化工进展 ›› 2019, Vol. 38 ›› Issue (06): 2726-2737.DOI: 10.16085/j.issn.1000-6613.2018-2051
含气页岩中水分赋存与分布的研究进展
罗翠娟1,张登峰1( ),赵春鹏2,3,4,伦增珉2,3,4,王海涛2,3,4,李艳红1,杨劲1
),赵春鹏2,3,4,伦增珉2,3,4,王海涛2,3,4,李艳红1,杨劲1
- 1. 昆明理工大学化学工程学院,云南 昆明 650500
2. 中国石油化工股份有限公司石油勘探开发研究院,北京 100083
3. 页岩油气富集机理与有效开发国家重点实验室,北京 100083
4. 中国石化页岩油气勘探开发重点;实验室,北京 100083
-
收稿日期:2018-10-16出版日期:2019-06-05发布日期:2019-06-05 -
通讯作者:张登峰 -
作者简介:罗翠娟(1995—),女,硕士研究生,主要研究方向为非常规天然气开采与二氧化碳地质封存。 -
基金资助:中国石化页岩油气勘探开发重点实验室2018年度开放基金(G5800-18-ZS-KFZY007);国家自然科学基金(41762013,21766013);“十三五”国家科技重大专项子课题(2016ZX05060002)
Occurrence and distribution of moisture in gas shale reservoirs:
Cuijuan LUO1,Dengfeng ZHANG1( ),Chunpeng ZHAO2,3,4,Zengmin LUN2,3,4,Haitao WANG2,3,4,Yanhong LI1,Jin YANG1
),Chunpeng ZHAO2,3,4,Zengmin LUN2,3,4,Haitao WANG2,3,4,Yanhong LI1,Jin YANG1
- 1. Faculty of Chemical Engineering,Kunming University of Science and Technology,Kunming 650500,Yunnan,China
2. Exploration and Production Research Institute,SINOPEC,Beijing 100083,China
3. State Key Laboratory of Shale Oil and Gas Enrichment Mechanisms and Effective Development,Beijing 100083,China
