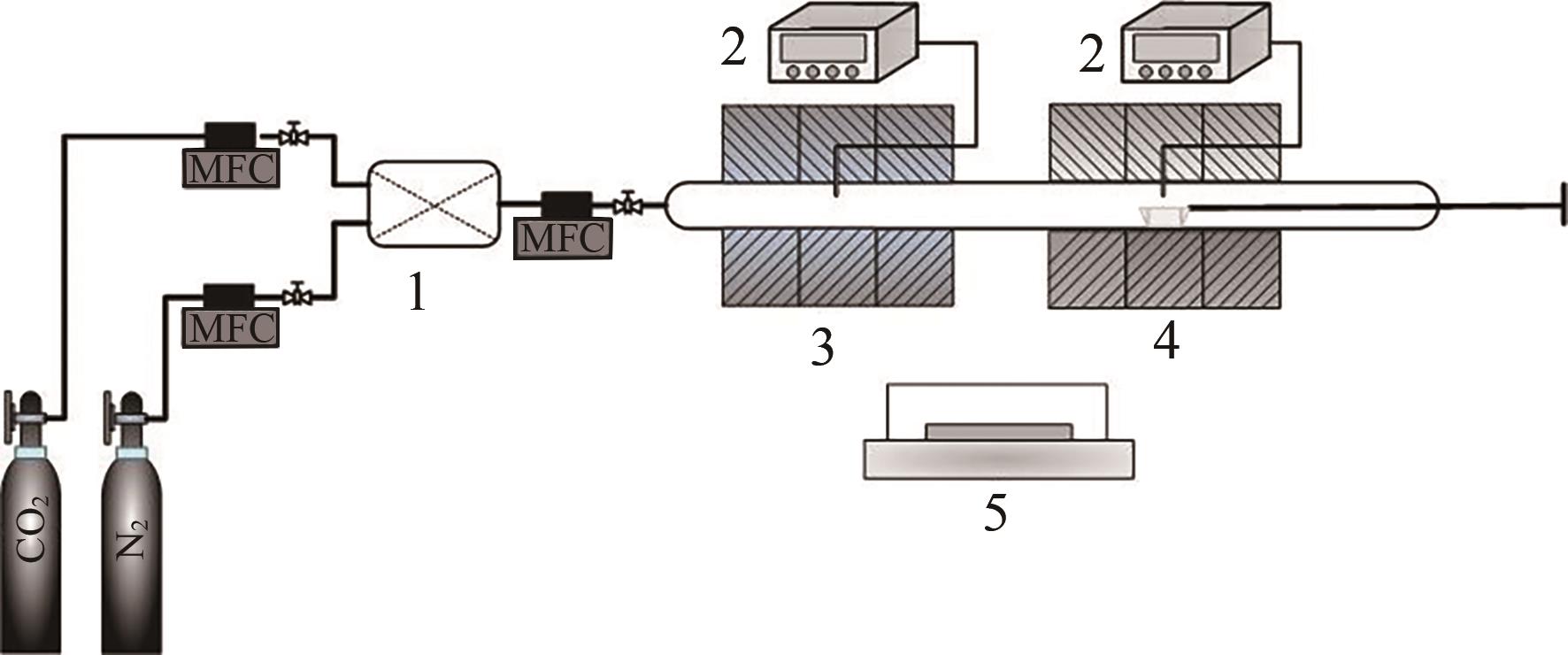
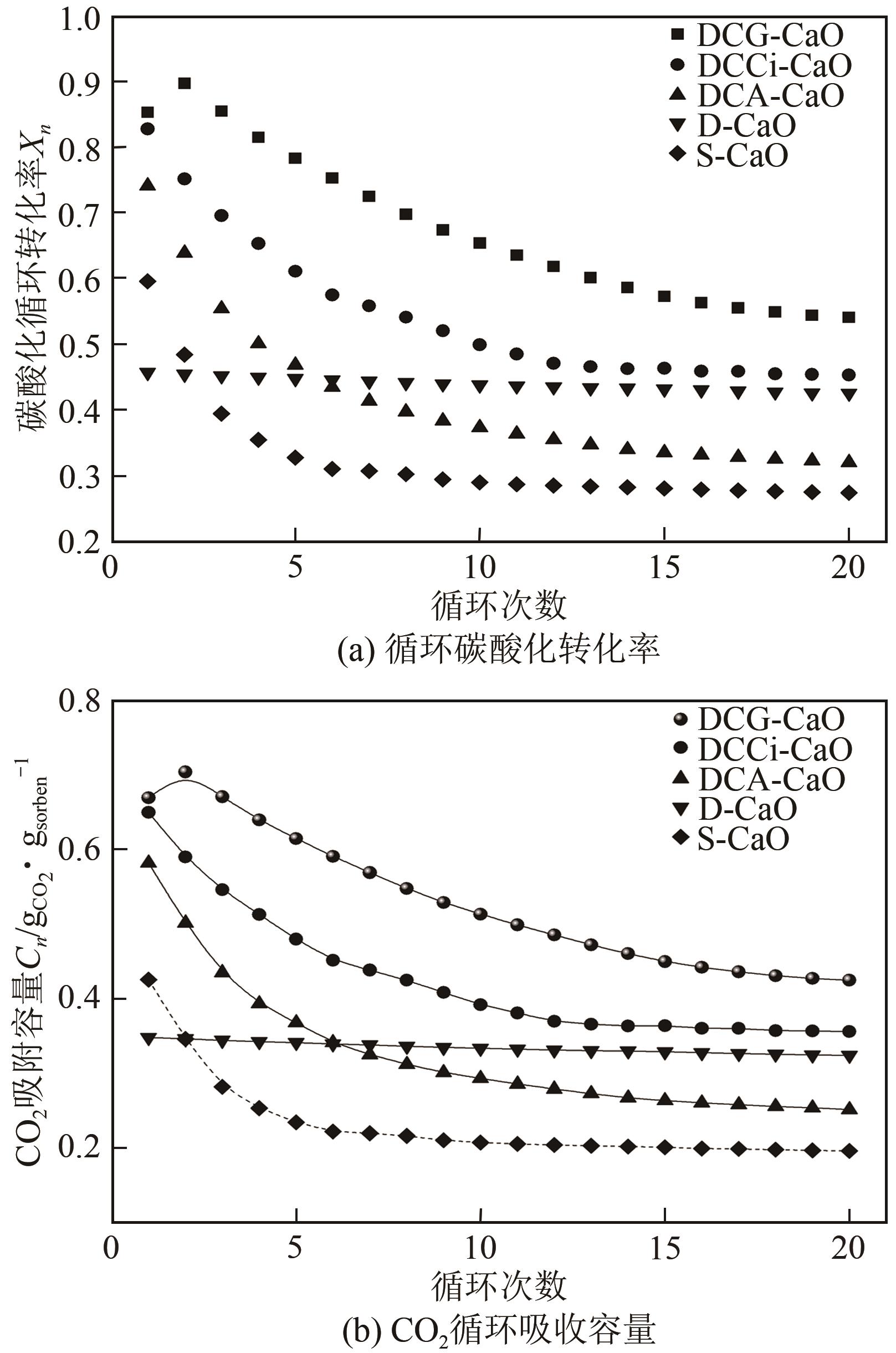
