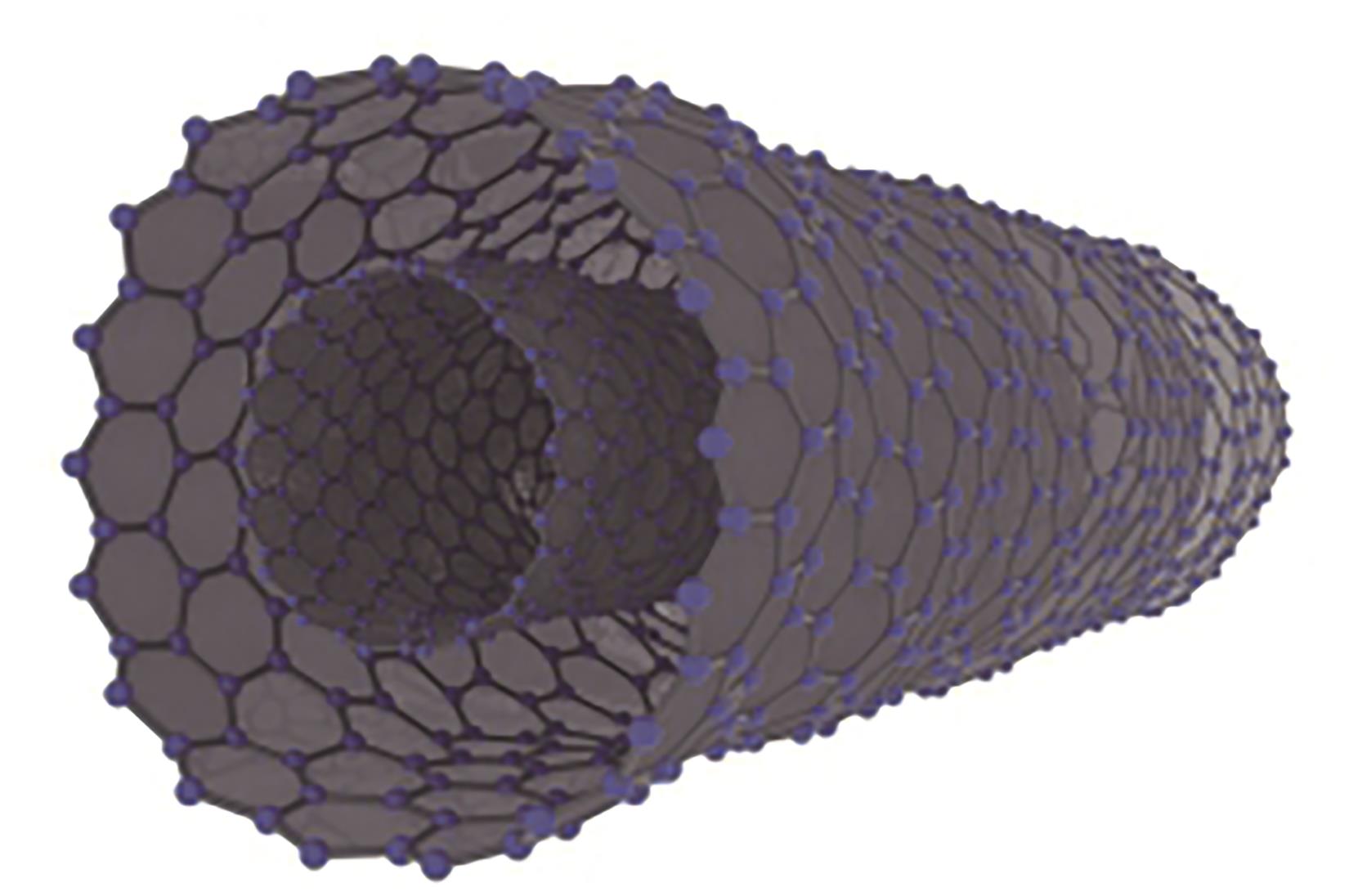
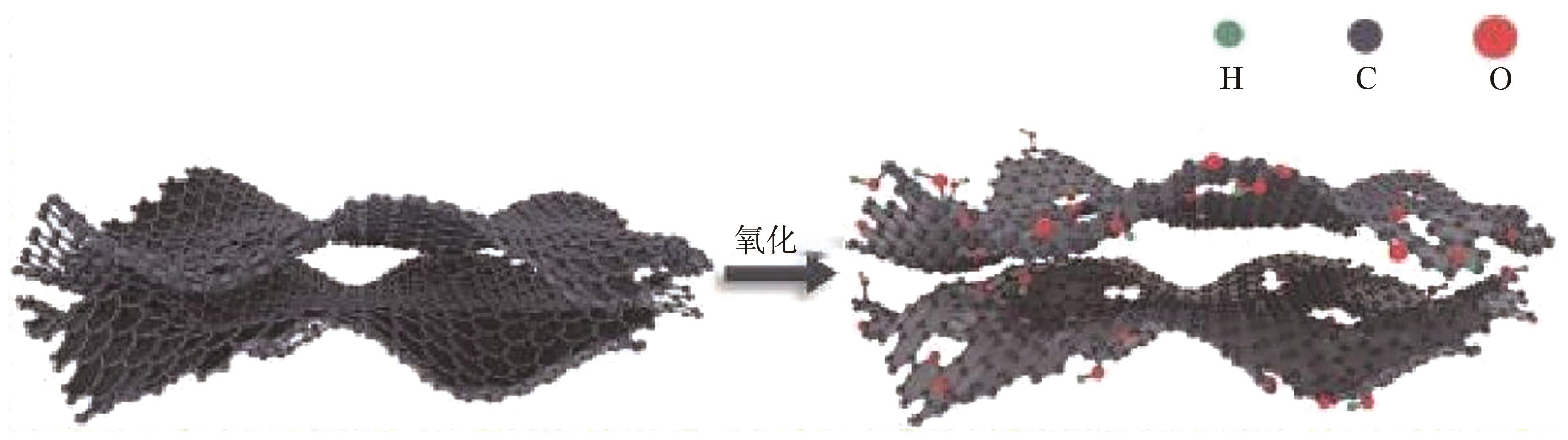
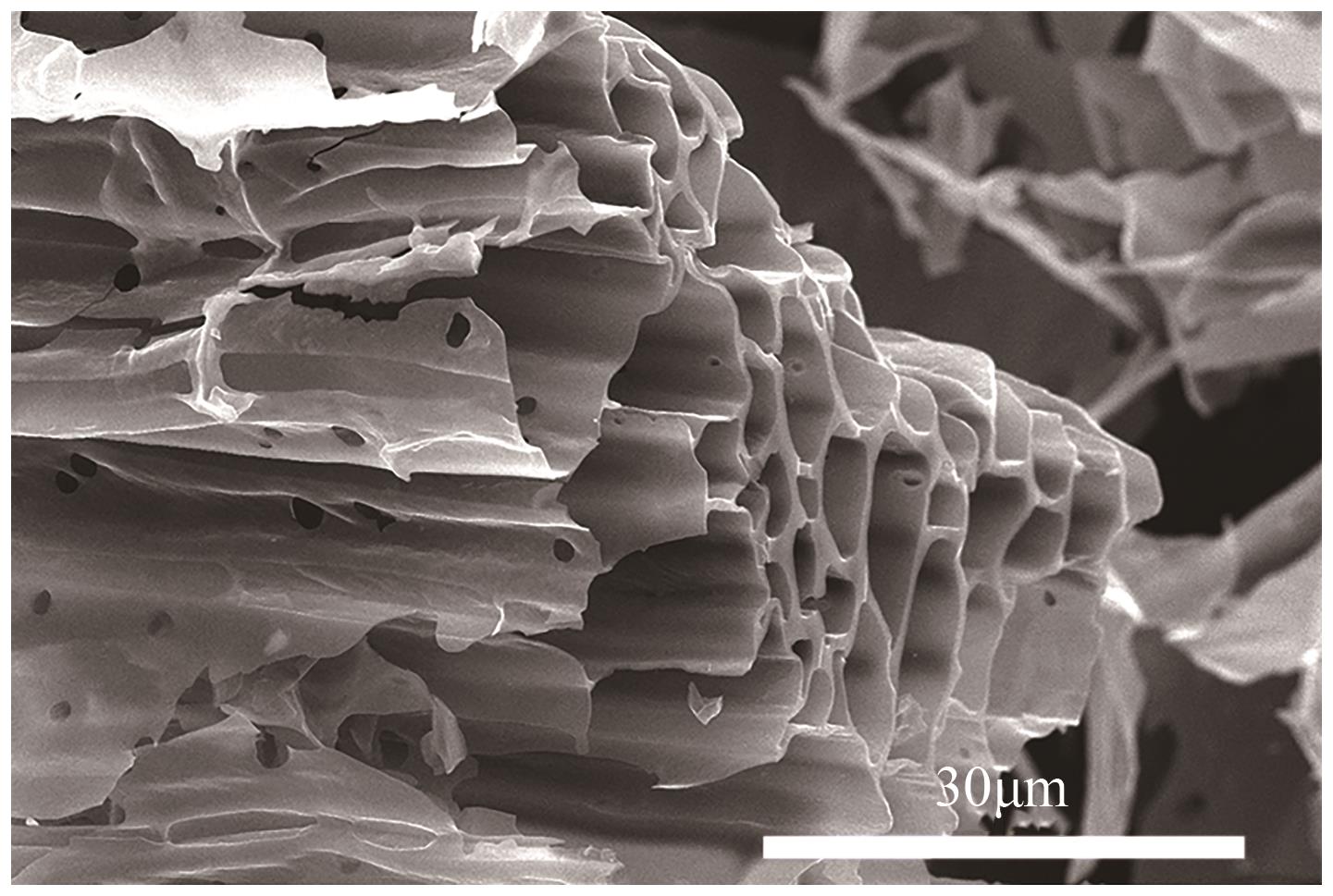
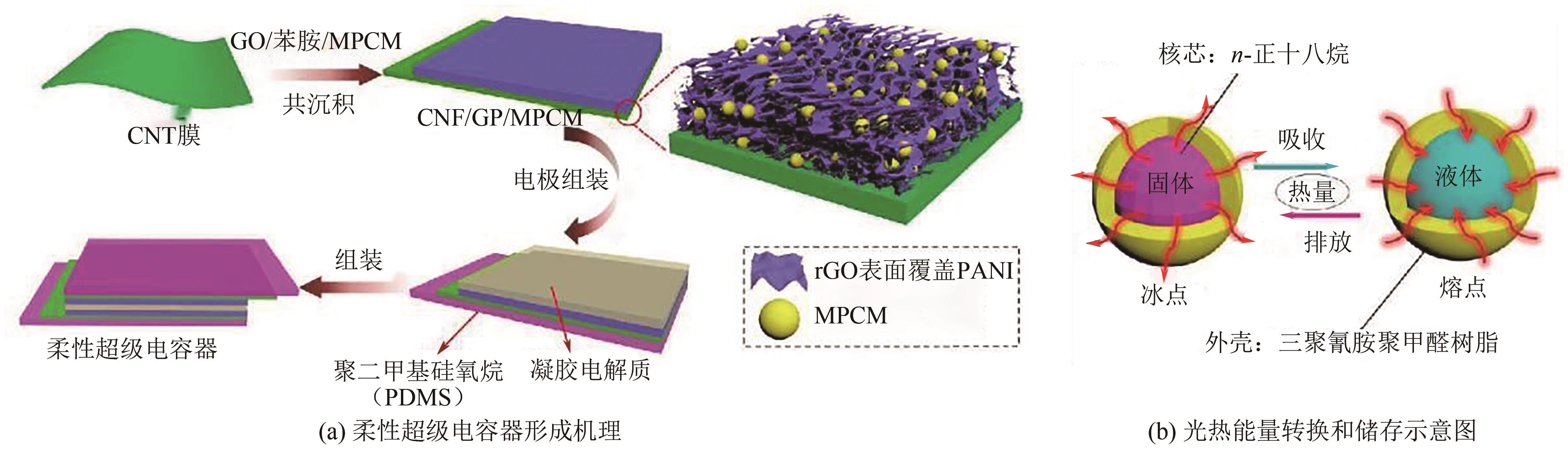
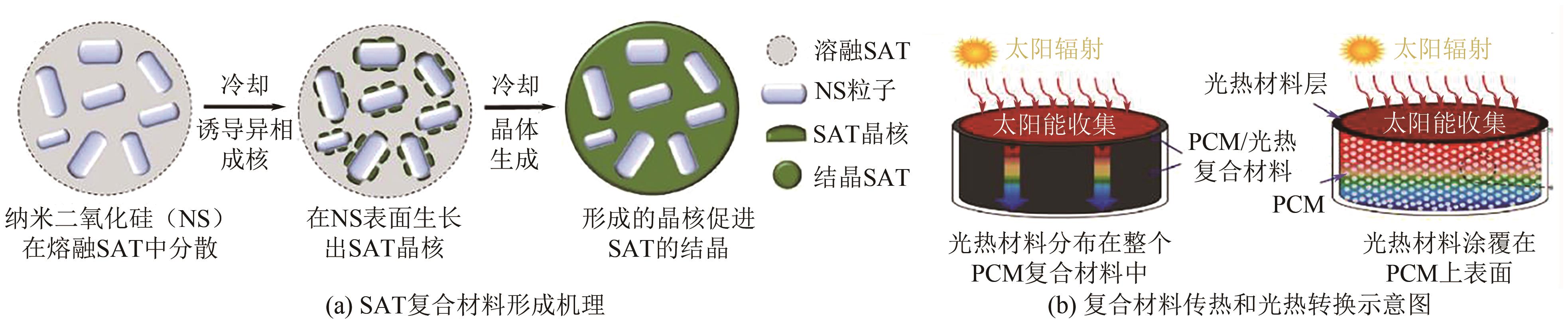
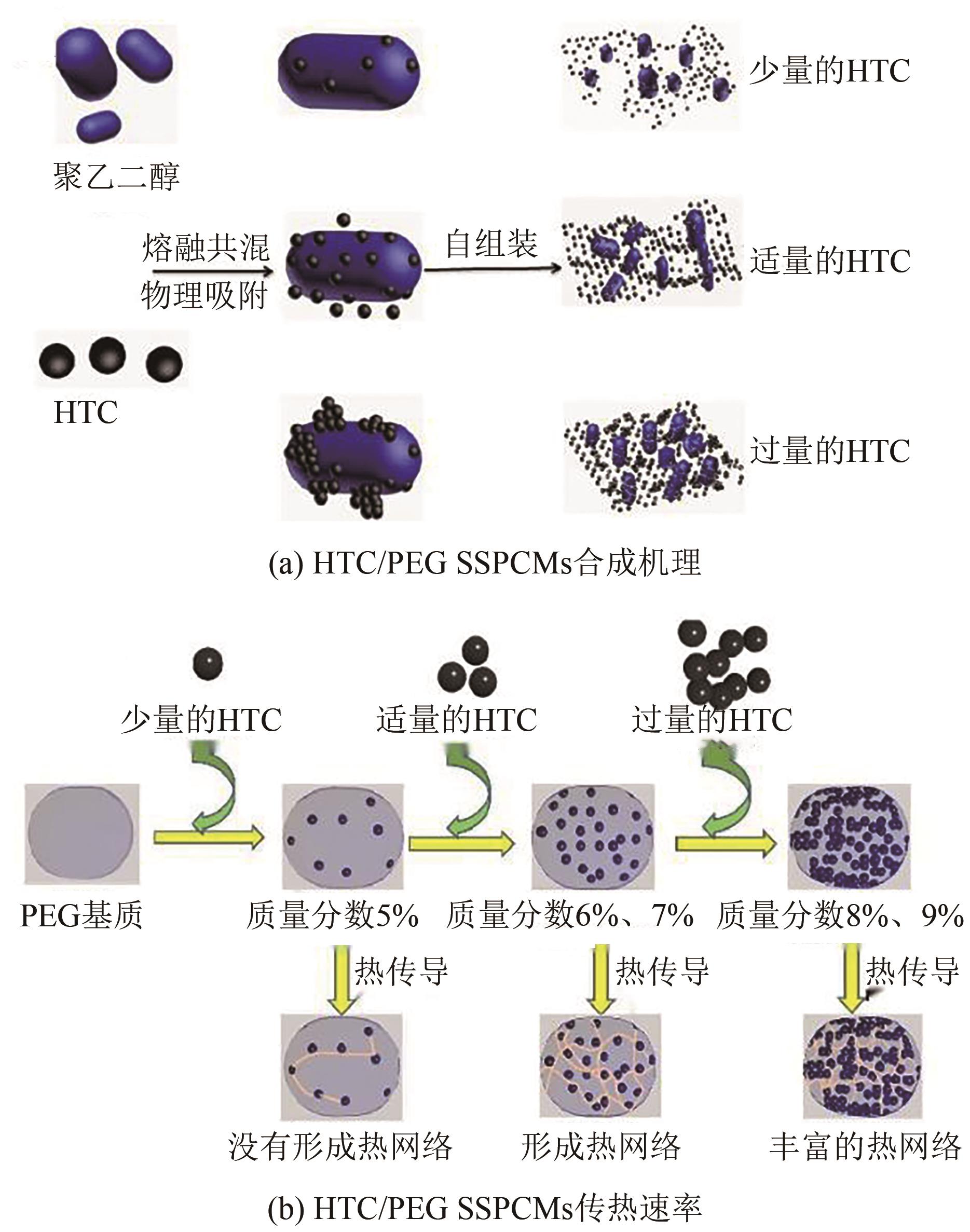
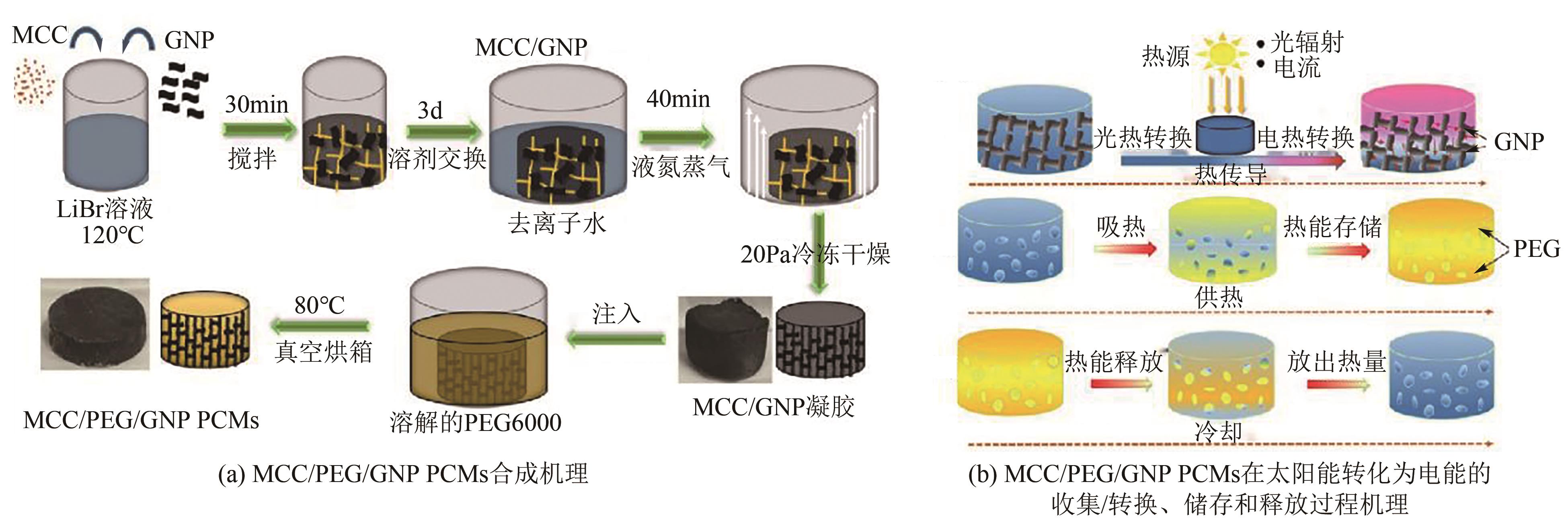
化工进展 ›› 2025, Vol. 44 ›› Issue (4): 2102-2118.DOI: 10.16085/j.issn.1000-6613.2024-0558
碳基定型复合相变材料的研究进展
安明泽1,2( ), 张兵兵1,2,3(
), 张兵兵1,2,3( ), 王盛1,2, 陈蔚洁1,2,3, 刘世旺4, 薛斌1,2, 徐国敏1,2, 秦舒浩1,2,4
), 王盛1,2, 陈蔚洁1,2,3, 刘世旺4, 薛斌1,2, 徐国敏1,2, 秦舒浩1,2,4
- 1.贵州省材料产业技术研究院,贵州 贵阳 550022
2.国家复合改性聚合物材料工程技术研究中心,贵州 贵阳 550014
3.贵州大学资源与环境工程学院,贵州 贵阳 550025