4. Key Laboratory of Shale Oil and Gas Exploration & Production, SINOPEC,Beijing 100083,China
-
Received:2018-10-16Online:2019-06-05Published:2019-06-05 -
Contact:Dengfeng ZHANG
摘要:
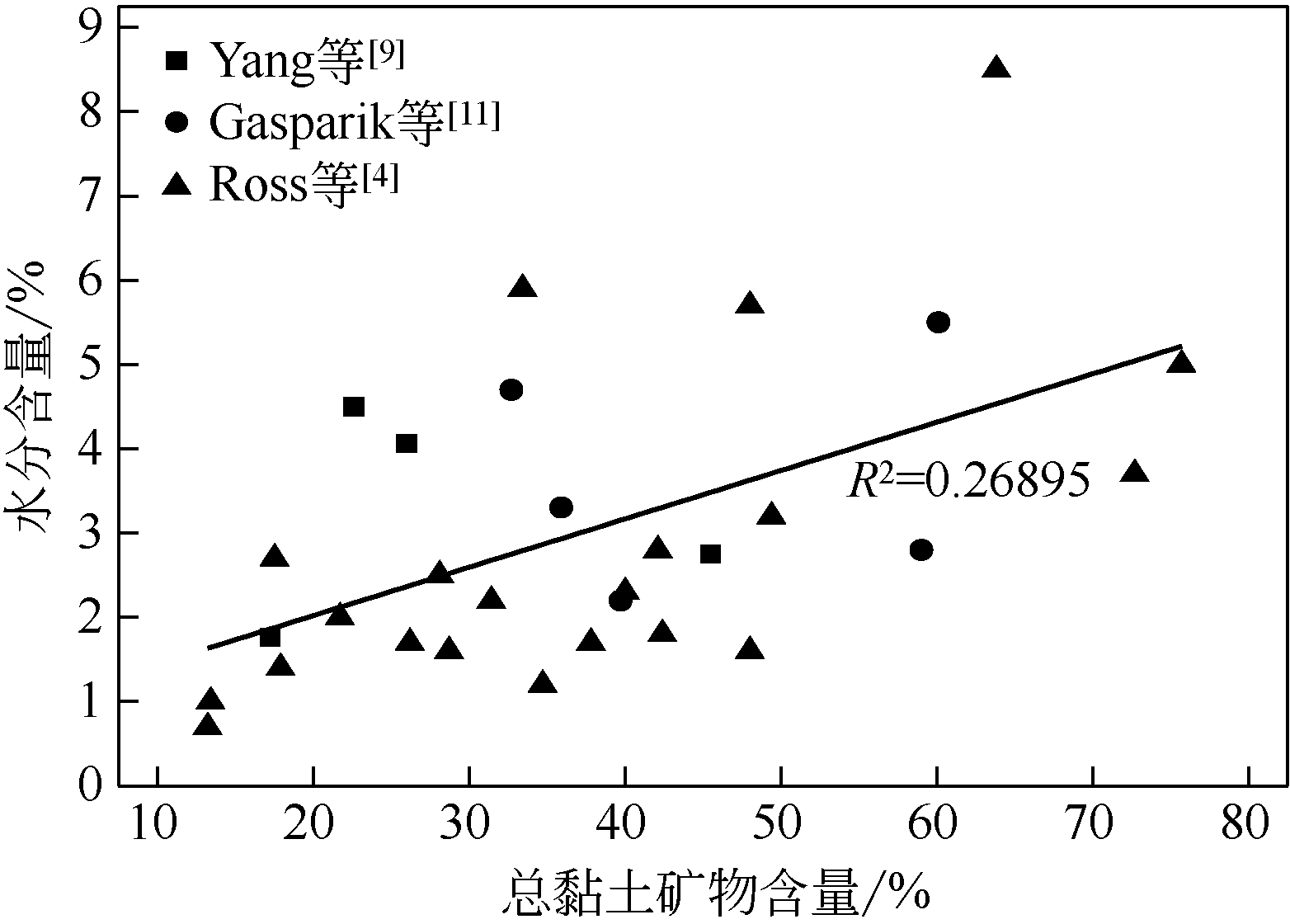
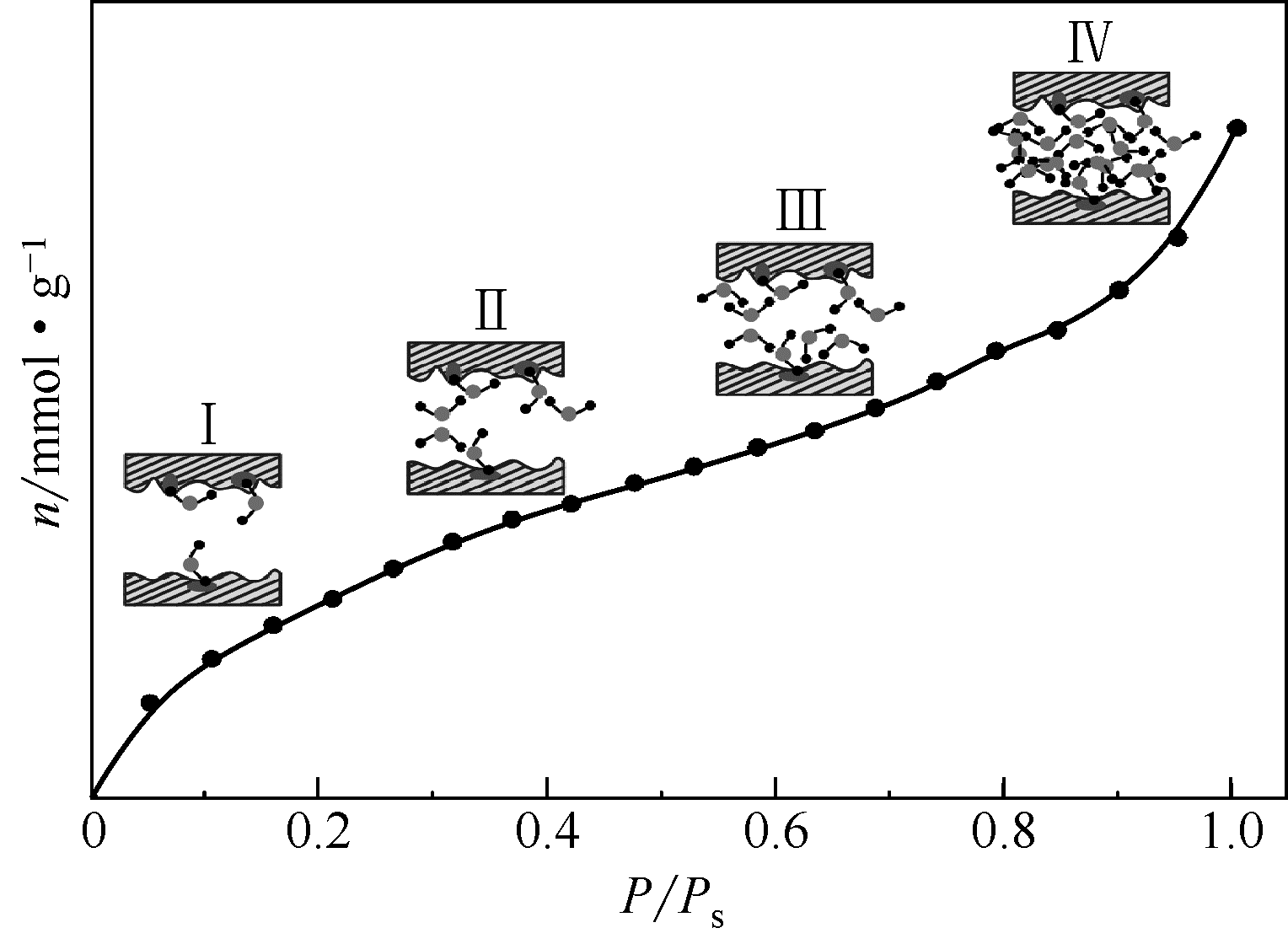
页岩气(主要组分为甲烷,CH4)作为一种新兴的非常规天然气,对于优化我国现行能源消费结构、缓解能源消耗过程中的环境污染问题具有重要意义。研究表明,含气页岩储层中页岩气主要以吸附态形式存在。影响含气页岩吸附性能的因素包括页岩自身理化性质和外部储层条件。其中,页岩中的水分是影响页岩气吸附/解吸的重要因素。因此,本文结合国内外相关研究工作,分析了含气页岩中水分的赋存与分布特征,归纳了页岩储层中水分的分析方法,指出了水分赋存与分布的后续研究方向。分析表明:①页岩中水分主要赋存于孔隙结构中,且无机孔隙中的水分赋存量比有机孔隙多;②水分子主要通过氢键吸附于有机孔隙的亲水性位点,以及经由氢键和表面作用力结合于黏土颗粒或孔隙表面;③水分含量与页岩黏土矿物含量及总有机碳(TOC)含量有关;④探明页岩中水分赋存与分布的实验表征手段包括水蒸气等温吸附、低温差示扫描量热、低场核磁共振、红外热成像和等离子体低温灰化等。虽然页岩中水分的研究已经引起国内外学者的关注,但是相比煤中水分的研究仍显不足。因此,本文指出后续需开展以下工作:探明水分在页岩中的无机矿物质空间和有机质空间的含量分布和空间分布特征;明确水分对页岩吸附/解吸CH4流体的作用规律;联用实验科学和理论模拟方法,探明水分对页岩吸附/解吸CH4流体的作用机理。
中图分类号:
引用本文
罗翠娟, 张登峰, 赵春鹏, 伦增珉, 王海涛, 李艳红, 杨劲. 含气页岩中水分赋存与分布的研究进展[J]. 化工进展, 2019, 38(06): 2726-2737.
Cuijuan LUO, Dengfeng ZHANG, Chunpeng ZHAO, Zengmin LUN, Haitao WANG, Yanhong LI, Jin YANG. Occurrence and distribution of moisture in gas shale reservoirs:[J]. Chemical Industry and Engineering Progress, 2019, 38(06): 2726-2737.
