglei, ZHU Yian, FAN Chen, et al. DFT study of propane dehydrogenation on Pt catalyst: effects of step sites[J]. Physical Chemistry Chemical Physics, 2011, 13(8): 3257-3267. |
| 10 | YANG Minglei, ZHU Jun, ZHU Yi’an, et al. Tuning selectivity and stability in propane dehydrogenation by shaping Pt particles: a combined experimental and DFT study[J]. Journal of Molecular Catalysis A: Chemical, 2014, 395: 329-336. |
| 11 | NIKNADDAF S, SOLTANI M, FARJOO A, et al. Modeling of coke formation and catalyst deactivation in propane dehydrogenation over a commercial Pt-Sn/γ-Al2O3 Catalyst[J]. Petroleum Science and Technology, 2013, 31(23): 2451-2462. |
| 12 | 张新平, 周兴贵, 袁渭康. 丙烷脱氢固定床反应器的动态模拟与优化[J]. 化工学报, 2009, 60(10): 2484-2489. |
| ZHANG Xinping, ZHOU Xinggui, YUAN Weikang. Dynamic simulation and optimization of fixed-bed reactor for propane dehydrogenation[J]. Journal of the Chemical Industry and Engineering Society of China, 2009, 60(10): 2484-2489. | |
| 13 | WANG Haizhi, SUN Lili, SUI Zhijun, et al. Coke formation on Pt-Sn/Al2O3 catalyst for propane dehydrogenation[J]. Industrial & Engineering Chemistry Research, 2018, 57(26): 8647-8654. |
| 14 | ZHU Jun, YANG Minglei, YU Yingda, et al. Size-dependent reaction mechanism and kinetics for propane dehydrogenation over Pt catalysts[J]. ACS Catalysis, 2015, 5(11): 6310-6319. |
| 15 | REDEKOP E A, SAERENS S, GALVITA V V, et al. Early stages in the formation and burning of graphene on a Pt/Mg(Al)O x dehydrogenation catalyst: a temperature- and time-resolved study[J]. Journal of Catalysis, 2016, 344: 482-495. |
| 16 | 李庆, 隋志军, 朱贻安, 等. Pt催化丙烷脱氢过程中结焦反应的粒径效应与Sn的作用[J]. 化工学报, 2013, 64(2): 524-531. |
| LI Qing, SUI Zhijun, ZHU Yi’an, et al. Formation of coke on Pt catalysts during propane dehydrogenation: effect of Pt particle size and Sn addition[J]. CIESC Journal, 2013, 64(2): 524-531. | |
| 17 | SHEN Liling, XIA Ke, LANG Wanzhong, et al. The effects of calcination temperature of support on PtIn/Mg(Al)O catalysts for propane dehydrogenation reaction[J]. Chemical Engineering Journal, 2017, 324: 336-346. |
| 18 | PHAM H N, SATTLER J J H B, WECKHUYSEN B M, et al. Role of Sn in the regeneration of Pt/γ-Al2O3 light alkane dehydrogenation catalysts[J]. ACS Catalysis, 2016, 6(4): 2257-2264. |