4.贵州大学材料与冶金学院,贵州 贵阳 550025
-
收稿日期:2024-04-03修回日期:2024-07-13出版日期:2025-04-25发布日期:2025-05-07 -
通讯作者:张兵兵 -
作者简介:安明泽(1992—),男,硕士,助理研究员,研究方向为碳基复合相变储能材料。E-mail:amz921810@163.com。 -
基金资助:贵州省基础研究(自然科学)项目(QKHJC-ZK[2023]YB158);贵州省科技计划(黔科合支撑[2022]一般219,黔科合服企[2021]9);贵州省黔东南州科技支撑计划(黔东南科合J字[2022]59)
Research progress on carbon-based stereotyped composite phase change materials
AN Mingze1,2( ), ZHANG Bingbing1,2,3(
), ZHANG Bingbing1,2,3( ), WANG Sheng1,2, CHEN Weijie1,2,3, LIU Shiwang4, XUE Bin1,2, XU Guomin1,2, QIN Shuhao1,2,4
), WANG Sheng1,2, CHEN Weijie1,2,3, LIU Shiwang4, XUE Bin1,2, XU Guomin1,2, QIN Shuhao1,2,4
- 1.Guizhou Material Industrial Technology Institute, Guiyang 550022, Guizhou, China
2.National Engineering Research Center for Compounding and Modification of Polymer Materials, Guiyang 550014, Guizhou, China
3.College of Resources and Environmental Engineering Department, Guizhou University, Guiyang 550025, Guizhou, China
4.College of Materials and Metallurgy, Guizhou University, Guiyang 550025, Guizhou, China
-
Received:2024-04-03Revised:2024-07-13Online:2025-04-25Published:2025-05-07 -
Contact:ZHANG Bingbing
摘要:
相变材料(PCMs)是指随温度变化而改变物质的物理性质并能提供潜热的材料,在太阳能热储存、电子设备、动力电池热管理、建筑温度控制等领域具有广泛的应用前景,在我国已经被列为国家级研发利用序列。