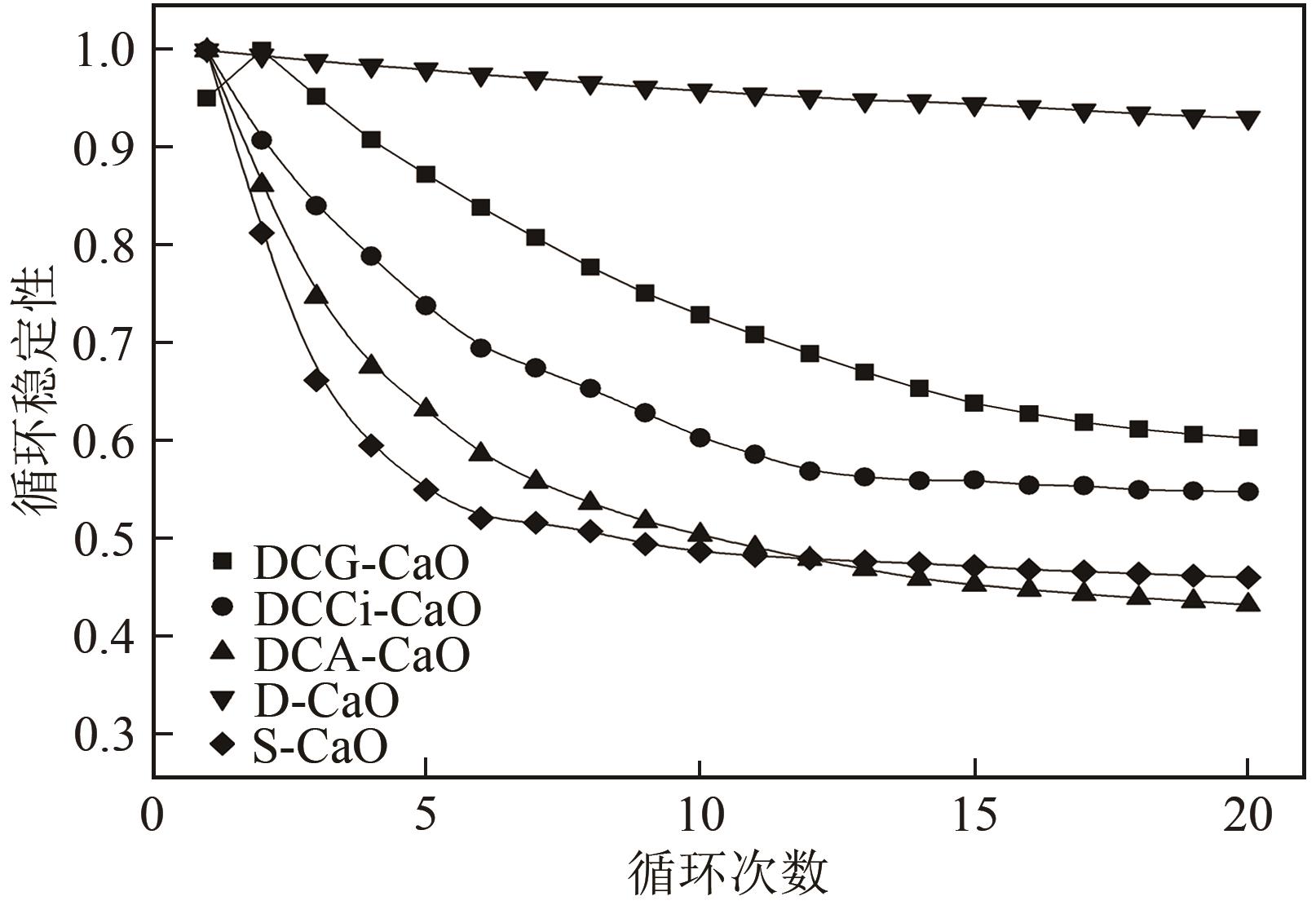