| 19 | NAWAZ Z, TANG X P, WANG Y, et al. Parametric characterization and influence of tin on the performance of Pt-Sn/SAPO-34 catalyst for selective propane dehydrogenation to propylene[J]. Industrial & Engineering Chemistry Research, 2010, 49(3): 1274-1280. |
| 20 | ZHANG Yiwei, ZHOU Yuming, QIU Anding, et al. Propane dehydrogenation on PtSn/ZSM-5 catalyst: effect of tin as a promoter[J]. Catalysis Communications, 2006, 7(11): 860-866. |
| 21 | DUAN Yongzheng, ZHOU Yuming, ZHANG Yiwei, et al. Propane dehydrogenation on PtSnNa/AlSBA-15 catalyst: influence of tin as a promoter[J]. China Petroleum Processing & Petrochemical Technology, 2012, 14(1): 37-45. |
| 22 | HUANG Li, ZHOU Shijian, ZHOU Yuming, et al. Effect of strontium addition to platinum catalyst for propane dehydrogenation[J]. China Petroleum Processing & Petrochemical Technology, 2012, 14(3): 75-82. |
| 23 | 马占华, 李帅, 姜爱晶, 等. 助剂Zn对PtSn/Al2O3催化剂丙烷脱氢性能的影响[J]. 化工进展, 2019, 38(8): 3670-3678. |
| MA Zhanhua, LI Shuai, JIANG Aijing, et al. Effects of Zn on catalytic performances of PtSn/Al2O3 in propane dehydrogenation[J]. Chemical Industry and Engineering Progress, 2019, 38(8): 3670-3678. | |
| 24 | WANG Tuo, JIANG Feng, LIU Gang, et al. Effects of Ga doping on Pt/CeO2-Al2O3 catalysts for propane dehydrogenation[J]. AIChE Journal, 2016, 62(12): 4365-4376. |
| 25 | LIU Xuan, ZHOU Yuming, ZHANG Yiwei, et al. Effect of Ga addition on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. China Petroleum Processing & Petrochemical Technology, 2011, 13(4): 45-52. |
| 26 | WANG Yansu, SUO Yujun, Xianwei LYU, et al. Enhanced performances of bimetallic Ga-Pt nanoclusters confined within silicalite-1 zeolite in propane dehydrogenation[J]. Journal of Colloid and Interface Science, 2021, 593: 304-314. |
| 27 | SEARLES K, CHAN K W, MENDES BURAK J A, et al. Highly productive propane dehydrogenation catalyst using silica-supported Ga-Pt nanoparticles generated from single-sites[J]. Journal of the American Chemical Society, 2018, 140(37): 11674-11679. |
| 28 | SUN Qiming, WANG Ning, FAN Qiyuan, et al. Subnanometer bimetallic platinum-zinc clusters in zeolites for propane dehydrogenation[J]. Angewandte Chemie International Edition, 2020, 59(44): 19450-19459. |
| 29 | TOLEK W, SURIYE K, PRASERTHDAM P, et al. Enhanced stability and propene yield in propane dehydrogenation on PtIn/Mg(Al)O catalysts with various in loadings[J]. Topics in Catalysis, 2018, 61(15/16/17): 1624-1632. |
