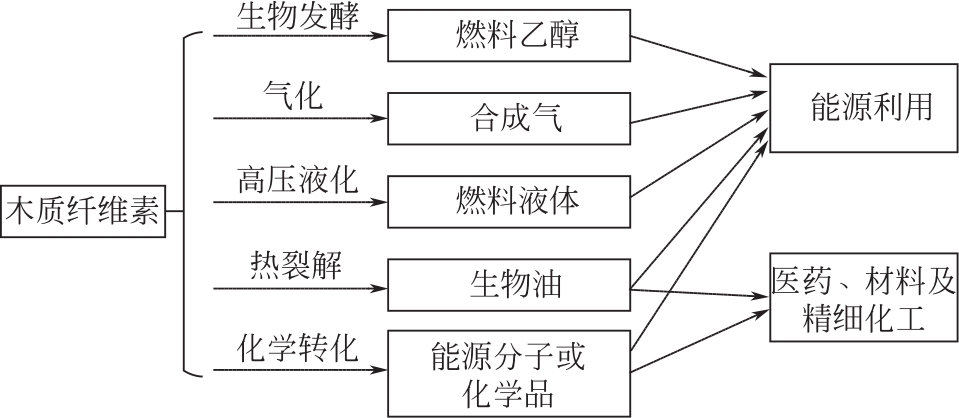
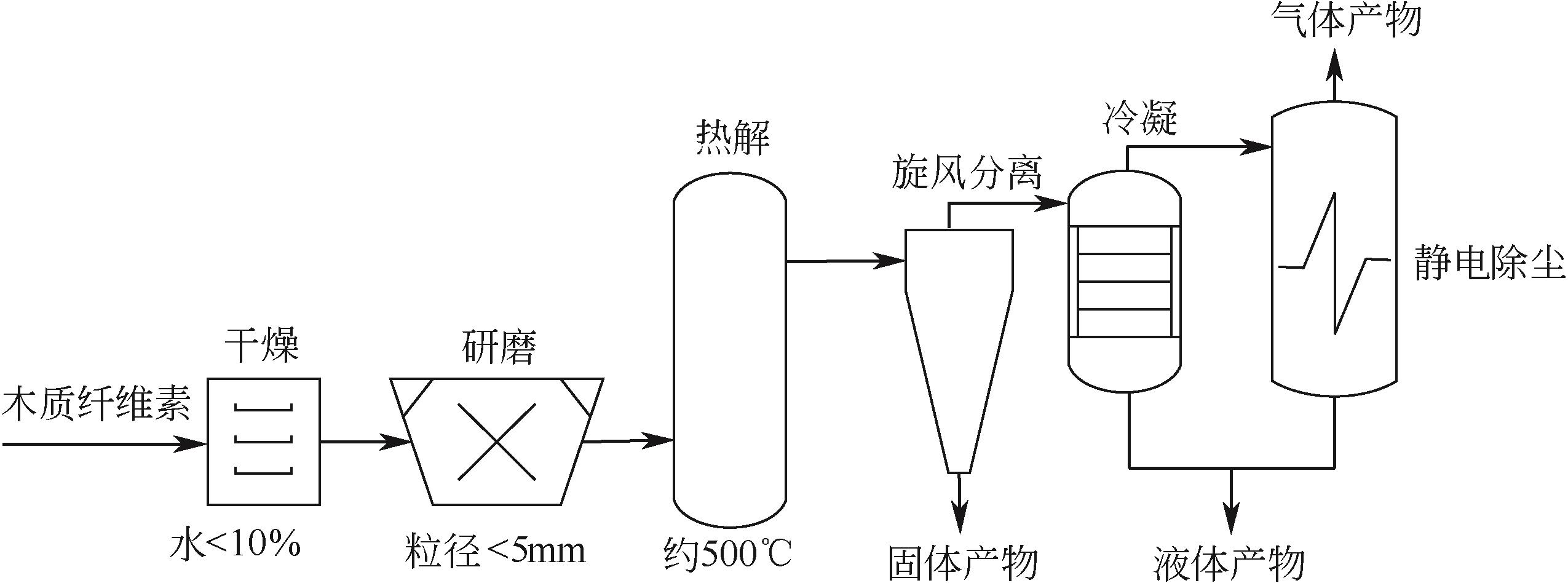
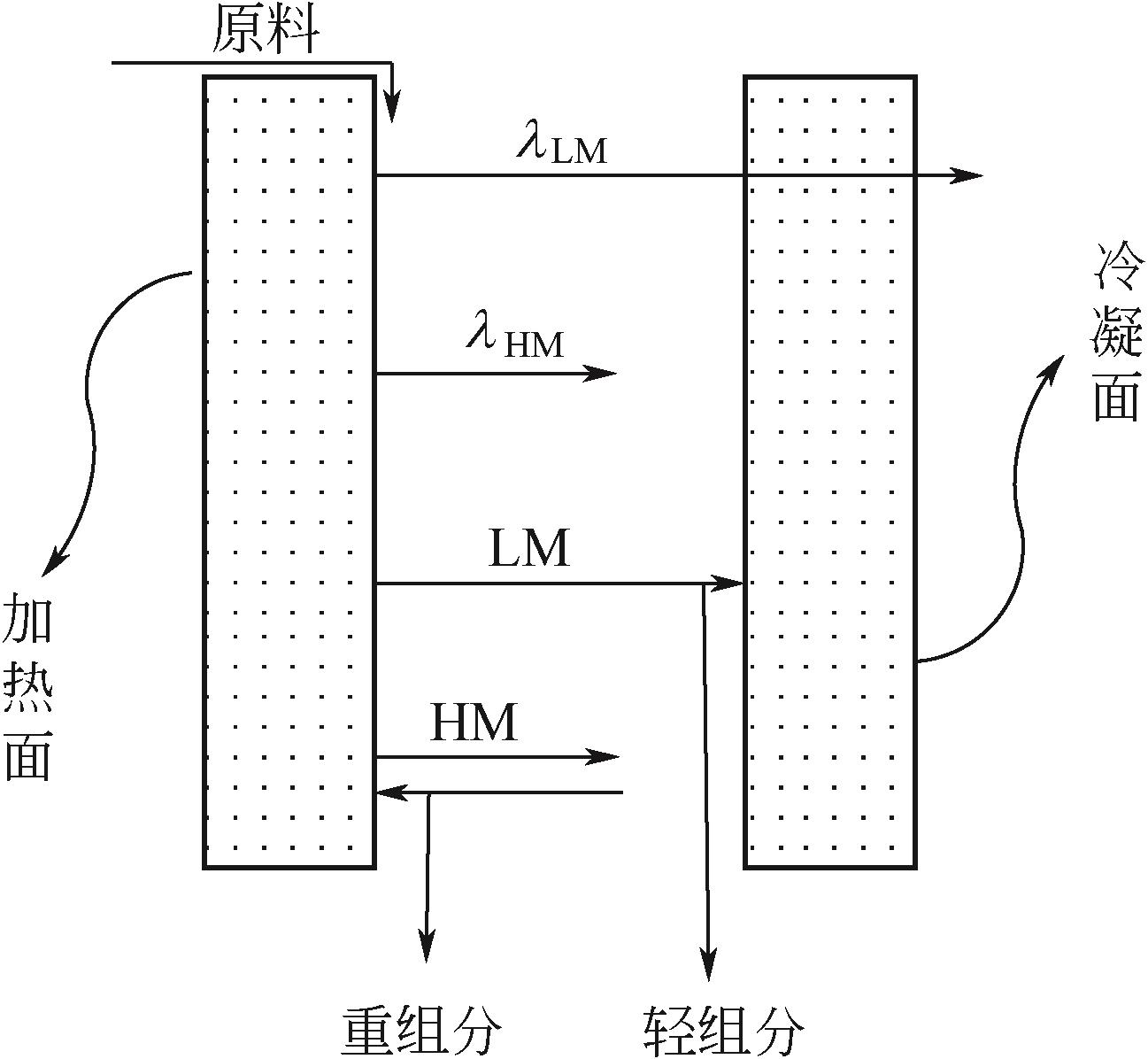
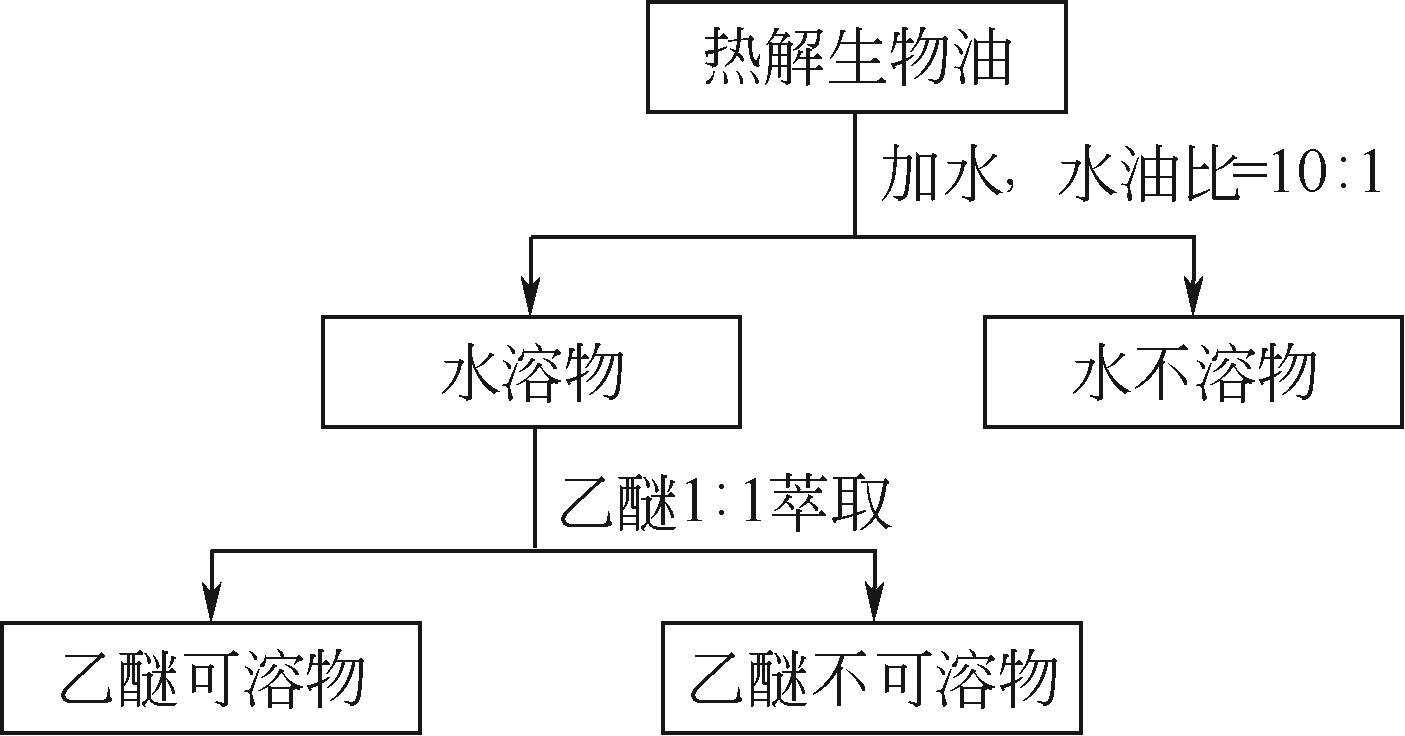
化工进展 ›› 2021, Vol. 40 ›› Issue (12): 6640-6655.DOI: 10.16085/j.issn.1000-6613.2020-2539
生物油组分分离与化学品提取的研究进展
- 中国石油大学(北京)重质油国家重点实验室,北京 102249
-
收稿日期:2020-12-21修回日期:2021-03-19出版日期:2021-12-05发布日期:2021-12-21 -
通讯作者:孟祥海 -
作者简介:耿风华(1981—),女,博士研究生。E-mail:gengfenghua_16@163.com 。
Progress in the separation of components and extraction of chemicals from bio-oils
GENG Fenghua( ), ZHANG Rui, LIU Haiyan, MENG Xianghai(
), ZHANG Rui, LIU Haiyan, MENG Xianghai( )
)
- State Key Laboratory of Heavy Oil Processing, China University of Petroleum (Beijing), Beijing 102249, China
-
Received:2020-12-21Revised:2021-03-19Online:2021-12-05Published:2021-12-21 -
Contact:MENG Xianghai
摘要:
生物质在高温无氧条件下热解可以生成富含高附加值化学品和燃油成分的生物油。有效分离技术和高效提取手段的发展是生物油质量提升的关键。基于此,本文在介绍生物油性质与生物质快速热解工艺的同时,对目前国内外的生物油分离技术如蒸馏、液-液萃取、柱色谱、超临界萃取、膜分离等进行了较为详细的分析和评述。常规蒸馏和溶剂萃取等技术,工艺成熟、操作简单,但存在生物油的热敏性差、萃取剂回收难度大和污染严重等问题;分子蒸馏技术分离过程安全环保,但工艺复杂,设备成本高;超临界萃取和膜分离等技术安全环保,技术成熟,具有较大的潜力。文章还综述了目前生物油中具有高附加值的组分和单一化学品的分离提取研究进展,为生物油的有效分离和高效利用提供了理论参考,也为未来生物油分离技术的发展提供了研发方向。
中图分类号:
引用本文
耿风华, 张睿, 刘海燕, 孟祥海. 生物油组分分离与化学品提取的研究进展[J]. 化工进展, 2021, 40(12): 6640-6655.
GENG Fenghua, ZHANG Rui, LIU Haiyan, MENG Xianghai. Progress in the separation of components and extraction of chemicals from bio-oils[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6640-6655.
| 热解方式 | 热解条件 | 质量分数/% | ||
|---|---|---|---|---|
| 液体 | 固体 | 气体 | ||
| 快速热解 | 温度500℃,气体停留时间<2s | 75 | 12 | 13 |
| 中间方式 | 温度400℃,气体停留时间5~20s | 40(两相) | 40 | 20 |
| 慢速热解(碳化) | 温度400℃,气体停留时间几小时 | 30(两相) | 35 | 35 |
| 气化(有氧) | 温度750~900℃,气体停留时间5s | 0 | 2 | 98 |
| 干燥(慢) | 温度250~300℃,固体停留时间30min | 5~15 | 70~80 | 15 |
表1 木材(干木基)通过不同的热解方式得到的典型产品及产率[9]
| 热解方式 | 热解条件 | 质量分数/% | ||
|---|---|---|---|---|
| 液体 | 固体 | 气体 | ||
| 快速热解 | 温度500℃,气体停留时间<2s | 75 | 12 | 13 |
| 中间方式 | 温度400℃,气体停留时间5~20s | 40(两相) | 40 | 20 |
| 慢速热解(碳化) | 温度400℃,气体停留时间几小时 | 30(两相) | 35 | 35 |
| 气化(有氧) | 温度750~900℃,气体停留时间5s | 0 | 2 | 98 |
| 干燥(慢) | 温度250~300℃,固体停留时间30min | 5~15 | 70~80 | 15 |
| 技术工艺 | 研发单位 | 生产规模/t·d-1 |
|---|---|---|
| 涡旋反应器热解 | 美国国家可再生能源实验室(NREL) | 0.48 |
| 烧蚀热解 | 美国国家可再生能源实验室(NREL) | 0.48 |
| 旋转锥式反应热解 | 荷兰Twente大学 | 50 |
| 沸腾流化床热解 | 加拿大Waterloo大学 | 100 |
| 循环流化床热解 | 加拿大Ensyn工程协会 | 70 |
| 热循环真空热解 | 加拿大Institute Pyrovac Inc | 1.2 |
| 携带床反应器热解 | 美国Georgia工程学院 | 1.08 |
| 奥格窑热解 | 加拿大WWTC | 1.01 |
| 旋风热解 | 加拿大Jacques | 1.32 |
表2 国外具有代表性的快速热解工艺[13-15]
| 技术工艺 | 研发单位 | 生产规模/t·d-1 |
|---|---|---|
| 涡旋反应器热解 | 美国国家可再生能源实验室(NREL) | 0.48 |
| 烧蚀热解 | 美国国家可再生能源实验室(NREL) | 0.48 |
| 旋转锥式反应热解 | 荷兰Twente大学 | 50 |
| 沸腾流化床热解 | 加拿大Waterloo大学 | 100 |
| 循环流化床热解 | 加拿大Ensyn工程协会 | 70 |
| 热循环真空热解 | 加拿大Institute Pyrovac Inc | 1.2 |
| 携带床反应器热解 | 美国Georgia工程学院 | 1.08 |
| 奥格窑热解 | 加拿大WWTC | 1.01 |
| 旋风热解 | 加拿大Jacques | 1.32 |
| 物理性质 | 生物油 | 重质油 |
|---|---|---|
| 水含量/% | 15~30 | 0.1 |
| pH | 2.5 | — |
| 相对密度 | 1.2 | 0.94 |
| 元素组成/% | ||
| C | 54~58 | 85 |
| H | 5.5~7.0 | 11 |
| O | 35~40 | 1.0 |
| N | 0~0.2 | 0.3 |
| 灰分 | 0~0.2 | 0.1 |
| 热值/MJ·kg-1 | 16~19 | 40 |
| 黏度/(50℃)cP | 40~100 | 180 |
| 固体含量/% | 0.2~1 | 1 |
表3 木材热解生物油与重质燃料油的典型性质[19]
| 物理性质 | 生物油 | 重质油 |
|---|---|---|
| 水含量/% | 15~30 | 0.1 |
| pH | 2.5 | — |
| 相对密度 | 1.2 | 0.94 |
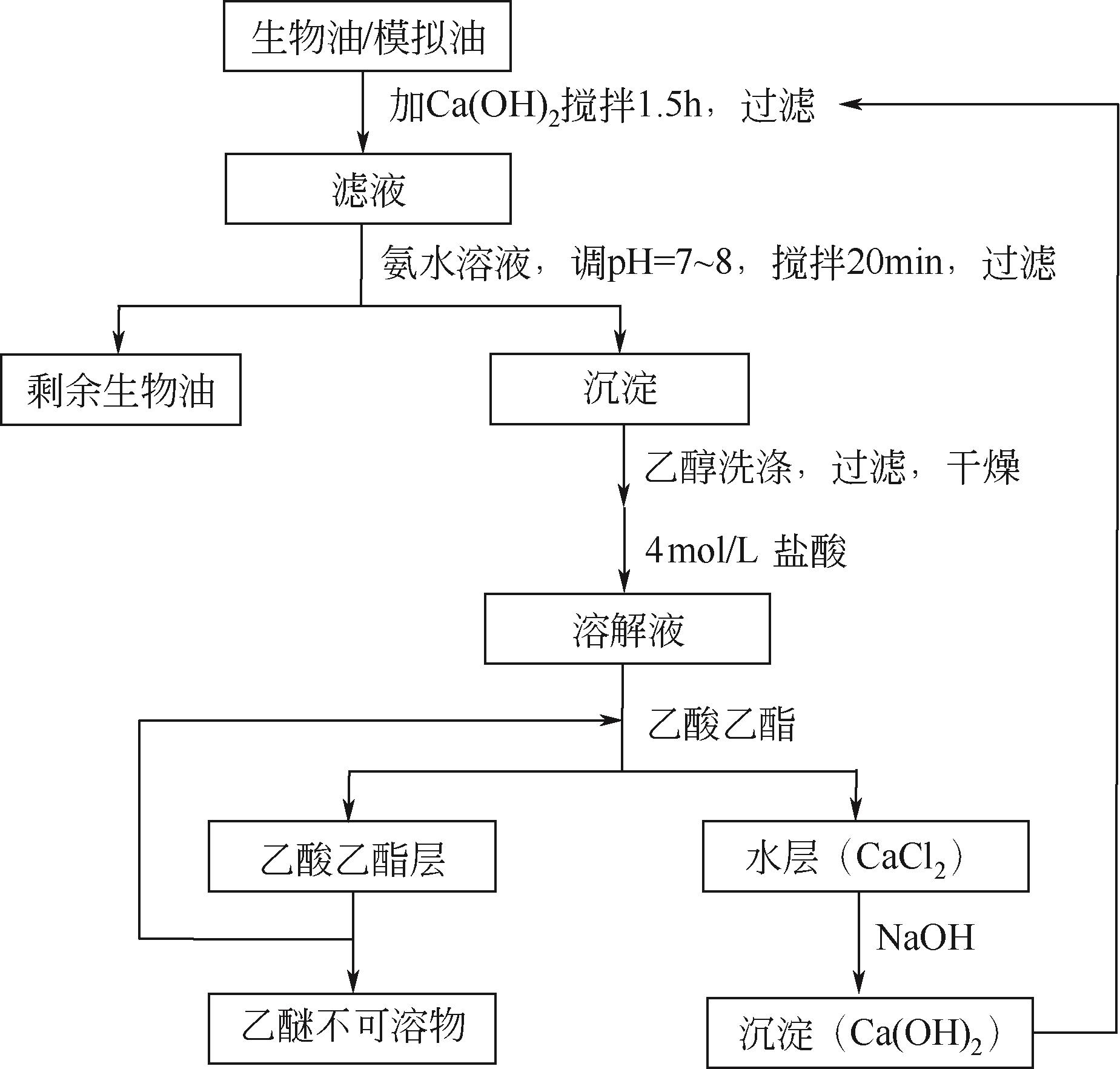
| 元素组成/% | ||
| C | 54~58 | 85 |