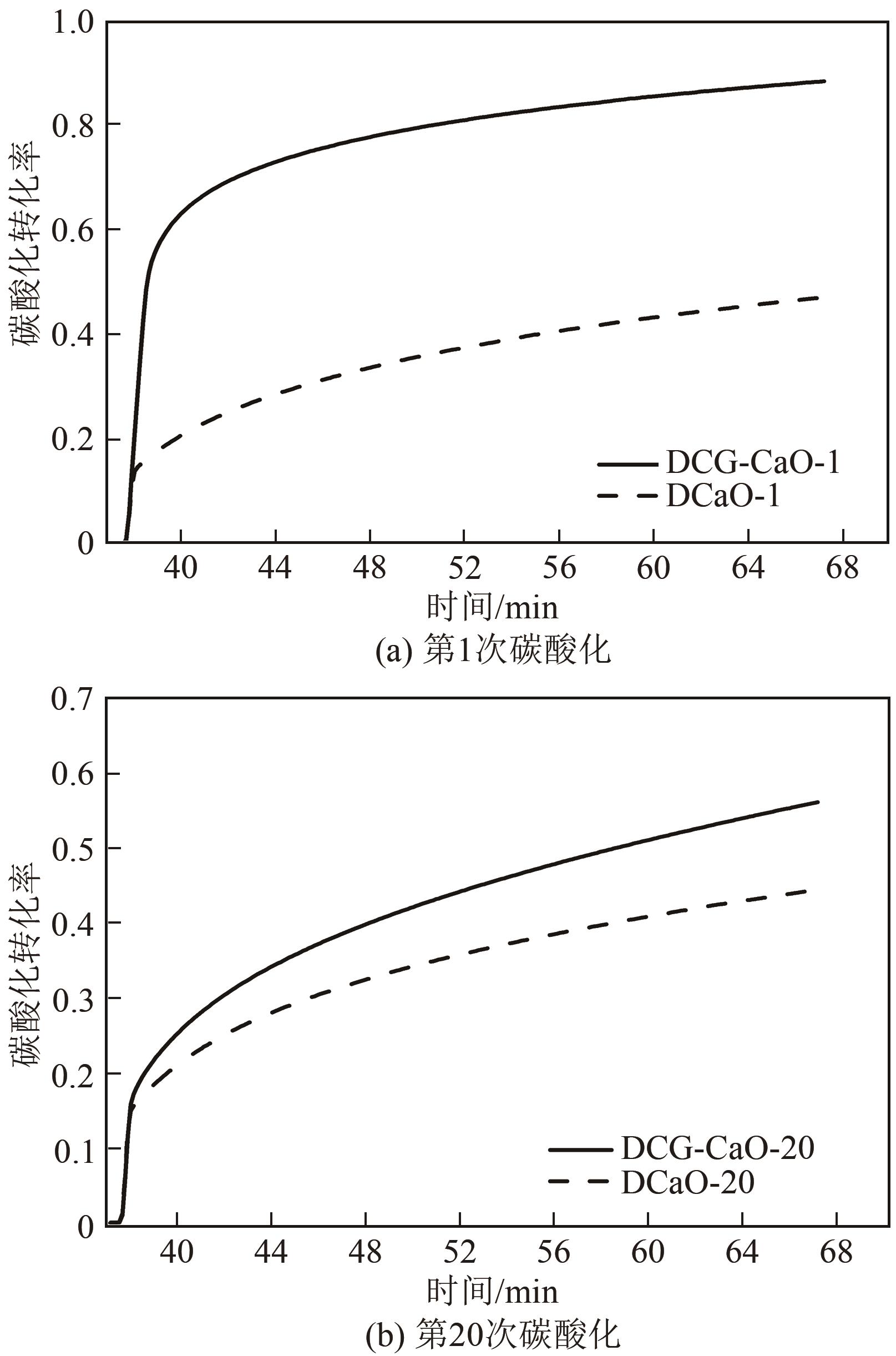
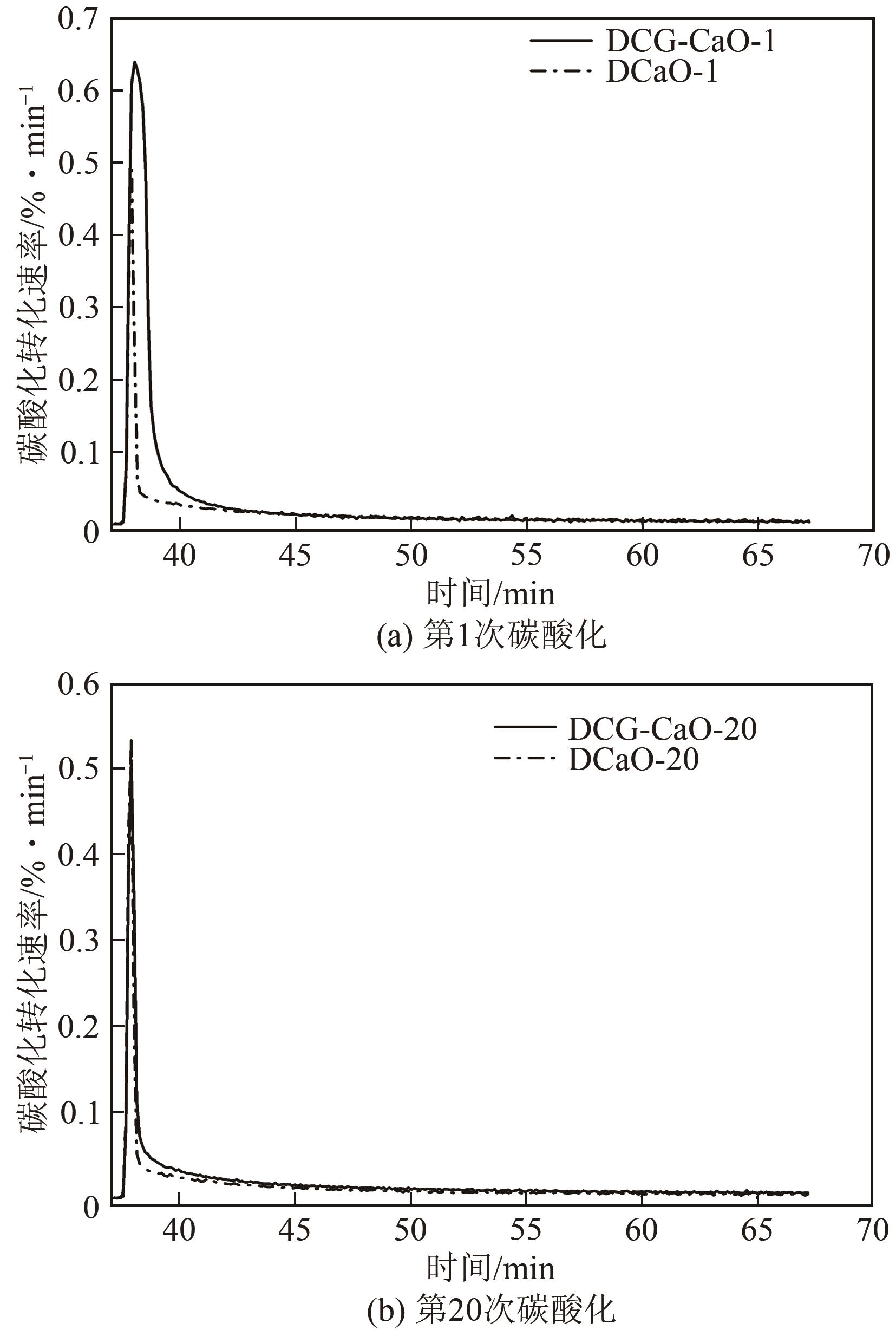
