化工进展 ›› 2022, Vol. 41 ›› Issue (8): 4530-4543.DOI: 10.16085/j.issn.1000-6613.2021-1940
废锂离子电池正极材料中锂元素选择性回收的研究进展
王玥1,2( ), 郑晓洪2,3(
), 郑晓洪2,3( ), 陶天一2, 刘秀庆4, 李丽1(
), 陶天一2, 刘秀庆4, 李丽1( ), 孙峙2
), 孙峙2
- 1.北京理工大学材料学院,北京 100081
2.中国科学院过程工程研究所,绿色过程与工程重点实验室,北京市
1.过程污染控制工程技术研究中心,北京 100190,中国地质大学(北京)材料科学与工程学院,北京非金属矿物
1.与固体废物材料利用重点实验室,矿物材料国家重点实验室,北京 100083,华友资源再生科技有限公司 ;浙江 衢州 324000
-
收稿日期:2021-09-09修回日期:2021-12-20出版日期:2022-08-25发布日期:2022-08-22 -
通讯作者:郑晓洪,李丽 -
作者简介:王玥(1997—),女,硕士研究生,研究方向为锂离子电池资源化回收。E-mail:1679441065@qq.com 。 -
基金资助:国家自然科学基金(52002371);中国科学院绿色过程制造创新研究院项目(LAGM-2019-A15);中科院过程工程研究所南京绿色制造产业创新研究院重点研发项目(E0010715);中国科学院重点部署项目(ZDRM-CN-2020-1)
Review on selective recovery of lithium from cathode materials in spent lithium-ion batteries
WANG Yue1,2( ), ZHENG Xiaohong2,3(
), ZHENG Xiaohong2,3( ), TAO Tianyi2, LIU Xiuqing4, LI Li1(
), TAO Tianyi2, LIU Xiuqing4, LI Li1( ), SUN Zhi2
), SUN Zhi2
- 1.School of Materials Science & Engineering, Beijing Institute of Technology, Beijing 100081, China
2.Beijing Engineering Research Center of Process Pollution Control, Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
3.Beijing Key Laboratory of Materials Utilization of Nonmetallic Minerals and Solid Wastes, National Laboratory of Mineral Materials, School of Materials Science and Technology, China University of Geosciences (Beijing), Beijing 100083, China
4.Quzhou Huayou Resource Recycling Technology Company Limited, Quzhou 324000, Zhejiang, China
-
Received:2021-09-09Revised:2021-12-20Online:2022-08-25Published:2022-08-22 -
Contact:ZHENG Xiaohong,LI Li
摘要:
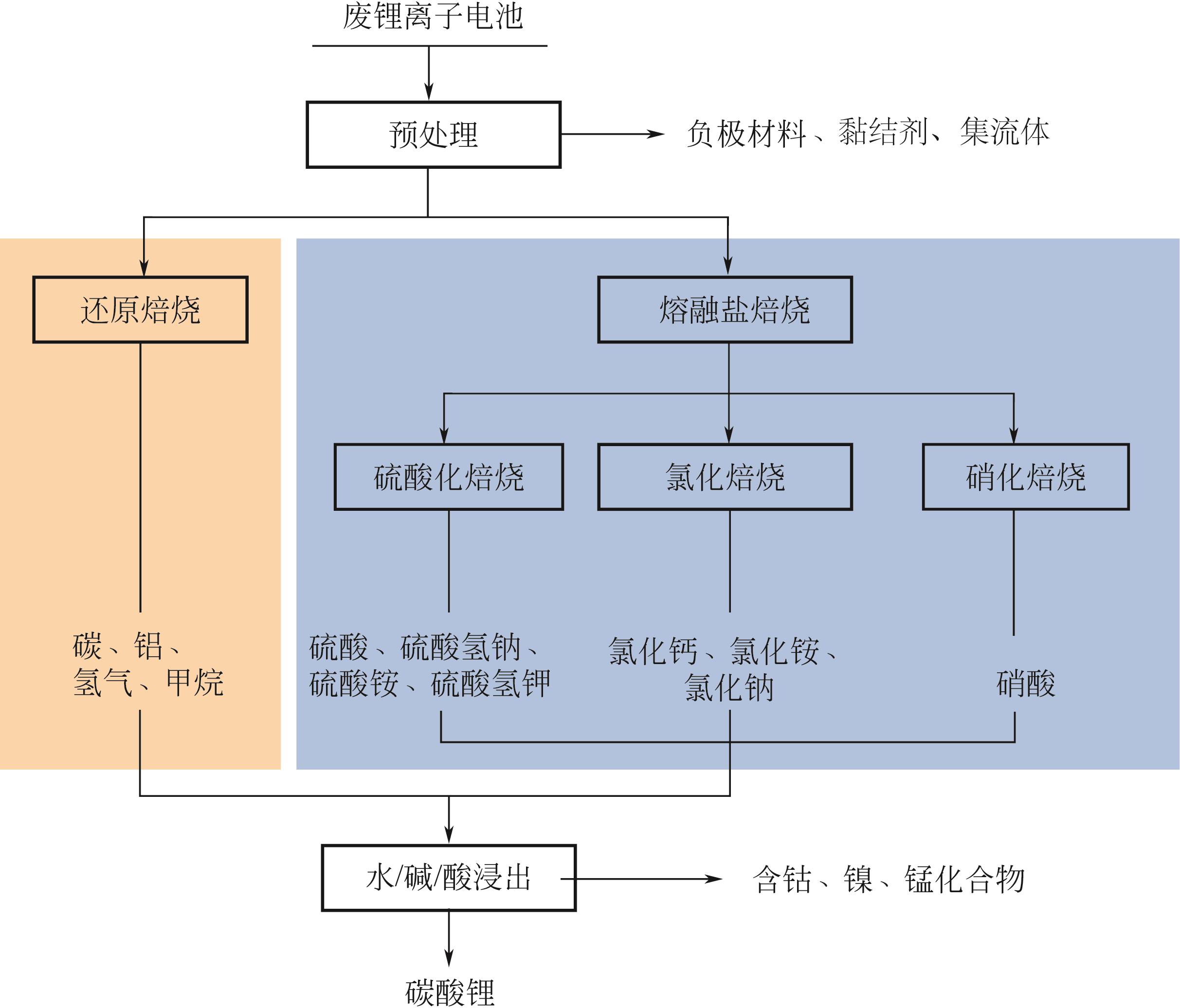
随着新能源汽车市场的蓬勃发展,锂离子电池作为新能源汽车的关键部件,面临着关键金属资源尤其是锂资源供给不足的风险,回收废锂离子电池中所含的二次锂资源将成为解决锂资源供需问题、推动行业可持续发展的重要途经。因此为实现废锂离子电池中锂元素的高效提取,分步或优先提取的选择性提锂工艺备受研究者们关注。本文介绍了火法、湿法、机械化学法和电化学法四种当前主流的选择性提锂工艺,在阐述其基础反应机理的基础上,总结归纳了各工艺最新的研究成果,并从提取过程中的工艺能耗、物耗、回收率、选择性、环境影响等多个角度对各工艺的优势和不足进行了深入分析。最后,对废锂离子电池中有价金属资源化回收的发展趋势及前景进行了展望,为未来研发更加清洁高效的回收工艺提供参考。
中图分类号:
引用本文
王玥, 郑晓洪, 陶天一, 刘秀庆, 李丽, 孙峙. 废锂离子电池正极材料中锂元素选择性回收的研究进展[J]. 化工进展, 2022, 41(8): 4530-4543.
WANG Yue, ZHENG Xiaohong, TAO Tianyi, LIU Xiuqing, LI Li, SUN Zhi. Review on selective recovery of lithium from cathode materials in spent lithium-ion batteries[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4530-4543.
| 国家 | 储量/104t |
|---|---|
| 阿根廷 | 1930 |
| 玻利维亚 | 2100 |
| 智利 | 960 |
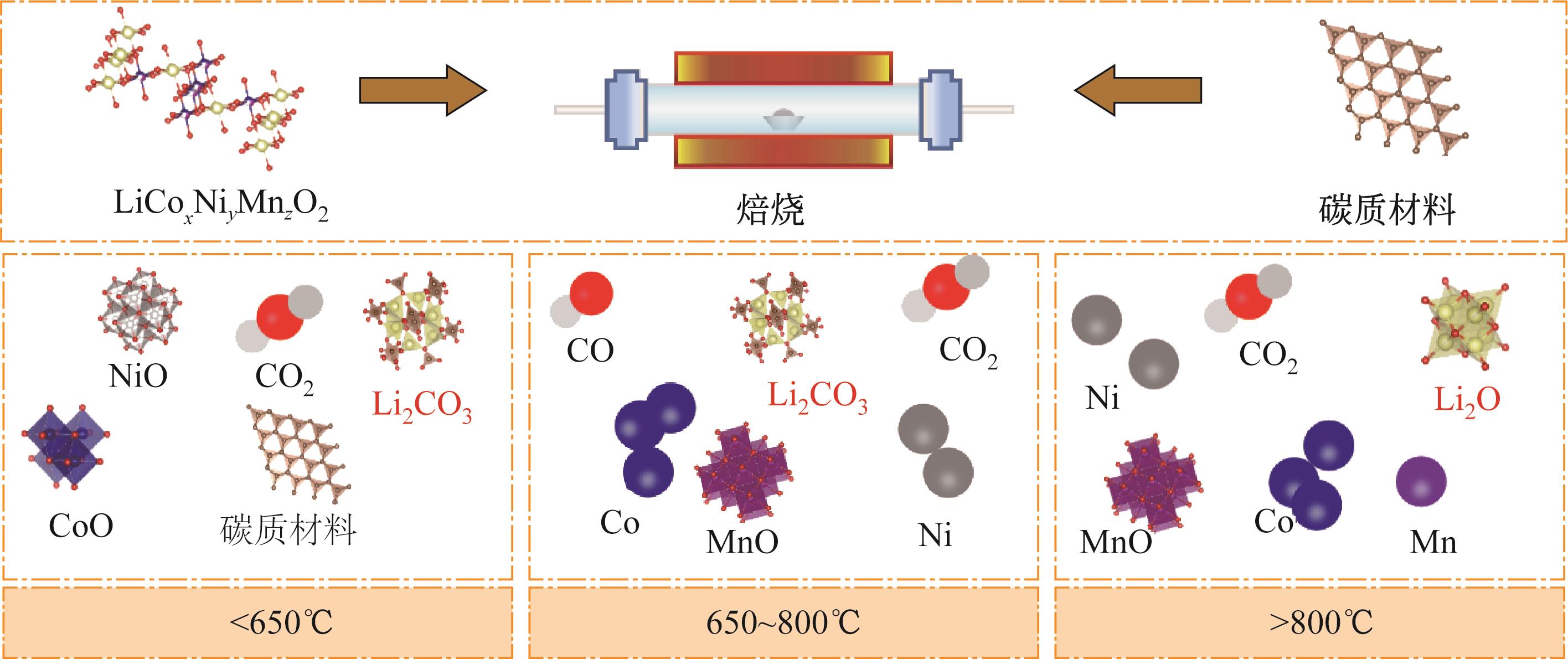
| 澳大利亚 | 640 |
| 美国 | 790 |
| 中国 | 510 |
| 刚果 | 300 |
表1 2020年世界各国锂资源分布[10]
| 国家 | 储量/104t |
|---|---|
| 阿根廷 | 1930 |
| 玻利维亚 | 2100 |
| 智利 | 960 |
| 澳大利亚 | 640 |
| 美国 | 790 |
| 中国 | 510 |
| 刚果 | 300 |
| 企业 | 所属国家 | 回收工艺 | 回收产品 |
|---|---|---|---|
| AEA | 英国 | 电化学 | 氧化钴、氢氧化锂 |
| Recupyl | 法国 | 湿法 | 氢氧化钴、碳酸锂/磷酸锂 |
| Accurec | 德国 | 火法 | 钴基合金、富锂残渣 |
| Umicore | 比利时 | 火法 | 高值合金(钴/镍/铜)、富锂残渣 |
| Inmetco | 美国 | 火法 | 钴基合金 |
表2 全球废锂离子电池回收工艺[27-29]
| 企业 | 所属国家 | 回收工艺 | 回收产品 |
|---|---|---|---|
| AEA | 英国 | 电化学 | 氧化钴、氢氧化锂 |
| Recupyl | 法国 | 湿法 | 氢氧化钴、碳酸锂/磷酸锂 |
| Accurec | 德国 | 火法 | 钴基合金、富锂残渣 |
| Umicore | 比利时 | 火法 | 高值合金(钴/镍/铜)、富锂残渣 |
| Inmetco | 美国 | 火法 | 钴基合金 |
| 电池 | 浸出试剂 | 温度/℃ | 时间/min | 浸出效率 | 参考文献 |
|---|---|---|---|---|---|
| LiCoO2 | 磷酸+葡萄糖 | 80 | 120 | Li 100%,Co 98% | [ |