然而,相变材料容易出现渗漏、热导率低、光吸收弱等问题,阻碍了其更广泛的应用和发展。为了克服这些固有问题,提高热物理性能,将碳基材料作为封装载体构建形状稳定的定型复合相变材料可以有效防止固-液相变渗漏,提高潜热性能。本文综述了碳纳米管、石墨烯、活性炭、生物炭等多孔碳支撑材料作为相变材料封装载体的主要研究进展,介绍了不同多孔碳材料作为封装载体对相变复合材料的热导率、潜热、相变温度、过冷度、形状稳定性和热循环稳定性等物理性能的影响,总结了不同多孔碳基复合相变材料在潜热性能方面的研究动态和各行业中的应用。最后,对多孔碳基材料在未来发展中的研究和面临的挑战进行了总结和展望。
中图分类号:
引用本文
安明泽, 张兵兵, 王盛, 陈蔚洁, 刘世旺, 薛斌, 徐国敏, 秦舒浩. 碳基定型复合相变材料的研究进展[J]. 化工进展, 2025, 44(4): 2102-2118.
AN Mingze, ZHANG Bingbing, WANG Sheng, CHEN Weijie, LIU Shiwang, XUE Bin, XU Guomin, QIN Shuhao. Research progress on carbon-based stereotyped composite phase change materials[J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2102-2118.