| 30 | XIA Ke, LANG Wanzhong, LI Peipei, et al. Analysis of the catalytic activity induction and deactivation of PtIn/Mg(Al)O catalysts for propane dehydrogenation reaction[J]. RSC Advances, 2015, 5(79): 64689-64695. |
| 31 | WANG Xuchun, YANG Di, XU Yong, et al. Colloidal synthesis of Pt-In bimetallic nanoparticles for propane dehydrogenation[J]. Canadian Journal of Chemistry, 2017, 95(11): 1135-1140. |
| 32 | TOLEK W, SURIYE K, PRASERTHDAM P, et al. Effect of preparation method on the Pt-In modified Mg(Al)O catalysts over dehydrogenation of propane[J]. Catalysis Today, 2020, 358: 100-108. |
| 33 | LIU Xue, LANG Wanzhong, LONG Liuliu, et al. Improved catalytic performance in propane dehydrogenation of PtSn/γ-Al2O3 catalysts by doping indium[J]. Chemical Engineering Journal, 2014, 247: 183-192. |
| 34 | YU Changlin, XU Hengyong, CHEN Xirong, et al. Preparation, characterization, and catalytic performance of PtZn-Sn/SBA-15 catalyst for propane dehydrogenation[J]. Journal of Fuel Chemistry and Technology, 2010, 38(3): 308-312. |
| 35 | WU Xiaoping, ZHANG Qiao, CHEN Lungang, et al. Enhanced catalytic performance of PtSn catalysts for propane dehydrogenation by a Zn-modified Mg(Al)O support[J]. Fuel Processing Technology, 2020, 198: 106222. |
| 36 | CHEN Chong, SUN Minglei, HU Zhongpan, et al. New insight into the enhanced catalytic performance of ZnPt/HZSM-5 catalysts for direct dehydrogenation of propane to propylene[J]. Catalysis Science & Technology, 2019, 9(8): 1979-1988. |
| 37 | ROCHLITZ L, SEARLES K, ALFKE J, et al. Silica-supported, narrowly distributed, subnanometric Pt-Zn particles from single sites with high propane dehydrogenation performance[J]. Chemical Science, 2020, 11(6): 1549-1555. |
| 38 | ALY M, FORNERO E L, LEON-GARZON A R, et al. Effect of boron promotion on coke formation during propane dehydrogenation over Pt/γ-Al2O3 catalysts[J]. ACS Catalysis, 2020, 10(9): 5208-5216. |
| 39 | DE COLA P L, GL SER R, WEITKAMP J. Non-oxidative propane dehydrogenation over Pt-Zn-containing zeolites[J]. Applied Catalysis A: General, 2006, 306: 85-97. |
| 40 | ZHANG Bofeng, LI Guozhu, ZHAI Ziwei, et al. PtZn intermetallic nanoalloy encapsulated in silicalite-1 for propane dehydrogenation[J]. AIChE Journal, 2021, 67(7): e17295. |