| LiNi x Co y Mn z O2 | 硫酸+亚硫酸氢钠 | 95 | 240 | Li 96.7%,Co 91.6%,Ni 96.4%,Mn 87.9% | [ |
| LiCoO2 | 柠檬酸+过氧化氢 | 90 | 60 | Li 100%,Co 99% | [ |
| LiNi x Co y Mn z O2 | 甲酸+过氧化氢 | 60 | 120 | Li 100%,Co 85%,Ni 85%,Mn 85% | [ |
| LiCoO2 | 盐酸 | 80 | 90 | Li 100%,Co 100% | [ |
表3 废锂离子电池中有价金属浸出的优化工艺参数及浸出效率
| 电池 | 浸出试剂 | 温度/℃ | 时间/min | 浸出效率 | 参考文献 |
|---|---|---|---|---|---|
| LiCoO2 | 磷酸+葡萄糖 | 80 | 120 | Li 100%,Co 98% | [ |
| LiNi x Co y Mn z O2 | 硫酸+亚硫酸氢钠 | 95 | 240 | Li 96.7%,Co 91.6%,Ni 96.4%,Mn 87.9% | [ |
| LiCoO2 | 柠檬酸+过氧化氢 | 90 | 60 | Li 100%,Co 99% | [ |
| LiNi x Co y Mn z O2 | 甲酸+过氧化氢 | 60 | 120 | Li 100%,Co 85%,Ni 85%,Mn 85% | [ |
| LiCoO2 | 盐酸 | 80 | 90 | Li 100%,Co 100% | [ |
| 工艺 | 优点 | 缺点 |
|---|---|---|
| 火法 | 工艺流程短(焙烧+浸出+沉淀),锂回收率高(≥90%),选择性高(≥99%),提锂条件温和(常温浸出) | 能耗高(焙烧温度普遍大于500℃),环境风险高(CO2、SO x 、NO x 、HCl尾气排放),高温下锂易以气态形式挥发,易损失 |
| 湿法 | 工艺流程短(浸出+沉淀),锂回收率高(≥92%),浸出温度低(30~90℃),能耗低,工艺流程简单,易于工业化应用 | 易造成其他金属的浸出,选择性低;药剂消耗量大,需消耗大量的酸/碱,环境风险高(H2SO4、HCl、HNO3酸雾排放,高盐废水排放) |
| 机械化学法 | 工艺流程短(焙烧+浸出+沉淀),锂回收率高(≥92%),选择性高(≥99%),提锂条件温和(常温浸出) | 设备规模化受限,药剂消耗量大,需添加大量的助磨剂,工艺流程会产生高盐废水 |
| 电化学法 | 工艺流程短(浸出+沉淀),锂回收率高(≥98%),选择性高(≥99%),提锂条件温和(常温浸出),无化学试剂消耗 | 设备规模化受限,电能消耗较高 |
表4 废锂离子电池中选择性提锂方法对比
| 工艺 | 优点 | 缺点 |
|---|---|---|
| 火法 | 工艺流程短(焙烧+浸出+沉淀),锂回收率高(≥90%),选择性高(≥99%),提锂条件温和(常温浸出) | 能耗高(焙烧温度普遍大于500℃),环境风险高(CO2、SO x 、NO x 、HCl尾气排放),高温下锂易以气态形式挥发,易损失 |
| 湿法 | 工艺流程短(浸出+沉淀),锂回收率高(≥92%),浸出温度低(30~90℃),能耗低,工艺流程简单,易于工业化应用 | 易造成其他金属的浸出,选择性低;药剂消耗量大,需消耗大量的酸/碱,环境风险高(H2SO4、HCl、HNO3酸雾排放,高盐废水排放) |
| 机械化学法 | 工艺流程短(焙烧+浸出+沉淀),锂回收率高(≥92%),选择性高(≥99%),提锂条件温和(常温浸出) | 设备规模化受限,药剂消耗量大,需添加大量的助磨剂,工艺流程会产生高盐废水 |
| 电化学法 | 工艺流程短(浸出+沉淀),锂回收率高(≥98%),选择性高(≥99%),提锂条件温和(常温浸出),无化学试剂消耗 | 设备规模化受限,电能消耗较高 |
| 1 | 黄健航, 王永刚, 夏永姚. 新型储能化学电源研究进展[J]. 电源技术, 2020, 44(6): 793-798. |
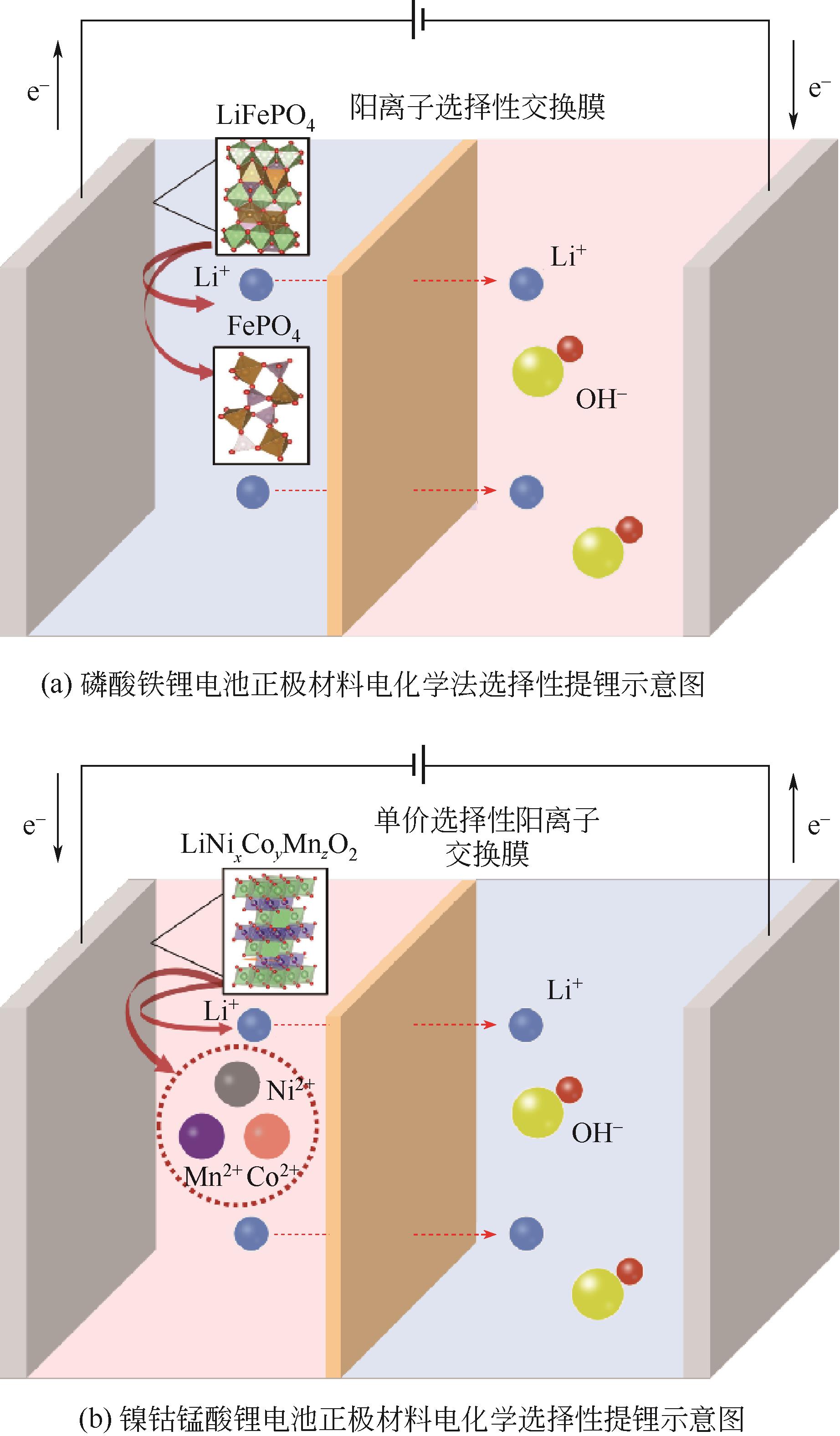
| HUANG Jianhang, WANG Yonggang, XIA Yongyao. Research progress of new energy storage electrochemical power sources[J]. Chinese Journal of Power Sources, 2020, 44(6): 793-798. | |
| 2 | CHAYAMBUKA K, MULDER G, DANILOV D L, et al. Sodium-ion battery materials and electrochemical properties reviewed[J]. Advanced Energy Materials, 2018, 8(16): 1800079. |
| 3 | ARSHAD F, LI L, AMIN K, et al. A comprehensive review of the advancement in recycling the anode and electrolyte from spent lithium ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(36): 13527-13554. |
| 4 | SANTANA I L, MOREIRA T F M, LELIS M F F, et al. Photocatalytic properties of Co3O4/LiCoO2 recycled from spent lithium-ion batteries using citric acid as leaching agent[J]. Materials Chemistry and Physics, 2017, 190: 38-44. |
| 5 | ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. |
| 6 | 罗明增, 周柯, 吴珏, 等. 二次电池储能机理的电化学谱学研究进展[J]. 厦门大学学报(自然科学版), 2020, 59(5): 726-746. |
| LUO Mingzeng, ZHOU Ke, WU Jue, et al. Research progress of electrochemical spectroscopy on energy storage mechanism of rechargeable batteries[J]. Journal of Xiamen University (Natural Science), 2020, 59(5): 726-746. | |
| 7 | RAZMJOU A, ASADNIA M, HOSSEINI E, et al. Design principles of ion selective nanostructured membranes for the extraction of lithium ions[J]. Nature Communications, 2019, 10: 5793. |