| CNTs载体 | 相变材料 | 制备方法 | 相变温度Tm/℃ | 过冷度 ΔT/℃ | 相变潜热 ΔHm/J·g-1 | 热导率K/W·(m·K)-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| S-MWCNTs:φ=815nm,L=0.52µm | 石蜡 | 溶液吸附 | 57.7 | 7.1 | 178.2 | 0.324 | [ |
| L-MWCNTs:φ=3050nm,L=515µm | 58.2 | 6.9 | 177.4 | 0.309 | |||
| SWCNTs:φ=1.5nm,L>5µm | 56.8 | 6.3 | 146.1 | 0.550 | [ | ||
| SWCNTs:φ=1~2nm,L=0.5~2μm | 丙烯酸十六酯 | 表面接枝 | 36.7 | 13.0 | 52.0 | 0.468 | [ |
| MWCNTs:φ=8nm,L=0.5~2μm | 38.0 | 11.2 | 40.0 | 0.877 | |||
| 氨基-SWCNTs:φ<2nm,L=1~3μm | PEG6000 | 溶液吸附 | 53.9 | 27.7 | 125.1 | 0.421 | [ |
MWCNTs:φ=10~30nm,L=10~30μm, SBET>60m2/g | 蜂蜡 | 真空注入 | 60.6 | 3.6 | 126.0 | 0.410 | [ |
| 三维CNTs海绵 | 癸二酸 | 溶液吸附 | 121.1 | 0.4 | 131.8 | 7.270 | [ |
| PEG | 水热还原 | 43.5 | — | 120.0 | 0.310 | [ | |
| CNTs/MOF(金属有机骨架):φ=30~50nm,L=10~20μm | PEG2000 | 真空注入 | 52.4 | 23 | 96.2 | 0.464 | [ |
| SWCNTs/聚苯乙烯泡沫(PS):φ=2nm,L=30μm | 石蜡 | 38.0~47.0 | 1.0~2.0 | 124.9 | 0.400 | [ | |
| MWCNTs:φ内=30~50nm,φ外=50~60nm,L=5~30μm,SBET=120m2/g | 硬脂酸 | 59.3 | 11.4 | 91.94 | 7.159 | [ | |
| CNTs-Cu/Cu2O:φ=20~30nm | 石蜡 | 水热还原结合原位沉积 | 61.2 | — | 81.3141 | 0.632 | [ |
表1 碳纳米管定型复合相变材料的制备方法与热性能
| CNTs载体 | 相变材料 | 制备方法 | 相变温度Tm/℃ | 过冷度 ΔT/℃ | 相变潜热 ΔHm/J·g-1 | 热导率K/W·(m·K)-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| S-MWCNTs:φ=815nm,L=0.52µm | 石蜡 | 溶液吸附 | 57.7 | 7.1 | 178.2 | 0.324 | [ |
| L-MWCNTs:φ=3050nm,L=515µm | 58.2 | 6.9 | 177.4 | 0.309 | |||
| SWCNTs:φ=1.5nm,L>5µm | 56.8 | 6.3 | 146.1 | 0.550 | [ | ||
| SWCNTs:φ=1~2nm,L=0.5~2μm | 丙烯酸十六酯 | 表面接枝 | 36.7 | 13.0 | 52.0 | 0.468 | [ |
| MWCNTs:φ=8nm,L=0.5~2μm | 38.0 | 11.2 | 40.0 | 0.877 | |||
| 氨基-SWCNTs:φ<2nm,L=1~3μm | PEG6000 | 溶液吸附 | 53.9 | 27.7 | 125.1 | 0.421 | [ |
MWCNTs:φ=10~30nm,L=10~30μm, SBET>60m2/g | 蜂蜡 | 真空注入 | 60.6 | 3.6 | 126.0 | 0.410 | [ |
| 三维CNTs海绵 | 癸二酸 | 溶液吸附 | 121.1 | 0.4 | 131.8 | 7.270 | [ |
| PEG | 水热还原 | 43.5 | — | 120.0 | 0.310 | [ | |
| CNTs/MOF(金属有机骨架):φ=30~50nm,L=10~20μm | PEG2000 | 真空注入 | 52.4 | 23 | 96.2 | 0.464 | [ |
| SWCNTs/聚苯乙烯泡沫(PS):φ=2nm,L=30μm | 石蜡 | 38.0~47.0 | 1.0~2.0 | 124.9 | 0.400 | [ | |
| MWCNTs:φ内=30~50nm,φ外=50~60nm,L=5~30μm,SBET=120m2/g | 硬脂酸 | 59.3 | 11.4 | 91.94 | 7.159 | [ | |
| CNTs-Cu/Cu2O:φ=20~30nm | 石蜡 | 水热还原结合原位沉积 | 61.2 | — | 81.3141 | 0.632 | [ |
| 石墨烯载体 | 相变材料 | 制备方法 | 相变温度Tm/℃ | 过冷度 ΔT/℃ | 相变潜热 ΔHm/J·g-1 | 热导率K/W·(m·K)-1 | 文献 |
|---|---|---|---|---|---|---|---|
| GNPs:SBET=300/500/750m2/g | 棕榈酸 | 真空注入 | 61.16 | 1.35 | 188.98 | 2.11 | [ |
| 1.01 | |||||||
| 0.96 | |||||||
| GNPs:H<6μm,SBET=300m2/g | 蜂蜡 | 溶液吸附 | 62.42 | 10.37 | 186.74 | 2.8 | [ |
| C18-rGO | 石蜡 | 55.7 | 2.8 | 167.4 | — | [ | |
| E2C16-g-GO | 二甘醇十六烷基醚 | 化学接枝 | 43.3 | 23.9 | 70 | — | [ |
| PDA-rGO | 环氧化甲氧基聚乙二醇 | 60.3 | 20.7 | 168.2 | — | [ | |
| GA | 月桂酸 | 真空注入 | 43.27 | 9 | 179.1 | 0.15 | [ |
| 43.31 | 13.9 | 205.2 | 1.207 | [ | |||
| CNTs与GNPs共混:HGNPs=4~7nm,LCNTs=10μm,φCNTs=11nm | 月桂酸 | 溶液吸附 | 40.8 | 9 | 198 | 0.87 | [ |
| CNTs-GA | 石蜡 | 真空注入 | 46.56 | 2.74 | 245.5 | 0.836 | [ |
| GO-MEPCM | 41.08 | 8.84 | 202.8 | — | [ | ||
| GO-MEPCM | 正十二醇 | 26 | — | 170 | 0.279 | [ |
表2 石墨烯基复合相变材料的制备方法与热性能
| 石墨烯载体 | 相变材料 | 制备方法 | 相变温度Tm/℃ | 过冷度 ΔT/℃ | 相变潜热 ΔHm/J·g-1 | 热导率K/W·(m·K)-1 | 文献 |
|---|---|---|---|---|---|---|---|
| GNPs:SBET=300/500/750m2/g | 棕榈酸 | 真空注入 | 61.16 | 1.35 | 188.98 | 2.11 | [ |
| 1.01 | |||||||
| 0.96 | |||||||