| H | 5.5~7.0 | 11 |
| O | 35~40 | 1.0 |
| N | 0~0.2 | 0.3 |
| 灰分 | 0~0.2 | 0.1 |
| 热值/MJ·kg-1 | 16~19 | 40 |
| 黏度/(50℃)cP | 40~100 | 180 |
| 固体含量/% | 0.2~1 | 1 |
| 化合物 | 质量分数/% | 化合物 | 质量分数/% | 化合物 | 质量分数/% |
|---|---|---|---|---|---|
| 羧酸类 | 5.0~20 | 酚类 | 5.0~20 | 呋喃甲醛 | 0.1~1.1 |
| 甲酸 | 0.3~9.1 | 苯酚 | 0.1~3.8 | 康醇 | 0.1~5.2 |
| 乙酸 | 0.5~12 | 2-甲氧基苯酚 | 0.1~1.1 | 5-羟甲基-2-糠醛 | 0.3~2.2 |
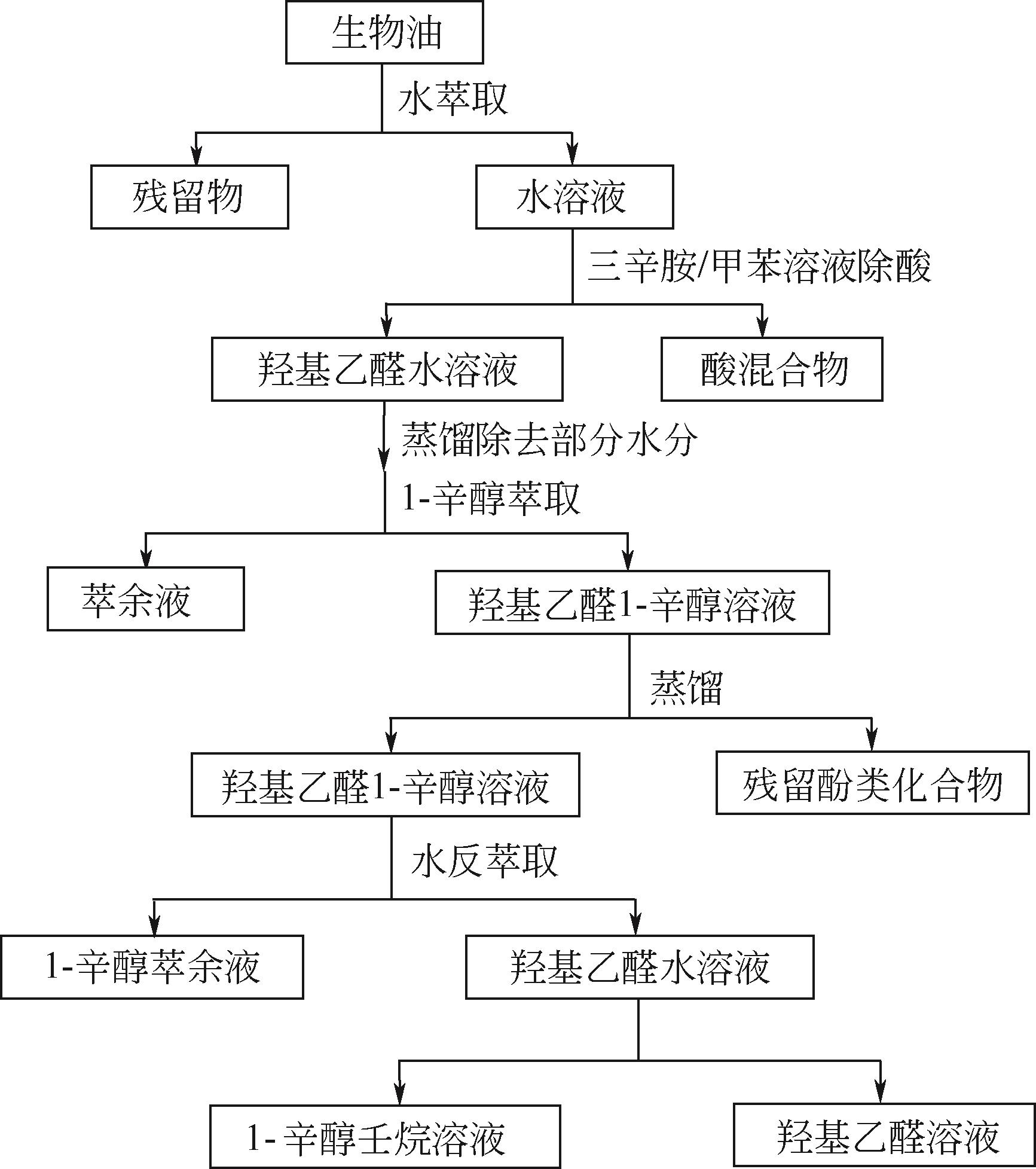
| 丙酸 | 0.1~1.8 | 4-甲基愈创木酚 | 0.1~1.9 | 醇类 | 1.0~7.0 |
| 醛类 | 3.0~15 | 丁子香酚 | 0.1~2.3 | 甲醇 | 0.4~2.4 |
| 甲醛 | 0.1~3.3 | 异丁子香酚 | 0.1~7.2 | 乙醇 | 0.6~1.4 |
| 乙二醛 | 0.9~4.6 | 二甲氧基苯酚类 | 1.0~7.0 | 乙二醇 | 0.7~2.0 |
| 羟基乙醛 | 0.9~13 | 2,6-二甲氧基苯酚 | 0.7~4.8 | 酯类 | 0.5~3.0 |
| 酮类 | 4.0~15 | 丁香醛 | 0.1~1.5 | 其他 | 5.0~10 |
| 丙酮 | 2.8 | 丙基丁香醛 | 0.1~1.5 | 甲苯 | 0.1~0.8 |
| 羟基丙酮 | 0.7~7.4 | 呋喃类 | 5.0~10 | 左旋葡聚糖 | 0.4~1.4 |
| 甲基环戊酮 | 0.1~1.9 | 2-呋喃酮 | 0.1~1.1 | 未确定 | 0.5~5.0 |
表4 生物油中部分典型化合物组成[22]
| 化合物 | 质量分数/% | 化合物 | 质量分数/% | 化合物 | 质量分数/% |
|---|---|---|---|---|---|
| 羧酸类 | 5.0~20 | 酚类 | 5.0~20 | 呋喃甲醛 | 0.1~1.1 |
| 甲酸 | 0.3~9.1 | 苯酚 | 0.1~3.8 | 康醇 | 0.1~5.2 |
| 乙酸 | 0.5~12 | 2-甲氧基苯酚 | 0.1~1.1 | 5-羟甲基-2-糠醛 | 0.3~2.2 |
| 丙酸 | 0.1~1.8 | 4-甲基愈创木酚 | 0.1~1.9 | 醇类 | 1.0~7.0 |
| 醛类 | 3.0~15 | 丁子香酚 | 0.1~2.3 | 甲醇 | 0.4~2.4 |
| 甲醛 | 0.1~3.3 | 异丁子香酚 | 0.1~7.2 | 乙醇 | 0.6~1.4 |
| 乙二醛 | 0.9~4.6 | 二甲氧基苯酚类 | 1.0~7.0 | 乙二醇 | 0.7~2.0 |
| 羟基乙醛 | 0.9~13 | 2,6-二甲氧基苯酚 | 0.7~4.8 | 酯类 | 0.5~3.0 |
| 酮类 | 4.0~15 | 丁香醛 | 0.1~1.5 | 其他 | 5.0~10 |
| 丙酮 | 2.8 | 丙基丁香醛 | 0.1~1.5 | 甲苯 | 0.1~0.8 |
| 羟基丙酮 | 0.7~7.4 | 呋喃类 | 5.0~10 | 左旋葡聚糖 | 0.4~1.4 |
| 甲基环戊酮 | 0.1~1.9 | 2-呋喃酮 | 0.1~1.1 | 未确定 | 0.5~5.0 |
| 分离技术 | 优点 | 不足 |
|---|---|---|
| 减压蒸馏 | 工艺简单,避免使用挥发性有机溶剂 | 热敏性物质易发生化学反应 |
| 分子蒸馏 | 避免使用挥发性溶剂,无污染 | 设备体积庞大,投资高,生产能力小 |
| 分级冷凝 | 热解产物直接分离,避免二次消耗 | 工艺复杂,且固体杂质含量高 |
| 液-液萃取 | 常规有机溶剂萃取操作简单,成本低;离子液体萃取选择性高,安全环保 | 常规有机溶剂萃取使用大量挥发性溶剂,污染大,且选择性差;离子液体萃取经济成本高 |
| 超临界萃取 | 相对绿色环保,产品纯度高 | 设备运行成本高,难以规模化 |
| 柱色谱分离 | 选择性好,产品纯度高 | 洗脱剂用量大,部分组分因难以洗脱而损失 |
| 膜分离 | 避免使用挥发性溶剂,相对绿色环保,选择性高 | 膜制备困难,成本高 |
表5 生物油分离技术特点
| 分离技术 | 优点 | 不足 |
|---|---|---|
| 减压蒸馏 | 工艺简单,避免使用挥发性有机溶剂 | 热敏性物质易发生化学反应 |
| 分子蒸馏 | 避免使用挥发性溶剂,无污染 | 设备体积庞大,投资高,生产能力小 |
| 分级冷凝 | 热解产物直接分离,避免二次消耗 | 工艺复杂,且固体杂质含量高 |
| 液-液萃取 | 常规有机溶剂萃取操作简单,成本低;离子液体萃取选择性高,安全环保 | 常规有机溶剂萃取使用大量挥发性溶剂,污染大,且选择性差;离子液体萃取经济成本高 |
| 超临界萃取 | 相对绿色环保,产品纯度高 | 设备运行成本高,难以规模化 |
| 柱色谱分离 | 选择性好,产品纯度高 | 洗脱剂用量大,部分组分因难以洗脱而损失 |
| 膜分离 | 避免使用挥发性溶剂,相对绿色环保,选择性高 | 膜制备困难,成本高 |
| 1 | 陈茹茹, 王雪, 吕兴梅, 等. 离子液体在生物质转化中的应用与研究进展[J]. 轻工学报, 2019, 34(3): 1-20. |
| CHEN Ruru, WANG Xue, Xingmei LYU, et al. Application and progress of ionic liquid in biomass conversion[J]. Journal of Light Industry, 2019, 34(3): 1-20. | |
| 2 | 郭艳, 王垚, 魏飞, 等. 生物质快速裂解液化技术的研究进展[J]. 化工进展, 2001, 20(8): 13-17. |
| GUO Yan, WANG Yao, WEI Fei, et al. Research progress in biomass flash pyrolysis technology for liquids production[J]. Chemical Industry and Engineering Progress, 2001, 20(8): 13-17. | |
| 3 | 候其东, 鞠美庭. 秸秆类生物质资源化技术研究前沿和发展趋势[J]. 环境保护, 2020, 48(18): 65-70. |
| HOU Qidong, JU Meiting. Frontiers and trend of straw biomass utilization technology[J]. Environmental Protection, 2020, 48(18): 65-70. | |
| 4 | 王刚, 梁小蕊, 薛钦昭, 等. 生物质高压液化技术影响因素分析及展望[J]. 可再生能源, 2008, 26(4): 31-34, 40. |
| WANG Gang, LIANG Xiaorui, XUE Qinzhao, et al. Analysis on the influencing factors of biomass high-pressure liquefaction technology and its prospect[J]. Renewable Energy Resources, 2008, 26(4): 31-34, 40. | |