| 8 | YANG S X, ZHANG F, DING H P, et al. Lithium metal extraction from seawater[J]. Joule, 2018, 2(9): 1648-1651. |
| 9 | ZENG X L, LI J H. Emerging anthropogenic circularity science: principles, practices, and challenges[J]. iScience, 2021, 24(3): 102237. |
| 10 | The United States Geological Survey(USGS). Mineral commodity summaries [EB/OL]. [2021-08-12]. . |
| 11 | 韩佳欢, 乜贞, 方朝合, 等. 中国锂资源供需现状分析[J]. 无机盐工业, 2021, 53(12): 61-66. |
| HAN Jiahuan, NIE Zhen, FANG Chaohe, et al. Analysis of existing circumstance of supply and demand on China’s lithium resources[J]. Inorganic Chemicals Industry, 2021, 53(12): 61-66. | |
| 12 | 马哲, 李建武. 中国锂资源供应体系研究: 现状、问题与建议[J]. 中国矿业, 2018, 27(10): 1-7. |
| MA Zhe, LI Jianwu. Analysis of China’s lithium resources supply system: status, issues and suggestions[J]. China Mining Magazine, 2018, 27(10): 1-7. | |
| 13 | 齐帅军, 肖克炎, 丁建华, 等. 中国锂矿资源分布和潜力分析[J]. 矿床地质, 2014, 33(S1): 809-810. |
| QI Shuaijun, XIAO Keyan, DING Jianhua, et al. Analysis of distribution and potential on China’s lithium resources[J]. Mineral Deposits, 2014, 33(S1): 809-810. | |
| 14 | ZOU H Y, GRATZ E, APELIAN D, et al. A novel method to recycle mixed cathode materials for lithium ion batteries[J]. Green Chemistry, 2013, 15(5): 1183. |
| 15 | DUNN J B, GAINES L, KELLY J C, et al. Life cycle analysis summary for automotive lithium-ion battery production and recycling[M]. Cham: Springer International Publishing, 2016: 73-79. |
| 16 | HOSSAIN R, KUMAR U, SAHAJWALLA V. Selective thermal transformation of value added cobalt from spent lithium-ion batteries[J]. Journal of Cleaner Production, 2021, 293: 126140. |
| 17 | HU J T, ZHANG J L, LI H X, et al. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries[J]. Journal of Power Sources, 2017, 351: 192-199. |
| 18 | HU X F, MOUSA E, TIAN Y, et al. Recovery of Co, Ni, Mn, and Li from Li-ion batteries by smelting reduction-Part I: A laboratory-scale study[J]. Journal of Power Sources, 2021, 483: 228936. |
| 19 | ATIA T A, ELIA G, HAHN R, et al. Closed-loop hydrometallurgical treatment of end-of-life lithium ion batteries: towards zero-waste process and metal recycling in advanced batteries[J]. Journal of Energy Chemistry, 2019, 35: 220-227. |
| 20 | 黎华玲, 陈永珍, 宋文吉, 等. 湿法回收退役三元锂离子电池有价金属的研究进展[J]. 化工进展, 2019, 38(2): 921-932. |
| LI Hualing, CHEN Yongzhen, SONG Wenji, et al. Research progress on the recovery of valuable metals in retired LiNi x Co y Mn z O2 batteries by wet process[J]. Chemical Industry and Engineering Progress, 2019, 38(2): 921-932. | |
| 21 | GUO M M, LI K, LIU L Z, et al. Resource utilization of spent ternary lithium-ions batteries: synthesis of highly active manganese-based perovskite catalyst for toluene oxidation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 102: 268-275. |
| 22 | FAN E S, LI L, ZHANG X X, et al. Selective recovery of Li and Fe from spent lithium-ion batteries by an environmentally friendly mechanochemical approach[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 11029-11035. |
| 23 | WANG M M, TAN Q Y, HUANG Q F, et al. Converting spent lithium cobalt oxide battery cathode materials into high-value products via a mechanochemical extraction and thermal reduction route[J]. Journal of Hazardous Materials, 2021, 413: 125222. |