| 页岩样品 | 相对湿度/% | 含水率/% | n L,dry/mmol·g-1 | n L,moist/mmol·g-1 | 吸附温度/K | n L降幅/% | 文献 |
|---|---|---|---|---|---|---|---|
| CN_11 | 33 | 0.72 | 0.18 | 0.15 | 312.15 | 16.67 | [ |
| CN_11 | 53 | 1.05 | 0.18 | 0.14 | 312.15 | 22.22 | [ |
| CN_11 | 75 | 1.58 | 0.18 | 0.10 | 312.15 | 44.44 | [ |
| CN_11 | 97 | 4.06 | 0.18 | 0.08 | 312.15 | 55.56 | [ |
| CN_22 | 97 | 0.98 | 0.11 | 0.06 | 312.15 | 45.45 | [ |
| CN_33 | 97 | 1.53 | 0.12 | 0.05 | 312.15 | 58.33 | [ |
| CQ_14 | 97 | 2.50 | 0.22 | 0.08 | 312.15 | 63.64 | [ |
| Bossier | 97 | 7.05 | 0.11 | 0.02 | 318.15 | 81.82 | [ |
| Haynesville | 97 | 4.58 | 0.09 | 0.04 | 318.15 | 55.56 | [ |
| unnamed | 97 | 2.69 | 0.22 | 0.10 | 318.15 | 54.55 | [ |
| LOS-1 | 97 | 1.94 | 0.08 | 0.02 | 318.15 | 75.00 | [ |
| LOS-2 | 97 | 5.81 | 0.14 | 0.08 | 318.15 | 42.86 | [ |
| LOS-3 | 97 | 4.12 | 0.23 | 0.18 | 318.15 | 21.74 | [ |
表1 页岩中水分对其饱和CH4吸附容量(n L)的影响
| 页岩样品 | 相对湿度/% | 含水率/% | n L,dry/mmol·g-1 | n L,moist/mmol·g-1 | 吸附温度/K | n L降幅/% | 文献 |
|---|---|---|---|---|---|---|---|
| CN_11 | 33 | 0.72 | 0.18 | 0.15 | 312.15 | 16.67 | [ |
| CN_11 | 53 | 1.05 | 0.18 | 0.14 | 312.15 | 22.22 | [ |
| CN_11 | 75 | 1.58 | 0.18 | 0.10 | 312.15 | 44.44 | [ |
| CN_11 | 97 | 4.06 | 0.18 | 0.08 | 312.15 | 55.56 | [ |
| CN_22 | 97 | 0.98 | 0.11 | 0.06 | 312.15 | 45.45 | [ |
| CN_33 | 97 | 1.53 | 0.12 | 0.05 | 312.15 | 58.33 | [ |
| CQ_14 | 97 | 2.50 | 0.22 | 0.08 | 312.15 | 63.64 | [ |
| Bossier | 97 | 7.05 | 0.11 | 0.02 | 318.15 | 81.82 | [ |
| Haynesville | 97 | 4.58 | 0.09 | 0.04 | 318.15 | 55.56 | [ |
| unnamed | 97 | 2.69 | 0.22 | 0.10 | 318.15 | 54.55 | [ |
| LOS-1 | 97 | 1.94 | 0.08 | 0.02 | 318.15 | 75.00 | [ |
| LOS-2 | 97 | 5.81 | 0.14 | 0.08 | 318.15 | 42.86 | [ |
| LOS-3 | 97 | 4.12 | 0.23 | 0.18 | 318.15 | 21.74 | [ |
| 孔隙类型 | 成因机制 | 孔径 | 常见分布特征 |
|---|---|---|---|
| 有机质孔 | 有机质成熟生烃 | 2~1000nm | 常以近球形、椭圆形、凹坑状或片麻状等分布于热演化程度较高的有机质中 |
| 无机孔 | |||
| 粒间孔 | 矿物颗粒堆积形成 | 5~1200nm | 多见于软硬颗粒接触面和黏土矿物聚合体中 |
| 粒内孔 | 矿物成岩转化 | 8~100nm | 多见于层状或薄片状黏土矿物颗粒层间 |
| 晶间孔 | 晶体生长不紧密堆积 | 5~200nm | 见于骨架颗粒或胶结物晶体接触面 |
| 溶蚀孔 | 溶蚀作用 | 200~1200nm | 见于长石、方解石等化学性质不稳定矿物中 |
| 微裂缝 | |||
| 层间页理缝 | 沉积成岩及构造作用 | 10nm~60μm | 多数被完全填充 |
| 层面滑移缝 | 沉积成岩及构造作用 | 10nm~40μm | |
| 成岩收缩缝 | 成岩作用 | 5nm~100μm | |
| 有机质演化异常压裂缝 | 有机质演化局部异常压力作用 | 5nm~100μm | 裂缝面不规整,多充填有机质 |
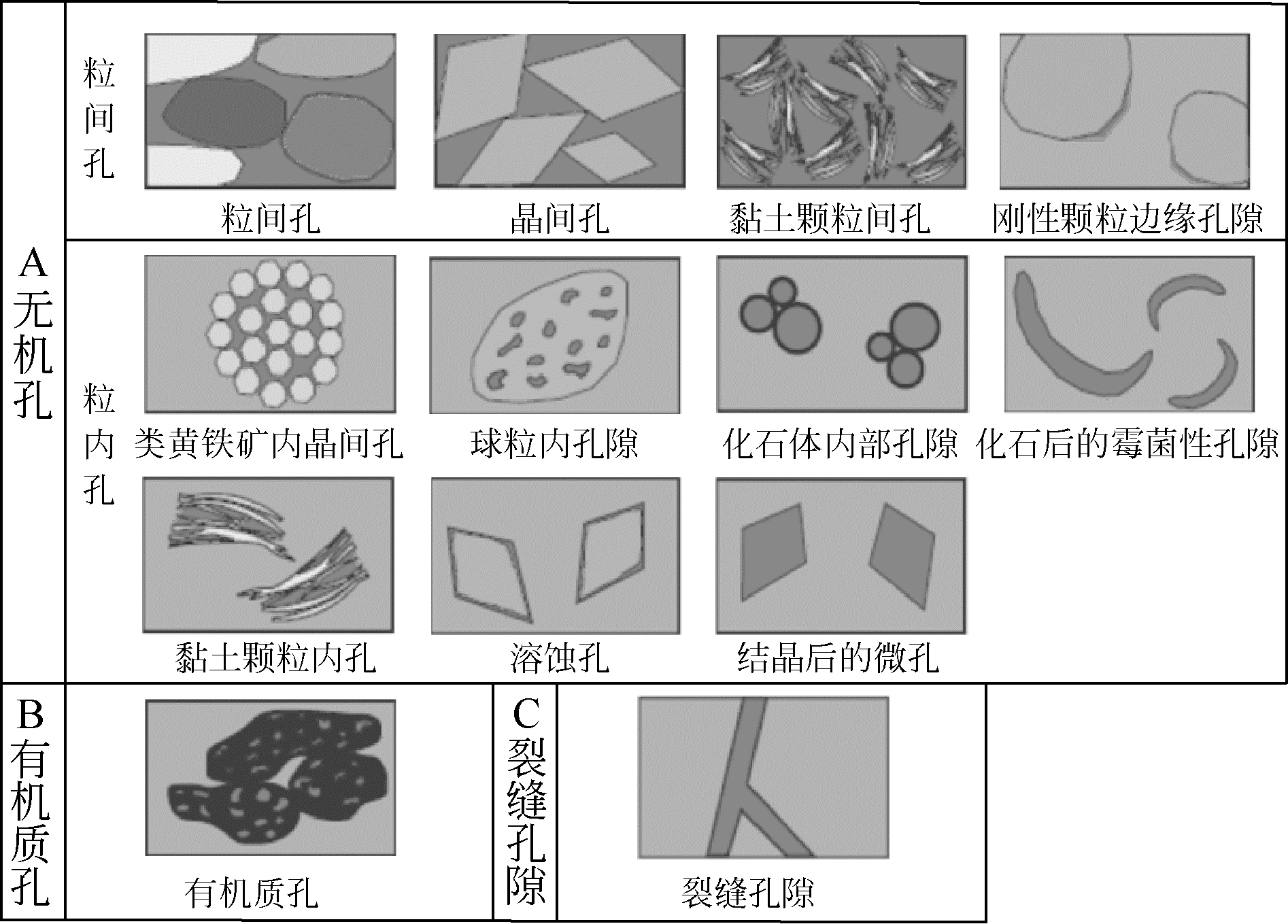
表2 页岩孔隙类型[16]
| 孔隙类型 | 成因机制 | 孔径 | 常见分布特征 |
|---|---|---|---|
| 有机质孔 | 有机质成熟生烃 | 2~1000nm | 常以近球形、椭圆形、凹坑状或片麻状等分布于热演化程度较高的有机质中 |
| 无机孔 | |||
| 粒间孔 | 矿物颗粒堆积形成 | 5~1200nm | 多见于软硬颗粒接触面和黏土矿物聚合体中 |
| 粒内孔 | 矿物成岩转化 | 8~100nm | 多见于层状或薄片状黏土矿物颗粒层间 |
| 晶间孔 | 晶体生长不紧密堆积 | 5~200nm | 见于骨架颗粒或胶结物晶体接触面 |
| 溶蚀孔 | 溶蚀作用 | 200~1200nm | 见于长石、方解石等化学性质不稳定矿物中 |
| 微裂缝 | |||
| 层间页理缝 | 沉积成岩及构造作用 | 10nm~60μm | 多数被完全填充 |
| 层面滑移缝 | 沉积成岩及构造作用 | 10nm~40μm | |
| 成岩收缩缝 | 成岩作用 | 5nm~100μm | |
| 有机质演化异常压裂缝 | 有机质演化局部异常压力作用 | 5nm~100μm | 裂缝面不规整,多充填有机质 |
| 类型 | 定义 | 数学表达式 | 说明 |
|---|---|---|---|
| Π m | 存在于中性分子或原子之间的一种弱碱性的电性吸引力 | ∏ m(h)= | 分子间作用力是分离压力中被研究最多的部分[ Melrose[ |
| Π e | 在电解质水溶液中具有相似或相反电荷的两个表面之间的相互用[ | | ε 0在真空中是电常数,F·m-1;ε是液体的相对介电常数,量纲为1;ζ 1和ζ 2分别是固-液和液-气界面的电位,mV。通常,分离压力的静电力分量决定了黏土表面水膜的稳定性 |
| Π s | 具有改变水分子结构的边界层被称为水化层。两个界面的水化层相互靠近时,将发生重叠并导致两个界面的排斥或吸引[ | ∏ s(h)=ke | 结构力通常是距离小于5nm的短程相互作用力[ Churaev[ |
表3 分子间作用力、静电力和结构力
| 类型 | 定义 | 数学表达式 | 说明 |
|---|---|---|---|
| Π m | 存在于中性分子或原子之间的一种弱碱性的电性吸引力 | ∏ m(h)= | 分子间作用力是分离压力中被研究最多的部分[ Melrose[ |
| Π e | 在电解质水溶液中具有相似或相反电荷的两个表面之间的相互用[ | | ε 0在真空中是电常数,F·m-1;ε是液体的相对介电常数,量纲为1;ζ 1和ζ 2分别是固-液和液-气界面的电位,mV。通常,分离压力的静电力分量决定了黏土表面水膜的稳定性 |
| Π s | 具有改变水分子结构的边界层被称为水化层。两个界面的水化层相互靠近时,将发生重叠并导致两个界面的排斥或吸引[ | ∏ s(h)=ke | 结构力通常是距离小于5nm的短程相互作用力[ Churaev[ |
| 矿物质 | 孔隙形态 | 孔隙类型 | 比表面积 /m2·g-1 | 单层含水量/g水·g黏土 -1 |
|---|---|---|---|---|
| 高岭石 | 狭缝状、不规则形状 | 大孔、中孔 | 11~15 | 0.022±0.010 |
| 伊利石 | 矩形、三角形、狭缝状 | 大孔、毛细孔 | 21~30 | 0.065±0.032 |
