化工进展 ›› 2025, Vol. 44 ›› Issue (11): 6716-6729.DOI: 10.16085/j.issn.1000-6613.2024-1684
• 资源与环境化工 • 上一篇
粉煤灰基硅酸镁纳米片的制备及其亚甲基蓝吸附性能
刘倩( ), 李梦茹, 白守礼, 冯拥军(
), 李梦茹, 白守礼, 冯拥军( ), 唐平贵(
), 唐平贵( ), 李殿卿
), 李殿卿
- 北京化工大学化工资源有效利用全国重点实验室,北京 100029
-
收稿日期:2024-10-20修回日期:2024-12-18出版日期:2025-11-25发布日期:2025-12-08 -
通讯作者:冯拥军,唐平贵 -
作者简介:刘倩(2000—),女,硕士研究生,研究方向为无机功能材料。E-mail:2022201016@buct.edu.cn。 -
基金资助:国家重点研发计划(2022YFA1503400);国家重点研发计划(2023YFC3903700);国家自然科学基金(U1507119);国家自然科学基金(21627813)
Preparation and adsorption performance to methylene blue of fly ash based magnesium silicate nanosheets
LIU Qian( ), LI Mengru, BAI Shouli, FENG Yongjun(
), LI Mengru, BAI Shouli, FENG Yongjun( ), TANG Pinggui(
), TANG Pinggui( ), LI Dianqing
), LI Dianqing
- State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China
-
Received:2024-10-20Revised:2024-12-18Online:2025-11-25Published:2025-12-08 -
Contact:FENG Yongjun, TANG Pinggui
摘要:
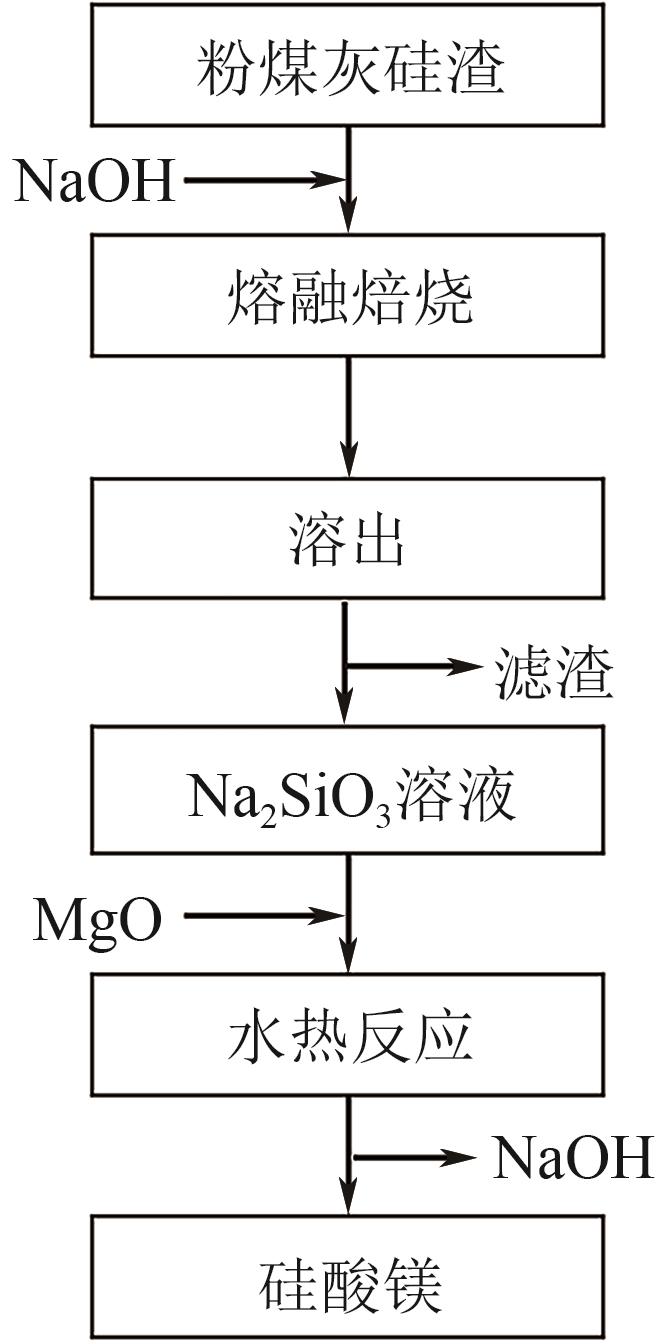
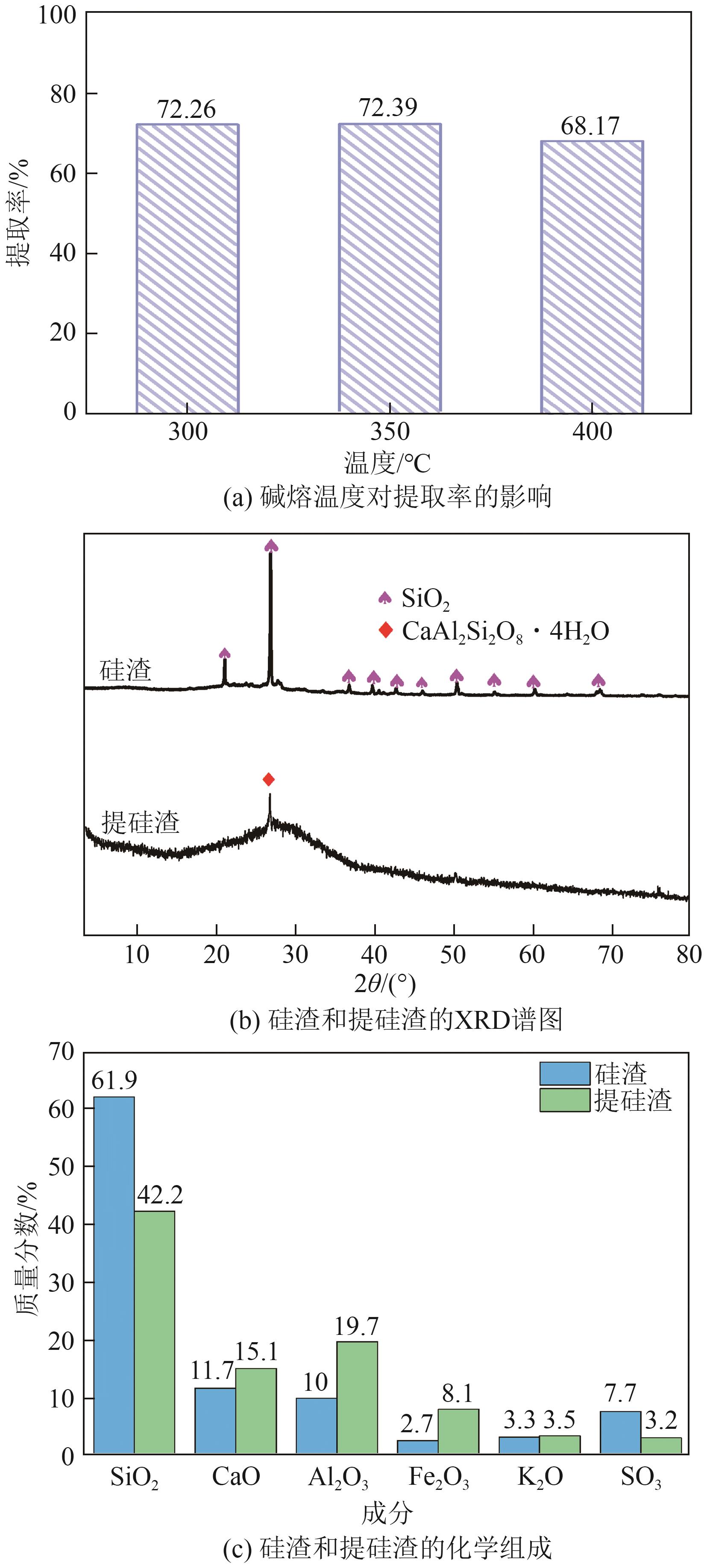
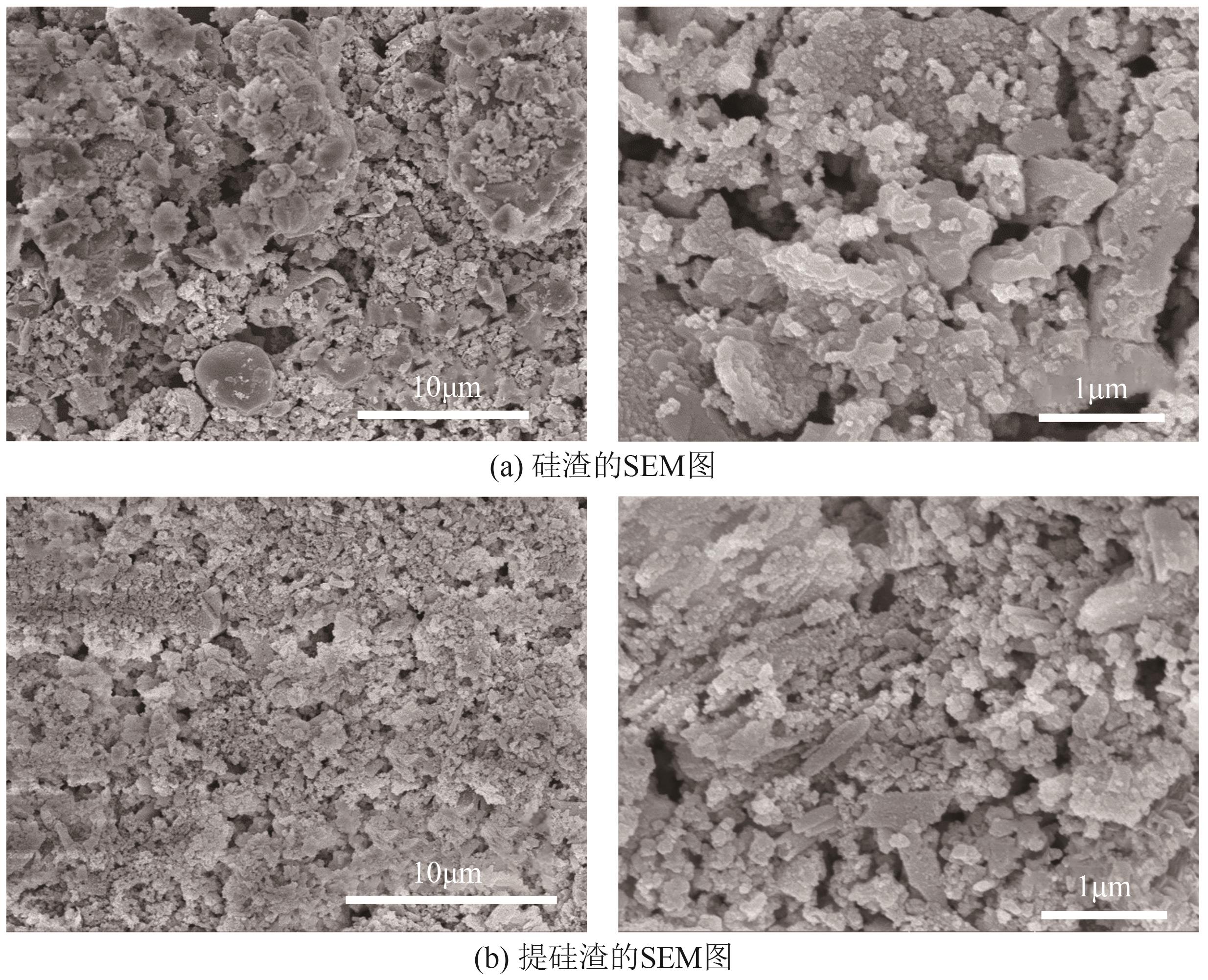
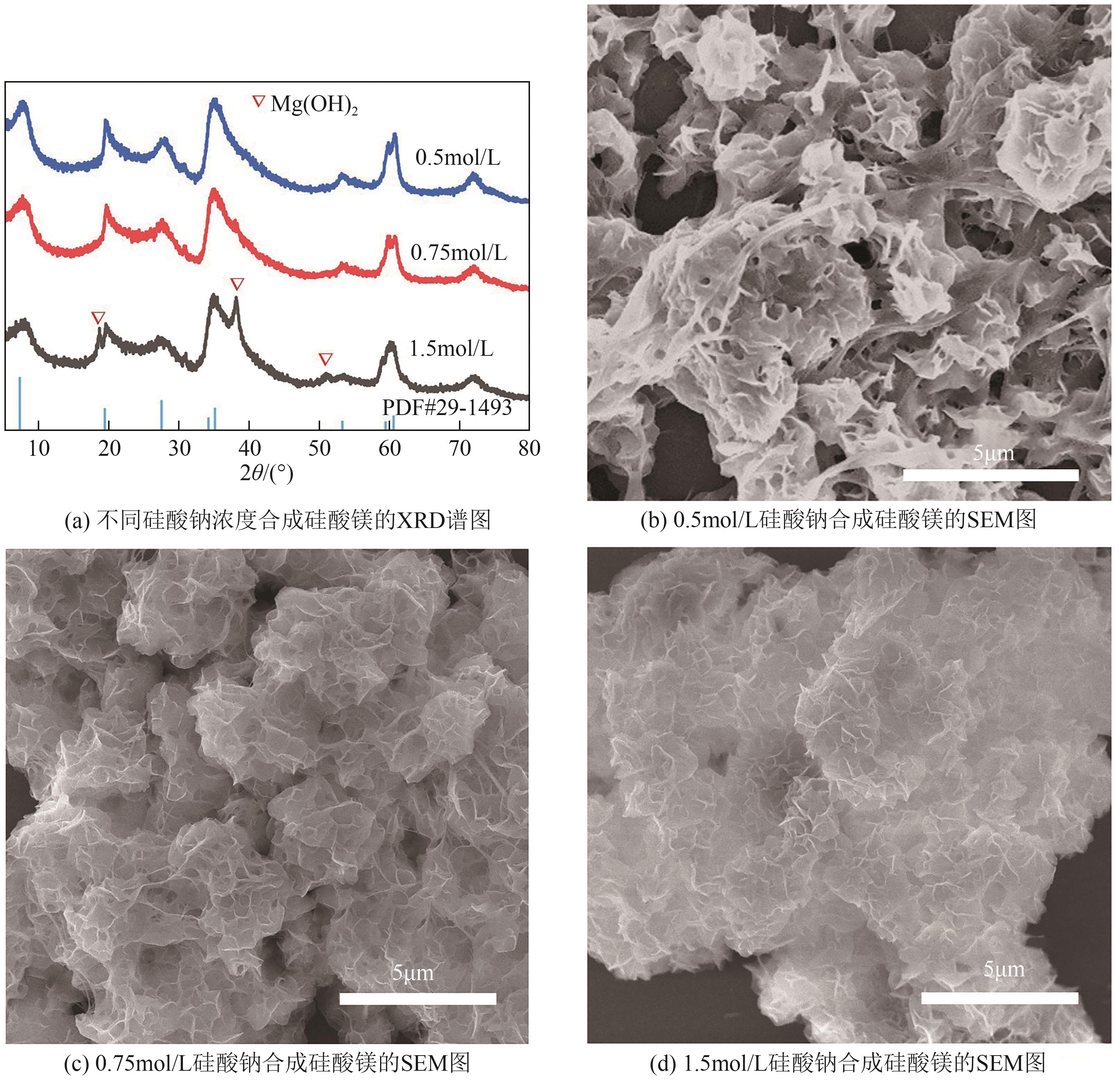
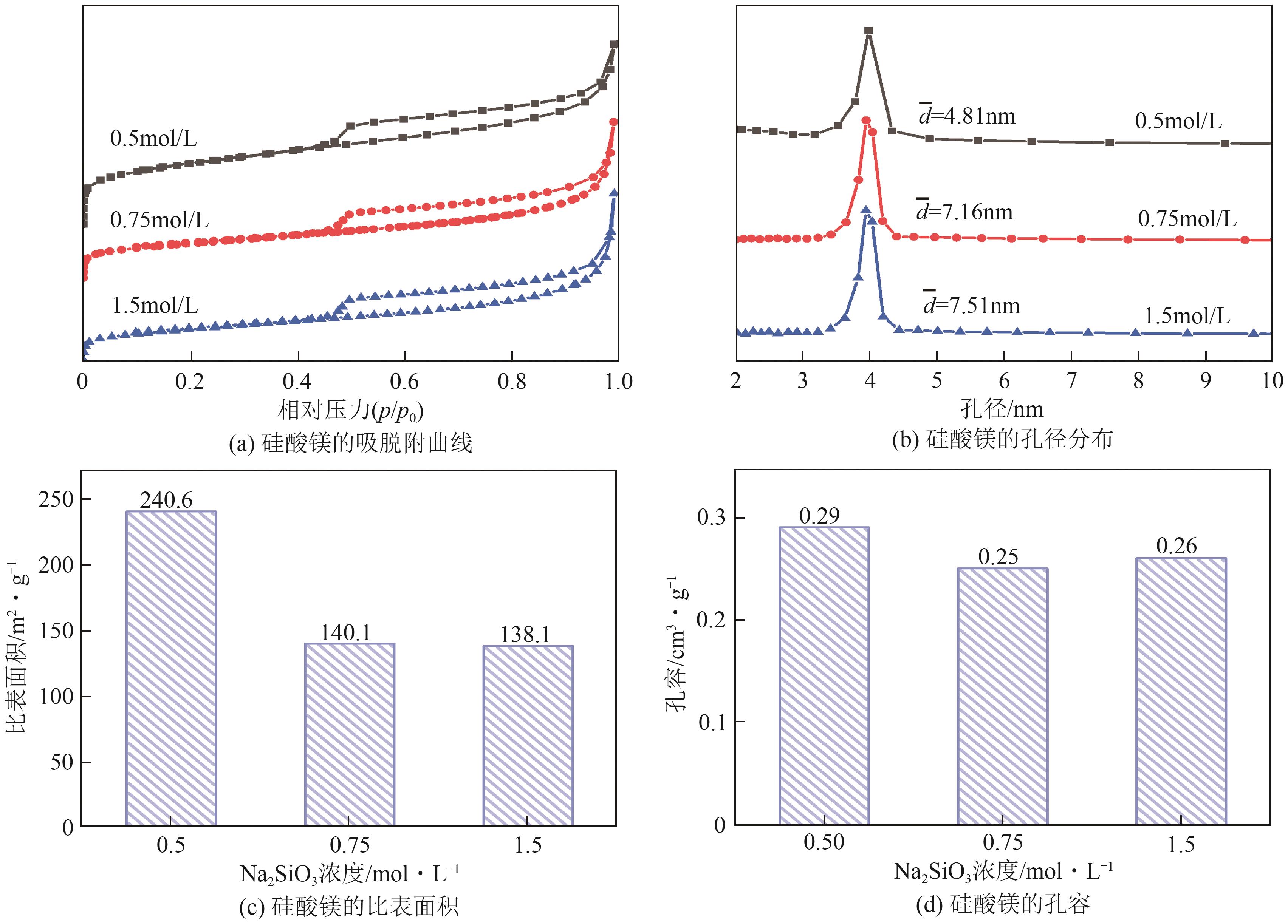
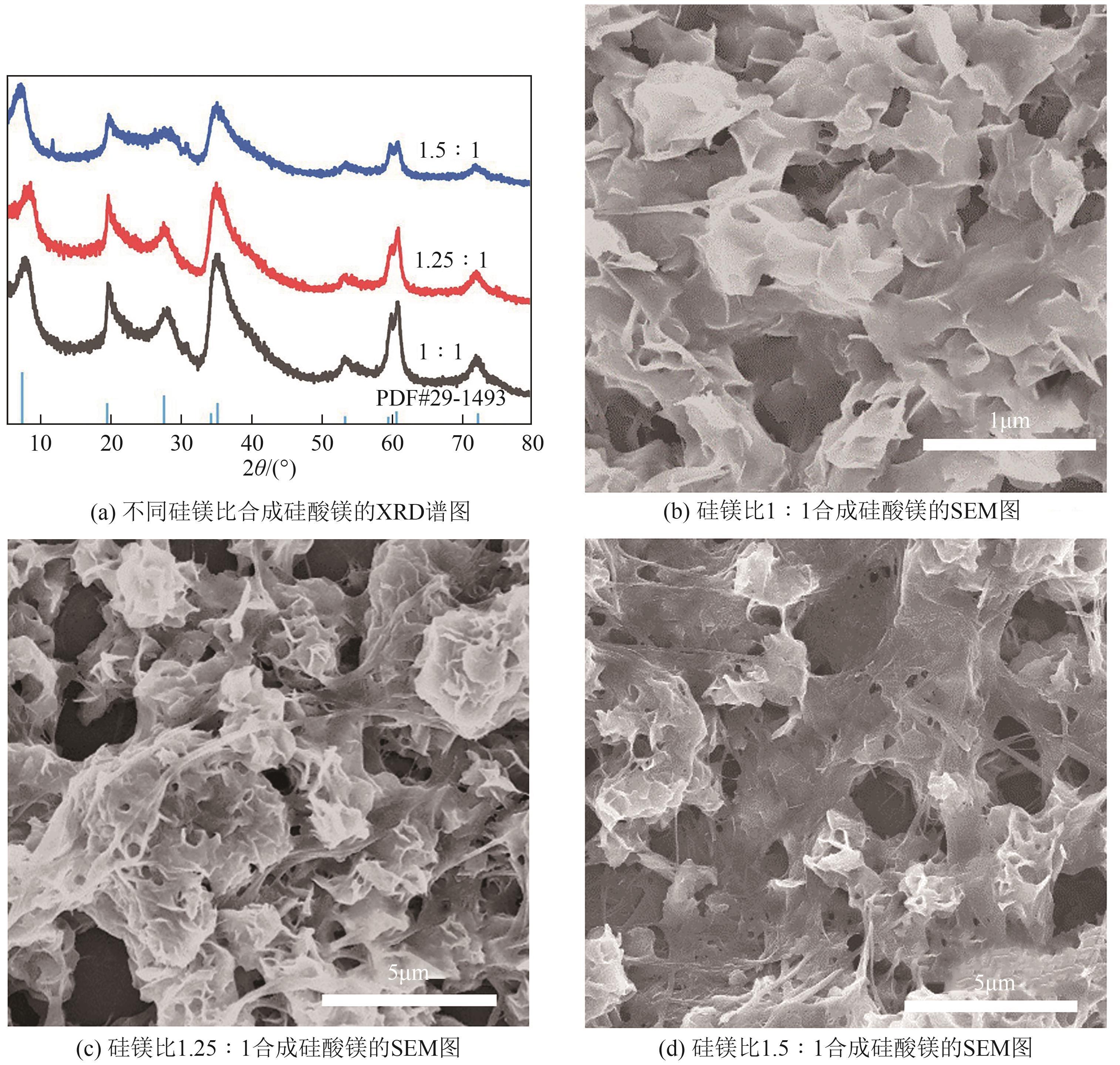
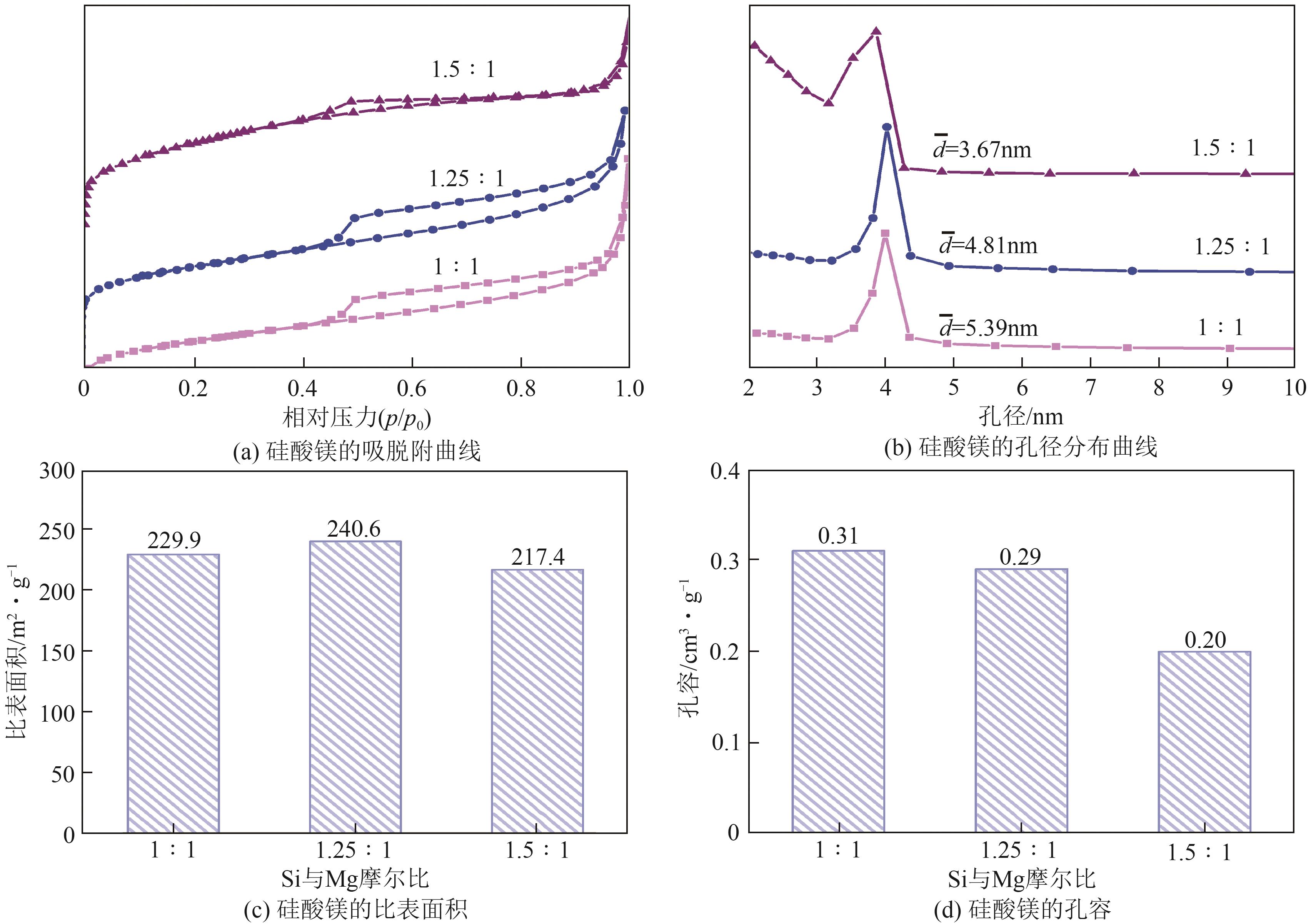
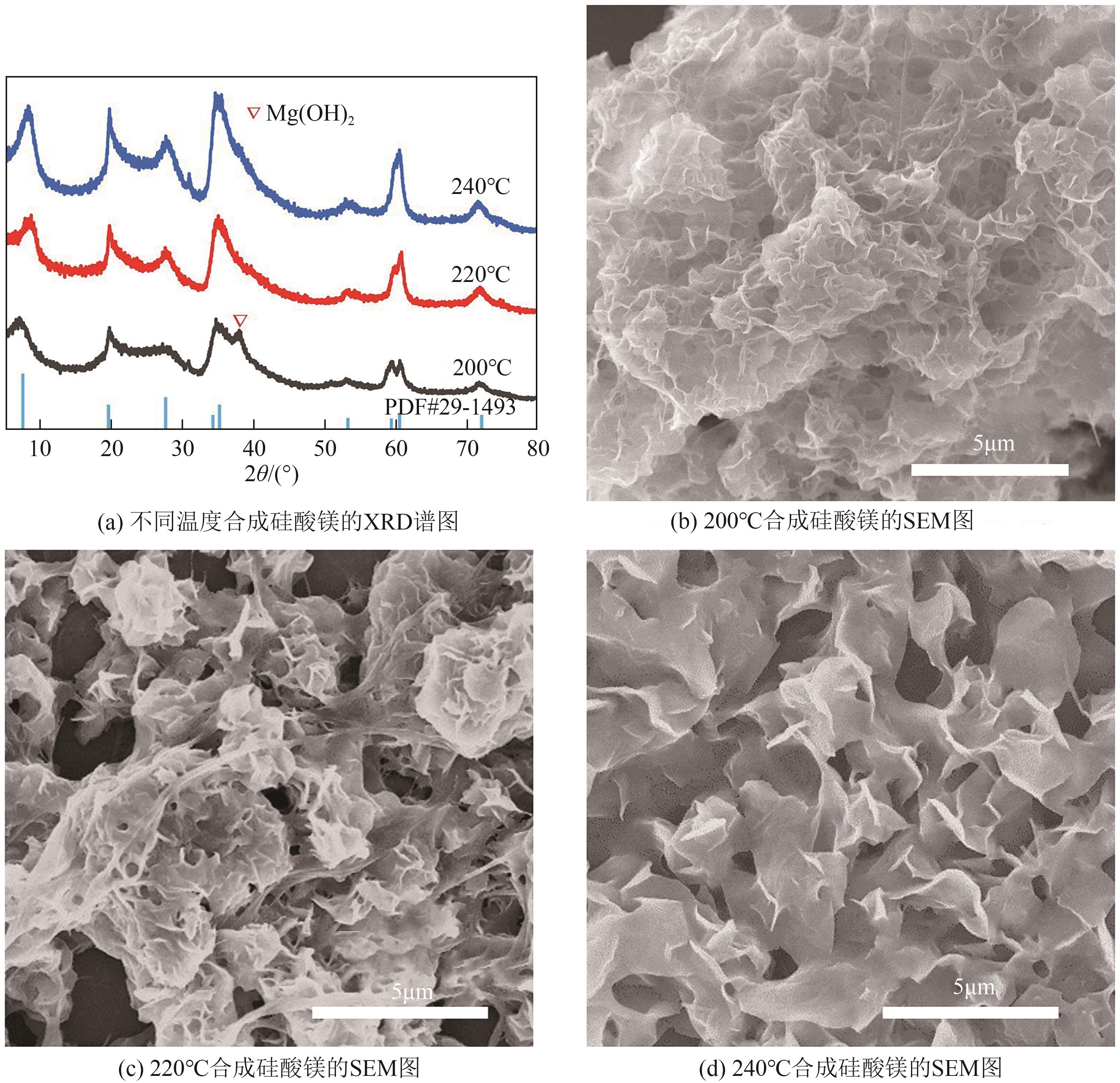
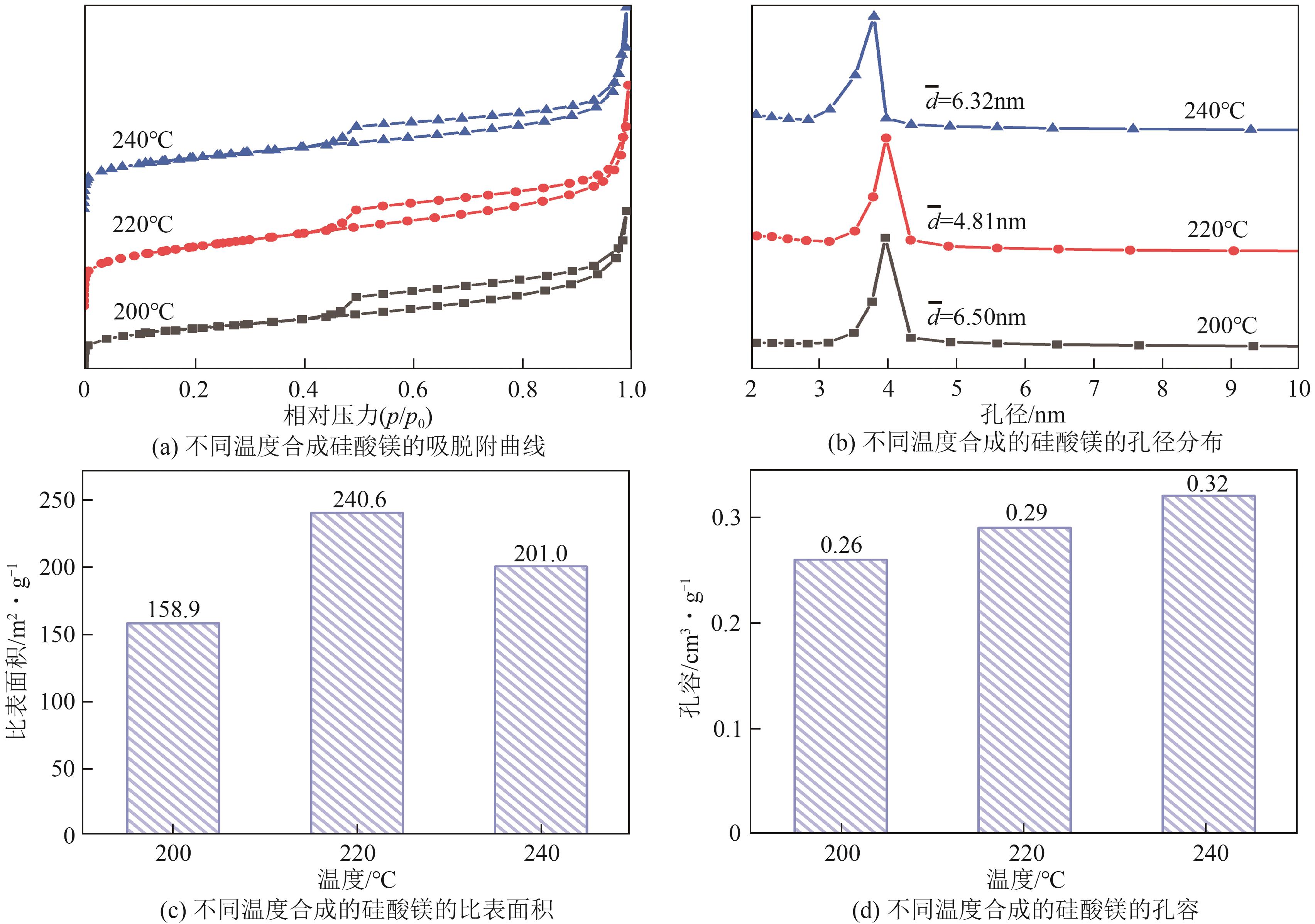
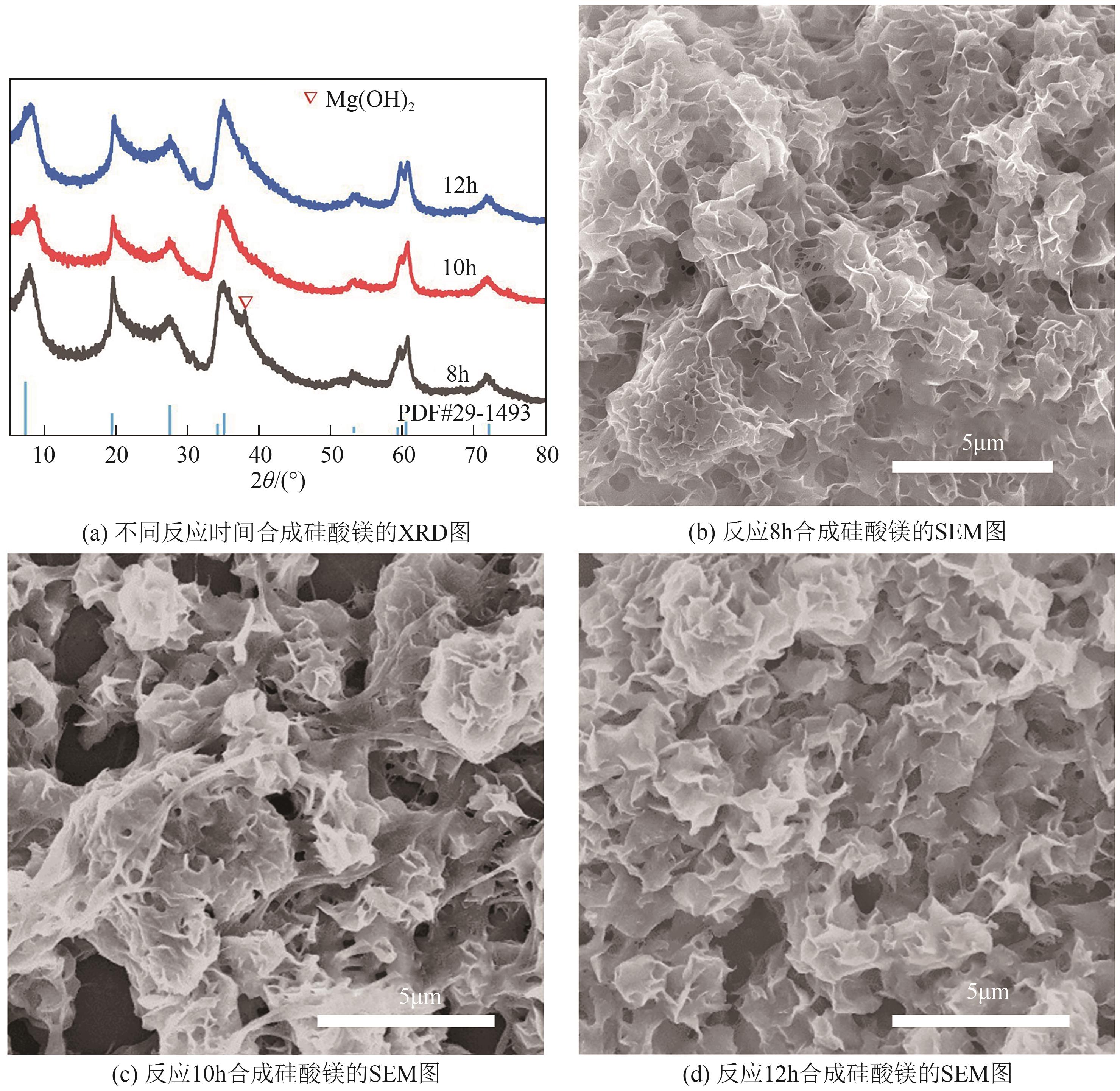
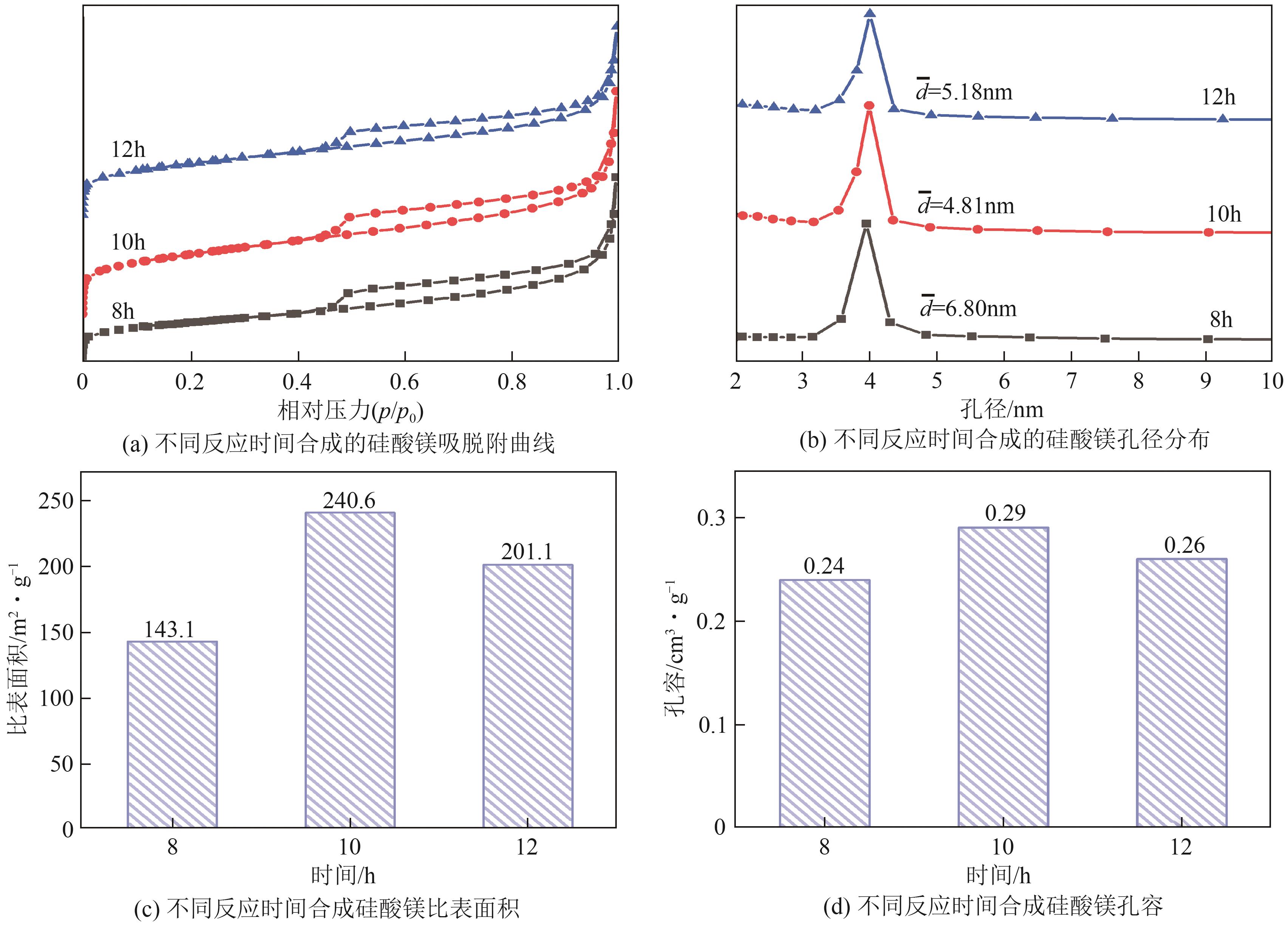
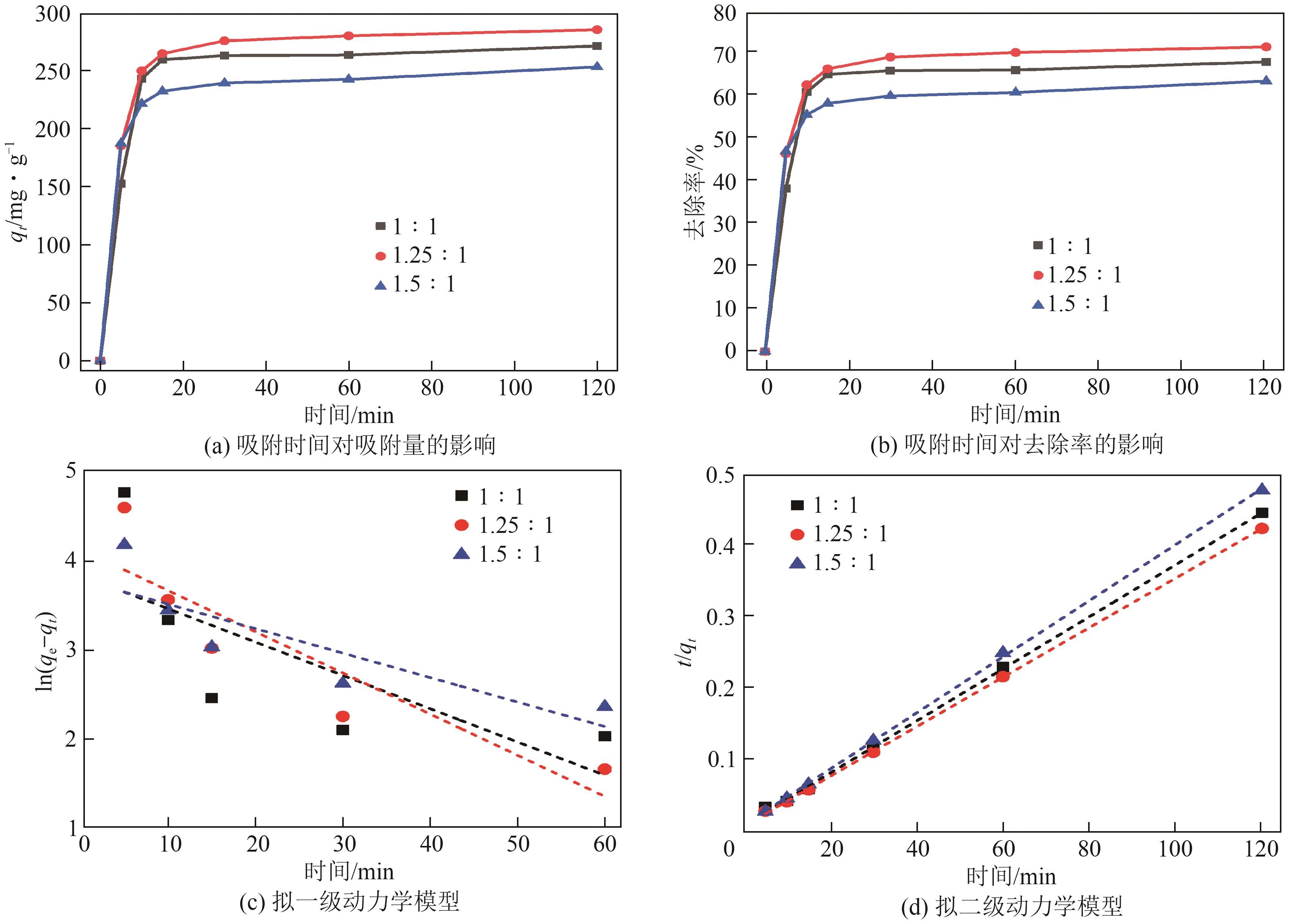
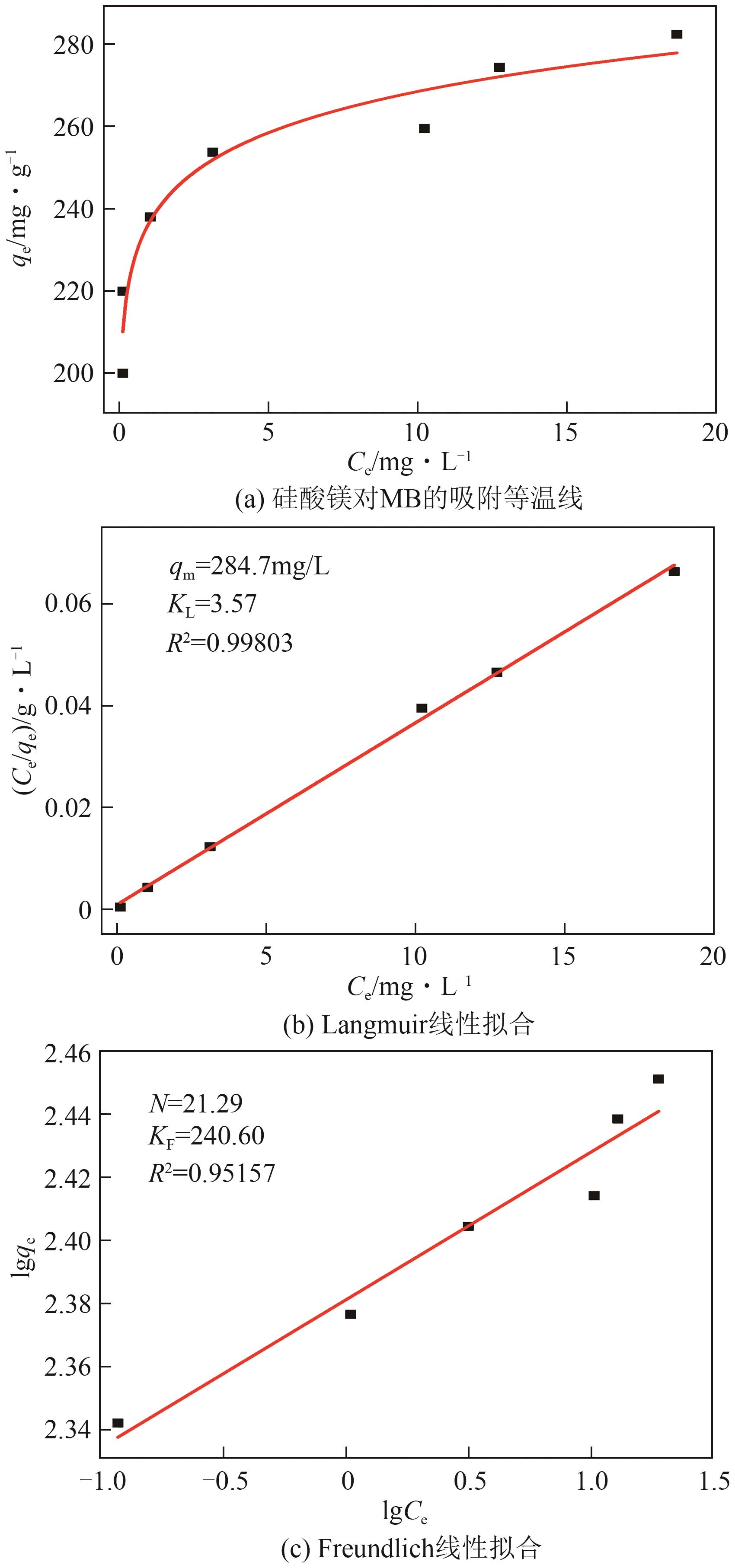
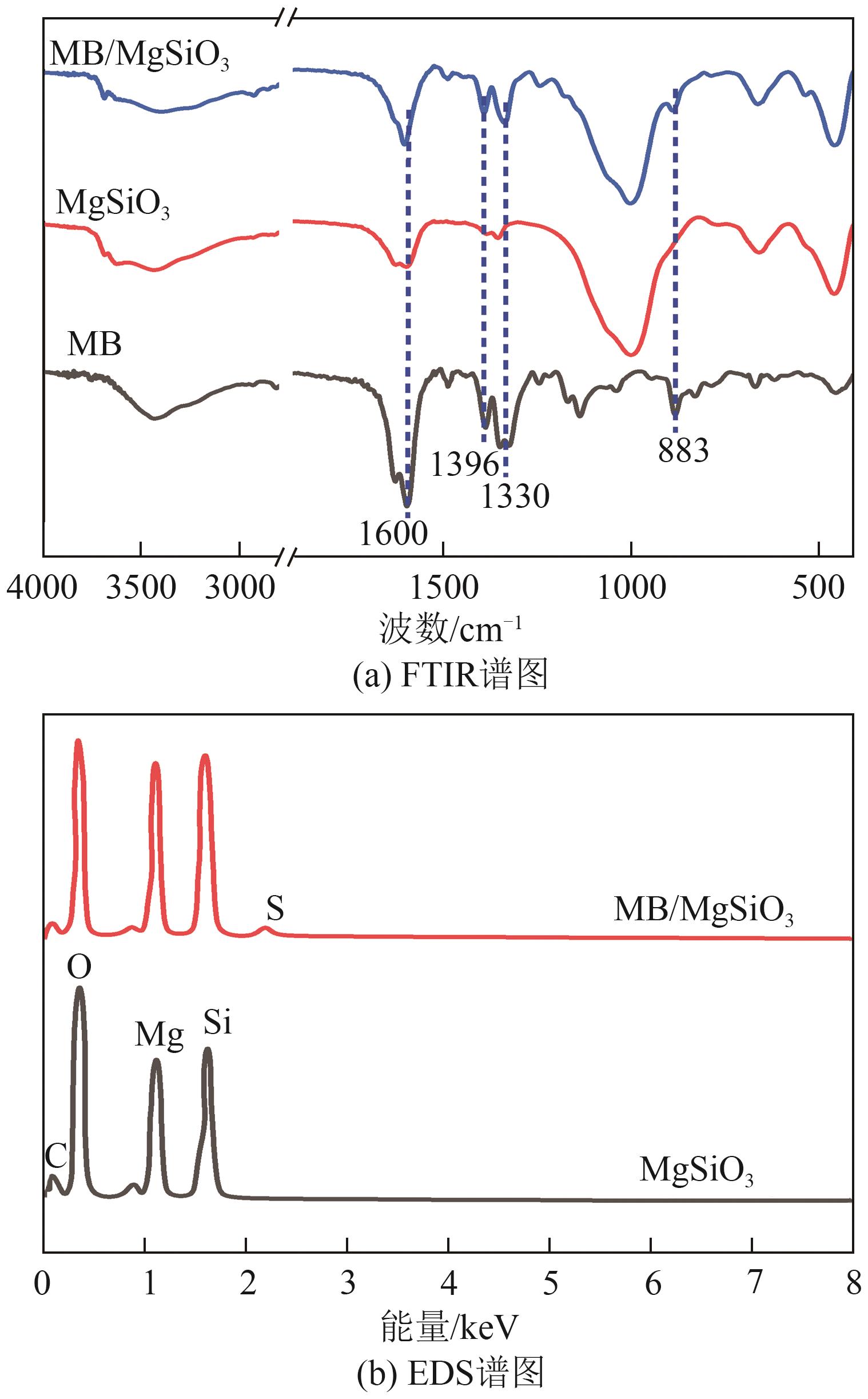
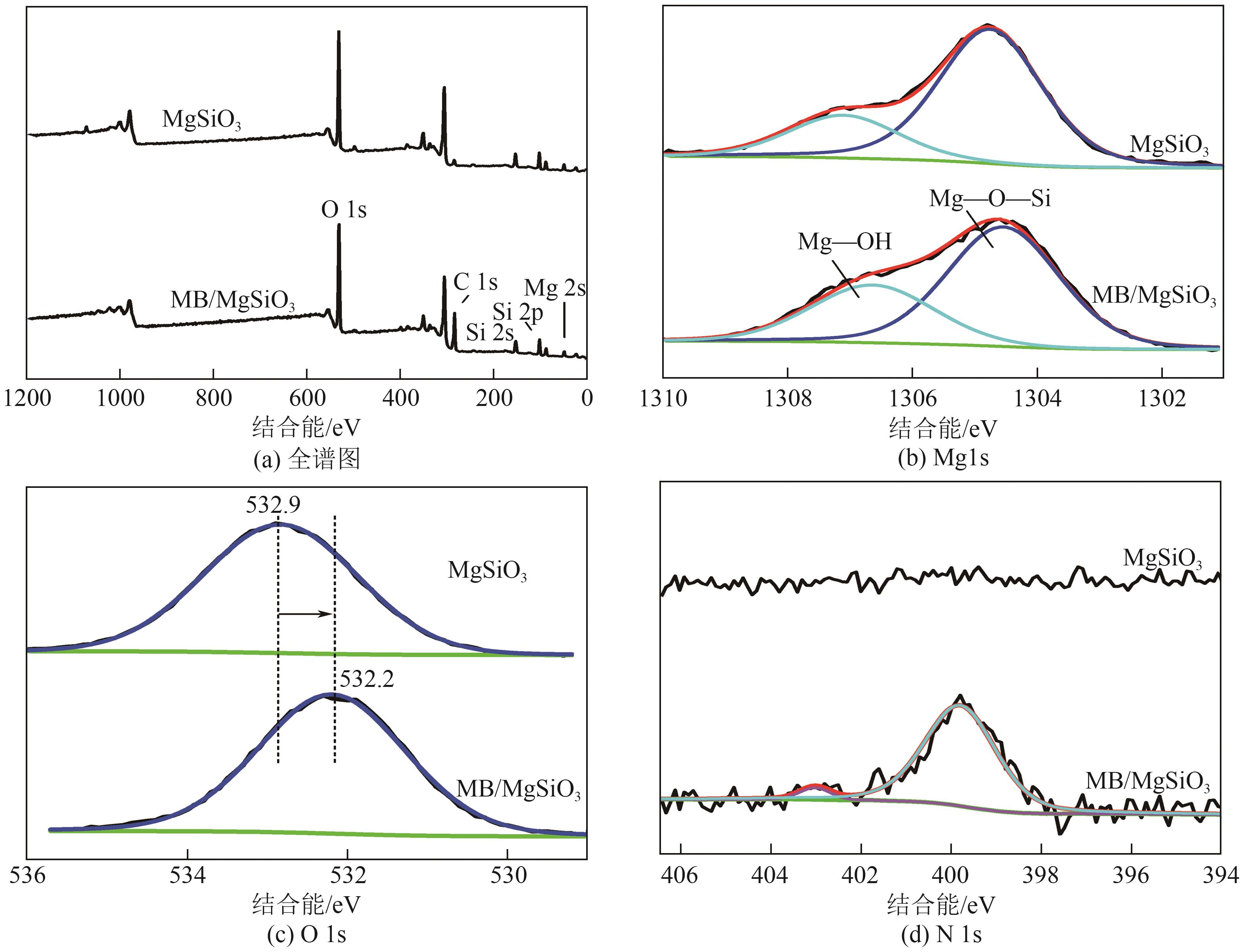