| GNPs:H<6μm,SBET=300m2/g | 蜂蜡 | 溶液吸附 | 62.42 | 10.37 | 186.74 | 2.8 | [ |
| C18-rGO | 石蜡 | 55.7 | 2.8 | 167.4 | — | [ | |
| E2C16-g-GO | 二甘醇十六烷基醚 | 化学接枝 | 43.3 | 23.9 | 70 | — | [ |
| PDA-rGO | 环氧化甲氧基聚乙二醇 | 60.3 | 20.7 | 168.2 | — | [ | |
| GA | 月桂酸 | 真空注入 | 43.27 | 9 | 179.1 | 0.15 | [ |
| 43.31 | 13.9 | 205.2 | 1.207 | [ | |||
| CNTs与GNPs共混:HGNPs=4~7nm,LCNTs=10μm,φCNTs=11nm | 月桂酸 | 溶液吸附 | 40.8 | 9 | 198 | 0.87 | [ |
| CNTs-GA | 石蜡 | 真空注入 | 46.56 | 2.74 | 245.5 | 0.836 | [ |
| GO-MEPCM | 41.08 | 8.84 | 202.8 | — | [ | ||
| GO-MEPCM | 正十二醇 | 26 | — | 170 | 0.279 | [ |
| 活性炭基体 | 相变材料 | 制备方法 | 相变温度Tm/℃ | 过冷度 ΔT/℃ | 相变潜热 ΔHm/J·g-1 | 热导率K/W·(m·K)-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 椰子壳AC:SBET=1197.67m2/g,R=26.4Å | 聚乙二醇 | 浸渍吸附法 | 62 | 20.5 | 90.2 | — | [ |
| 大麻纤维AC | 油酸-癸酸 | 7.53 | 2.57 | 52.7 | 0.312 | [ | |
| 月桂酸-肉豆蔻酸 | 38.16 | 5.62 | 61.67 | — | [ | ||
| 木质AC:φ=0.075mm | 十六醇-肉豆蔻酸 | 42.38 | 4.06 | 76.24 | — | [ | |
| 泥炭土AC:SBET=893m2/g,R=22Å | 正十八烷 | 30.9 | 6.8 | 95.4 | 0.165 | [ | |
| 棕榈仁壳AC:SBET=1169m2/g | — | — | 57.56 | — | [ | ||
| 棕榈仁壳AC:SBET=183~1169m2/g | 28.8 | — | 87.42 | — | [ | ||
| 木质AC:SBET=1468m2/g,R=5.8Å | — | — | 101.8 | 0.168 | [ | ||
| 棕榈仁壳AC | 石蜡 | 29.2 | 2.4 | 57.3 | 1.17 | [ | |
| 木质AC/膨胀石墨/剥离石墨 | 正十八烷 | 30.65 | 8.09 | 72.45 | — | [ | |
| 木质AC:φ>0.075mm | 月桂醇-辛酸 | 真空注入 | 0.21 | 2.44 | 28.08 | — | [ |
表3 活性炭基定型复合相变材料的制备方法与热性能
| 活性炭基体 | 相变材料 | 制备方法 | 相变温度Tm/℃ | 过冷度 ΔT/℃ | 相变潜热 ΔHm/J·g-1 | 热导率K/W·(m·K)-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 椰子壳AC:SBET=1197.67m2/g,R=26.4Å | 聚乙二醇 | 浸渍吸附法 | 62 | 20.5 | 90.2 | — | [ |
| 大麻纤维AC | 油酸-癸酸 | 7.53 | 2.57 | 52.7 | 0.312 | [ | |
| 月桂酸-肉豆蔻酸 | 38.16 | 5.62 | 61.67 | — | [ | ||
| 木质AC:φ=0.075mm | 十六醇-肉豆蔻酸 | 42.38 | 4.06 | 76.24 | — | [ | |
| 泥炭土AC:SBET=893m2/g,R=22Å | 正十八烷 | 30.9 | 6.8 | 95.4 | 0.165 | [ | |
| 棕榈仁壳AC:SBET=1169m2/g | — | — | 57.56 | — | [ | ||
| 棕榈仁壳AC:SBET=183~1169m2/g | 28.8 | — | 87.42 | — | [ | ||
| 木质AC:SBET=1468m2/g,R=5.8Å | — | — | 101.8 | 0.168 | [ | ||
| 棕榈仁壳AC | 石蜡 | 29.2 | 2.4 | 57.3 | 1.17 | [ | |
| 木质AC/膨胀石墨/剥离石墨 | 正十八烷 | 30.65 | 8.09 | 72.45 | — | [ | |
| 木质AC:φ>0.075mm | 月桂醇-辛酸 | 真空注入 | 0.21 | 2.44 | 28.08 | — | [ |
| 生物炭基体 | PCMs | 导热增强剂 | 制备方法 | 相变温度Tm/℃ | 过冷度 ΔT/℃ | 相变潜热 ΔHm/J·g-1 | 热导率K/W·(m·K)-1 | 参考文献 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 生物质 | SBET/m2·g-1 | R/nm | ||||||||
| 松果 | 289.69 | 3.078 | 棕榈酸 | — | 真空注入 | 59.25 | 0.12 | 84.74 | 0.393 | [ |
| 竹子 | — | 十六醇-硬脂酸 | — | 浸渍吸附 | 45.29 | — | 68.85 | — | [ | |
| 云杉木 | 454 | 3.1~6.4 | 十二烷、十四烷、十八烷 | — | 真空注入 | 31.1 | — | 91.5 | — | [ |
| 冻干马铃薯 | 42.6 | 204.7 | 聚乙二醇4000 | — | 浸渍吸附 | 51.67 | 17.76 | 91.8 | — | [ |
| 杏仁壳 | 291.21 | 2.33 | 聚乙二醇 | — | 真空注入 | 62.76 | 30.63 | 82.73 | 0.402 | [ |
| 针叶木 | 7.3 | 36.6 | 正二十烷 | — | 37 | 7.1 | 53.4 | — | [ | |
| 小麦秸秆 | 11.9 | 34.1 | — | 浸渍吸附 | 37 | 8.8 | 75 | — | ||
| 冻干马铃薯 | — | — | PEG | — | 浸渍吸附 | 56.5 | 18.6 | 158.8 | 4.489 | [ |
| 冻干白萝卜 | — | — | — | 真空注入 | 56.5 | 18.6 | 159.7 | 1.746 | ||
| 蔗糖@MgO | 869~1101 | 16~30 | 石蜡 | — | 浸渍吸附 | — | — | 182.0 | — | [ |
| 芒草、油菜和污泥 | 7.4~77.6 | — | 正十二醇和 正十二烷 | — | 超声浸渍吸附 | 8.2~24.7 | — | 73.7 90.5 | — | [ |
| 梧桐木 | — | 10~20 | 月桂酸 | — | 41 | 2.1 | 177.9 | — | [ | |
| 枣核 | 412.1 | 3.816 | PEG4000 | — | 真空注入 | 52.5 | — | 112.3 | 0.54 | [ |