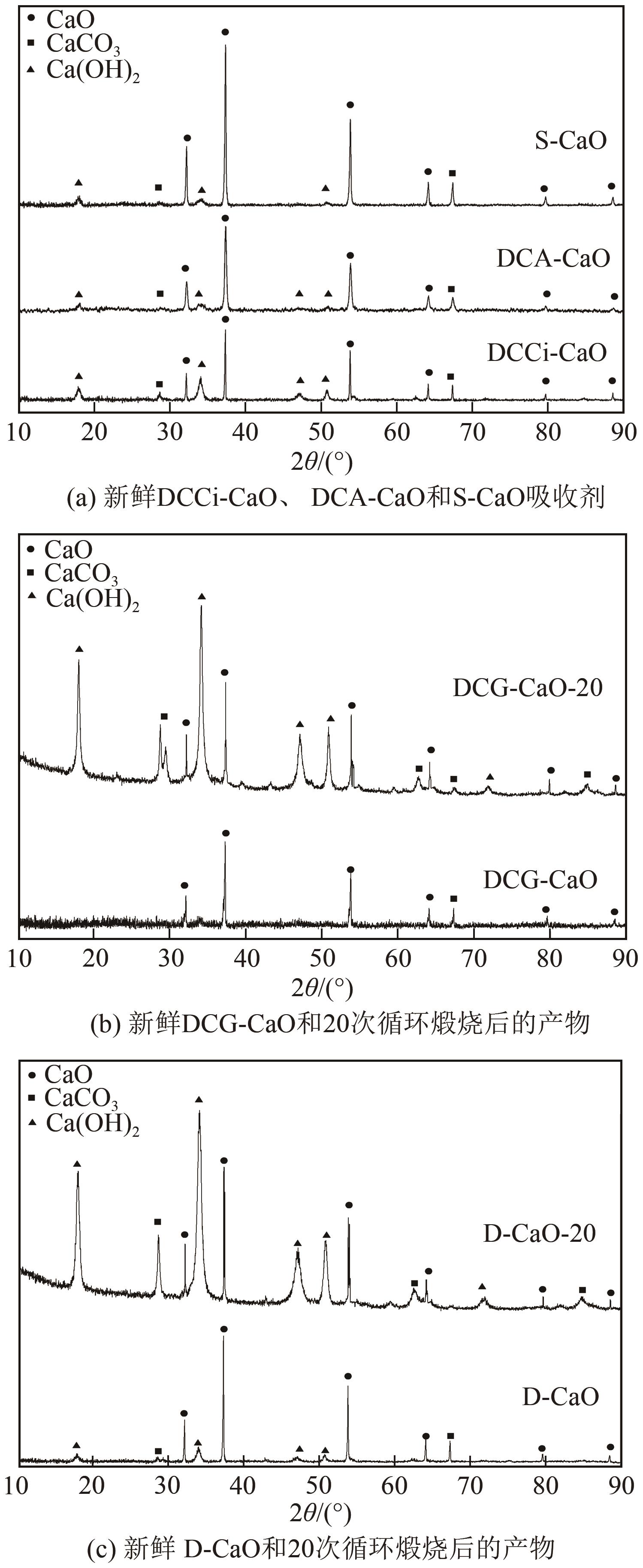