| 蒙脱石 | 狭缝状、圆形 | 大孔、中孔、毛细孔、微孔 | 26~50 | 0.063±0.036 |
| 石英 | — | — | 0.02 | — |
表4 不同黏土矿物孔隙结构参数[40,47]
| 矿物质 | 孔隙形态 | 孔隙类型 | 比表面积 /m2·g-1 | 单层含水量/g水·g黏土 -1 |
|---|---|---|---|---|
| 高岭石 | 狭缝状、不规则形状 | 大孔、中孔 | 11~15 | 0.022±0.010 |
| 伊利石 | 矩形、三角形、狭缝状 | 大孔、毛细孔 | 21~30 | 0.065±0.032 |
| 蒙脱石 | 狭缝状、圆形 | 大孔、中孔、毛细孔、微孔 | 26~50 | 0.063±0.036 |
| 石英 | — | — | 0.02 | — |
| 温度/℃ | 相对湿度/% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KOH | LiCl·xH2O | CH3COOK | MgCl2·6H2O | K2CO3·2H2O | Mg(NO3)2·H2O | NaBr | KI | NaCl | (NH4)2SO4 | KCl | KNO3 | K2SO4 | |
| 25 | 8 | 11 | 23 | 33 | 43 | 53 | 58 | 69 | 75 | 81 | 84 | 94 | 97 |
| 30 | 7 | 11 | 22 | 32 | 43 | 51 | 56 | 68 | 75 | 81 | 84 | 92 | 97 |
| 40 | 6 | 11 | — | 32 | — | 48 | 53 | 66 | 75 | 80 | 82 | 89 | 96 |
表5 饱和盐溶液在不同温度下的相对湿度[53]
| 温度/℃ | 相对湿度/% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KOH | LiCl·xH2O | CH3COOK | MgCl2·6H2O | K2CO3·2H2O | Mg(NO3)2·H2O | NaBr | KI | NaCl | (NH4)2SO4 | KCl | KNO3 | K2SO4 | |
| 25 | 8 | 11 | 23 | 33 | 43 | 53 | 58 | 69 | 75 | 81 | 84 | 94 | 97 |
| 30 | 7 | 11 | 22 | 32 | 43 | 51 | 56 | 68 | 75 | 81 | 84 | 92 | 97 |
| 40 | 6 | 11 | — | 32 | — | 48 | 53 | 66 | 75 | 80 | 82 | 89 | 96 |
| 模型 | 数学形式 | 模型参数 | 参考文献 |
|---|---|---|---|
| BET | | M m,C ① | [ |
| Guggenheim-Anderson-de-Boer (GAB) | M=M m Cka w/(1-ka w)[1+(c-1)ka w] | M m,C,k ② | [ |
| Halsey | a w=exp(-a/RT | a,r ③ | [ |
| Oswin | M=A | A,m ④ | [ |
表6 描述页岩对水蒸气等温吸附性能的模型
| 模型 | 数学形式 | 模型参数 | 参考文献 |
|---|---|---|---|
| BET | | M m,C ① | [ |
| Guggenheim-Anderson-de-Boer (GAB) | M=M m Cka w/(1-ka w)[1+(c-1)ka w] | M m,C,k ② | [ |
| Halsey | a w=exp(-a/RT | a,r ③ | [ |
| Oswin | M=A | A,m ④ | [ |
| 1 | 张烈辉,唐洪明,陈果,等 .川南下志留统龙马溪组页岩吸附特征及控制因素[J]. 天然气工业,2014,34(12):63-69. |
| ZHANG L H , TANG H M , CHEN G ,et al . Adsorption capacity and controlling factors of the lower Silurian Longmaxi Shale Play in southern Sichuan basin[J]. Natural Gas Industry,2014,34(12):63-69. | |
| 2 | CURTIS J B . Fractured shale-gas systems[J]. AAPG Bulletin,2002,86(11):1921-1938. |
| 3 | LI J , LI X F , WANG X Z ,et al . Water distribution characteristic and effect on methane adsorption capacity in shale clay[J].International Journal of Coal Geology,2016,159:135-154. |
| 4 | ROSS D J K , BUSTIN R M .Shale gas potential of the Lower Jurassic Gordondale Member, northeastern British Columbia, Canada[J].Bulletin of Canadian Petroleum Geology,2007,55(1):51-75. |
| 5 | JI W M , SONG Y , JIANG Z X ,et al . Geological controls and estimation algorithms of lacustrine shale gas adsorption capacity: a case study of the Triassic strata in the southeastern Ordos basin, China[J]. International Journal of Coal Geology,2014,134/135:61-73. |
| 6 | 霍培丽,张登峰,王倩倩,等 .页岩吸附性能及作用规律[J]. 化工进展,2016,35(1):74-82. |
| HUO P L , ZHANG D F , WANG Q Q ,et al . Perspective of adsorption performance of shale[J]. Chemical Industry and Engineering Progress,2016,35(1):74-82. | |
| 7 | 谢松彬,姚艳斌,陈基瑜,等 .煤储层微小孔孔隙结构的低场核磁共振研究[J]. 煤炭学报,2015,40(s1):170-176. |
| XIE S B , YAO Y B , CHEN J Y ,et al . Research of micro-pore structure in coal reservoir using low-field NMR[J]. Journal of China Coal Society,2015,40(s1):170-176. | |
| 8 | 陈尚斌,朱炎铭,王红岩,等 .川南龙马溪组页岩气储层纳米孔隙结构特征及其成藏意义[J]. 煤炭学报,2012,3:438-444. |
| CHEN S B , ZHU Y M , WANG H Y ,et al . Structure characteristics and accumulation significance of nanopores in Longmaxi shale gas reservoir in the southern Sichuan basin[J]. Journal of China Coal Society,2012,3:438-444. | |
| 9 | YANG F , XIE C J , NING Z F ,et al . High-pressure methane sorption on dry and moisture-equilibrated shales[J]. Energy & Fuels,2016,31(1):482-492. |
| 10 | MERKEL A , FINK R , LITTKE R . The role of pre-adsorbed water on methane sorption capacity of bossier and haynesville shales[J].International Journal of Coal Geology,2015,147:1-8. |