粉煤灰的资源化利用对社会和环境的可持续发展具有重要意义。本文以粉煤灰基硅渣为原料,采用NaOH碱熔法提取SiO2,在碱熔温度为300℃、碱与灰质量比为2∶1、反应时间为2h的条件下,SiO2的提取率达到72.26%;进而以提硅得到的Na2SiO3溶液和MgO为原料,通过水热反应制备硅酸镁纳米片,考察了Na2SiO3溶液浓度、Si与Mg摩尔比、反应温度及反应时间对硅酸镁纳米片的组成、晶相结构和孔道结构的影响规律。结果表明,在Na2SiO3浓度为0.50mol/L、Si与Mg摩尔比为1.25∶1、反应温度为220℃和反应时间为10h的条件下制备了结晶度较好、比表面积达240.6m2/g的介孔硅酸镁纳米片;其对亚甲基蓝(MB)具有较快的吸附速率,吸附量达285.0mg/g,对MB的吸附行为符合准二级动力学模型和Langmuir等温吸附模型。
中图分类号:
引用本文
刘倩, 李梦茹, 白守礼, 冯拥军, 唐平贵, 李殿卿. 粉煤灰基硅酸镁纳米片的制备及其亚甲基蓝吸附性能[J]. 化工进展, 2025, 44(11): 6716-6729.
LIU Qian, LI Mengru, BAI Shouli, FENG Yongjun, TANG Pinggui, LI Dianqing. Preparation and adsorption performance to methylene blue of fly ash based magnesium silicate nanosheets[J]. Chemical Industry and Engineering Progress, 2025, 44(11): 6716-6729.
| 样品 | 准一级吸附动力学模型 | 准二级吸附动力学模型 | ||||
|---|---|---|---|---|---|---|
| q1/mg·g-1 | k1/min-1 | R2 | q2/mg·g-1 | k2/ g·mg-1·min-1 | R2 | |
| MgSiO3(1∶1) | 46.64 | 3.75×10-2 | 0.3659 | 275.5 | 1.72×10-3 | 0.9992 |
| MgSiO3(1.25∶1) | 62.53 | 4.63×10-2 | 0.7430 | 289.9 | 1.80×10-3 | 0.9998 |
| MgSiO3(1.5∶1) | 44.45 | 2.75×10-2 | 0.6320 | 255.8 | 2.03×10-3 | 0.9997 |
表1 MgSiO3吸附MB的拟一级动力学和拟二级动力学参数
| 样品 | 准一级吸附动力学模型 | 准二级吸附动力学模型 | ||||
|---|---|---|---|---|---|---|
| q1/mg·g-1 | k1/min-1 | R2 | q2/mg·g-1 | k2/ g·mg-1·min-1 | R2 | |
| MgSiO3(1∶1) | 46.64 | 3.75×10-2 | 0.3659 | 275.5 | 1.72×10-3 | 0.9992 |
| MgSiO3(1.25∶1) | 62.53 | 4.63×10-2 | 0.7430 | 289.9 | 1.80×10-3 | 0.9998 |
| MgSiO3(1.5∶1) | 44.45 | 2.75×10-2 | 0.6320 | 255.8 | 2.03×10-3 | 0.9997 |
| 吸附剂 | 比表面积/m2·g-1 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|
| 碱活化MgSiO3 | 8.6 | 74.6 | [ |
| SiO2@MgSiO3 | 588 | 299 | [ |