| 枣核 | 1132.7 | 3.398 | 铜微球 | 52 | — | 108.2 | 0.63 | |||
| 水葫芦 | 14.001 | 11.92 | 石蜡 | 铝粉 | 浸渍吸附 | 57.67 | — | 179.4 | 0.349 | [ |
| 竹子 | 485.6 | 正十二烷 | MWCNTs | 真空浸渍吸附 | -7.7 | — | 127.4 | — | [ | |
| 杨木粉生物炭 | — | PEG10000 | Fe3O4/石墨烯纳米片 | 超声辅助 浸渍吸附 | 55.21 | 18.66 | 98.12 | 0.225 | [ | |
| 松果、松木锯末、造纸厂污泥 | 53.83~86.43 | 17.16~11.27 | 椰子油 | — | 22.71~24.85 | — | 74.6 | 0.034 | [ | |
| 稻壳 | 8.586 | 2.35~4.75 | 椰子油 | — | 真空注入 | 22.95 | 19.92 | 70.08 | 0.2 | [ |
| 棕榈油 | — | 3.68 | 1.44 | 25.24 | 0.23 | |||||
| 棕榈蜡 | — | 52.23 | 1.39,22.13 | 92.13 | 0.11 | |||||
| 大豆蜡 | — | 16 | 19.52,34.1 | 89.91 | 0.16 | |||||
表4 生物炭基定型复合相变材料的制备方法与热性能
| 生物炭基体 | PCMs | 导热增强剂 | 制备方法 | 相变温度Tm/℃ | 过冷度 ΔT/℃ | 相变潜热 ΔHm/J·g-1 | 热导率K/W·(m·K)-1 | 参考文献 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 生物质 | SBET/m2·g-1 | R/nm | ||||||||
| 松果 | 289.69 | 3.078 | 棕榈酸 | — | 真空注入 | 59.25 | 0.12 | 84.74 | 0.393 | [ |
| 竹子 | — | 十六醇-硬脂酸 | — | 浸渍吸附 | 45.29 | — | 68.85 | — | [ | |
| 云杉木 | 454 | 3.1~6.4 | 十二烷、十四烷、十八烷 | — | 真空注入 | 31.1 | — | 91.5 | — | [ |
| 冻干马铃薯 | 42.6 | 204.7 | 聚乙二醇4000 | — | 浸渍吸附 | 51.67 | 17.76 | 91.8 | — | [ |
| 杏仁壳 | 291.21 | 2.33 | 聚乙二醇 | — | 真空注入 | 62.76 | 30.63 | 82.73 | 0.402 | [ |
| 针叶木 | 7.3 | 36.6 | 正二十烷 | — | 37 | 7.1 | 53.4 | — | [ | |
| 小麦秸秆 | 11.9 | 34.1 | — | 浸渍吸附 | 37 | 8.8 | 75 | — | ||
| 冻干马铃薯 | — | — | PEG | — | 浸渍吸附 | 56.5 | 18.6 | 158.8 | 4.489 | [ |
| 冻干白萝卜 | — | — | — | 真空注入 | 56.5 | 18.6 | 159.7 | 1.746 | ||
| 蔗糖@MgO | 869~1101 | 16~30 | 石蜡 | — | 浸渍吸附 | — | — | 182.0 | — | [ |
| 芒草、油菜和污泥 | 7.4~77.6 | — | 正十二醇和 正十二烷 | — | 超声浸渍吸附 | 8.2~24.7 | — | 73.7 90.5 | — | [ |
| 梧桐木 | — | 10~20 | 月桂酸 | — | 41 | 2.1 | 177.9 | — | [ | |
| 枣核 | 412.1 | 3.816 | PEG4000 | — | 真空注入 | 52.5 | — | 112.3 | 0.54 | [ |
| 枣核 | 1132.7 | 3.398 | 铜微球 | 52 | — | 108.2 | 0.63 | |||
| 水葫芦 | 14.001 | 11.92 | 石蜡 | 铝粉 | 浸渍吸附 | 57.67 | — | 179.4 | 0.349 | [ |
| 竹子 | 485.6 | 正十二烷 | MWCNTs | 真空浸渍吸附 | -7.7 | — | 127.4 | — | [ | |
| 杨木粉生物炭 | — | PEG10000 | Fe3O4/石墨烯纳米片 | 超声辅助 浸渍吸附 | 55.21 | 18.66 | 98.12 | 0.225 | [ | |
| 松果、松木锯末、造纸厂污泥 | 53.83~86.43 | 17.16~11.27 | 椰子油 | — | 22.71~24.85 | — | 74.6 | 0.034 | [ | |
| 稻壳 | 8.586 | 2.35~4.75 | 椰子油 | — | 真空注入 | 22.95 | 19.92 | 70.08 | 0.2 | [ |
| 棕榈油 | — | 3.68 | 1.44 | 25.24 | 0.23 | |||||
| 棕榈蜡 | — | 52.23 | 1.39,22.13 | 92.13 | 0.11 | |||||
| 大豆蜡 | — | 16 | 19.52,34.1 | 89.91 | 0.16 | |||||
| 复合相变材料 | 制备方法 | 形态稳定性说明 | 参考文献 |
|---|---|---|---|
| 脂肪酸/CNTs | 将脂肪酸接枝到CNTs上,并与脂肪酸混合 | 纳米填料在复合材料结构内均匀分布 | [ |
| 1-十八醇(OD)/MWCNT | ①OD在65°C下加热并与MWCNT结合,然后在75°C下回流48h | OD接枝的MWCNT复合材料由于MWCNT和OD之间较强的胶体引力而具备良好的形态稳定性 | [ |
| ②将OD接枝的MWCNT与OD在振荡器中混合30min | 纳米填料均匀分布在整个OD表面 | ||
| 石蜡/铜泡沫和石蜡/镍泡沫 | 采用真空浸渍法 | 无论使用何种类型的金属泡沫,石蜡都与金属泡沫完全相容 | [ |
| 石蜡(n-壬烷)/剥落石墨纳米片(xGnP) | 将混合石蜡与xGnP或石墨烯在热甲苯中制备复合材料,然后进行溶剂萃取和真空干燥 | 所研究的纳米石墨颗粒对PCMs的吸收能力有显著差异,石墨烯作为导电填料不能很好吸收大部分石蜡,然而,石蜡在石墨薄片间被有效吸收 | [ |
| 赤藓糖醇/碳纤维 | 采用熔融分散法(MD)结合新型热压法(HP)进行研究 | 采用熔融分散法制备复合材料时,碳纤维分散均匀;在热压法下,碳纤维出现在相变材料的颗粒附近 | [ |
表5 不同PCMs复合相变材料的制备及形态稳定性总结分析
| 复合相变材料 | 制备方法 | 形态稳定性说明 | 参考文献 |
|---|---|---|---|
| 脂肪酸/CNTs | 将脂肪酸接枝到CNTs上,并与脂肪酸混合 | 纳米填料在复合材料结构内均匀分布 | [ |
| 1-十八醇(OD)/MWCNT | ①OD在65°C下加热并与MWCNT结合,然后在75°C下回流48h | OD接枝的MWCNT复合材料由于MWCNT和OD之间较强的胶体引力而具备良好的形态稳定性 | [ |
| ②将OD接枝的MWCNT与OD在振荡器中混合30min | 纳米填料均匀分布在整个OD表面 | ||
| 石蜡/铜泡沫和石蜡/镍泡沫 | 采用真空浸渍法 | 无论使用何种类型的金属泡沫,石蜡都与金属泡沫完全相容 | [ |