| 11 | GASPARIK M , GHANIZADEH A , GENSTERBLUM Y ,et al . “Multi-temperature” method for high-pressure sorption measurements on moist shales[J]. Review of Scientific Instruments,2013,84(8):1-9. |
| 12 | MERKEL A , FINK R , LITTKE R . High pressure methane sorption characteristics of lacustrine shales from the midland valley basin, Scotland[J]. Fuel,2016,182:361-372. |
| 13 | ZOLFAGHARI A , DEHGHANPOUR H , HOLYK J . Water sorption behaviour of gas shales: I. Role of clays[J].International Journal of Coal Geology,2017,179:130-138. |
| 14 | FENG D , LI X F , WANG X Z ,et al . Water adsorption and its impact on the pore structure characteristics of shale clay[J].Applied Clay Science,2018,155:126-138. |
| 15 | LOUCKS R G , REED R M , RUPPEL S C ,et al . Spectrum of pore types and networks in mudrocks and a descriptive classification for matrix-related mudrock pores[J]. AAPG Bulletin,2012,96(6):1071-1098. |
| 16 | 郭旭升,李宇平,刘若冰,等 .四川盆地焦石坝地区龙马溪组页岩微观孔隙结构特征及其控制因素[J]. 天然气工业,2014,34(6):9-16. |
| GUO X S , LI Y P , LIU R B ,et al . Characteristics and controlling factors of micro-pore structures of Longmaxi Shale Play in the Jiaoshiba area,Sichuan basin[J]. Natural Gas Industry,2014,34(6):9-16. | |
| 17 | JIAO K , YAO S P , LIU C ,et al . The characterization and quantitative analysis of nanopores in unconventional gas reservoirs utilizing FESEM-FIB and image processing: an example from the lower Silurian Longmaxi Shale, upper Yangtze region, China[J]. International Journal of Coal Geology,2014,128/129:1-11. |
| 18 | YANG Y F , YAO J , WANG C C ,et al . New pore space characterization method of shale matrix formation by considering organic and inorganic pores[J].Journal of Natural Gas Science and Engineering,2015,27:496-503. |
| 19 | RYAN B . A discussion on moisture in coal implication for coalbed gas and coal utilization[J]. Mines and Petroleum Resources,2006,1:139-149. |
| 20 | HUO P L , ZHANG D F , YANG Z ,et al .CO2 geological sequestration: displacement behavior of shale gas methane by carbon dioxide injection[J]. International Journal of Greenhouse Gas Control,2017,66:48-59. |
| 21 | GASPARIK M , GHANIZADEH A , BERTIER P ,et al . High-pressure methane sorption isotherms of black shales from the Netherlands[J].Energy & Fuels,2012,26(8):4995-5004. |
| 22 | LI J , LI X F , WU K L ,et al .Water sorption and distribution characteristics in clay and shale: effect of surface force[J].Energy & Fuels,2016,30(11):8863-8874. |
| 23 | HU Y N , DEVEGOWDA D , STRIOLO A ,et al .Microscopic dynamics of water and hydrocarbon in shale-kerogen pores of potentially mixed wettability[J]. SPE Journal,2015,20(1):112-124. |
| 24 | 张时音,桑树勋,杨志刚 .液态水对煤吸附甲烷影响的机理分析[J].中国矿业大学学报,2009,38(5):707-712. |
| ZHANG S Y , SANG S X , YANG Z G . Mechanism analysis on the effect of liquid water on coal adsorbing methane[J]. Journal of China University of Mining & Technology,2009,38(5):707-712. | |
| 25 | JIN Z H , FIROOZABADI A . Effect of water on methane and carbon dioxide sorption in clay minerals by Monte Carlo simulations[J].Fluid Phase Equilibria,2014,382:10-20. |
| 26 | MAO J D , FANG X W , LAN Y Q ,et al . Chemical and nanometer-scale structure of kerogen and its change during thermal maturation investigated by advanced solid-state 13C NMR spectroscopy[J].Geochimica et Cosmochimica Acta,2010,74(7):2110-2127. |
| 27 | TONG J H , HAN X X , WANG S ,et al . Evaluation of structural characteristics of Huadian oil shale kerogen using direct techniques (solid-state 13C NMR, XPS, FT-IR, and XRD)[J].Energy & Fuels,2011,25(9):4006-4013. |
| 28 | 夏阳超,刘晓阳,刘生玉 .褐煤表面含氧官能团对水分子的吸附机理[J].煤炭转化,2016,39(4):1-5,9. |
| XIA Y C , LIU X Y , LIU S Y . Adsorption mechanism of water molecule onto oxygen containing functional groups of lignite[J]. Coal Conversion,2016,39(4):1-5,9. | |
| 29 | 吴大清,刁桂仪,魏俊峰,等 .矿物表面基团与表面作用[J].高校地质学报,2000,6(4):225-232. |