| MgSiO3 | 582.3 | 234.19 | [ |
| MgSiO3 | 521 | 207 | [ |
| MgSiO3纳米管 | 539.4 | 175.13 | [ |
| MgSiO3纳米管 | 293 | 188 | [ |
| 棉/MgSiO3复合膜 | 523.1 | 194.4 | [ |
| MgSiO3 | 240.6 | 285.0 | 本文 |
表2 不同MgSiO3吸附剂对MB的吸附量对比
| 吸附剂 | 比表面积/m2·g-1 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|
| 碱活化MgSiO3 | 8.6 | 74.6 | [ |
| SiO2@MgSiO3 | 588 | 299 | [ |
| MgSiO3 | 582.3 | 234.19 | [ |
| MgSiO3 | 521 | 207 | [ |
| MgSiO3纳米管 | 539.4 | 175.13 | [ |
| MgSiO3纳米管 | 293 | 188 | [ |
| 棉/MgSiO3复合膜 | 523.1 | 194.4 | [ |
| MgSiO3 | 240.6 | 285.0 | 本文 |
| [1] | 陈岚, 权宇珩, 李志勇, 等. 超声波辅助粉煤灰去除水中亚甲基蓝染料的动力学分析[J]. 化工学报, 2019, 70(7): 2708-2716. |
| CHEN Lan, QUAN Yuheng, LI Zhiyong, et al. Kinetic analysis of removal of methylene blue using fly ash assisted by ultrasound from aqueous solution[J]. CIESC Journal, 2019, 70(7): 2708-2716. | |
| [2] | 王倩, 李神勇, 康帅, 等. 粉煤灰分质高效利用预处理技术的研究进展[J]. 化工学报, 2023, 74(3): 1010-1032. |
| WANG Qian, LI Shenyong, KANG Shuai, et al. Research progress of pretreatment technology for efficient utilization of coal ash[J]. CIESC Journal, 2023, 74(3): 1010-1032. | |
| [3] | 张世蕊, 范朕连, 宋慧平, 等. 粉煤灰负载光催化材料的研究进展[J]. 化工进展, 2024, 43(7): 4043-4058. |
| ZHANG Shirui, FAN Zhenlian, SONG Huiping, et al. Research progress of fly ash supported photocatalytic materials[J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4043-4058. | |
| [4] | 张国卿, 宋舒波, 王兴瑞, 等. 煤固废基分子筛的制备及其应用进展[J]. 化工进展, 2024, 43(5): 2311-2323. |
| ZHANG Guoqing, SONG Shubo, WANG Xingrui, et al. Recent advances in the synthesis and application of zeolites from coal-based solid wastes[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2311-2323. | |