| 5 | 吴志强, 张博, 杨伯伦. 生物质化学链转化技术研究进展[J]. 化工学报, 2019, 70(8): 2835-2853. |
| WU Zhiqiang, ZHANG Bo, YANG Bolun. Research progress on biomass chemical-looping conversion technology[J]. CIESC Journal, 2019, 70(8): 2835-2853. | |
| 6 | 许从峰, 张方政, 张伟, 等. 合成微生物群落用于木质纤维素生物质转化的研究进展[J]. 微生物学通报, 2020, 47(10): 3431-3441. |
| XU Congfeng, ZHANG Fangzheng, ZHANG Wei, et al. Synthetic microbial consortia for lignocellulosic biomass conversion[J]. Microbiology China, 2020, 47(10): 3431-3441. | |
| 7 | 张齐生, 马中青, 周建斌. 生物质气化技术的再认识[J]. 南京林业大学学报(自然科学版), 2013, 37(1): 1-10. |
| ZHANG Qisheng, MA Zhongqing, ZHOU Jianbin. History, challenge and solution of biomass gasification: a review[J]. Journal of Nanjing Forestry University (Natural Sciences Edition), 2013, 37(1): 1-10. | |
| 8 | STRADAL J A, UNDERWOOD G L. Process for prodcing hydroxyacetaldehyde US5393542[P]. 1995-02-28. |
| 9 | BRIDGWATER T. Challenges and opportunities in fast pyrolysis of biomass: part Ⅰ[J]. Johnson Matthey Technology Review, 2018, 62(1): 118-130. |
| 10 | HUBER G W, CORTRIGHT R D, DUMESIC J A. Renewable alkanes by aqueous-phase reforming of biomass-derived oxygenates[J]. Angewandte Chemie International Edition, 2004, 43(12): 1549-1551. |
| 11 | MOHAN D, PITTMAN C U, STEELE P H. Pyrolysis of wood/biomass for bio-oil: a critical review[J]. Energy & Fuels, 2006, 20(3): 848-889. |
| 12 | BUTLER E, DEVLIN G, MEIER D, et al. A review of recent laboratory research and commercial developments in fast pyrolysis and upgrading[J]. Renewable and Sustainable Energy Reviews, 2011, 15(8): 4171-4186. |
| 13 | MEIER D, FAIX O. State of the art of applied fast pyrolysis of lignocellulosic materials: a review[J]. Bioresource Technology, 1999, 68(1): 71-77. |
| 14 | PATHAK H, SINGH R, BHATIA A, et al. Recycling of rice straw to improve wheat yield and soil fertility and reduce atmospheric pollution[J]. Paddy and Water Environment, 2006, 4(2): 111-117. |
| 15 | DAI X W, WU C Z, LI H B, et al. The fast pyrolysis of biomass in CFB reactor[J]. Energy & Fuels, 2000, 14(3): 552-557. |
| 16 | 何敏. 澳大利亚小桉树流化床快速热解的研究[D]. 太原: 太原理工大学, 2012. |
| HE Min. Study on fast pyrolysis of Australian mallee biomass in fluidised-bed reactor[D]. Taiyuan: Taiyuan University of Technology, 2012. | |
| 17 | 田原宇, 乔英云. 生物质液化技术面临的挑战与技术选择[J]. 中外能源, 2014, 19(2): 19-24. |
| TIAN Yuanyu, QIAO Yingyun. Challenges and technical options of biomass liquefaction technology[J]. Sino-Global Energy, 2014, 19(2): 19-24. | |
| 18 | 孙洋洲, 丁一. 生物质快速热解技术进展和发展前景分析[J]. 现代化工, 2016, 36(6): 28-31, 33. |
| SUN Yangzhou, DING Yi. Development and prospects of fast pyrolysis technology for biomass[J]. Modern Chemical Industry, 2016, 36(6): 28-31, 33. | |
| 19 | CZERNIK S, BRIDGWATER A V. Overview of applications of biomass fast pyrolysis oil[J]. Energy & Fuels, 2004, 18(2): 590-598. |
| 20 | OASMAA A, KYT M, SIPIL K. Pyrolysis oil combustion tests in an industrial boiler[M]//Progress in Thermochemical Biomass Conversion. Oxford, UK: Blackwell Science Ltd., 2001: 1468-1481. |
| 21 | STRENZIOK R, HANSEN U, KNSTNER H. Combustion of bio-oil in a gas turbine[M]//Progress in Thermochemical Biomass Conversion. Oxford, UK: Blackwell Science Ltd., 2001: 1452-1458. |
| 22 | MANIATIS K, BUEKENS A. Fast pyrolysis of biomass[M]//Research in Thermochemical Biomass Conversion. Dordrecht: Springer Netherlands, 1988: 179-191. |