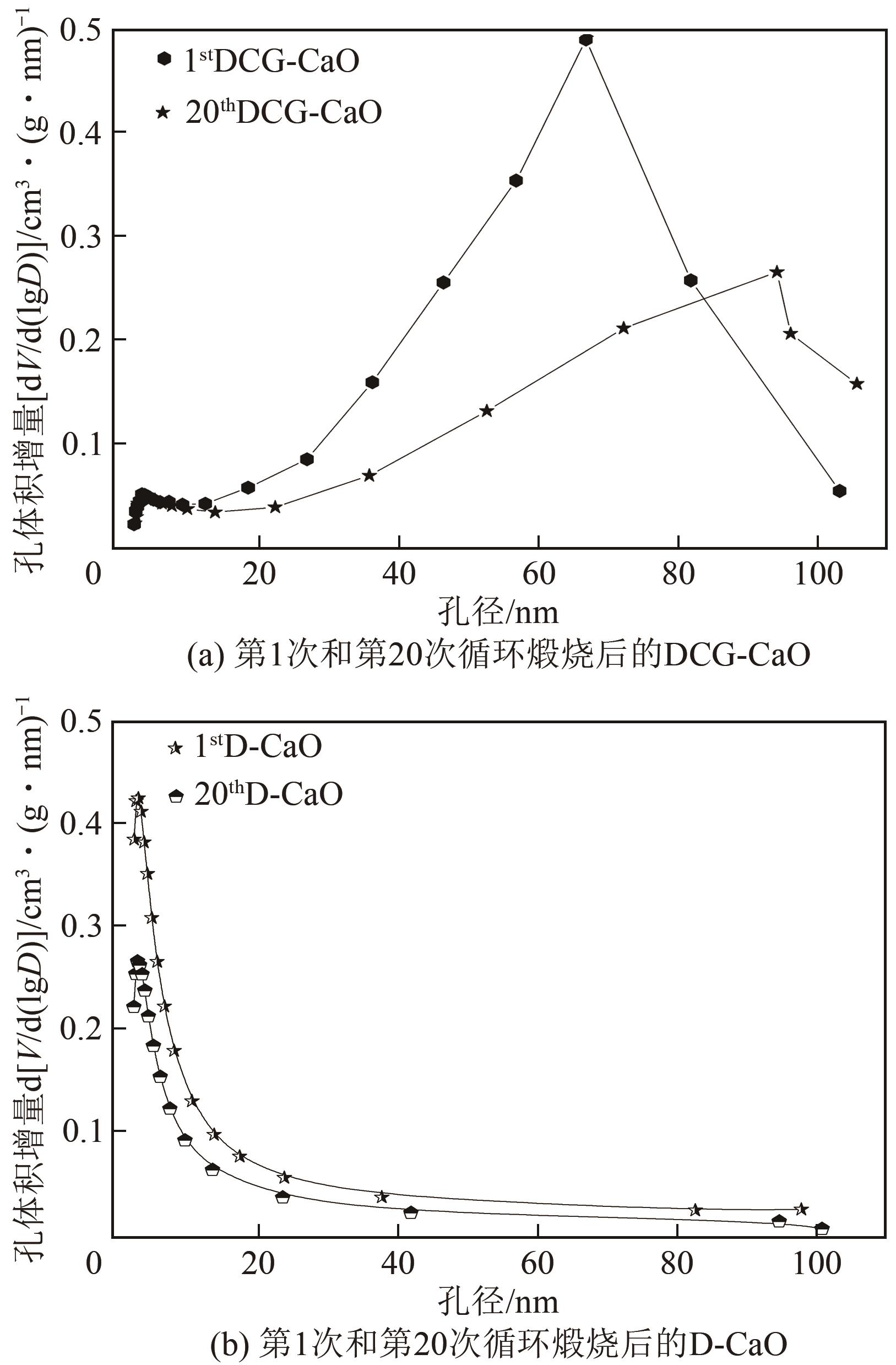
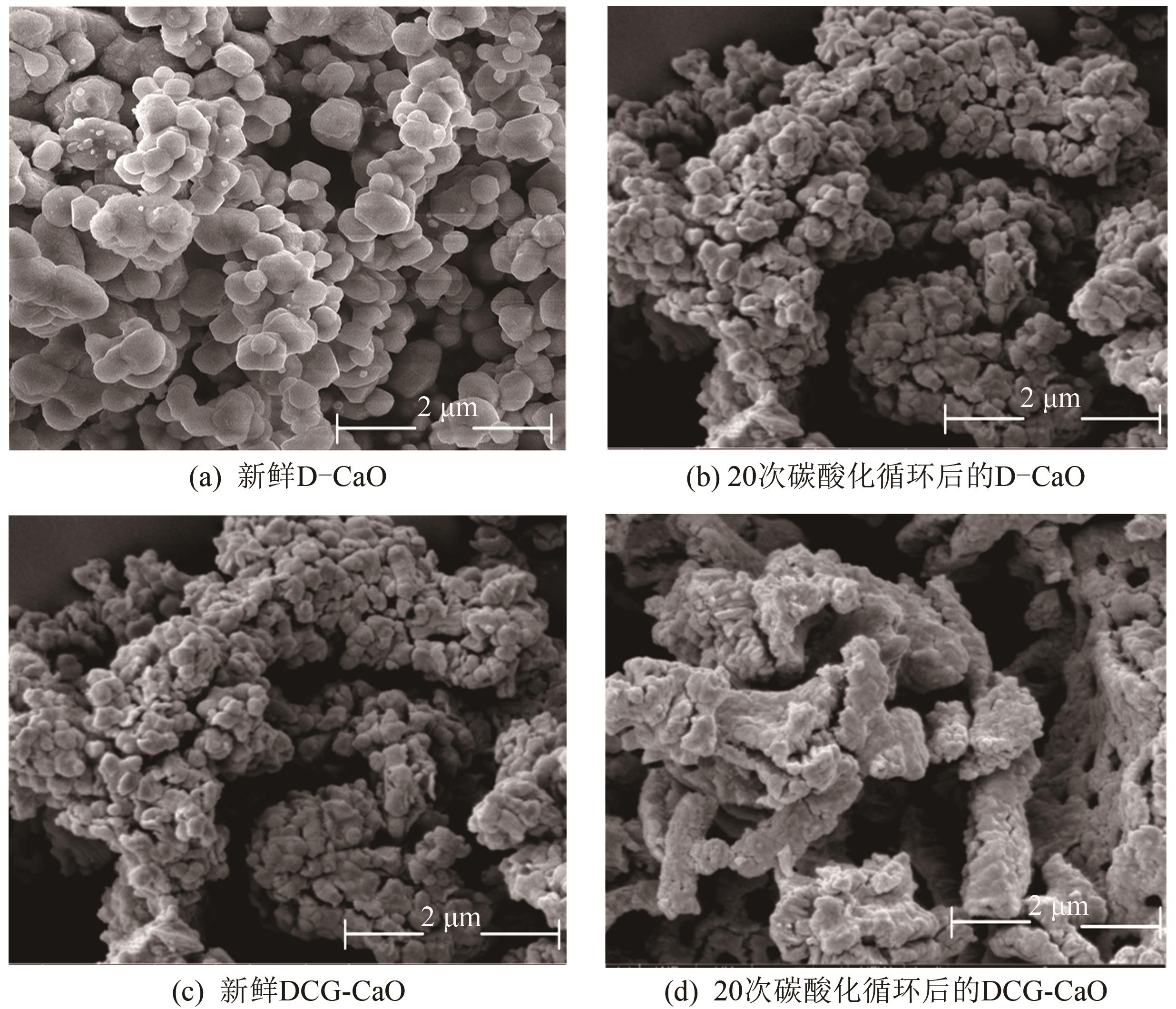
| 1 |
黄翔, 陶蕾, 杨燃, 等. 复合乳酸菌发酵蛋壳制备乳酸钙[J]. 食品科学, 2019, 40(20): 159-165.
