化工进展 ›› 2019, Vol. 38 ›› Issue (05): 2320-2328.DOI: 10.16085/j.issn.1000-6613.2018-2339
乙醇催化重整产氢催化剂及催化反应机制
陈俊1( ),舒红飞1,阮祝华1,倪嘉琪1,鲁礼林1,2(
),舒红飞1,阮祝华1,倪嘉琪1,鲁礼林1,2( ),刘义1
),刘义1
- 1. 武汉科技大学化学与化工学院,煤转化与新型炭材料湖北省重点实验室,湖北 武汉 430081
2. 武汉科技大学省部共建耐火材料与冶金国家重点实验室,湖北 武汉 430081
-
收稿日期:2018-11-30修回日期:2019-02-15出版日期:2019-05-05发布日期:2019-05-05 -
通讯作者:鲁礼林 -
作者简介:<named-content content-type="corresp-name">陈俊</named-content>(1996—),男,硕士研究生,研究方向为能源催化。E-mail:<email>ylsn99866@sina.com</email>。 -
基金资助:国家自然科学基金(21671154,U1732147)
Catalysts and catalytic mechanism for hydrogen production from ethanol steam reforming (ESR)
Jun CHEN1( ),Hongfei SHU1,Zhuhua RUAN1,Jiaqi NI1,Lilin LU1,2(
),Hongfei SHU1,Zhuhua RUAN1,Jiaqi NI1,Lilin LU1,2( ),Yi LIU1
),Yi LIU1
- 1. Hubei Province Key Laboratory of Coal Conversion and New Carbon Materials, School of Chemistry and Chemical Engineering, Wuhan University of Science and Technology, Wuhan 430081, Hubei, China
2. State Key Laboratory of Refractories and Metallurgy, Wuhan University of Science and Technology, Wuhan 430081, Hubei, China
-
Received:2018-11-30Revised:2019-02-15Online:2019-05-05Published:2019-05-05 -
Contact:Lilin LU
摘要:
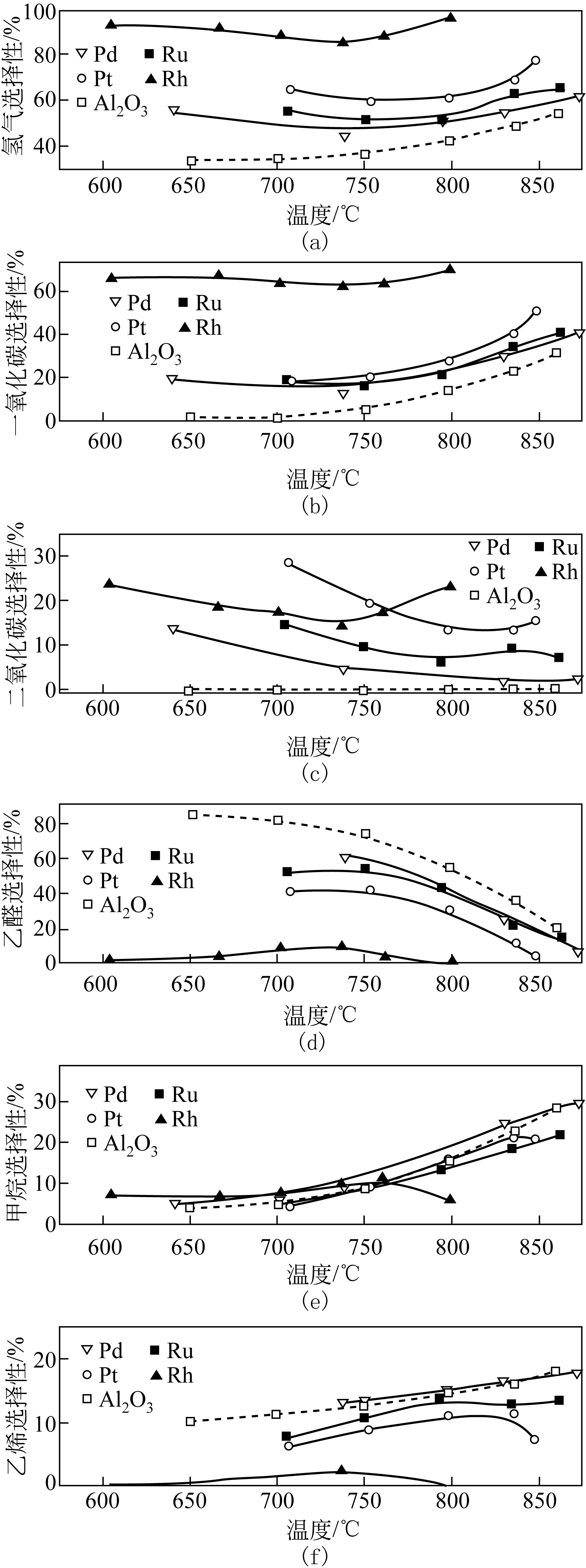
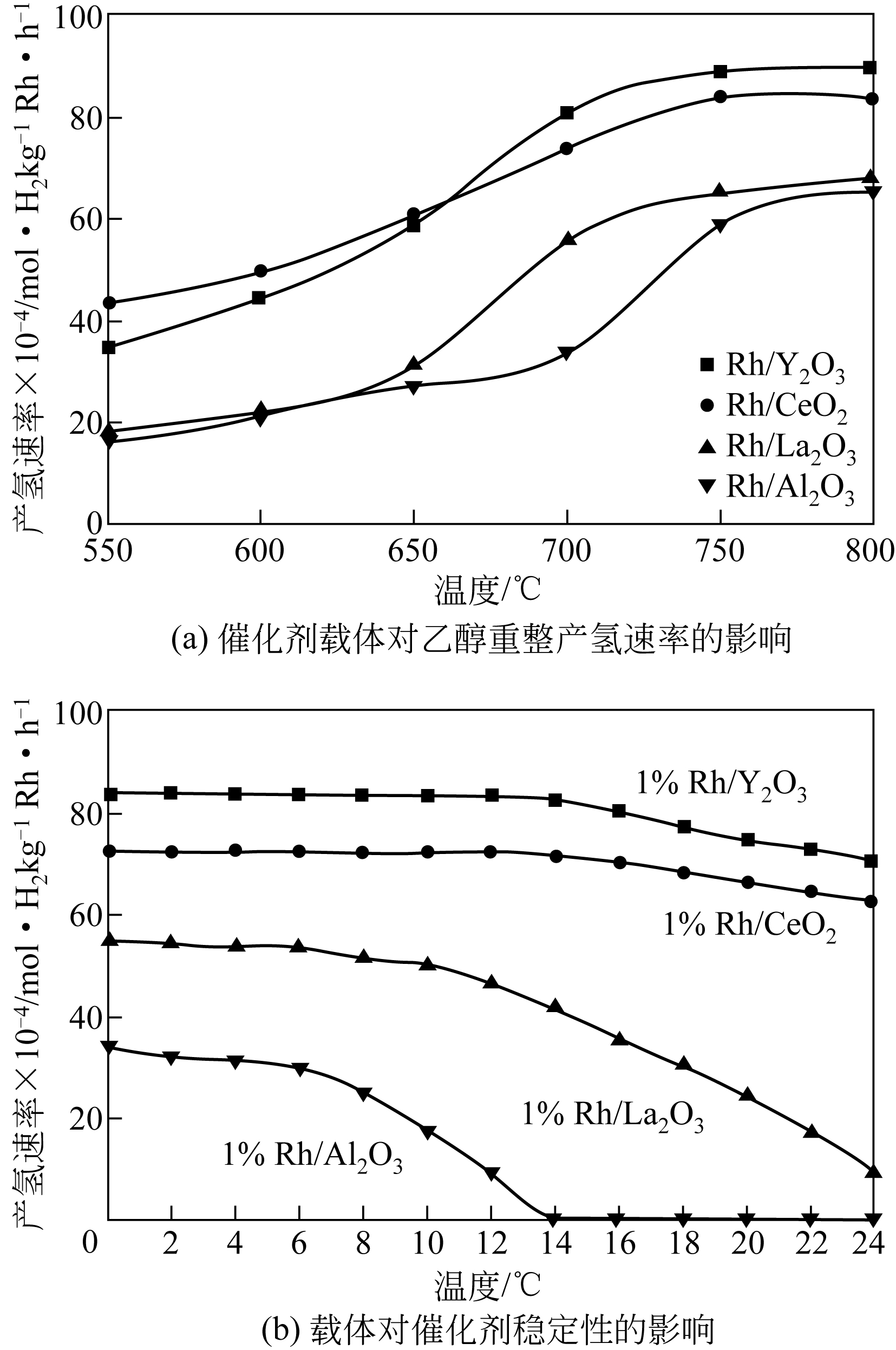
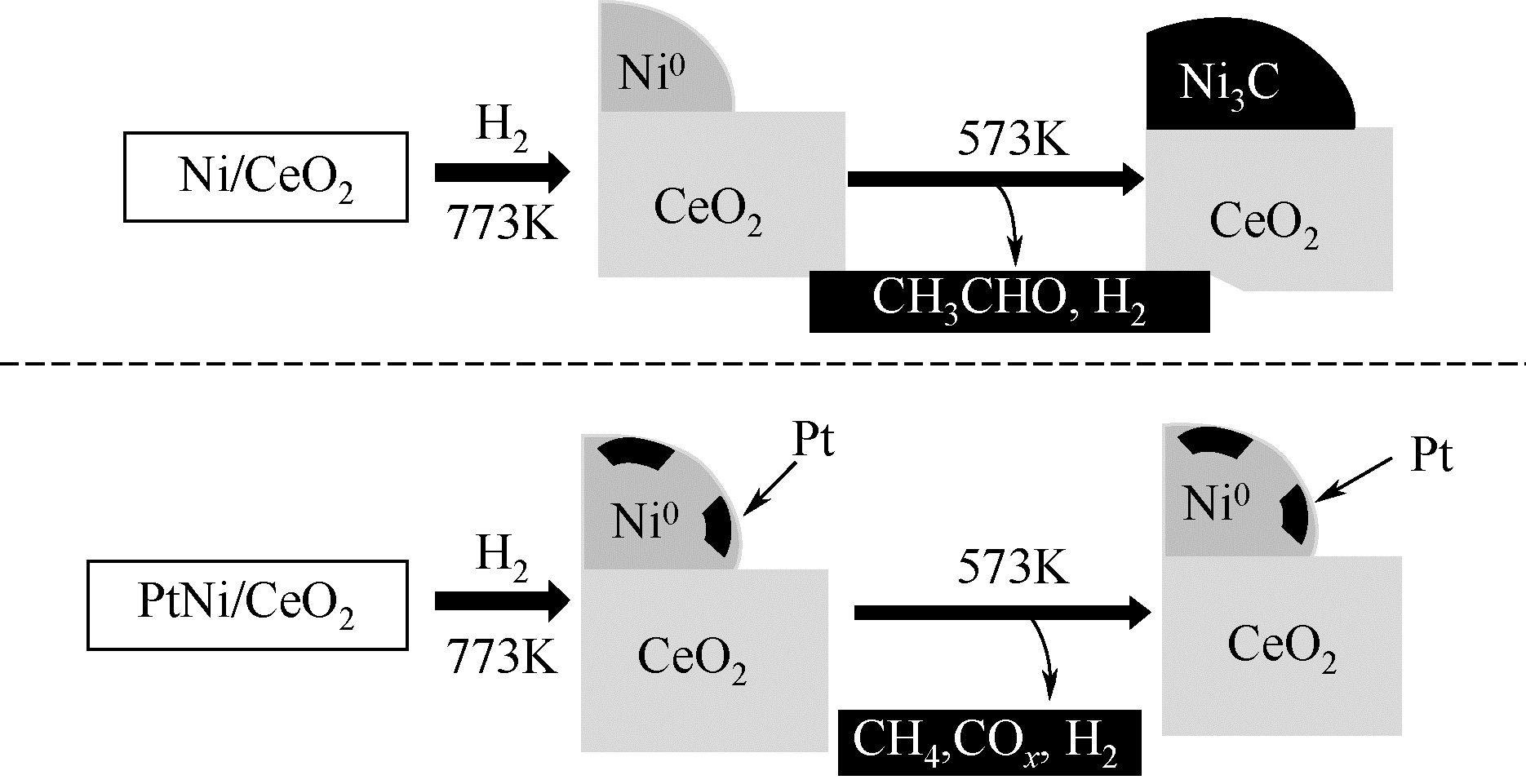
以乙醇为原料催化乙醇重整反应产氢由于具有效率高、毒性低以及乙醇可持续性生产等优点,被认为是一种极具工业化应用前景的制氢方法,但现存的乙醇重整反应催化剂存在催化效率和产氢选择性低、稳定性差等缺点,阻碍了乙醇重整制氢技术的广泛应用。本文主要综述了乙醇重整制氢金属催化剂的研究进展,着重阐述了金属元素种类、催化剂载体、反应温度和原料水醇比等对乙醇转化率和产物选择性的影响,归纳分析了乙醇重整过程中催化剂稳定性的影响因素和提高催化剂稳定性的方法和措施,并对乙醇重整制氢反应机制的研究工作进行了总结。基于乙醇重整产氢催化剂的研究现状,提出开发高效稳定催化剂的关键在于系统研究催化乙醇重整反应机制以及催化反应过程中催化剂与载体的协同效应对催化剂性能的影响。
中图分类号:
引用本文
陈俊, 舒红飞, 阮祝华, 倪嘉琪, 鲁礼林, 刘义. 乙醇催化重整产氢催化剂及催化反应机制[J]. 化工进展, 2019, 38(05): 2320-2328.
Jun CHEN, Hongfei SHU, Zhuhua RUAN, Jiaqi NI, Lilin LU, Yi LIU. Catalysts and catalytic mechanism for hydrogen production from ethanol steam reforming (ESR)[J]. Chemical Industry and Engineering Progress, 2019, 38(05): 2320-2328.
| 1 | YADAV M , XU Q . Liquid-phase chemical hydrogen storage materials[J]. Energy Environmental Science, 2012, 5: 9698-9725. |