| 23 | CAPUNITAN J A, CAPAREDA S C. Characterization and separation of corn stover bio-oil by fractional distillation[J]. Fuel, 2013, 112: 60-73. |
| 24 | NAM H, CHOI J, CAPAREDA S C. Comparative study of vacuum and fractional distillation using pyrolytic microalgae (Nannochloropsis oculata) bio-oil[J]. Algal Research, 2016, 17: 87-96. |
| 25 | PEDERSEN T H, JENSEN C U, SANDSTRÖM L, et al. Full characterization of compounds obtained from fractional distillation and upgrading of a HTL biocrude[J]. Applied Energy, 2017, 202: 408-419. |
| 26 | DENG L, YAN Z, FU Y, et al. Green solvent for flash pyrolysis oil separation[J]. Energy & Fuels, 2009, 23:3337-3338. |
| 27 | MAHFUD F H, MELIÁN-CABRERA I, MANURUNG R, et al. Biomass to fuels: upgrading of flash pyrolysis oil by reactive distillation using a high boiling alcohol and acid catalysts[J]. Process Safety and Environmental Protection, 2007, 85(5): 466-472. |
| 28 | WANG S R, GU Y L, LIU Q, et al. Separation of bio-oil by molecular distillation[J]. Fuel Processing Technology, 2009, 90(5): 738-745. |
| 29 | GUO Z G, WANG S R, GU Y L, et al. Separation characteristics of biomass pyrolysis oil in molecular distillation[J]. Separation and Purification Technology, 2010, 76(1): 52-57. |
| 30 | WANG Y R, WANG S R, LENG F R, et al. Separation and characterization of pyrolytic lignins from the heavy fraction of bio-oil by molecular distillation[J]. Separation and Purification Technology, 2015, 152: 123-132. |
| 31 | 郭祚刚, 王树荣, 朱颖颖, 等. 生物油酸性组分分离精制研究[J]. 燃料化学学报, 2009, 37(1): 49-52. |
| GUO Zuogang, WANG Shurong, ZHU Yingying, et al. Separation of acid compounds for refining biomass pyrolysis oil[J]. Journal of Fuel Chemistry and Technology, 2009, 37(1): 49-52. | |
| 32 | POLLARD A S, ROVER M R, BROWN R C. Characterization of bio-oil recovered as stage fractions with unique chemical and physical properties[J]. Journal of Analytical and Applied Pyrolysis, 2012, 93: 129-138. |
| 33 | 隋海清. 生物质热解气分级冷凝机制及其产物的应用研究[D]. 武汉: 华中科技大学, 2016. |
| SUI Haiqing. Study on the mechanism of fractional condensation for pyrolysis vapors and the products application[D]. Wuhan: Huazhong University of Science and Technology, 2016. | |
| 34 | 吕东灿, 刘运权, 王夺, 等. 生物油萃取分离技术的研究进展[J]. 化工进展, 2012, 31(7): 1425-1431. |
| Dongcan LYU, LIU Yunquan, WANG Duo, et al. Research progress in separation of bio-oils by extraction methods[J]. Chemical Industry and Engineering Progress, 2012, 31(7): 1425-1431. | |
| 35 | OASMAA A, KUOPPALA E, GUST S, et al. Fast pyrolysis of forestry residue. 1. Effect of extractives on phase separation of pyrolysis liquids[J]. Energy & Fuels, 2002, 17(1):1-12. |
| 36 | GARCIA-PEREZ M, CHAALA A, PAKDEL H, et al. Characterization of bio-oils in chemical families[J]. Biomass and Bioenergy, 2007, 31(4): 222-242. |
| 37 | SIPILÄ K, KUOPPALA E, FAGERNÄS L, et al. Characterization of biomass-based flash pyrolysis oils[J]. Biomass and Bioenergy, 1998, 14(2): 103-113. |
| 38 | BA T Y, CHAALA A, GARCIA P M, et al. Colloidal properties of bio-oils obtained by vacuum pyrolysis of softwood bark. Characterization of water-soluble and water-insoluble fractions[J]. Energy & Fuels, 2004, 18(3): 704-712. |
| 39 | 金文彬, 李雪楠, 张依, 等. 离子液体在结构相似物分离中的进展[J]. 中国科学: 化学, 2016, 46(12): 1251-1263. |