| [5] | 马子然, 王宝冬, 路光杰, 等. 粉煤灰基SAPO-34分子筛脱硝催化剂的合成及其脱硝性能[J]. 化工进展, 2020, 39(10): 4051-4060. |
| MA Ziran, WANG Baodong, LU Guangjie, et al. Preparation and performance of SAPO-34 based SCR catalyst derived from fly ash[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4051-4060. | |
| [6] | YADAV Virendra Kumar, FULEKAR Madhusudan Hiraman. Advances in methods for recovery of ferrous, alumina, and silica nanoparticles from fly ash waste[J]. Ceramics, 2020, 3(3): 384-420. |
| [7] | LIU Huidong. Conversion of harmful fly ash residue to zeolites: Innovative processes focusing on maximum activation, extraction, and utilization of aluminosilicate[J]. ACS Omega, 2022, 7(23): 20347-20356. |
| [8] | JU Tongyao, JIANG Jianguo, MENG Yuan, et al. An investigation of the effect of ultrasonic waves on the efficiency of silicon extraction from coal fly ash[J]. Ultrasonics Sonochemistry, 2020, 60: 104765. |
| [9] | TAN Miaomiao, LI Xiangyu, FENG Yu, et al. Fly ash-derived mesoporous silica with large pore volume for augmented CO2 capture[J]. Fuel, 2023, 351: 128874. |
| [10] | YAN Kezhou, ZHANG Ting, LIU Dandan, et al. Strengthening desilication of coal fly ash by alkaline leaching with the addition of ethylene diamine tetraacetic acid[J]. Minerals Engineering, 2023, 201: 108219. |
| [11] | YADAV Virendra Kumar, AMARI Abdelfattah, WANALE Shivraj Gangadhar, et al. Synthesis of floral-shaped nanosilica from coal fly ash and its application for the remediation of heavy metals from fly ash aqueous solutions[J]. Sustainability, 2023, 15(3): 2612. |