| 41 | XIE Linjun, CHAI Yuchao, SUN Lanlan, et al. Optimizing zeolite stabilized Pt-Zn catalysts for propane dehydrogenation[J]. Journal of Energy Chemistry, 2021, 57: 92-98. |
| 42 | ZHANG Yiwei, ZHOU Yuming, HUANG Li, et al. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation[J]. Chemical Engineering Journal, 2015, 270: 352-361. |
| 43 | MA Zhanhua, WANG Jun, LI Jun, et al. Propane dehydrogenation over Al2O3 supported Pt nanoparticles: effect of cerium addition[J]. Fuel Processing Technology, 2014, 128: 283-288. |
| 44 | ZHOU Shijian, ZHOU Yuming, SHI Junjun, et al. Synthesis of Ce-doped mesoporous γ-alumina with enhanced catalytic performance for propane dehydrogenation[J]. Journal of Materials Science, 2015, 50(11): 3984-3993. |
| 45 | YU Changlin, GE Qingjie, XU Hengyong, et al. Effects of Ce addition on the Pt-Sn/γ-Al2O3 catalyst for propane dehydrogenation to propylene[J]. Applied Catalysis A: General, 2006, 315: 58-67. |
| 46 | ZHANG Yiwei, ZHOU Yuming, SHI Junjun, et al. Propane dehydrogenation over PtSnNa/La-doped Al2O3 catalyst: effect of La content[J]. Fuel Processing Technology, 2013, 111: 94-104. |
| 47 | SUN Guodong, ZHAO Zhijian, MU Rentao, et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation[J]. Nature Communications, 2018, 9: 4454. |
| 48 | LEE H, KIM W I, JUNG K D, et al. Effect of Cu promoter and alumina phases on Pt/Al2O3 for propane dehydrogenation[J]. Korean Journal of Chemical Engineering, 2017, 34(5): 1337-1345. |
| 49 | ZHANG Xiaotong, HE Ning, LIU Chunyan, et al. Pt-Cu alloy nanoparticles encapsulated in silicalite-1 molecular sieve: coke-resistant catalyst for alkane dehydrogenation[J]. Catalysis Letters, 2019, 149(4): 974-984. |
| 50 | HAN Zhiping, LI Shuirong, JIANG Feng, et al. Propane dehydrogenation over Pt-Cu bimetallic catalysts: the nature of coke deposition and the role of copper[J]. Nanoscale, 2014, 6(17): 10000-10008. |
| 51 | MATA-MARTINEZ A, JIMENEZ-LAM S A, TALAVERA-LÓPEZ A, et al. The effect of Sn content in a Pt/KIT-6 catalyst over its performance in the dehydrogenation of propane[J]. International Journal of Chemical Reactor Engineering, 2018, 16(10). |
| 52 | VU B K, SONG M B, AHN I Y, et al. Location and structure of coke generated over Pt-Sn/Al2O3 in propane dehydrogenation[J]. Journal of Industrial and Engineering Chemistry, 2011, 17(1): 71-76. |