| 2 | MATTOS L V , JACOBS G , DAVIS B H , et al . Production of hydrogen from ethanol: review of reaction mechanism and catalyst deactivation[J]. Chemical Reviews, 2012, 112(7): 4094-4123. |
| 3 | DAVIDSON S D , ZHANG H , SUN J , et al . Supported metal catalysts for alcohol/sugar alcohol steam reforming[J]. Dalton Transactions, 2014, 43(31): 11782-11802. |
| 4 | CONTRERAS J L , SALMONES J , COLIN-LUNA J A , et al . Catalysts for H2 production using the ethanol steam reforming (a review)[J]. International Journal of Hydrogen Energy, 2014, 39(33): 18835-18853. |
| 5 | HARYANTO A , FERNANDO S , MURALI N , et al . Current status of hydrogen production techniques by steam reforming of ethanol: a review[J]. Energy Fuels, 2005, 19(5): 2098-2106. |
| 6 | VAIDYA P D , RODRIGUES A E . Insight into steam reforming of ethanol to produce hydrogen for fuel cells[J]. Chemical Engineering Journal, 2006, 117(1): 39-49. |
| 7 | NI M , LEUNG D Y C , LEUNG M K H . A review on reforming bio-ethanol for hydrogen production[J]. International Journal of Hydrogen Energy, 2007, 32(15): 3238-3247. |
| 8 | PISCINA P R , HOMS N . Use of biofuels to produce hydrogen (reformation processes)[J]. Chemical Society Reviews, 2008, 37(11): 2459-2467. |
| 9 | LIGURAS D K , KONDARIDES D I , VERYKIOS X E . Production of hydrogen for fuel cells by steam reforming of ethanol over supported noble metal catalysts[J]. Applied Catalysis B: Environmental, 2003, 43(4): 345-354. |
| 10 | CAVALLARO S , CHIODO V , FRENI S , et al . Performance of Rh/Al2O3 catalyst in the steam reforming of ethanol for MCFC[J]. Applied Catalysis A: General, 2003, 249(1): 119-128. |
| 11 | LOPEZ E , DIVINS N J , LLORCA J . Hydrogen production from ethanol over Pd-Rh/CeO2 with a metallic membrane reactor[J]. Catalysis Today, 2012, 193(15): 145-150. |
| 12 | COBO M , PIERUCCINI D , ABELLO R , et al . Steam reforming of ethanol over bimetallic RhPt/La2O3: long-term stability under favorable reaction conditions[J]. International Journal of Hydrogen Energy, 2013, 38(14): 5580-5593. |