| 24 | LI Z, HE L H, ZHU Y F, et al. A green and cost-effective method for production of LiOH from spent LiFePO4 [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(42): 15915-15926. |
| 25 | LI Z, LIU D F, XIONG J C, et al. Selective recovery of lithium and iron phosphate/carbon from spent lithium iron phosphate cathode material by anionic membrane slurry electrolysis[J]. Waste Management, 2020, 107: 1-8. |
| 26 | LYU H, HUANG H J, HUANG C, et al. Electric field driven de-lithiation: a strategy towards comprehensive and efficient recycling of electrode materials from spent lithium ion batteries[J]. Applied Catalysis B: Environmental, 2021, 283: 119634. |
| 27 | GEORGI-MASCHLER T, FRIEDRICH B, WEYHE R, et al. Development of a recycling process for Li-ion batteries[J]. Journal of Power Sources, 2012, 207: 173-182. |
| 28 | LIU C W, LIN J, CAO H B, et al. Recycling of spent lithium-ion batteries in view of lithium recovery: a critical review[J]. Journal of Cleaner Production, 2019, 228: 801-813. |
| 29 | ZENG X L, LI J H, SINGH N. Recycling of spent lithium-ion battery: a critical review[J]. Critical Reviews in Environmental Science and Technology, 2014, 44(10): 1129-1165. |
| 30 | XIAO J F, LI J, XU Z M. Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy[J]. Journal of Hazardous Materials, 2017, 338: 124-131. |
| 31 | QIU R J, HUANG Z, ZHENG J Y, et al. Energy models and the process of fluid-magnetic separation for recovering cobalt micro-particles from vacuum reduction products of spent lithium ion batteries[J]. Journal of Cleaner Production, 2021, 279: 123230. |
| 32 | TANG Y Q, XIE H W, ZHANG B L, et al. Recovery and regeneration of LiCoO2-based spent lithium-ion batteries by a carbothermic reduction vacuum pyrolysis approach: controlling the recovery of CoO or Co[J]. Waste Management, 2019, 97: 140-148. |
| 33 | DIAZ F, WANG Y, MOORTHY T, et al. Degradation mechanism of nickel-cobalt-aluminum (NCA) cathode material from spent lithium-ion batteries in microwave-assisted pyrolysis[J]. Metals, 2018, 8(8): 565. |
| 34 | ZHAO Y Z, LIU B G, ZHANG L B, et al. Microwave-absorbing properties of cathode material during reduction roasting for spent lithium-ion battery recycling[J]. Journal of Hazardous Materials, 2020, 384: 121487. |
| 35 | ZHAO Y Z, LIU B G, ZHANG L B, et al. Microwave pyrolysis of macadamia shells for efficiently recycling lithium from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2020, 396: 122740. |
| 36 | WU Z, ZHU H, BI H, et al. Recycling of electrode materials from spent lithium-ion power batteries via thermal and mechanical treatments[J]. Waste Management & Research, 2021, 39(4): 607-619. |
| 37 | LOMBARDO G, EBIN B, FOREMAN M R St J, et al. Chemical transformations in Li-ion battery electrode materials by carbothermic reduction[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(16): 13668-13679. |
| 38 | LIU P C, XIAO L, CHEN Y F, et al. Recovering valuable metals from LiNi x Co y Mn1- x- y O2 cathode materials of spent lithium ion batteries via a combination of reduction roasting and stepwise leaching[J]. Journal of Alloys and Compounds, 2019, 783: 743-752. |