| WU D Q , DIAO G Y , WEI J F ,et al . Surface function groups and surface reactions of minerals[J]. Geological Journal of China Universities,2000,6(4):225-232. | |
| 30 | DERJAGUIN B V , CHURAEV N V , MULLER V M ,et al . Surface forces[M]. New York: Consultants Bureau,Wiley,1987. |
| 31 | PAUNOVA V N , DIMOVA R I , KRALCHEVSKY P A ,et al . The hydration repulsion between charged surfaces as an interplay of volume exclusion and dielectric saturation effects[J]. Journal of Colloid and Interface Science,1996,182(1):239-248. |
| 32 | IWAMATSU M , HORII K . Capillary condensation and adhesion of two wetter surfaces[J].Journal of Colloid and Interface Science,1996,182(2):400-406. |
| 33 | TULLER M ,OR D, DUDLEY L M . Adsorption and capillary condensation in porous media: liquid retention and interfacial configurations in angular pores[J].Water Resources Research,1999,35(7):1949-1964. |
| 34 | MATTIA D , STAROV V , SEMENOV S . Thickness, stability and contact angle of liquid films on and inside nanofibres, nanotubes and nanochannels[J].Journal of Colloid and Interface Science,2012,384:149-156. |
| 35 | MELROSE J C . Interpretation of mixed wettability states in reservoir rock[C]//Society of Petroleum Engineers.APE Annual Technical Conference and Exhibition. New Orleans, Louisiana,1982. |
| 36 | TAKAHASHI S , KOVSCEK A R . Wettability estimation of low-permeability, siliceous shale using surface forces[J].Journal of Petroleum Science and Engineering,2010,75(1/2):33-43. |
| 37 | KHILAR K C , FOGLER H S .Migrations of fines in porous media (Chapter 3)[M].Boston: Kluwer Academic Publishers,2010. |
| 38 | CHURAEV N V . Contact angles and surface forces[J].Advances in Colloid and Interface Science,1995,58(2):87-118. |
| 39 | CHURAEV N V . The relation between colloid stability and wetting[J].Journal of Colloid and Interface Science,1995,172(2):479-484. |
| 40 | 王茂桢,柳少波,任拥军,等 .页岩气储层粘土矿物孔隙特征及其甲烷吸附作用[J].地质论评,2015,61(1):207-216. |
| WANG M Z , LIU S B , REN Y J ,et al . Pore characteristics and methane adsorption of clay minerals in shale gas reservoir[J]. Geological Review,2015,61(1):207-216. | |
| 41 | CHAVEZ-PAEZ M , WORKUM K V , PABLO L D ,et al . Monte Carlo simulations of Wyoming sodium montmorillonite hydrates[J]. Journal of Chemical Physics,2001,114(3):1405-1413. |
| 42 | LEIGH M C , JACKSON W R , CHAFFEE A L ,et al . Understanding brown coal-water interactions to reduce carbon dioxide emissions[C]//221st National Meeting of the American-Chemical-Society,San Diego,CA. 2002:203-250. |
| 43 | MULLER E A , RULL L F , VEGA L F ,et al . Adsorption of water on activated carbons: a molecular simulation study[J].Journal of Physical Chemistry,1996,100:1189-1196. |
| 44 | SKAAR C . Wood water relations[M]. Berlin:Springer Verlag,1988. |
| 45 | CASES J M , VILLIERAS F . The mechanisms of ionic and non-ionic surfactants adsorption-abstraction on heterogeneous surfaces[J]. Langmuir,1992,8(2):26. |
| 46 | YANG F , NING Z F , YANG Q W ,et al . Pore structure characteristics of lower Silurian shales in the southern Sichuan basin, China: insights to pore development and gas storage mechanism[J].International Journal of Coal Geology,2016,156:12-24. |
| 47 | HATCH C D , WIESE J S , CRANE C C ,et al . Water adsorption on clay minerals as a function of relative humidity: application of BET and Freundlich adsorption models[J]. Langmuir,2012,28(3):1790-1803. |
| 48 | 刘长龄 .我国耐火粘土的矿物类型和化学成分[J].硅酸盐学报,1965,4(3):198-202,213-214. |
| LIU C L . Mineral types and chemical composition of refractory clay in China[J]. Journal of the Chinese Ceramic Society,1965,4(3):198-202,213-214. | |
| 49 | 陈扬杰 .论内蒙老石旦软质粘土矿床的成因[J].西安矿业学院学报,1991,4:25-30. |
| CHEN Y J . On the origin of Laoshidan soft clay deposit in Neimenggu[J]. Journal of Xi’an Mining Industry,1991,4:25-30. | |