| 13 | KOH A C W, LEONG W K , CHEN L , et al . Highly efficient ruthenium and ruthenium-platinum cluster-derived nanocatalysts for hydrogen production via ethanol steam reforming[J]. Catalysis Communications, 2008, 9(1): 170-175. |
| 14 | AUPRETRE F , DESCORME C , DUPREZ D . Bio-ethanol catalytic steam reforming over supported metal catalysts[J]. Catalysis Communications, 2002, 3(6): 263-267. |
| 15 | FRUSTERI F , FRENI S , SPADARO L , et al . H2 production for MC fuel cell by steam reforming of ethanol over MgO supported Pd, Rh, Ni and Co catalysts[J]. Catalysis Communications, 2004, 5(10): 611-615. |
| 16 | CONTRERAS J L , TAPIA C , FUENTES G A , et al . Equilibrium compostion of ethanol steam reforming reaction to produce H2 applied to Ni, Co, and Pt/hydrotalcite-WO x catalysts[J]. International Journal of Hydrogen Energy, 2014, 39(29): 16608-16618. |
| 17 | 王拓,田昊,张成喜,等 . 乙醇蒸汽重整制氢镍基催化剂的尺度效应与构效关系[J]. 科学通报, 2015, 60(33): 3230-3238. |
| WANG T , TIAN H , ZHANG C X , et al . Particle size effect and structure-function relationship of Ni-based steam reforming catalysts[J]. Chinese Science Bulletin, 2015, 60(33): 3230-3238. | |
| 18 | HULL S , TRAWCZYNSKI J . Steam reforming of ethanol on zinc containing catalysts with spinel stucture[J]. International Journal of Hydrogen Energy, 2014, 39(9): 4259-4265. |
| 19 | 李宝茹,殷雪梅,吴旭,等 . Ni-Fe/蒙脱土催化剂催化乙醇水蒸气重整制氢的研究[J]. 燃料化学学报, 2016, 44(8): 993-1000. |
| LI B R , YIN X M , WU X , et al . Montmorillonite supported Ni-Fe catalysts for hydrogen production from steam reforming of ethanol[J]. Journal of Fuel Chemistry and Technology, 2016, 44(8): 993-1000. | |
| 20 | LI L , TANG D W , SONG Y C , et al . Hydrogen production from ethanol steam reforming on Ni-Ce/MMT catalysts[J]. Energy, 2018, 149(15): 937-943. |
| 21 | PANAGIOTOPOULOU P , VERYKIOS X E . Mechanistic aspects of the low temperature steam reforming of ethanol over supported Pt catalysts[J]. International Journal of Hydrogen Energy, 2012, 37(21): 16333-16345. |