| 53 | DENG L D, ZHOU Z J, SHISHIDO T. Behavior of active species on Pt-Sn/SiO2 catalyst during the dehydrogenation of propane and regeneration[J]. Applied Catalysis A: General, 2020, 606: 117826. |
| 54 | WANG Lei, WANG Yang, ZHANG Changwu, et al. A boron nitride nanosheet-supported Pt/Cu cluster as a high-efficiency catalyst for propane dehydrogenation[J]. Catalysis Science & Technology, 2020, 10(5): 1248-1255. |
| 55 | REN Guoqing, PEI Guangxian, REN Yujing, et al. Effect of group ⅠB metals on the dehydrogenation of propane to propylene over anti-sintering Pt/MgAl2O4 [J]. Journal of Catalysis, 2018, 366: 115-126. |
| 56 | PURDY S C, GHANEKAR P, MITCHELL G, et al. Origin of electronic modification of platinum in a Pt3V alloy and its consequences for propane dehydrogenation catalysis[J]. ACS Applied Energy Materials, 2020, 3(2): 1410-1422. |
| 57 | WU J, MALLIKARJUN SHARADA S, HO C, et al. Ethane and propane dehydrogenation over PtIr/Mg(Al)O[J]. Applied Catalysis A: General, 2015, 506: 25-32. |
| 58 | NASERI M, TAHRIRI ZANGENEH F, TAEB A. The effect of Ce, Zn and Co on Pt-based catalysts in propane dehydrogenation[J]. Reaction Kinetics, Mechanisms and Catalysis, 2019, 126(1): 477-495. |
| 59 | QIU Yi, LI Xinyi, ZHANG Yuanyuan, et al. Various metals (Ce, In, La, and Fe) promoted Pt/Sn-SBA-15 as highly stable catalysts for propane dehydrogenation[J]. Industrial & Engineering Chemistry Research, 2019, 58(25): 10804-10818. |
| 60 | ZHOU Shuai, LIU Shuangfei, JING Fangli, et al. Effects of dopants in PtSn/M-silicalite-1 on structural property and on catalytic propane dehydrogenation performance[J]. ChemistrySelect, 2020, 5(14): 4175-4185. |
| 61 | JANG E J, LEE J, JEONG H Y, et al. Controlling the acid-base properties of alumina for stable PtSn-based propane dehydrogenation catalysts[J]. Applied Catalysis A: General, 2019, 572: 1-8. |
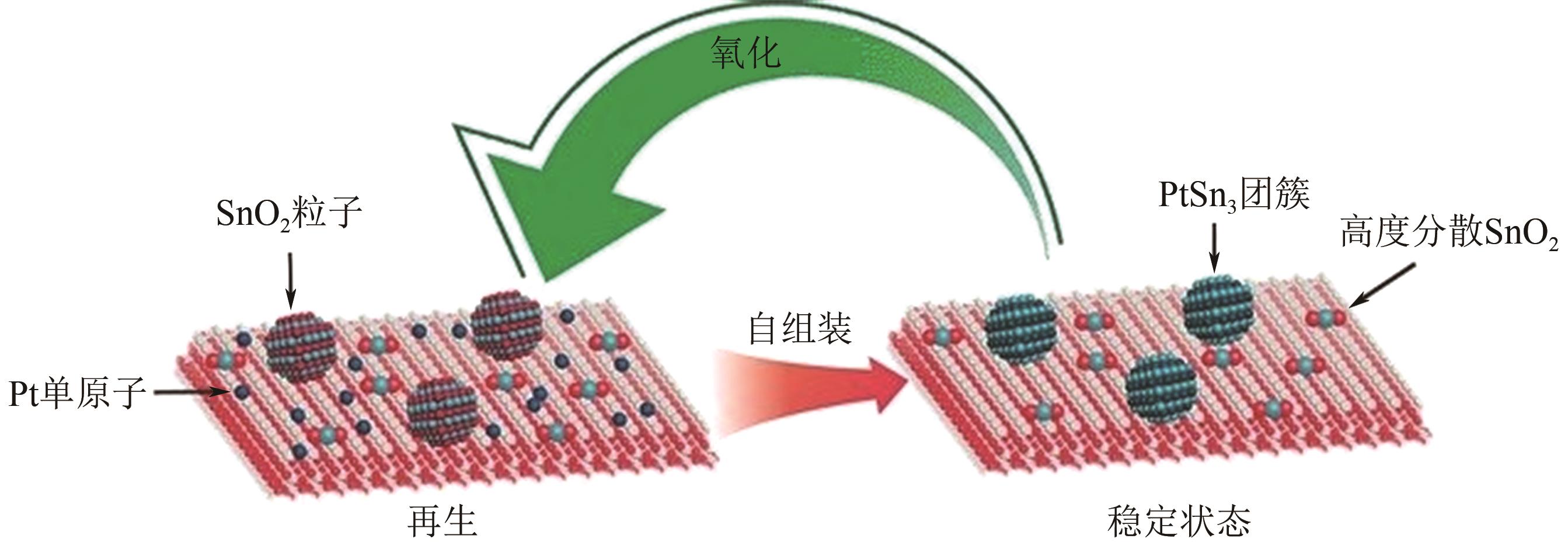
| 62 | XIONG H F, LIN S, GOETZE J, et al. Thermally stable and regenerable platinum-tin clusters for propane dehydrogenation prepared by atom trapping on Ceria[J]. Angewandte Chemie International Edition, 2017, 56(31): 8986-8991. |
| 63 | FAN Xiaoqiang, LI Jianmei, ZHAO Zhen, et al. Dehydrogenation of propane over PtSnAl/SBA-15 catalysts: Al addition effect and coke formation analysis[J]. Catalysis Science & Technology, 2015, 5(1): 339-350. |
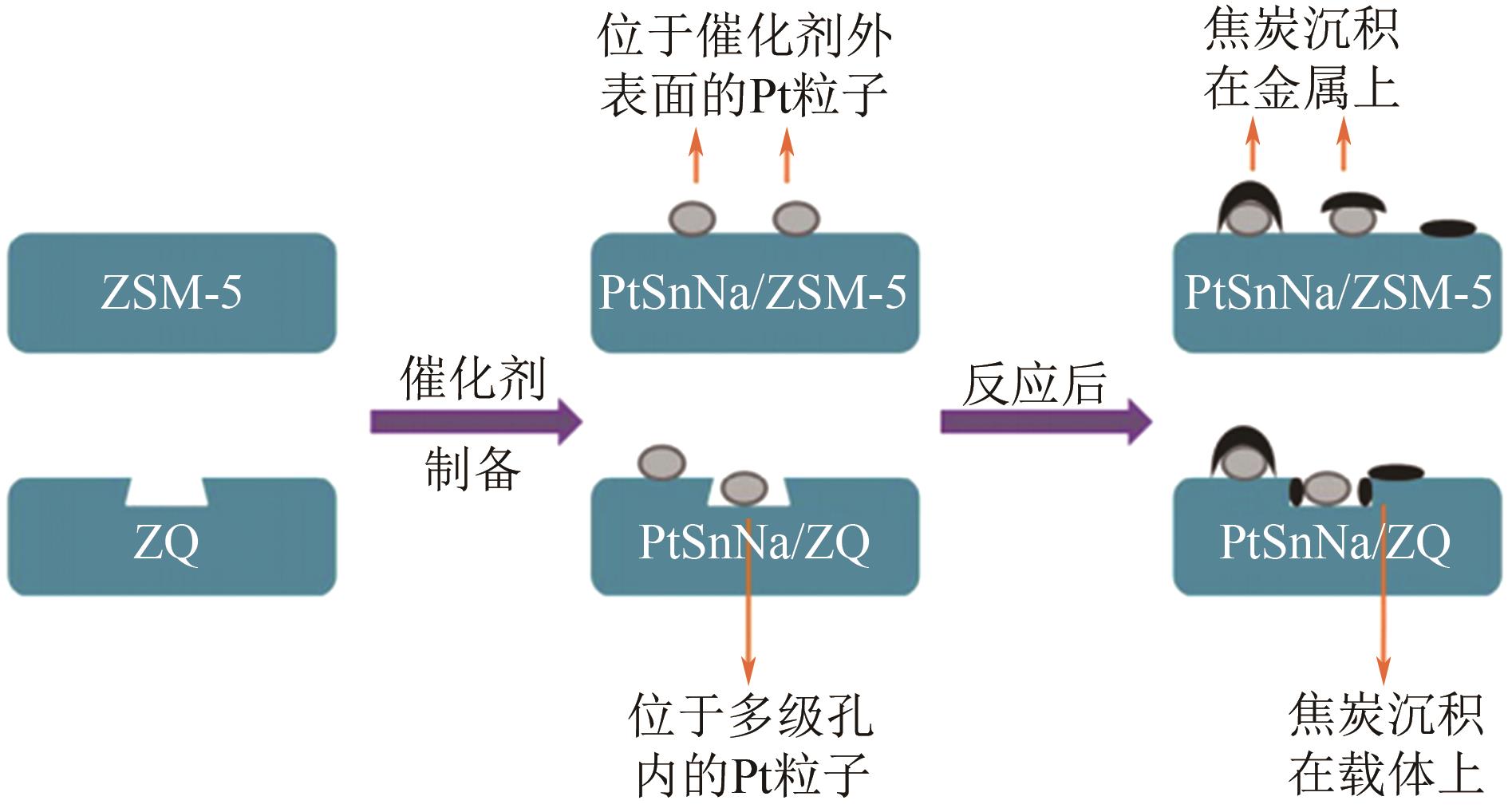