| 50 | ROSS D J K , BUSTIN R M . The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs[J].Marine and Petroleum Geology,2009,26(6):916-927. |
| 51 | GASPARIK M , BERTIER P , GENSTERBLUM Y ,et al . Geological controls on the methane storage capacity in organic-rich shales[J].International Journal of Coal Geology,2014,123:34-51. |
| 52 | 文进,林元哲,韩成赫,等 . SiO2纳米多孔调湿材料的制备与表征[J]. 合成技术及应用,2017,32(2):18-21. |
| WEN J , LIN Y Z , HAN C H ,et al . Preparation and characterization of SiO2 nanoporous humidity controller[J]. Synthetic Technology and Application,2017,32(2):18-21. | |
| 53 | ISO 483:2005(E).Plastics-small enclosures for conditioning and testing using aqueous solutions to maintain the humidity at a constant value[S]. International Standard,2005. |
| 54 | CHARRIERE D , BEHRA P . Water sorption on coals[J].Journal of Colloid and Interface Science,2010,344(2):460-467. |
| 55 | ZIMNY T , FINQUENEISEL G , COSSARUTTO L ,et al . Water vapor adsorption on activated carbon preadsorbed with naphtalene[J]. Journal of Colloidand Interface Science,2005,285(1):56-60. |
| 56 | SVÁBOVÁ M , WEISHAUPTOVÁ Z , PRIBYL O . Water vapour adsorption on coal[J]. Fuel,2011,90(5):1892-1899. |
| 57 | CEPONKUS J , ENGDAHL A , UVDAL P ,et al . Structure and dynamics of small water clusters, trapped in inert matrices[J]. Chemical Physics Letters,2013,581:1-9. |
| 58 | FLETCHER A J , UYGUR Y , THOMAS K M . Role of surface functional groups in the adsorption kinetics of water vapor on microporous activated carbons[J].Journal of Physical Chemistry C,2007,111(23):8349-8359. |
| 59 | OZBOY O , SAHBAZ F , KOKSEL H . Chemical and physical characterisation of sugar beet fiber[J].Acta Alimentaria,1998,27(2):137-148. |
| 60 | CHIRIFE J , IGLESIAS H A . Equations for fitting water sorption isotherms of foods: Part 1-a review[J].Journal of Food Technology,1978,13:159-174. |
| 61 | TSAMI E , KROKIDA M K , DROUZAS A E . Effect of drying method on the sorption characteristics of model fruit powders[J].Journal of Food Engineering,1998,38(4):381-392. |
| 62 | HALSEY G . Physical adsorption on non-uniform surfaces[J].The Journal of Chemical Physics,1948,16(10):931-937. |
| 63 | OSWIN C R . The kinetics of package life. Ⅲ. The isotherm[J]. Journal of the Society of Chemical Industry,1946(12):419-421. |
| 64 | 孙晓晓,姚艳斌,陈基瑜,等 .基于低场核磁共振的煤润湿性分析[J].现代地质,2015,1:190-197. |
| SUN X X , YAO Y B , CHEN J Y ,et al . Determination of coal wettability by using low-field nuclear magnetic resonance[J]. Geoscience,2015,1:190-197. | |
| 65 | 黄伟 .低场核磁共振系统的应用与研究[D].武汉:华中师范大学,2014. |
| HUANG W . The low-field NMR system’s application and research[D]. Wuhan:Central China Normal University,2014. | |
| 66 | ZHU H J , JU Y W , QI Y ,et al . Impact of tectonism on pore type and pore structure evolution in organic-rich shale: Implications for gas storage and migration pathways in naturally deformed rocks[J].Fuel,2018,228:272-289. |
| 67 | UNSWORTH J F , FOWLER C S , HEARD N A ,et al . Moisture in coal 1. Differentiation between forms of moisture by NMR and microwave attenuation techniques[J]. Fuel,1988,67:1111-1119. |
| 68 | 热依拉·阿布都瓦依提,马凤云,张翔,等 .低场核磁共振技术在煤炭岩相孔隙结构中的应用[J].核技术,2017,40(12):47-52. |
| RAHILA·A,MA F Y, ZHANG X ,et al . Application of low-field nuclear magnetic resonance technology in coal petrographic pore structure[J]. Nuclear Techniques,2017,40(12):47-52. | |
| 69 | SU S Y , JIANG Z X , SHAN X L ,et al . The wettability of shale by NMR measurements and its controlling factors[J].Journal of Petroleum Science and Engineering,2018,169:309-316. |
| 70 | 龚国波,孙伯勤,刘买力,等 .岩心孔隙介质中流体的核磁共振弛豫[J].波谱学杂志,2006,23(3):379-395. |
| GONG G B , SUN B Q , LIU M L ,et al . NMR relaxation of the fluid in rock porous media[J]. Chinese Journal of Magnetic Resonance,2006,23(3):379-395. | |