| 22 | CHEN L W , CHOONG C K S , ZHONG Z Y , et al . Carbon monoxide-free hydrogen production via low-temperature steam reforming of ethanol over iron-promoted Rh catalyst[J]. Journal of Catalysis, 2010, 276(2): 197-200. |
| 23 | LANG L , ZHAO S H , YIN X L , et al . Catalytic activities of K-modified zeolite ZSM-5 supported rhodium catalysts in low-temperature steam reforming of bioethanol[J]. International Journal of Hydrogen Energy, 2015, 40(32): 9924-9934. |
| 24 | WU X S , KAWI S . Steam reforming of ethanol to H2 over Rh/Y2O3: crucial roles of Y2O3 oxidizing ability, space velocity and H2/C[J]. Energy Environmental Science, 2010, 3: 334-342. |
| 25 | WANG F G , CAI W J , PROVENDIER H , et al . Hydrogen production from ethanol steam reforming over Ir/CeO2 catalysts: enhanced stability by PrO x promotion[J]. International Journal of Hydrogen Energy, 2011, 36(21): 13566-13574. |
| 26 | WANG F G , ZHANG L J , ZHU J Y , et al . Study on different CeO2 structure stability during ethanol steam reforming reaction over Ir/CeO2 nanocatalysts[J]. Applied Catalysis A: General, 2018, 564: 226-233. |
| 27 | SEKINE Y , NAKAZAWA Y , OYAMA K , et al . Effect of small amount of Fe addition on ethanol steam reforming over Co/ Al2O3 catalyst[J]. Applied Catalysis A: General, 2014, 472: 113-122. |
| 28 | KIM D, KWAK B S , MIN B K , et al . Characterization of Ni and W co-loaded SBA-15 catalyst and its hydrogen production catalytic ability on ethanol steam reforming reaction[J]. Applied Surface Science, 2015, 332: 736-746. |
| 29 | LI D , ZENG L , LI X Y , et al . Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation[J]. Applied Catalysis B: Environmental, 2015, 176/177: 532-541. |
| 30 | DAN M, MIHET M , TASNADI-ASZTALOS Z , et al . Hydrogen production by ethanol steam reforming on nickel catalysts: effect of support modification by CeO2 and La2O3 [J]. Fuel, 2015, 147: 260-268. |