| 64 | 邱安定, 李恩霞, 范以宁. 载体组成对负载型PtSn/ZSM-5催化剂上丙烷脱氢反应性能的影响[J]. 催化学报, 2007, 28(11): 970-974. |
| QIU Anding, LI Enxia, FAN Yining. Effect of support composition on catalytic performance of PtSn/ZSM-5 catalyst for propane dehydrogenation[J]. Chinese Journal of Catalysis, 2007, 28(11): 970-974. | |
| 65 | WANG Yongjuan, ZHOU Yuming, ZHOU Shijian, et al. Effect of morphological structure of PtSnNa/ZSM-5 on its catalytic performance in propane dehydrogenation[J]. China Petroleum Processing & Petrochemical Technology, 2020, 22(1): 87-97. |
| 66 | ZHOU Shijian, ZHOU Yuming, ZHANG Yiwei, et al. The synthesis of new coke-resistant support and its application in propane dehydrogenation to propene[J]. Journal of Chemical Technology & Biotechnology, 2016, 91(4): 1072-1081. |
| 67 | NAWAZ Z, TANG X P, ZHANG Q, et al. SAPO-34 supported Pt-Sn-based novel catalyst for propane dehydrogenation to propylene[J]. Catalysis Communications, 2009, 10(14): 1925-1930. |
| 68 | XUE Mengwei, ZHOU Yuming, HUANG Li, et al. Effect of mischmetal addition on catalytic performance of PtSnNa/ZSM-5 for propane dehydrogenation[J]. China Petroleum Processing & Petrochemical Technology, 2011, 13(3): 47-52. |
| 69 | ZHANG Yiwei, ZHOU Yuming, SHI Junjun, et al. Comparative study of bimetallic Pt-Sn catalysts supported on different supports for propane dehydrogenation[J]. Journal of Molecular Catalysis A: Chemical, 2014, 381: 138-147. |
| 70 | GUNTIDA A, WANNAKAO S, PRASERTHDAM P, et al. Acidic nanomaterials (TiO2, ZrO2, and Al2O3) are coke storage components that reduce the deactivation of the Pt-Sn/γ-Al2O3 catalyst in propane dehydrogenation[J]. Catalysis Science & Technology, 2020, 10(15): 5100-5112. |
| 71 | JIANG Feng, ZENG Liang, LI Shuirong, et al. Propane dehydrogenation over Pt/TiO2-Al2O3 Catalysts[J]. ACS Catalysis, 2015, 5(1): 438-447. |
| 72 | ZHAO Shiyong, XU Bolian, YU Lei, et al. Honeycomb-shaped PtSnNa/γ-Al2O3/cordierite monolithic catalyst with improved stability and selectivity for propane dehydrogenation[J]. Chinese Chemical Letters, 2018, 29(6): 884-886. |
| 73 | JUNG J W, KIM W I, KIM J R, et al. Effect of direct reduction treatment on Pt-Sn/Al2O3 catalyst for propane dehydrogenation[J]. Catalysts, 2019, 9(5): 446. |
| 74 | SUN Huaqian, ZHANG Yaoyuan, LI Yuming, et al. Synergistic construction of bifunctional and stable Pt/HZSM-5-based catalysts for efficient catalytic cracking of n-butane[J]. Nanoscale, 2021, 13(9): 5103-5114. |