| JIN Wenbin, LI Xuenan, ZHANG Yi, et al. Separation of structurally-related compounds with ionic liquids[J]. Scientia Sinica (Chimica), 2016, 46(12): 1251-1263. | |
| 40 | 杨启炜, 鲍宗必, 邢华斌, 等. 离子液体萃取分离结构相似化合物研究进展[J]. 化工进展, 2019, 38(1): 91-99. |
| YANG Qiwei, BAO Zongbi, XING Huabin, et al. Research progress on the extractive separation of structurally-related compounds by ionic liquids[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 91-99. | |
| 41 | 张香平, 白银鸽, 闫瑞一, 等. 离子液体萃取分离有机物研究进展[J]. 化工进展, 2016, 35(6): 1587-1605. |
| ZHANG Xiangping, BAI Yinge, YAN Ruiyi, et al. Progress in ionic liquids for extraction of organic compounds[J]. Chemical Industry and Engineering Progress, 2016, 35(6): 1587-1605. | |
| 42 | HOU Y, REN Y, PENG W, et al. Separation of phenols from oil using imidazolium-based ionic liquids[J]. Industrial & Engineering Chemistry Research, 2013, 52(50): 18071-18075. |
| 43 | JIAO T T, LI C S, ZHUANG X L, et al. The new liquid-liquid extraction method for separation of phenolic compounds from coal tar[J]. Chemical Engineering Journal, 2015, 266: 148-155. |
| 44 | JIAO T, ZHUANG X, HE H Y, et al. Separation of phenolic compounds from coal tar via liquid–liquid extraction using amide compounds[J]. Industrial & Engineering Chemistry Research, 2015, 54(9): 2573-2579. |
| 45 | SIDEK N, MANAN N S A, MOHAMAD S. Efficient removal of phenolic compounds from model oil using benzyl imidazolium-based ionic liquids[J]. Journal of Molecular Liquids, 2017, 240: 794-802. |
| 46 | LI Z Y, LI R P, YUAN X Q, et al. Anionic structural effect in liquid-liquid separation of phenol from model oil by choline carboxylate ionic liquids[J]. Green Energy & Environment, 2019, 4(2): 131-138. |
| 47 | SHI L J, SHEN B X, WANG G Q. Removal of naphthenic acids from beijiang crude oil by forming ionic liquids[J]. Energy & Fuels, 2008, 22(6): 4177-4181. |
| 48 | SUN Y, SHI L. Basic ionic liquids with imidazole anion: new reagents to remove naphthenic acids from crude oil with high total acid number[J]. Fuel, 2012, 99: 83-87. |
| 49 | ANDERSON K, ATKINS M P, GOODRICH P, et al. Naphthenic acid extraction and speciation from Doba crude oil using carbonate-based ionic liquids[J]. Fuel, 2015, 146: 60-68. |
| 50 | NASIR SHAH S, KALLIDANTHIYIL CHELLAPPAN L, GONFA G, et al. Extraction of naphthenic acid from highly acidic oil using phenolate based ionic liquids[J]. Chemical Engineering Journal, 2016, 284: 487-493. |
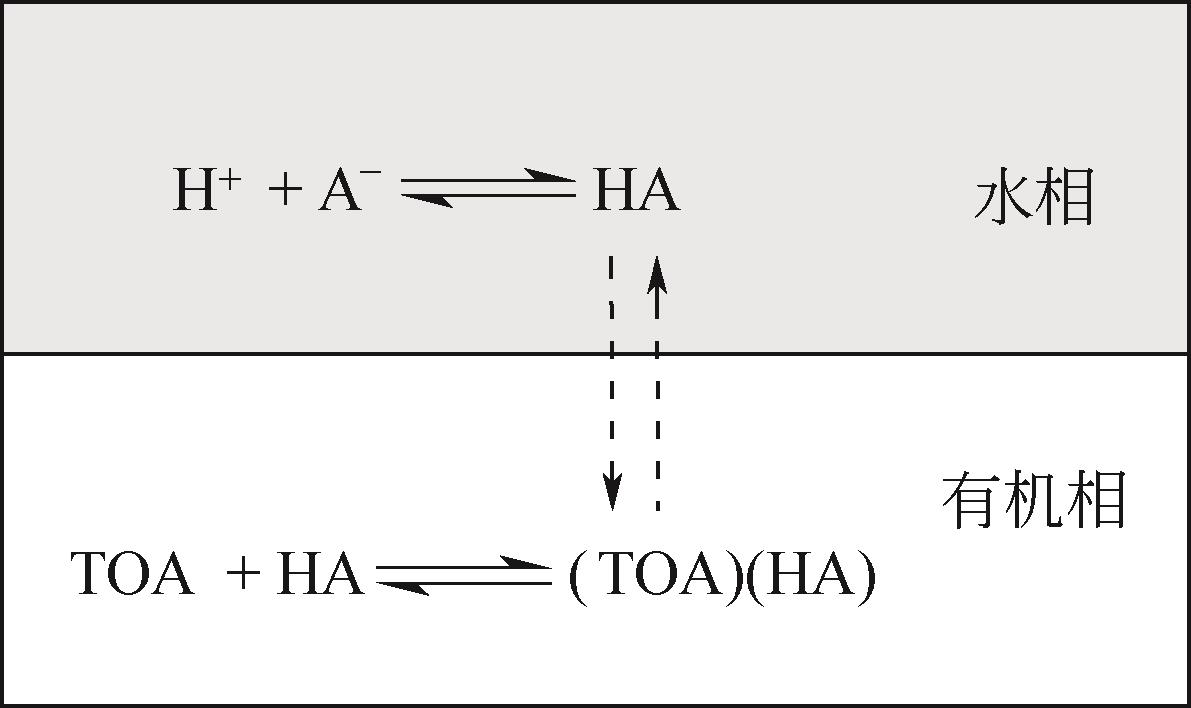
| 51 | MAHFUD F H, GEEL F P VAN, VENDERBOSCH R H, et al. Acetic acid recovery from fast pyrolysis oil. an exploratory study on liquid-liquid reactive extraction using aliphatic tertiary amines[J]. Separation Science and Technology, 2008, 43(11/12): 3056-3074. |
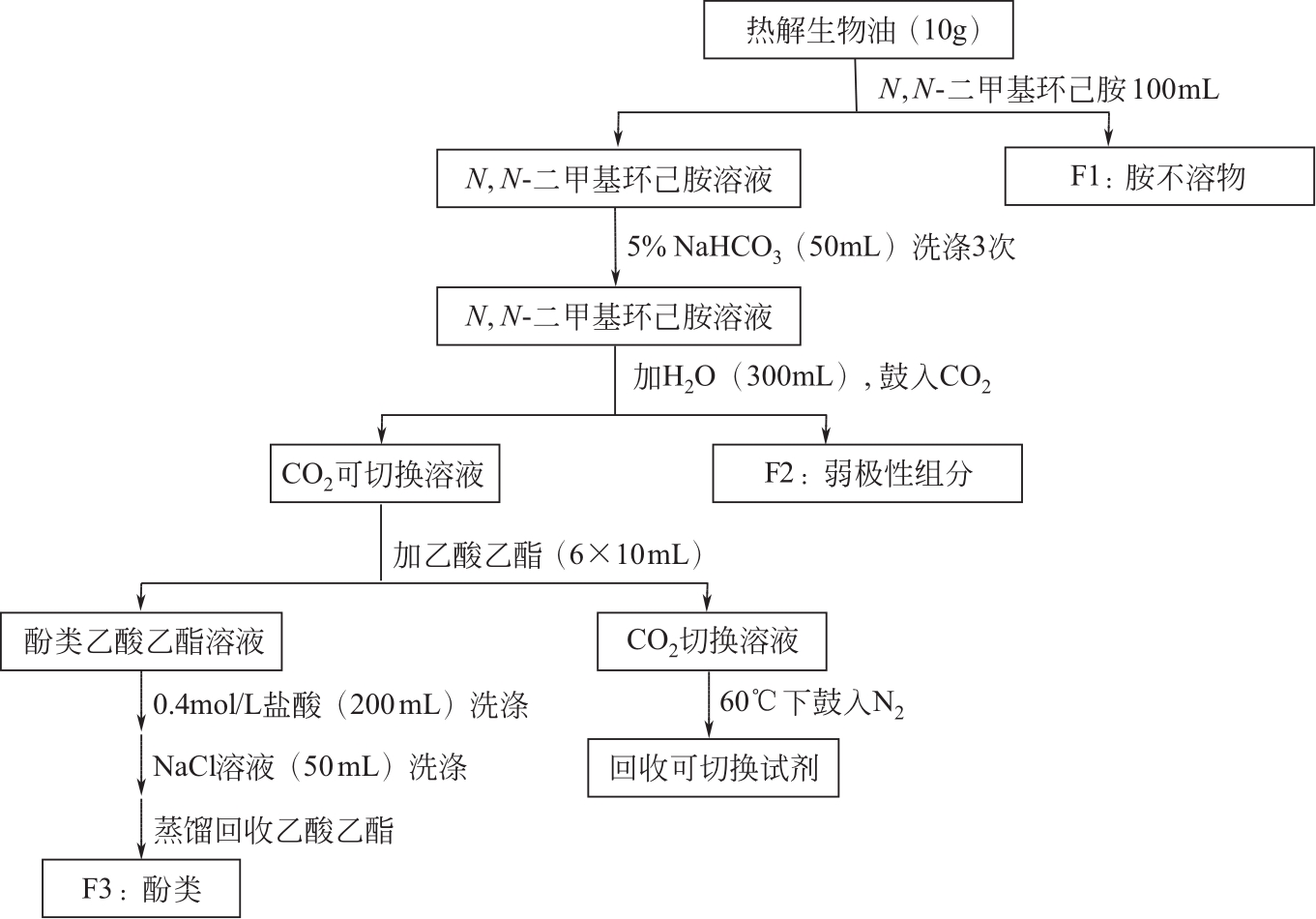
| 52 | FU D B, FARAG S, CHAOUKI J, et al. Extraction of phenols from lignin microwave-pyrolysis oil using a switchable hydrophilicity solvent[J]. Bioresource Technology, 2014, 154: 101-108. |
| 53 | ROUT P K, NAIK M K, NAIK S N, et al. Supercritical CO2Fractionation of bio-oil produced from mixed biomass of wheat and wood sawdust[J]. Energy & Fuels, 2009, 23(12): 6181-6188. |
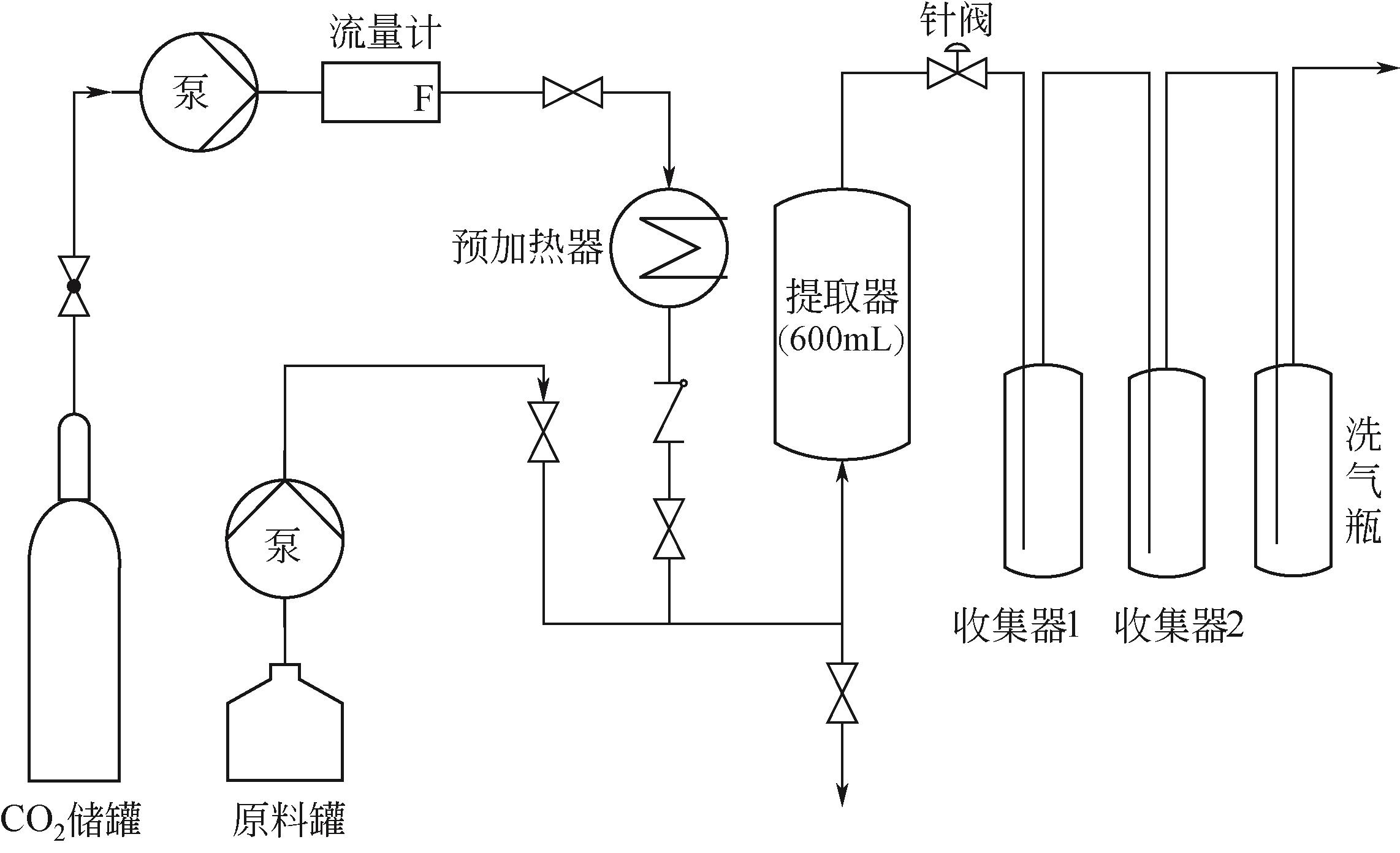
| 54 | 崔洪友, 王景华, 魏书芹, 等. 超临界CO2萃取分离生物油[J]. 山东理工大学学报(自然科学版), 2010, 24(6): 1-5, 10. |
| CUI Hongyou, WANG Jinghua, WEI Shuqin, et al. Supercritical CO2 extraction of bio-oil[J]. Journal of Shandong University of Technology (Natural Science Edition), 2010, 24(6): 1-5, 10. | |