| 31 | FERENCZ Z , ERDOHELYI A , BAAN K , et al . Effect of support and Rh additive on Co-based catalysts in the ethanol steam reforming reaction[J]. ACS Catalysis, 2014, 4(4): 1205-1218. |
| 32 | MEMON M Z , ZHAO X , SIKARWAR V S , et al . Alkali metal CO2 sorbents and the resulting metal carbonates: potential for process intensification of sorption-enhanced steam reforming[J]. Environmental Science Technology, 2017, 51(1): 12-27. |
| 33 | HAN S J , SONG J H , YOO J, et al . Sorption-enhanced hydrogen production by steam reforming of ethanol over mesoporous Co/CaO-Al2O3 xerogel catalysts: effect of Ca/Al molar ratio[J]. International Journal of Hydrogen Energy, 2017, 42(9): 5886-5898. |
| 34 | COMPAGNONI M , TRIPODI A , ROSSETTI I . Parametric study and kinetic testing for ethanol steam reforming[J]. Applied Catalysis B: Environmental, 2017, 203: 899-909. |
| 35 | SILVA A M , SOUZA K R , JACOBS G , et al . Steam and CO2 reforming of ethanol over Rh/CeO2 catalyst[J]. Applied Catalysis B: Environmental, 2011, 102(1/2): 94-109. |
| 36 | WU Y J , SANTOS J C , LI P , et al . Simplified kinetic model for steam reforming of ethanol on a Ni/Al2O3 catalyst[J]. The Canadian Journal of Chemical Engineering, 2014, 92: 116-130. |
| 37 | HE S F , MEI Z Q , LIU N S , et al . Ni/SBA-15 catalysts for hydrogen production by ethanol steam reforming: effect of nickel precursor[J]. International Journal of Hydrogen Energy, 2017, 42(21): 14429-14438. |
| 38 | KWAK B S , LEE G, PARK S M , et al . Effect of MnO x in the catalytic stabilization of Co2MnO4 spinel during the ethanol steam reforming reaction[J]. Applied Catalysis A: General, 2015, 503: 165-175. |
| 39 | WU G W , LI S R , ZHANG C X , et al . Glycerol steam reforming over perovskite-derived nickel-based catalysts[J]. Applied Catalysis B: Environmental, 2014, 144: 277-285. |
| 40 | ZENG G M , LI Y D , OLSBYE U . Kinetic and process study of ethanol steam reforming over Ni/Mg(Al)O catalysts: the initial steps[J]. Catalysis Today, 2016, 259(2): 312-322. |