| [12] | JU Tongyao, MENG Yuan, HAN Siyu, et al. A green and multi-win strategy for coal fly ash disposal by CO2 fixation and mesoporous silica synthesis[J]. Science of the Total Environment, 2023, 883: 163822. |
| [13] | BAO Jing, FENG Yongjun, PAN Yong, et al. Modified approaches to prepare nano-magnesium silicates with hierarchical pore structure and their performance towards adsorption of Cd2+ [J]. Environmental Science and Pollution Research International, 2023, 30(38): 89784-89793. |
| [14] | GHODS Bahare, REZAEI Mehran, MESHKANI Fereshteh. Synthesis of nanostructured magnesium silicate with high surface area and mesoporous structure[J]. Ceramics International, 2016, 42(6): 6883-6890. |
| [15] | ZHAO Rui, LI Yanzi, SUN Bolun, et al. Highly flexible magnesium silicate nanofibrous membranes for effective removal of methylene blue from aqueous solution[J]. Chemical Engineering Journal, 2019, 359: 1603-1616. |
| [16] | WANG Bin, GAO Kai, WANG Yujie, et al. Synergistic dispersion, adsorption, and anti-wear effects of magnesium silicate hydroxide nanomaterials and carboxylic acid[J]. Applied Surface Science, 2024, 665: 160373. |
| [17] | XING Lang, LI Xinran, CAO Pengxu, et al. Stepwise extraction and utilization of silica and alumina from coal fly ash by mild hydrothermal process[J]. Process Safety and Environmental Protection, 2024, 182: 918-929. |
| [18] | WANG Xuekai, WANG Jinshu, TENG Weili, et al. Fabrication of highly efficient magnesium silicate and its adsorption behavior towards Cr(Ⅵ)[J]. Microporous and Mesoporous Materials, 2021, 323: 111196. |