| 71 | 苏怀兴 .褐煤中水分存在形式的实验研究[D].鞍山:辽宁科技大学,2014. |
| SU H X . An experimental study on the existing form of water in lignite[D]. Anshan:University of Science and Technology Liaoning,2014. | |
| 72 | NORINAGA K , KUMAGAI H , J-I HAYASHI ,et al . Classification of water sorbed in coal on the basis of congelation characteristics[J].Energy & Fuels,1998,12(3):574-579. |
| 73 | KOK M V, SENGULER I . Geological and thermal characterization of Eskisehir region oil shales[J].Journal of Thermal Analysis and Calorimetry,2014,116(1):367-372. |
| 74 | LIU Q Q , HAN X X , LI Q Y ,et al .TG-DSC analysis of pyrolysis process of two Chinese oil shales[J].Journal of Thermal Analysis and Calorimetry,2014,116(1):511-517. |
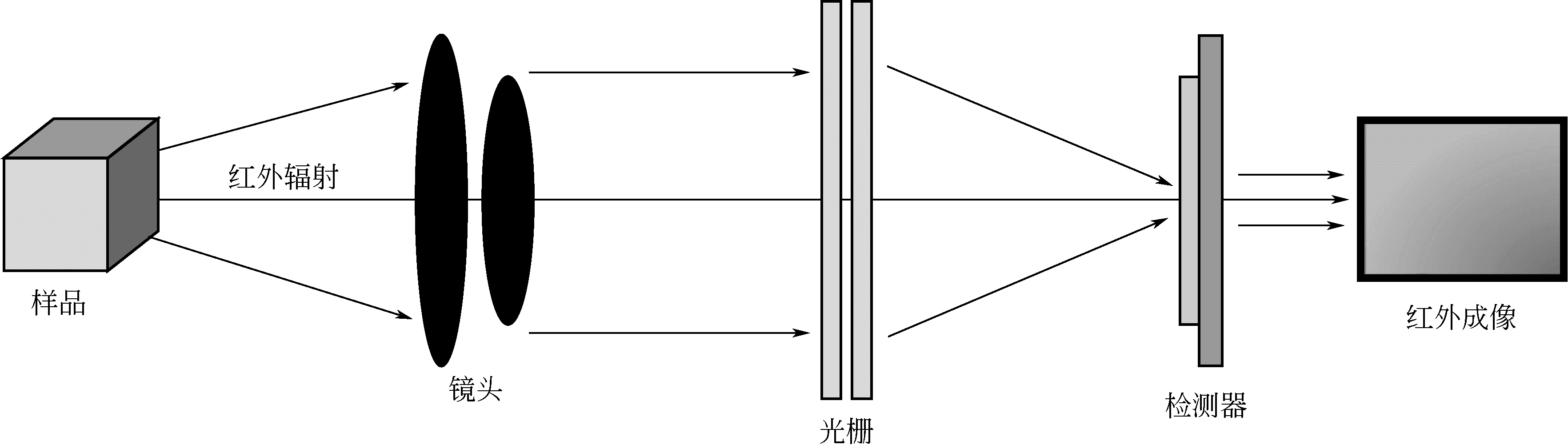
| 75 | 吕事桂,刘学业 .红外热像检测技术的发展和研究现状[J]. 红外技术,2018,40(3):214-219. |
| LÜ S G , LIU X Y . Development and research status of infrared thermal image detection technology[J]. Infrared Technology,2018,40(3):214-219. | |
| 76 | 刘颖韬,郭广平,曾智,等 .红外热像无损检测技术的发展历程、现状和趋势[J].无损检测,2017,39(8):63-70. |
| LIU Y T , GUO G P , ZENG Z ,et al . The development history, status and trends of infrared thermographic nondestructive testing[J]. Nondestructive Testing,2017,39(8):63-70. | |
| 77 | 张城 .红外热成像技术原理及应用前景[J].数字通信世界,2017,2:126-127. |
| ZHANG C . Principle and application prospect of infrared thermal imaging technology[J]. Digital Communication World,2017,2:126-127. | |
| 78 | 李朋朋,康敬东,赵晓蕾 .红外热成像技术及其在沥青路面施工中的应用[J].建设机械技术与管理,2015,28(11):59-62. |
| LI P P , KANG J D , ZHAO X L . Application of the infrared thermal imaging technology in the asphalt pavement construction[J]. Construction Machinery Technology and Management,2015,28(11):59-62. | |
| 79 | 穗积启一郎,廖时萱,李彩娥 .等离子体灰化法的原理和应用[J].国外医学.药学分册,1980,3:164-168. |
| HOZUMI K , LIAO S X , LI C E . Principle and application of plasma ashing method[J]. Foreign Medicine. Pharmacology,1980,3:164-168. | |
| 80 | 柏静儒,潘思慧,王擎,等 .低温灰化中不同有机质的内蒙古油页岩热解特性[J].化工进展,2017,36(7):2428-2435. |
| BAI J R , PAN S H , WANG Q ,et al . Pyrolysis characteristics of Inner Mongolia oil shales with different organic matter contents[J]. Chemical Industry and Engineering Progress,2017,36(7):2428-2435. | |
| 81 | 金梅荪 .等离子体低温灰化技术的进展及其应用[J].辐射防护通讯,1989,4:1-8. |
| JIN M S . Advances and applications of plasma low temperature ashing technology[J]. Radiation Protection Communication,1989,4:1-8. | |
| 82 | 杨燕梅,杨欣华,刘青,等 .灰化温度对准东煤灰组分分析的影响[J].煤炭学报,2016,41(10):2441-2447. |
| YANG Y M , YANG X H , LIU Q ,et al . Effect of ashing temperature on analysis of Zhundong coal ash[J]. Journal of China Coal Society,2016,41(10):2441-2447. |
| [1] | 赵晨, 苗天泽, 张朝阳, 洪芳军, 汪大海. 负压状态窄缝通道乙二醇水溶液传热特性[J]. 化工进展, 2023, 42(S1): 148-157. |
| [2] | 王家庆, 宋广伟, 李强, 郭帅成, DAI Qingli. 橡胶混凝土界面改性方法及性能提升路径[J]. 化工进展, 2023, 42(S1): 328-343. |
| [3] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [4] | 王莹, 韩云平, 李琳, 李衍博, 李慧丽, 颜昌仁, 李彩侠. 城市污水厂病毒气溶胶逸散特征研究现状与未来展望[J]. 化工进展, 2023, 42(S1): 439-446. |
| [5] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [6] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [7] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [8] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [9] | 王少凡, 周颖, 郝康安, 黄安荣, 张如菊, 吴翀, 左晓玲. 具有pH响应性的自愈合蓝光水凝胶[J]. 化工进展, 2023, 42(9): 4837-4846. |
| [10] | 李东泽, 张祥, 田键, 胡攀, 姚杰, 朱林, 卜昌盛, 王昕晔. 基于水泥窑脱硝的碳基还原NO x 研究进展[J]. 化工进展, 2023, 42(9): 4882-4893. |
| [11] | 王晨, 白浩良, 康雪. 大功率UV-LED散热与纳米TiO2光催化酸性红26耦合系统性能[J]. 化工进展, 2023, 42(9): 4905-4916. |
| [12] | 李由, 吴越, 钟禹, 林琦璇, 任俊莉. 酸性熔盐水合物预处理麦秆高效制备木糖及其对酶解效率的影响[J]. 化工进展, 2023, 42(9): 4974-4983. |
| [13] | 王琦, 寇丽红, 王冠宇, 王吉坤, 刘敏, 李兰廷, 王昊. 焦化废水生物出水中可溶解性有机物的分子识别[J]. 化工进展, 2023, 42(9): 4984-4993. |
| [14] | 王雪婷, 顾霞, 徐先宝, 赵磊, 薛罡, 李响. 水热预处理对餐厨垃圾厌氧发酵产戊酸的影响[J]. 化工进展, 2023, 42(9): 4994-5002. |
| [15] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||