| 41 | ROMERO A , JABBAGY M , LABORDE M , et al . Ni(II)-Mg(II)-Al(III) catalysts for hydrogen production from ethanol steam reforming: influence of the Mg content[J]. Applied Catalysis A: General, 2014, 470: 398-404. |
| 42 | ROSSETTI I , LASSO J , NICHELE V , et al . Silica and zirconia supported catalysts for the low-temperature ethanol steam reforming[J]. Applied Catalysis B: Environmental, 2014, 150/151: 257-267. |
| 43 | NICHELE V , SIGNORETTO M , PINNA F , et al . Ni/ZrO2 catalysts in ethanol steam reforming: Inhibition of coke formation by CaO-doping[J]. Applied Catalysis B: Environmental, 2014, 150/151: 12-20. |
| 44 | ZHAO L , WEI Y , HUANG Y M , et al . La1- x K x Fe0.7Ni0.3O3 catalyst for ethanol steam reforming-The effect of K-doping[J]. Catalysis Today, 2016, 259(2): 430-437. |
| 45 | SILVA A L M , BREEJEN J P , MATTOS L V , et al . Cobalt particle size effects on catalytic performance for ethanol steam reforming-smaller is better[J]. Journal of Catalysis, 2014, 318: 67-74. |
| 46 | WANG Z J , WANG C X , CHEN S Q , et al . Co-Ni bimetal catalyst supported on perovskite-type oxide for steam reforming of ethanol to produce hydrogen[J]. International Journal of Hydrogen Energy, 2014, 39(11): 5644-5652. |
| 47 | KLOUZ V , FIERRO V , DENTON P , et al . Ethanol reforming for hydrogen production in a hybrid electric vehicle: process optimisation[J]. Journal of Power Source, 2002, 105(1): 26-34. |
| 48 | TRANE-RESTRUP R , DAHL S , JENSEN A D . Steam reforming of ethanol over Ni-based catalysts: effect of feed composition on catalyst stability[J]. International Journal of Hydrogen Energy, 2014, 39(15): 7735-7746. |
| 49 | BEDNARCZUK L , PISCINA P R , HOMS N . H2-production from CO2-assisted ethanol steam reforming: the regeneration of Ni-based catalysts[J]. International Journal of Hydrogen Energy, 2015, 40(15): 5256-5263. |
| 50 | MONDAL T , PANT K K , DALAI A K . Oxidative and non-oxidative steam reforming of crude bio-ethanol for hydrogen production over Rh promoted Ni/CeO2-ZrO2 catalyst[J]. Applied Catalysis A: General, 2015, 499: 19-31. |