| [19] | HE Zhendong, REN Bozhi, HURSTHOUSE Andrew, et al. Efficient removal of Cd(Ⅱ) using SiO2-Mg(OH)2 nanocomposites derived from sepiolite[J]. International Journal of Environmental Research and Public Health, 2020, 17(7): 2223. |
| [20] | SUN Zhiwei, LIU Yanhua, SRINIVASAKANNAN Chandrasekar. One-pot fabrication of rod-like magnesium silicate and its adsorption for Cd2+ [J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104380. |
| [21] | PAYAN François, ISSA Albert, KRAFFT Jean-Marc, et al. Controlling magnesium silicates coprecipitation conditions: A tool to tune their surface acid-base reactivity[J]. Catalysts, 2023, 13(11): 1393. |
| [22] | SUN Zhiwei, LIU Yanhua, HONG Wei. Facile synthesis of porous hydrated magnesium silicate adsorbent from ordinary silica gel[J]. Materials Letters, 2020, 272: 127886. |
| [23] | WANG Weixue, CHEN Zhe, ZHOU Haijiang, et al. Two-dimensional lamellar magnesium silicate with large spacing as an excellent adsorbent for uranium immobilization[J]. Environmental Science: Nano, 2018, 5(10): 2406-2414. |
| [24] | Kardelen KAYA-ÖZKIPER, UZUN Alper, Sezen SOYER-UZUN. A novel alkali activated magnesium silicate as an effective and mechanically strong adsorbent for methylene blue removal[J]. Journal of Hazardous Materials, 2022, 424: 127256. |
| [25] | SUN Zhiwei, HUANG Di, DUAN Xinhui, et al. Functionalized nanoflower-like hydroxyl magnesium silicate for effective adsorption of aflatoxin B1[J]. Journal of Hazardous Materials, 2020, 387: 121792. |