| 55 | FENG Y S, MEIER D. Extraction of value-added chemicals from pyrolysis liquids with supercritical carbon dioxide[J]. Journal of Analytical and Applied Pyrolysis, 2015, 113: 174-185. |
| 56 | FENG Y S, MEIER D. Supercritical carbon dioxide extraction of fast pyrolysis oil from softwood[J]. The Journal of Supercritical Fluids, 2017, 128: 6-17. |
| 57 | CHENG T T, HAN Y H, ZHANG Y F, et al. Molecular composition of oxygenated compounds in fast pyrolysis bio-oil and its supercritical fluid extracts[J]. Fuel, 2016, 172: 49-57. |
| 58 | MONTESANTOS N, PEDERSEN T H, NIELSEN R P, et al. Supercritical carbon dioxide fractionation of bio-crude produced by hydrothermal liquefaction of pinewood[J]. The Journal of Supercritical Fluids, 2019, 149: 97-109. |
| 59 | 张素萍, 颜涌捷, 任铮伟, 等. 生物质快速裂解液体产物的分析[J]. 华东理工大学学报, 2001, 27(6): 666-668. |
| ZHANG Suping, YAN Yongjie, REN Zhengwei, et al. Analysis of liquid product obtained by the fast pyrolysis of biomass[J]. Journal of East China University of Science and Technology, 2001, 27(6): 666-668. | |
| 60 | ATEŞ F, PÜTÜN E, PÜTÜN A E. Fast pyrolysis of sesame stalk: yields and structural analysis of bio-oil[J]. Journal of Analytical and Applied Pyrolysis, 2004, 71(2): 779-790. |
| 61 | PINHEIRO A, HUDEBINE D, DUPASSIEUX N, et al. Membrane fractionation of biomass fast pyrolysis oil and impact of its presence on a petroleum gas oil hydrotreatment[J]. Oil & Gas Science and Technology-Rev. IFP Energies Nouvelles, 2013, 68(5): 815-828. |
| 62 | HYÖTYLÄINEN T, ANDERSSON T, JUSSILA M, et al. Determination of phenols in pyrolysis oil by on-line coupled microporous membrane liquid-liquid extraction and multidimensional liquid chromatography[J]. Journal of Separation Science, 2001, 24(7): 544-550. |
| 63 | AMEN-CHEN C, PAKDEL H, ROY C. Production of monomeric phenols by thermochemical conversion of biomass: a review[J]. Bioresource Technology, 2001, 79(3): 277-299. |
| 64 | GÜLLÜ D, DEMIRBAŞ A. Biomass to methanol via pyrolysis process[J]. Energy Conversion and Management, 2001, 42(11): 1349-1356. |
| 65 | ZAKZESKI J, BRUIJNINCX P C, JONGERIUS A L, et al. The catalytic valorization of lignin for the production of renewable chemicals[J]. Chemical Reviews, 2010, 110(6): 3552-3599. |
| 66 | BRIDGWATER T. Challenges and opportunities in fast pyrolysis of biomass: part II[J]. Johnson Matthey Technology Review, 2018, 62(2): 150-160. |
| 67 | 吕东灿, 刘运权, 王夺, 等. 生物油的分离及其产品应用[J]. 林产化学与工业, 2013, 33(4): 137-143. |
| Dongcan LYU, LIU Yunquan, WANG Duo, et al. Separation of chemicals from bio-oil and their application prospects[J]. Chemistry and Industry of Forest Products, 2013, 33(4): 137-143. | |
| 68 | 江洪明, 赵增立, 常胜, 等. 生物油中有机酸的去除及利用进展[J]. 化工进展, 2012, 31(9): 1926-1935. |
| JIANG Hongming, ZHAO Zengli, CHANG Sheng, et al. Removal and utilization of organic acids in bio-oil[J]. Chemical Industry and Engineering Progress, 2012, 31(9): 1926-1935. | |
| 69 | 何寿林, 杨昌炎, 关媛. 生物油分离制备化学品和燃油的研究进展[J]. 现代化工, 2008, 28(S2): 79-82, 84. |
| HE Shoulin, YANG Changyan, GUAN Yuan. Advance in fractional separation of bio-oil for chemicals and fuel oil[J]. Modern Chemical Industry, 2008, 28(S2): 79-82, 84. | |
| 70 | YAMAN S. Pyrolysis of biomass to produce fuels and chemical feedstocks[J]. Energy Conversion and Management, 2004, 45(5): 651-671. |