| 51 | VOZNIUK O , AGNOLI S , ARTIGLIA L , et al . Towards an improved process for hydrogen production: the chemical-loop reforming of ethanol[J]. Green Chemistry, 2016, 18: 1038-1050. |
| 52 | MORAES T S , NETO R C R , RIBEIRO M C , et al . Ethanol conversion at low temperature over CeO2-Supported Ni-based catalysts. Effect of Pt addition to Ni catalyst[J]. Applied Catalysis B: Environmental, 2016, 181: 754-768. |
| 53 | ABELLO S , BOLSHAK E , GISPERT-GUIRADO F , et al . Ternary Ni-Al-Fe catalysts for ethanol steam reforming[J]. Catalysis Science & Technology, 2014, 4: 1111-1122. |
| 54 | KRALEVA E , SOKOLOV S , NASILLO G , et al . Catalytic performance of CoAlZn and NiAlZn mixed oxides in hydrogen production by bio-ethanol partial oxidation[J]. International Journal of Hydrogen Energy, 2014, 39(1): 209-220. |
| 55 | MORETTI E , STORARO L , TALON A , et al . Ceria-zirconia based catalysts for ethanol steam reforming[J]. Fuel, 2015, 153: 166-175. |
| 56 | YU S W , HUANG H H , TANG C W , et al . The effect of accessible oxygen over Co3O4-CeO2 catalysts on the steam reforming of ethanol[J]. International Journal of Hydrogen Energy, 2014, 39(35): 20700-20711. |
| 57 | FRANCHINI C A , ARANZAEZ W , FARIAS A M D , et al . Ce-substituted LaNiO3 mixed oxides as catalyst precursors for glycerol steam reforming[J]. Applied Catalysis B: Environmental, 2014, 147: 193-202. |
| 58 | GUIL J M , HOMS N , LLORCA J , et al . Microcalorimetric and infrared studies of ethanol and acetaldehyde adsorption to investigate the ethanol steam reforming on supported cobalt catalysts[J]. The Journal of Physical Chemistry B, 2005, 109(21): 10813-10819. |
| 59 | CHOONG C , ZHONG Z Y , HUANG L , et al . Infrared evidence of a formate-intermediate mechanism over Ca-modified supports in low-temerature ethanol steam reforming[J]. ACS Catalysis, 2014, 4(7): 2359-2363. |
| 60 | SUN J M , KARIM A M , MEI D H , et al . New insights into reaction mechanisms of ethanol steam reforming on Co-ZrO2 [J]. Applied Catalysis B: Environmental, 2015, 162: 141-148. |