|
|
HUANG Xiang, TAO Lei, YANG Ran, et al. Preparation of calcium lactate from eggshell by mixed-culture fermentation of lactic acid bacteria[J]. Food Science, 2019, 40(20): 159-165.
|
| 2 |
马美湖, 邱宁, 黄茜, 等. 我国蛋品加工业发展情况及特点[J]. 农业工程技术, 2015, 35(5): 26-31.
|
|
MA Meihu, QIU Ning, HUANG Qian, et al. The development situation and characteristics of China’s egg processing industry[J]. Agriculture Engineering Technology, 2015, 35(5): 26-31.
|
| 3 |
KANNAN M B, RONAN K. Conversion of biowastes to biomaterial: an innovative waste management approach[J]. Waste Management, 2017, 67: 67-72.
|
| 4 |
YE M, SUN M M, CHEN X, et al. Feasibility of sulfate-calcined eggshells for removing pathogenic bacteria and antibiotic resistance genes from landfill leachates[J]. Waste Management, 2017, 63: 275-283.
|
| 5 |
WANG B W, SONG X Y, WANG Z H, et al. Preparation and application of the sol-gel combustion synthesis-made CaO/CaZrO3 sorbent for cyclic CO2 capture through the severe calcination condition[J]. Chinese Journal of Chemical Engineering, 2014, 22(9): 991-999.
|
| 6 |
孙超颖, 李英杰, 闫宪尧, 等. 钙循环辅集CO2后CaO的水合/脱水热化学储热性能[J].化工进展, 2020, 39(5): 1734-1743.
|
|
SUN Chaoying, LI Yingjie, YAN Xianyao, et al. Hydration/dehydration thermochemical heat storage performance of CaO from CO2 capture cyles[J]. Chemical Industry and Engineering Progress, 2020, 39(5): 1734-1743.
|
| 8 |
WITOON T. Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent[J]. Ceramics International, 2011, 37(8): 3291-3298.
|
| 9 |
SALAUDEEN S A, TASNIM S H, HEIDARI M, et al. Eggshell as a potential CO2 sorbent in the calcium looping gasification of biomass[J]. Waste Management, 2018, 80: 274-284.
|
| 10 |
MOHAMMADI M, LAHIJANI P, MOHAMED A R. Refractory dopant-incorporated CaO from waste eggshell as sustainable sorbent for CO2 capture: experimental and kinetic studies[J]. Chemical Engineering Journal, 2014, 243: 455-464.
|
| 11 |
IYER M V, FAN L S. High temperature CO capture using engineered eggshells: a route to carbon management: US7678351[P]. 2010-03-16.
|
| 12 |
OLIVARES-MARÍN M, CUERDA-CORREA E M, NIETO-SÁNCHEZ A, et al. Influence of morphology, porosity and crystal structure of CaCO3 precursors on the CO2 capture performance of CaO-derived sorbents[J]. Chemical Engineering Journal, 2013, 217: 71-81.
|
| 13 |
CASTILHO S, KIENNEMANN A, COSTA PEREIRA M F, et al. Sorbents for CO2 capture from biogenesis calcium wastes[J]. Chemical Engineering Journal, 2013, 226: 146-153.
|
| 14 |
SHAN S Y, MA A H, HU Y C, et al. Development of sintering-resistant CaO-based sorbent derived from eggshells and bauxite tailings for cyclic CO2 capture[J]. Environmental Pollution, 2016, 208: 546-552.