| 75 | KUMAR M S, HOLMEN A, CHEN D. The influence of pore geometry of Pt containing ZSM-5, Beta and SBA-15 catalysts on dehydrogenation of propane[J]. Microporous and Mesoporous Materials, 2009, 126(1/2): 152-158. |
| 76 | HUANG Li, ZHOU Yuming, ZHANG Yiwei, et al. Propane dehydrogenation over PtSnNa catalyst supported on La-ZSM-5 zeolite[J]. China Petroleum Processing and Petrochemical Technology, 2010, 12(3): 18-24. |
| 77 | ZHANG Yiwei, ZHOU Yuming, ZHANG Shaobo, et al. Catalytic structure and reaction performance of PtSnK/ZSM-5 catalyst for propane dehydrogenation: influence of impregnation strategy[J]. Journal of Materials Science, 2015, 50(19): 6457-6468. |
| 78 | FENG Jing, ZHANG Mingsen, YANG Yuanyi. Dehydrogenation of propane on Pt or PtSn catalysts with Al2O3 or SBA-15 support[J]. Chinese Journal of Chemical Engineering, 2014, 22(11/12): 1232-1236. |
| 79 | VU B K, BOK S M, AHN I Y, et al. Oxidation of coke formed over Pt-Al2O3 and Pt-SBA-15 in propane dehydrogenation[J]. Catalysis Letters, 2009, 133(3/4): 376-381. |
| 80 | HUANG Lihua, XU Bolian, YANG Lili, et al. Propane dehydrogenation over the PtSn catalyst supported on alumina-modified SBA-15[J]. Catalysis Communications, 2008, 9(15): 2593-2597. |
| 81 | XIA Ke, LANG Wanzhong, LI Peipei, et al. The properties and catalytic performance of PtIn/Mg(Al)O catalysts for the propane dehydrogenation reaction: effects of pH value in preparing Mg(Al)O supports by the co-precipitation method[J]. Journal of Catalysis, 2016, 338: 104-114. |
| 82 | ZHANG Ming, SONG Zhen, GUO Mengquan, et al. Effect of reduction atmosphere on structure and catalytic performance of PtIn/Mg(Al)O/ZnO for propane dehydrogenation[J]. Catalysts, 2020, 10(5): 485. |
| 83 | PERECHODJUK A, ZHANG Y Y, KONDRATENKO V A, et al. The effect of supported Rh, Ru, Pt or Ir nanoparticles on activity and selectivity of ZrO2-based catalysts in non-oxidative dehydrogenation of propane[J]. Applied Catalysis A: General, 2020, 602: 117731. |
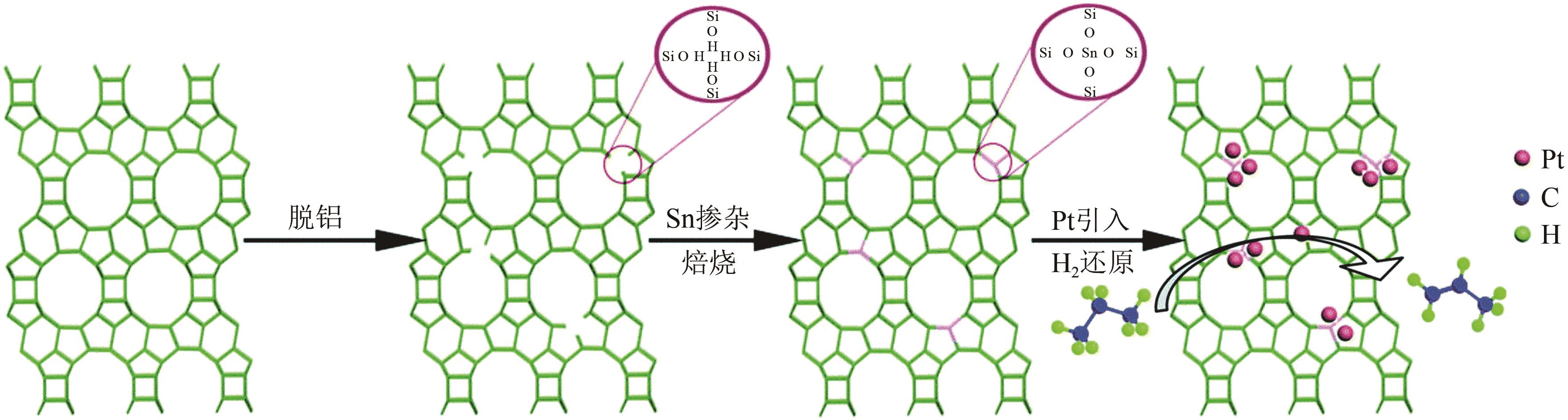
| 84 | WANG Yansu, HU Zhongpan, TIAN Wenwen, et al. Framework-confined Sn in Si-beta stabilizing ultra-small Pt nanoclusters as direct propane dehydrogenation catalysts with high selectivity and stability[J]. Catalysis Science & Technology, 2019, 9(24): 6993-7002. |