| 61 | LIU Z Y , XU W Q , YAO S Y , et al . Superior performance of Ni-W-Ce mixed-metal oxide catalysts for ethanol steam reforming: synergistic effects of W- and Ni-dopants[J]. Journal of Catalysis, 2015, 321: 90-99. |
| 62 | CROWLEY S , CASTALDI M J . Mechanistic insights into catalytic ethanol steam reforming using isotope-labeled reactants[J]. Angewandte Chemie International Edition, 2016, 55(36): 10650-10655. |
| 63 | SUTTON J E , PANAGIOTOPOULOU P , VERYKIOS X E , et al . Combined DFT, microkinetic, and experimental study of ethanol steam reforming on Pt[J]. The Journal of Physical Chemistry C, 2013, 117(9): 4691-4706. |
| 64 | ZHANG J , ZHONG Z Y , CAO X M , et al . Ethanol steam reforming on Rh catalysts: theoretical and experimental understanding[J]. ACS Catalysis, 2014, 4(2): 448-456. |
| 65 | LIN S , HUANG J , GAO X M , et al . Theoretical insight into the reaction mechanism of ethanol steam reforming on Co(0001)[J]. The Journal of Physical Chemistry C, 2015, 119(5): 2680-2691. |
| 66 | LI M R , CHEN J , WANG G C . Reaction mechanism of ethanol on model cobalt catalysts: DFT calculations[J]. The Journal of Physical Chemistry C, 2016, 120(26): 14198-14208. |
| 67 | ZANCHET D , SANTOS J B O , DAMYANOVA S , et al . Toward understanding metal-catalyzed ethanol reforming[J]. ACS Catalysis, 2015, 5(6): 3841-3863. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [6] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [7] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [10] | 盛维武, 程永攀, 陈强, 李小婷, 魏嘉, 李琳鸽, 陈险峰. 微气泡和微液滴双强化脱硫反应器操作分析[J]. 化工进展, 2023, 42(S1): 142-147. |
| [11] | 赵晨, 苗天泽, 张朝阳, 洪芳军, 汪大海. 负压状态窄缝通道乙二醇水溶液传热特性[J]. 化工进展, 2023, 42(S1): 148-157. |
| [12] | 舒斌, 陈建宏, 熊健, 吴其荣, 喻江涛, 杨平. 碳中和目标下推动绿色甲醇发展的必要性分析[J]. 化工进展, 2023, 42(9): 4471-4478. |
| [13] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [14] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [15] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||