| [26] | LI Qiang, ZHANG Jingjing, LU Qingshan, et al. Hydrothermal synthesis and characterization of ordered mesoporous magnesium silicate-silica for dyes adsorption[J]. Materials Letters, 2016, 170: 167-170. |
| [27] | TIAN Yaxi, CUI Guijia, LIU Yan, et al. Self-assembly synthesis of hollow double silica@mesoporous magnesium silicate magnetic hierarchical nanotubes with excellent performance for fast removal of cationic dyes[J]. Applied Surface Science, 2016, 387: 631-641. |
| [28] | ZHAO Wenting, FENG Ke, ZHANG Huan, et al. Sustainable green conversion of coal gangue waste into cost-effective porous multimetallic silicate adsorbent enables superefficient removal of Cd(Ⅱ) and dye[J]. Chemosphere, 2023, 324: 138287. |
| [29] | WANG Yongqiang, WANG Guozhong, WANG Hongqiang, et al. Chemical-template synthesis of micro/nanoscale magnesium silicate hollow spheres for waste-water treatment[J]. Chemistry—A European Journal, 2010, 16(11): 3497-3503. |
| [30] | YANG Jinbo, ZHANG Min, ZHANG Yanwei, et al. Facile synthesis of magnetic magnesium silicate hollow nanotubes with high capacity for removal of methylene blue[J]. Journal of Alloys and Compounds, 2017, 721: 772-778. |
| [31] | ZHENG Jun, CHENG Chao, YAN Ruiwen, et al. Synthesis of yolk-shell magnetic magnesium silicate with tunable yolk morphology for removal of methylene blue in water[J]. Journal of Alloys and Compounds, 2014, 596: 5-9. |
| [32] | BIAN Shaowei, HUANG Yali, YUE Yuan, et al. Porous cotton/magnesium silicate composite films as high-performance adsorbents for organic dye removal[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 625: 126751. |