| 39 | WANG W Q, ZHANG Y C, LIU X G, et al. A simplified process for recovery of Li and co from spent LiCoO2 cathode using Al foil as the in situ reductant[J]. ACS Sustainable Chemistry & Engineering, 2019, 7: 12222-12230. |
| 40 | WANG W Q, ZHANG Y C, ZHANG L, et al. Cleaner recycling of cathode material by in situ thermite reduction[J]. Journal of Cleaner Production, 2020, 249: 119340. |
| 41 | YANG C, ZHANG J L, YU B Y, et al. Recovery of valuable metals from spent LiNi x Co y Mn z O2 cathode material via phase transformation and stepwise leaching[J]. Separation and Purification Technology, 2021, 267: 118609. |
| 42 | LIU F P, PENG C, MA Q X, et al. Selective lithium recovery and integrated preparation of high-purity lithium hydroxide products from spent lithium-ion batteries[J]. Separation and Purification Technology, 2021, 259: 118181. |
| 43 | LIU P C, XIAO L, TANG Y W, et al. Study on the reduction roasting of spent LiNi x Co y Mn z O2 lithium-ion battery cathode materials[J]. Journal of Thermal Analysis and Calorimetry, 2019, 136(3): 1323-1332. |
| 44 | SAIT H H, SALEMA A A. Microwave dielectric characterization of Saudi Arabian date palm biomass during pyrolysis and at industrial frequencies[J]. Fuel, 2015, 161: 239-247. |
| 45 | MAO J K, LI J, XU Z M. Coupling reactions and collapsing model in the roasting process of recycling metals from LiCoO2 batteries[J]. Journal of Cleaner Production, 2018, 205: 923-929. |
| 46 | XIAO J F, LI J, XU Z M. Novel approach for in situ recovery of lithium carbonate from spent lithium ion batteries using vacuum metallurgy[J]. Environmental Science & Technology, 2017, 51(20): 11960-11966. |
| 47 | LI J, WANG G X, XU Z M. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries[J]. Journal of Hazardous Materials, 2016, 302: 97-104. |
| 48 | ZHANG H L, XU J Q, ZHANG J J. Surface-coated LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode materials by Al2O3, ZrO2, and Li2O-2B2O3 thin-layers for improving the performance of lithium ion batteries[J]. Frontiers in Materials, 2019, 6: 309. |
| 49 | XIAO J F, NIU B, XU Z M. Highly efficient selective recovery of lithium from spent lithium-ion batteries by thermal reduction with cheap ammonia reagent[J]. Journal of Hazardous Materials, 2021, 418: 126319. |
| 50 | YANG C, ZHANG J L, CAO Z H, et al. Sustainable and facile process for lithium recovery from spent LiNi x Co y Mn z O2 cathode materials via selective sulfation with ammonium sulfate[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(41): 15732-15739. |
| 51 | LIN J, LIU C W, CAO H B, et al. Environmentally benign process for selective recovery of valuable metals from spent lithium-ion batteries by using conventional sulfation roasting[J]. Green Chemistry, 2019, 21(21): 5904-5913. |
| 52 | LIN J, LI L, FAN E S, et al. Conversion mechanisms of selective extraction of lithium from spent lithium-ion batteries by sulfation roasting[J]. ACS Applied Materials & Interfaces, 2020, 12(16): 18482-18489. |