| 71 | MOTA F L, QUEIMADA A J, PINHO S P, et al. Aqueous solubility of some natural phenolic compounds[J]. Industrial & Engineering Chemistry Research, 2008, 47(15): 5182-5189. |
| 72 | JENNINGS A C. The determination of dihydroxy phenolic compounds in extracts of plant tissues[J]. Analytical Biochemistry, 1981, 118(2): 396-398. |
| 73 | SINGLETON V L, ORTHOFER R, LAMUELA-RAVENTÓS R M. 14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent[J]. Methods in Enzymology, 1999, 299: 152-178. |
| 74 | YEUNG S Y, LAN W H, HUANG C S, et al. Scavenging property of three cresol isomers against H2O2, hypochlorite, superoxide and hydroxyl radicals[J]. Food and Chemical Toxicology, 2002, 40(10): 1403-1413. |
| 75 | ARCHANA V, MEERA S BEGUM K M, ANANTHARAMAN N. Studies on removal of phenol using ionic liquid immobilized polymeric micro-capsules[J]. Arabian Journal of Chemistry, 2016, 9(3): 371-382. |
| 76 | MAGA J A, KATZ I. Simple phenol and phenolic compounds in food flavor[J]. Critical Reviews in Food Science and Nutrition, 1978, 10(4): 323-372. |
| 77 | WILLIAM F, FILBERT W N J. Manufacture of dinitro orth-ocresol[P]. US2256195. 1941-9-16. |
| 78 | GALLIVAN R M, MATSCHEI P K. Fractionation of oil obtained by pyrolysis of lignocellulosic materials to recover a phenolic fraction for use in making phenol-formaldehyde resins: US4209647[P]. 1980-06-24. |
| 79 | SINGRU R N, ZADE A B, GURNULE W B. Synthesis, characterization, and thermal degradation studies of copolymer resin derived fromp-cresol, melamine, and formaldehyde[J]. Journal of Applied Polymer Science, 2008, 109(2): 859-868. |
| 80 | CHUM H L, BLACK S K. Process for fractionating fast-pyrolysis oils, and products derived therefrom: US4942269[P]. 1990-07-17. |
| 81 | WANG D, LI D B, LIU Y Q, et al. Study of a new complex method for extraction of phenolic compounds from bio-oils[J]. Separation and Purification Technology, 2014, 134: 132-138. |
| 82 | WANG S R, WANG Y R, CAI Q J, et al. Multi-step separation of monophenols and pyrolytic lignins from the water-insoluble phase of bio-oil[J]. Separation and Purification Technology, 2014, 122: 248-255. |
| 83 | YANG H M, ZHAO W, NORINAGA K, et al. Separation of phenols and ketones from bio-oil produced from ethanolysis of wheat stalk[J]. Separation and Purification Technology, 2015, 152: 238-245. |
| 84 | POZO C DEL, BARTROLÍ J, PUY N, et al. Separation of value-added chemical groups from bio-oil of olive mill waste[J]. Industrial Crops and Products, 2018, 125: 160-167. |
| 85 | MANTE O D, THOMPSON S J, SOUKRI M, et al. Isolation and purification of monofunctional methoxyphenols from loblolly pine biocrude[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(2): 2262-2269. |
| 86 | CESARI L, CANABADY-ROCHELLE L, MUTELET F. Separation of phenols from lignin pyrolysis oil using ionic liquid[J]. Separation and Purification Technology, 2019, 209: 528-534. |
| 87 | 吕东灿, 刘运权, 王夺. 生物油中络合萃取乙酸的研究[J]. 化学工业与工程, 2013, 30(6): 32-36. |
| Dongcan LYU, LIU Yunquan, WANG Duo. Extracting acetic acid from bio-oil using TOA[J]. Chemical Industry and Engineering, 2013, 30(6): 32-36. | |
| 88 | OH S J, CHOI G G, KIM J S. Production of acetic acid-rich bio-oils from the fast pyrolysis of biomass and synthesis of calcium magnesium acetate deicer[J]. Journal of Analytical and Applied Pyrolysis, 2017, 124: 122-129. |