| [1] | 王瑞琪, 刘浩伟, 孙彦丽, 李荣花, 王政, 吴玉花, 吴建波, 张慧, 白红存. 面向高效储氢MOFs的设计构筑与性能调控研究现状分析及展望[J]. 化工进展, 2025, 44(S1): 323-339. |
| [2] | 刘颖, 包成, 张欣欣. 用于氢气提纯的改性载铜活性炭[J]. 化工进展, 2025, 44(S1): 413-421. |
| [3] | 王睿, 王海澜, 戴若彬, 王志伟. 工业废水深度处理反渗透膜硅污染研究进展:机理、影响因素与控制策略[J]. 化工进展, 2025, 44(9): 5315-5326. |
| [4] | 孙梦圆, 陆诗建, 刘玲, 薛艳阳, 张云蓉, 董琦, 康国俊. 金属有机框架及衍生物在碳捕集领域的研究进展[J]. 化工进展, 2025, 44(9): 5339-5350. |
| [5] | 卢永琦, 肖嘉宁, 迭庆杞, 徐思琪, 黄瑞潇, 孔祥蕊, 杨玉飞. 堆存粉煤灰长期淋溶过程污染物释放特征与环境风险评估[J]. 化工进展, 2025, 44(9): 5479-5490. |
| [6] | 杨证禄, 杨立峰, 路晓飞, 锁显, 张安运, 崔希利, 邢华斌. 机器学习加速多孔吸附剂筛选发现的研究进展[J]. 化工进展, 2025, 44(8): 4288-4301. |
| [7] | 仇玉静, 刘畅, 罗国华, 董森, 李建华. 脱除苯中二硫化碳吸附剂的制备及其吸附性能[J]. 化工进展, 2025, 44(4): 2374-2382. |
| [8] | 孙雅娟, 段思宇, 张宏, 周冬冬, 路广军, 马志斌. 化学外加剂对固废基胶凝材料性能及水化行为的影响[J]. 化工进展, 2025, 44(3): 1739-1748. |
| [9] | 左骥, 罗莉, 谢永锴, 陈文尧, 钱刚, 周兴贵, 段学志. 甲醇无氧脱氢制甲醛Cu催化剂的粒径效应[J]. 化工进展, 2025, 44(3): 1347-1354. |
| [10] | 倪鹏, 王先泓, 黄钰涵, 马晓彤, 马子轸, 谈琰, 张华伟, 刘亭. 活性炭类和磁性金属类吸附剂喷射脱汞技术应用对比及最新进展[J]. 化工进展, 2025, 44(1): 513-524. |
| [11] | 张炜, 黄赳, 朱晓芳, 李鹏. 凹凸棒石基钴钨水滑石吸附铅的性能及机理[J]. 化工进展, 2025, 44(1): 596-606. |
| [12] | 闻静, 张红婴, 张屹东, 许润泽. 月桂酸-石蜡二元共晶和纳米SiO2气凝胶新型建筑储能材料的研制和性能表征[J]. 化工进展, 2025, 44(1): 388-397. |
| [13] | 刘丽, 冯博, 文洋, 古启雄. 硅基介孔材料的合成、功能化及对金属的吸附研究进展[J]. 化工进展, 2024, 43(9): 5063-5078. |
| [14] | 阳梦萍, 孙军军, 张晨曦, 薛昊龙, 肖长发. 聚丙烯/聚硅氧烷-二氧化硅中空纤维膜的制备与性能分析[J]. 化工进展, 2024, 43(9): 5106-5112. |
| [15] | 杨新衡, 纪志永, 郭志远, 刘萁, 张盼盼, 汪婧, 刘杰, 毕京涛, 赵颖颖, 袁俊生. 锂铝层状双金属氢氧化物的制备及其锂脱嵌过程[J]. 化工进展, 2024, 43(9): 5262-5274. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||