| 53 | DANG H, WANG B F, CHANG Z D, et al. Recycled lithium from simulated pyrometallurgical slag by chlorination roasting[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(10): 13160-13167. |
| 54 | PENG C, LIU F P, WANG Z L, et al. Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system[J]. Journal of Power Sources, 2019, 415: 179-188. |
| 55 | PRADHAN N, NAYAK R R, PRIYADARSHINI E, et al. Application of iron reducing bacteria for recovery of nickel and cobalt chromite overburden [C]//ⅩⅩⅥ International Mineral Processing Congress- IMPC 2012. India: 2012: 24-28. |
| 56 | 石剑锋, 王志兴, 胡启阳, 等. 硫酸氢铵硫酸化焙烧法红土镍矿提取镍钴[J]. 中国有色金属学报, 2013, 23(2): 510-515. |
| SHI Jianfeng, WANG Zhixing, HU Qiyang, et al. Recovery of nickel and cobalt from nickel laterite ore by sulfation roasting method using ammonium bisulfate[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(2): 510-515. | |
| 57 | KAR B B, SWAMY Y V. Some aspects of nickel extraction from chromitiferous overburden by sulphatization roasting[J]. Minerals Engineering, 2000, 13(14/15): 1635-1640. |
| 58 | PAULINO J F, BUSNARDO N G, AFONSO J C. Recovery of valuable elements from spent Li-batteries[J]. Journal of Hazardous Materials, 2008, 150(3): 843-849. |
| 59 | WANG D H, ZHANG X D, CHEN H J, et al. Separation of Li and Co from the active mass of spent Li-ion batteries by selective sulfating roasting with sodium bisulfate and water leaching[J]. Minerals Engineering, 2018, 126: 28-35. |
| 60 | XU P, LIU C W, ZHANG X H, et al. Synergic mechanisms on carbon and sulfur during the selective recovery of valuable metals from spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(5): 2271-2279. |
| 61 | FAN E S, LI L, LIN J, et al. Low-temperature molten-salt-assisted recovery of valuable metals from spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(19): 16144-16150. |
| 62 | XIAO J F, NIU B, SONG Q M, et al. Novel targetedly extracting lithium: an environmental-friendly controlled chlorinating technology and mechanism of spent lithium ion batteries recovery[J]. Journal of Hazardous Materials, 2021, 404: 123947. |
| 63 | HUANG Y, SHAO P H, YANG L M, et al. Thermochemically driven crystal phase transfer via chlorination roasting toward the selective extraction of lithium from spent LiNi1/3Co1/3Mn1/3O2 [J]. Resources, Conservation and Recycling, 2021, 174: 105757. |
| 64 | YAO Y L, ZHU M Y, ZHAO Z, et al. Hydrometallurgical processes for recycling spent lithium-ion batteries: a critical review[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 13611-13627. |
| 65 | ZHANG P W, YOKOYAMA T, ITABASHI O, et al. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries[J]. Hydrometallurgy, 1998, 47(2/3): 259-271. |
| 66 | GUO Y, LI F, ZHU H C, et al. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl)[J]. Waste Management, 2016, 51: 227-233. |
| 67 | YAO L, YAO H S, XI G X, et al. Recycling and synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries using D,L-malic acid[J]. RSC Advances, 2016, 6(22): 17947-17954. |
| 68 | SUN L, QIU K Q. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries[J]. Waste Management, 2012, 32(8): 1575-1582. |