|
| 15 |
杨彬, 余钟亮, 李春玉, 等. CeO2掺杂对CaO基吸收剂CO2捕获性能的影响[J]. 燃料化学学报, 2019, 47(3): 344-351.
|
|
YANG Bin, YU Zhongliang, LI Chunyu, et al. Influence of cerium doping on CO2 capture of CaO-based sorbents[J]. Journal of Fuel Chemistry and Technology, 2019, 47(3): 344-351.
|
| 16 |
耿一琪, 郭彦霞, 樊飙, 等. CaO基吸附剂捕集CO2及其抗烧结改性研究进展[J]. 燃料化学学报, 2021, 49(7): 998-1013.
|
|
GENG Yiqi, GUO Yanxia, FAN Biao, et al. Research progress of calcium-based adsorbents for CO2 capture and anti-sintering modification[J]. Journal of Fuel Chemistry and Technology, 2021, 49(7): 998-1013.
|
| 17 |
LIU W Q, LOW N W, FENG B, et al. Calcium precursors for the production of CaO sorbents for multicycle CO2 capture[J]. Environmental Science & Technology, 2010, 44(2): 841-847.
|
| 18 |
LIU W Q, FENG B, WU Y Q, et al. Synthesis of sintering-resistant sorbents for CO2 capture[J]. Environmental Science & Technology, 2010, 44(8): 3093-3097.
|
| 19 |
ERANS M, MANOVIC V, ANTHONY E J. Calcium looping sorbents for CO2 capture[J]. Applied Energy, 2016, 180: 722-742.
|
| 20 |
郭名女. 抗烧结钙基吸收剂同时捕集CO2/SO2的循环反应特性及动力学研究[D]. 重庆: 重庆大学, 2011.
|
|
GUO Mingnv. Cyclic reaction characteristic of co-capture CO2/SO2 and kinetic study for synthetized anti-sintering calcium-based sorbents[D]. Chongqing: Chongqing University, 2011.
|
| 21 |
刘小通. 改性钙基CO2高温吸附剂的研究[D]. 西安: 西北大学, 2017.
|
|
LIU Xiaotong. Study of modified CaO-based sorbents for CO2 at high temperature[D]. Xi’an: Northwest University, 2017.
|
| 22 |
任聃艳. 基于复合载体的钙铜联合循环性能研究[D]. 武汉: 华中科技大学, 2017.
|
|
REN Danyan. Development of combined calcium and copper chemical looping process using composite Cao/CuO-based materials[D]. Wuhan: Huazhong University of Science and Technology, 2017.
|
| 23 |
BARKER R. The reversibility of the reaction CaCO3 ⇄ CaO+CO2 [J]. Journal of Applied Chemistry and Biotechnology, 2007, 23(10): 733-742.
|
| 24 |
WANG B W, ZHENG Y, YAN R, et al. A new indicator for determining the fast chemical reaction stage of CaO carbonation with CO2 [J]. Asia-Pacific Journal of Chemical Engineering, 2007, 2(3): 197-202.
|
| 25 |
YANG Y D, LIU W Q, HU Y C, et al. Novel low cost Li4SiO4-based sorbent with naturally occurring wollastonite as Si-source for cyclic CO2 capture[J]. Chemical Engineering Journal, 2019, 374: 328-337.
|
| 26 |
史杰文. 基于钙基吸收剂的CO2、SO2和NO联合脱除研究[D]. 济南: 山东大学, 2018.
|
|
SHI Jiewen. Study on combined removal of CO2, SO2 and NO based on calcium-based sorbents[D]. Jinan: Shandong University, 2018.
|
| 27 |
彭微薇. 基于自组装模板法的新型CO2吸收剂性能研究[D]. 武汉: 华中科技大学, 2016.
|
|
PENG Weiwei. Research on novel CO2 sorbents based on self-assembly template synthesis method[D]. Wuhan: Huazhong University of Science and Technology, 2016.
|
| 28 |
SUN P, GRACE J R, LIM C J, et al. The effect of CaO sintering on cyclic CO2 capture in energy systems[J]. AIChE Journal, 2007, 53(9): 2432-2442.
|
| 29 |
ABANADES J C. The maximum capture efficiency of CO2 using a carbonation/calcination cycle of CaO/CaCO3 [J]. Chemical Engineering Journal, 2002, 90(3): 303-306.
|
 ), 姜涛1, 许炳辉1, 梁彦琛1, 郭超凡1, 李旭刚1, 周志永2
), 姜涛1, 许炳辉1, 梁彦琛1, 郭超凡1, 李旭刚1, 周志永2
 ), JIANG Tao1, XU Binghui1, LIANG Yanchen1, GUO Chaofan1, LI Xugang1, ZHOU Zhiyong2
), JIANG Tao1, XU Binghui1, LIANG Yanchen1, GUO Chaofan1, LI Xugang1, ZHOU Zhiyong2