| 89 | OH S J, CHOI G G, KIM J S. Preparation of calcium magnesium acetate deicer using raw acetic acid-rich bio-oil obtained from continuous two-stage pyrolysis of corncob[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(3): 4362-4369. |
| 90 | ZHANG Y, CUI H Y, YI W M, et al. Highly effective decarboxylation of the carboxylic acids in fast pyrolysis oil of rice husk towards ketones using CaCO3 as a recyclable agent[J]. Biomass and Bioenergy, 2017, 102: 13-22. |
| 91 | BADMAKHAND S, STEELE P H, INGRAM L L, et al. An exploratory study on the removal of acetic and formic acids from bio-oil[J]. BioResources, 2000, 4(4): 1319-1329. |
| 92 | BENNETT N M, HELLE S S, DUFF S J B. Extraction and hydrolysis of levoglucosan from pyrolysis oil[J]. Bioresource Technology, 2009, 100(23): 6059-6063. |
| 93 | LI Q, STEELE P H, MITCHELL B K, et al. The addition of water to extract maximum levoglucosan from the bio-oil produced via fast pyrolysis of pretreated loblolly pinewood[J]. BioResources, 2013, 8(2): 1868-1880. |
| 94 | WANG J Q, WEI Q, ZHENG J L, et al. Effect of pyrolysis conditions on levoglucosan yield from cotton straw and optimization of levoglucosan extraction from bio-oil[J]. Journal of Analytical and Applied Pyrolysis, 2016, 122: 294-303. |
| 95 | VITASARI C R, MEINDERSMA G W, DE HAAN A B. Glycolaldehyde co-extraction during the reactive extraction of acetic acid with tri-n-octylamine/2-ethyl-1-hexanol from a wood-based pyrolysis oil-derived aqueous phase[J]. Separation and Purification Technology, 2012, 95: 39-43. |
| 96 | VITASARI C R, MEINDERSMA G W, DE HAAN A B. Renewable glycolaldehyde isolation from pyrolysis oil-derived aqueous solution by reactive extraction with primary amines[J]. Separation and Purification Technology, 2012, 95: 103-108. |
| 97 | VITASARI C R, MEINDERSMA G W, DE HAAN A B. Laboratory scale conceptual process development for the isolation of renewable glycolaldehyde from pyrolysis oil to produce fermentation feedstock[J]. Green Chem., 2012, 14(2): 321-325. |
| 98 | LI X H, KERSTEN S R A, SCHUUR B. Extraction of acetic acid, glycolaldehyde and acetol from aqueous solutions mimicking pyrolysis oil cuts using ionic liquids[J]. Separation and Purification Technology, 2017, 175: 498-505. |
| [1] | 贺美晋. 分子管理在炼油领域分离技术中的应用和发展趋势[J]. 化工进展, 2023, 42(S1): 260-266. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [4] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [5] | 廖志新, 罗涛, 王红, 孔佳骏, 申海平, 管翠诗, 王翠红, 佘玉成. 溶剂脱沥青技术应用与进展[J]. 化工进展, 2023, 42(9): 4573-4586. |
| [6] | 邵志国, 任雯, 许世佩, 聂凡, 许毓, 刘龙杰, 谢水祥, 李兴春, 王庆吉, 谢加才. 终温对油基钻屑热解产物分布和特性影响[J]. 化工进展, 2023, 42(9): 4929-4938. |
| [7] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [8] | 潘宜昌, 周荣飞, 邢卫红. 高效分离同碳数烃的先进微孔膜:现状与挑战[J]. 化工进展, 2023, 42(8): 3926-3942. |
| [9] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [10] | 吴亚, 赵丹, 方荣苗, 李婧瑶, 常娜娜, 杜春保, 王文珍, 史俊. 用于复杂原油乳液的高效破乳剂开发及应用研究进展[J]. 化工进展, 2023, 42(8): 4398-4413. |
| [11] | 郑梦启, 王成业, 汪炎, 王伟, 袁守军, 胡真虎, 何春华, 王杰, 梅红. 菌藻共生技术在工业废水零排放中的应用与展望[J]. 化工进展, 2023, 42(8): 4424-4431. |
| [12] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [13] | 关红玲, 杨辉, 井红权, 刘玉琼, 谷守玉, 王好斌, 侯翠红. 木质素基控释材料及其在药物输送和肥料控释中的应用[J]. 化工进展, 2023, 42(7): 3695-3707. |
| [14] | 姚丽铭, 王亚琢, 范洪刚, 顾菁, 袁浩然, 陈勇. 餐厨垃圾处理现状及其热解技术研究进展[J]. 化工进展, 2023, 42(7): 3791-3801. |
| [15] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||