| 69 | FERREIRA D A, PRADOS L M Z, MAJUSTE D, et al. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries[J]. Journal of Power Sources, 2009, 187(1): 238-246. |
| 70 | PARK O K, CHO Y, LEE S, et al. Who will drive electric vehicles, olivine or spinel?[J]. Energy & Environmental Science, 2011, 4(5): 1621. |
| 71 | KUMAR J, NEIBER R R, PARK J, et al. Recent progress in sustainable recycling of LiFePO4-type lithium-ion batteries: strategies for highly selective lithium recovery[J]. Chemical Engineering Journal, 2021: 133993. |
| 72 | LI H, XING S Z, LIU Y, et al. Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 8017-8024. |
| 73 | LI L, GE J, CHEN R J, et al. Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries[J]. Waste Management, 2010, 30(12): 2615-2621. |
| 74 | LYU W, WANG Z H, ZHENG X H, et al. Selective recovery of lithium from spent lithium-ion batteries by coupling advanced oxidation processes and chemical leaching processes[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(13): 5165-5174. |
| 75 | MENG Q, ZHANG Y J, DONG P. Use of glucose as reductant to recover Co from spent lithium ions batteries[J]. Waste Management, 2017, 64: 214-218. |
| 76 | MESHRAM P, PANDEY B D, MANKHAND T R. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching[J]. Chemical Engineering Journal, 2015, 281: 418-427. |
| 77 | LI L, BIAN Y F, ZHANG X X, et al. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching[J]. Waste Management, 2018, 71: 362-371. |
| 78 | GAO W F, ZHANG X H, ZHENG X H, et al. Lithium carbonate recovery from cathode scrap of spent lithium-ion battery: a closed-loop process[J]. Environmental Science & Technology, 2017, 51(3): 1662-1669. |
| 79 | TAKACOVA Z, HAVLIK T, KUKURUGYA F, et al. Cobalt and lithium recovery from active mass of spent Li-ion batteries: theoretical and experimental approach[J]. Hydrometallurgy, 2016, 163: 9-17. |
| 80 | CHEN X P, LUO C B, ZHANG J X, et al. Sustainable recovery of metals from spent lithium-ion batteries: a green process[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(12): 3104-3113. |
| 81 | ZENG X L, LI J H, SHEN B Y. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid[J]. Journal of Hazardous Materials, 2015, 295: 112-118. |
| 82 | LI L, LU J, REN Y, et al. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries[J]. Journal of Power Sources, 2012, 218: 21-27. |
| 83 | YANG Y X, MENG X Q, CAO H B, et al. Selective recovery of lithium from spent lithium iron phosphate batteries: a sustainable process[J]. Green Chemistry, 2018, 20(13): 3121-3133. |
| 84 | CHEN L L, CHAO Y H, LI X W, et al. Engineering a tandem leaching system for the highly selective recycling of valuable metals from spent Li-ion batteries[J]. Green Chemistry, 2021, 23(5): 2177-2184. |
| 85 | NIINAE M, KOMATSU N, NAKAHIRO Y, et al. Preferential leaching of cobalt, nickel and copper from cobalt-rich ferromanganese crusts with ammoniacal solutions using ammonium thiosulfate and ammonium sulfite as reducing agents[J]. Hydrometallurgy, 1996, 40(1/2): 111-121. |
| 86 | ZHENG X H, GAO W F, ZHANG X H, et al. Spent lithium-ion battery recycling—Reductive ammonia leaching of metals from cathode scrap by sodium sulphite[J]. Waste Management, 2017, 60: 680-688. |