| 85 | SIDDIQI G, SUN P P, GALVITA V, et al. Catalyst performance of novel Pt/Mg(Ga)(Al)O catalysts for alkane dehydrogenation[J]. Journal of Catalysis, 2010, 274(2): 200-206. |
| 86 | 张海娟, 王振宇, 李江红, 等. 反应条件对丙烷脱氢催化剂积炭行为的影响[J]. 天然气化工(C1化学与化工), 2014, 39(2): 38-42. |
| ZHANG Haijuan, WANG Zhenyu, LI Jianghong, et al. Effect of reaction conditions on coke formation over the catalyst for propane dehydrogenation[J]. Natural Gas Chemical Industry, 2014, 39(2): 38-42. | |
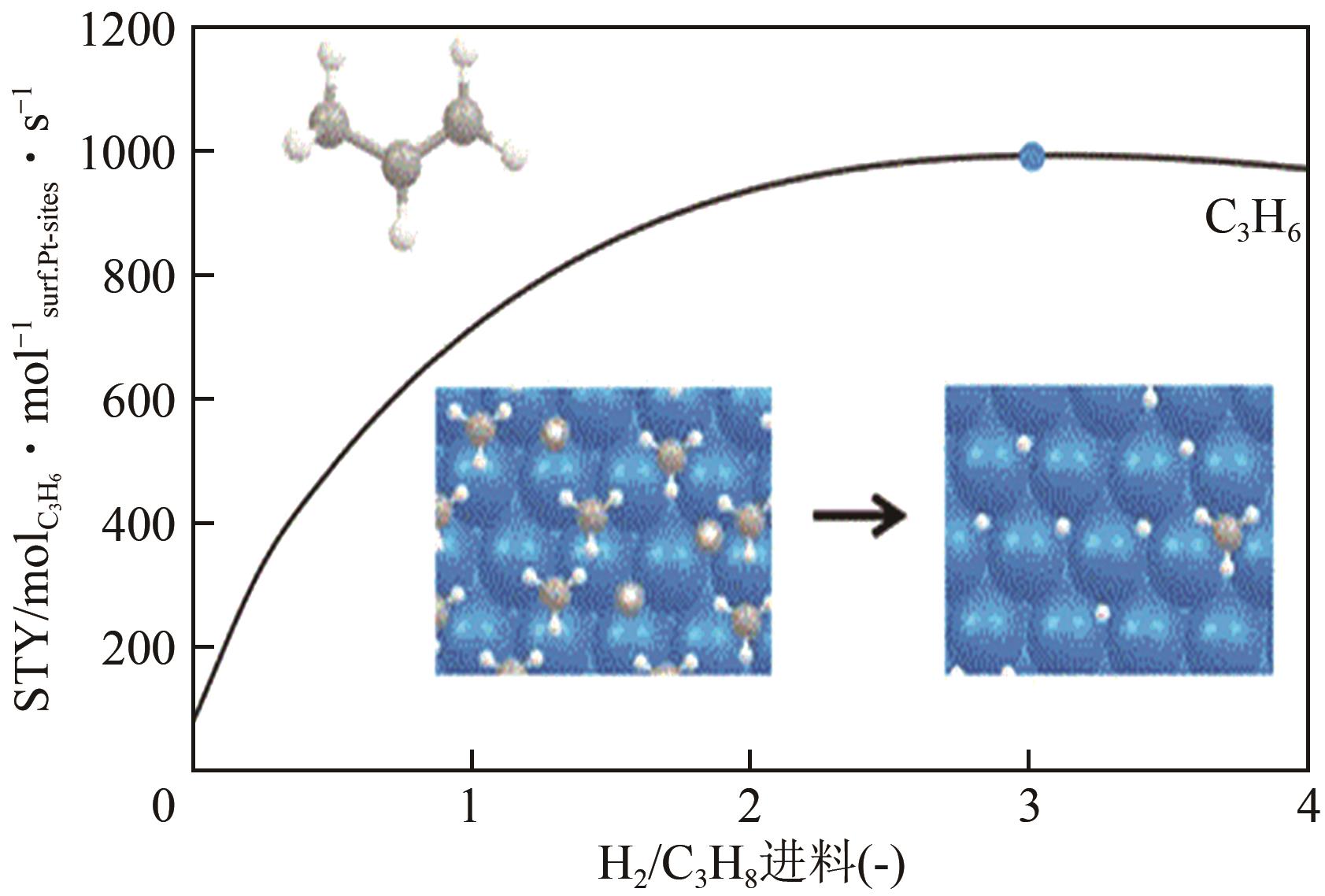
| 87 | SAERENS S, SABBE M K, GALVITA V V, et al. The positive role of hydrogen on the dehydrogenation of propane on Pt(111)[J]. ACS Catalysis, 2017, 7(11): 7495-7508. |
| 88 | GOMEZ E, XIE Z H, CHEN J G. The effects of bimetallic interactions for CO2-assisted oxidative dehydrogenation and dry reforming of propane[J]. AIChE Journal, 2019, 65(8): e16670. |
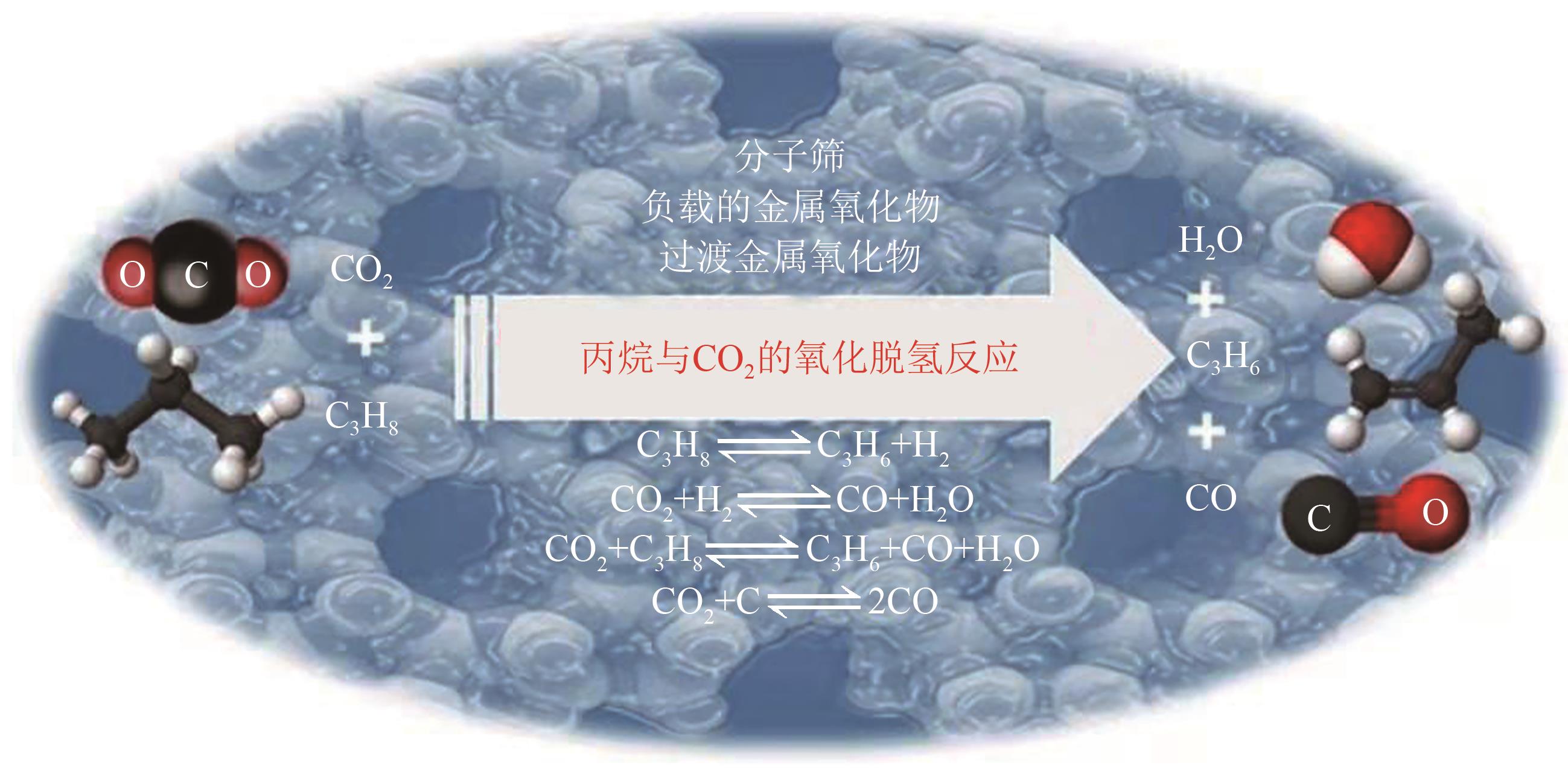
| 89 | ATANGA M A, REZAEI F, JAWAD A, et al. Oxidative dehydrogenation of propane to propylene with carbon dioxide[J]. Applied Catalysis B: Environmental, 2018, 220: 429-445. |
| 90 | REN Yingjie, ZHANG Fan, HUA Weiming, et al. ZnO supported on high silica HZSM-5 as new catalysts for dehydrogenation of propane to propene in the presence of CO2 [J]. Catalysis Today, 2009, 148(3/4): 316-322. |
| 91 | KOGAN S B, SCHRAMM H, HERSKOWITZ M. Dehydrogenation of propane on modified Pt/θ-alumina performance in hydrogen and steam environment[J]. Applied Catalysis A: General, 2001, 208(1/2): 185-191. |
| 92 | SHEINTUCH M, LIRON O, RICCA A, et al. Propane dehydrogenation kinetics on supported Pt catalyst[J]. Applied Catalysis A: General, 2016, 516: 17-29. |
| 93 | NAWAZ Z, WEI F. Hydrothermal study of Pt-Sn-based SAPO-34 supported novel catalyst used for selective propane dehydrogenation to propylene[J]. Journal of Industrial and Engineering Chemistry, 2010, 16(5): 774-784. |
| 94 | SHAN Yuling, ZHU Yian, SUI Zhijun, et al. Insights into the effects of steam on propane dehydrogenation over a Pt/Al2O3 catalyst[J]. Catalysis Science & Technology, 2015, 5(8): 3991-4000. |
| 95 | AVITHI KANNIAPPAN S, RAGULA U B R. Effect of reduction of Pt-Sn/α-Al2O3 on catalytic dehydrogenation of mixed-paraffin feed[J]. Catalysts, 2020, 10(1): 113. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||