| 87 | GUO Y G, LI Y G, LOU X Y, et al. Improved extraction of cobalt and lithium by reductive acid from spent lithium-ion batteries via mechanical activation process[J]. Journal of Materials Science, 2018, 53(19): 13790-13800. |
| 88 | WANG M M, TAN Q Y, LI J H. Unveiling the role and mechanism of mechanochemical activation on lithium cobalt oxide powders from spent lithium-ion batteries[J]. Environmental Science & Technology, 2018, 52(22): 13136-13143. |
| 89 | TAN Q Y, LI J H. Recycling metals from wastes: a novel application of mechanochemistry[J]. Environmental Science & Technology, 2015, 49(10): 5849-5861. |
| 90 | XIE J Y, HUANG K Y, NIE Z L, et al. An effective process for the recovery of valuable metals from cathode material of lithium-ion batteries by mechanochemical reduction[J]. Resources, Conservation and Recycling, 2021, 168: 105261. |
| 91 | LIU K, LIU L L, TAN Q Y, et al. Selective extraction of lithium from a spent lithium iron phosphate battery by mechanochemical solid-phase oxidation[J]. Green Chemistry, 2021, 23(3): 1344-1352. |
| 92 | LIU K, TAN Q Y, LIU L L, et al. Acid-free and selective extraction of lithium from spent lithium iron phosphate batteries via a mechanochemically induced isomorphic substitution[J]. Environmental Science & Technology, 2019, 53(16): 9781-9788. |
| 93 | WANG M M, TAN Q Y, LIU L L, et al. Selective regeneration of lithium from spent lithium-ion batteries using ionic substitution stimulated by mechanochemistry[J]. Journal of Cleaner Production, 2021, 279: 123612. |
| 94 | YU J Z, WANG X, ZHOU M Y, et al. A redox targeting-based material recycling strategy for spent lithium ion batteries[J]. Energy & Environmental Science, 2019, 12(9): 2672-2677. |
| 95 | LIU K, YANG S L, LAI F Y, et al. Innovative electrochemical strategy to recovery of cathode and efficient lithium leaching from spent lithium-ion batteries[J]. ACS Applied Energy Materials, 2020, 3(5): 4767-4776. |
| 96 | GMAR S, CHAGNES A. Recent advances on electrodialysis for the recovery of lithium from primary and secondary resources[J]. Hydrometallurgy, 2019, 189: 105124. |
| 97 | CHAN K H, MALIK M, AZIMI G. Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis[J]. Resources, Conservation and Recycling, 2022, 178: 106076. |
| 98 | LYU W, RUAN D S, ZHENG X H, et al. One-step recovery of valuable metals from spent lithium-ion batteries and synthesis of persulfate through paired electrolysis[J]. Chemical Engineering Journal, 2021, 421: 129908. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [4] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 李季桐, 王刚, 熊亚选, 徐钱. 不同工质单效吸收式制冷系统的能量和㶲分析[J]. 化工进展, 2023, 42(S1): 104-112. |
| [9] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [10] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [11] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [12] | 雷伟, 姜维佳, 王玉高, 和明豪, 申峻. N、S共掺杂煤基碳量子点的电化学氧化法制备及用于Fe3+检测[J]. 化工进展, 2023, 42(9): 4799-4807. |
| [13] | 钱思甜, 彭文俊, 张先明. PET熔融缩聚与溶液解聚形成环状低聚物的对比分析[J]. 化工进展, 2023, 42(9): 4808-4816. |
| [14] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [15] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||