| 石蜡(n-壬烷)/剥落石墨纳米片(xGnP) | 将混合石蜡与xGnP或石墨烯在热甲苯中制备复合材料,然后进行溶剂萃取和真空干燥 | 所研究的纳米石墨颗粒对PCMs的吸收能力有显著差异,石墨烯作为导电填料不能很好吸收大部分石蜡,然而,石蜡在石墨薄片间被有效吸收 | [ |
| 赤藓糖醇/碳纤维 | 采用熔融分散法(MD)结合新型热压法(HP)进行研究 | 采用熔融分散法制备复合材料时,碳纤维分散均匀;在热压法下,碳纤维出现在相变材料的颗粒附近 | [ |
| 复合相变材料 | 循环次数 | 相变温度Tm/℃ | 相变潜热 ΔHm/J·g-1 | 参考文献 |
|---|---|---|---|---|
| CA-H/EG | 0 | 25.04 | 177.8 | [ |
| 1000 | 24.61 | 171.5 | ||
| CA-PS/EG | 0 | 27.04 | 144.4 | [ |
| 1000 | 27.14 | 130.6 | ||
| OA-MA/EG | 0 | 6.8 | 136.3 | [ |
| 100 | 6.9 | 136.8 | ||
| MA-PA-SA/EG | 0 | 41.61 | 153.5 | [ |
| 500 | 41.18 | 152.6 | ||
| 1000 | 41.92 | 151.0 |
表6 复合相变材料的热稳定性
| 复合相变材料 | 循环次数 | 相变温度Tm/℃ | 相变潜热 ΔHm/J·g-1 | 参考文献 |
|---|---|---|---|---|
| CA-H/EG | 0 | 25.04 | 177.8 | [ |
| 1000 | 24.61 | 171.5 | ||
| CA-PS/EG | 0 | 27.04 | 144.4 | [ |
| 1000 | 27.14 | 130.6 | ||
| OA-MA/EG | 0 | 6.8 | 136.3 | [ |
| 100 | 6.9 | 136.8 | ||
| MA-PA-SA/EG | 0 | 41.61 | 153.5 | [ |
| 500 | 41.18 | 152.6 | ||
| 1000 | 41.92 | 151.0 |
| 1 | 王成君, 汪林强, 马晶, 等. 碳基复合相变材料的研究进展[J]. 化工进展, 2023, 42(12): 6383-6398. |
| WANG Chengjun, WANG Linqiang, MA Jing, et al. Research progress of carbon matrix composite phase change materials[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6383-6398. | |
| 2 | SHI Lei, HUANG Cunwen, ZHENG Nianben, et al. Thermal energy storage characteristics of carbon-based phase change composites for photo-thermal conversion[J]. Journal of Energy Storage, 2024, 77: 109892. |
| 3 | PRABHU Pravin, SAWANT Sanjay. Current developments in composite phase change materials for thermal energy storage application: A review[J]. Materials Today: Proceedings, 2023, 72: 810-816. |
| 4 | YANG Guijun, Yoon-Ji YIM, LEE Ji Won, et al. Carbon-filled organic phase-change materials for thermal energy storage: A review[J]. Molecules, 2019, 24(11): 2055. |
| 5 | XIANG Li, LUO Dajun, YANG Jingkui, et al. Preparation and comparison of properties of three phase change energy storage materials with hollow fiber membrane as the supporting carrier[J]. Polymers, 2019, 11(8): 1343. |
| 6 | FENG Lili, YU Runxiang, LI Yang, et al. Shape-stabilized phase change materials composed of polyethylene glycol and ordered mesoporous silica synthesized from fly ash[J]. Thermochimica Acta, 2023, 720: 179428. |
| 7 | SARAVANAN M, SUDALAI S, DHARANEESH A B, et al. An extensive review on mesoporous silica from inexpensive resources: Properties, synthesis, and application toward modern technologies[J]. Journal of Sol-Gel Science and Technology, 2023, 105(1): 1-29. |
| 8 | OLLA Chiara, CARBONARO Carlo Maria. The void side of silica: Surveying optical properties and applications of mesoporous silica[J]. Journal of Physics: Condensed Matter, 2024, 36(25): 253002. |
| 9 | JIN Jiao, LIU Lang, LIU Ruohua, et al. Preparation and thermal performance of binary fatty acid with diatomite as form-stable composite phase change material for cooling asphalt pavements[J]. Construction and Building Materials, 2019, 226: 616-624. |
| 10 | YI Hao, ZHAN Weiquan, ZHAO Yunliang, et al. A novel core-shell structural montmorillonite nanosheets/stearic acid composite PCM for great promotion of thermal energy storage properties[J]. Solar Energy Materials and Solar Cells, 2019, 192: 57-64. |
| 11 | GUO Qijing, YI Hao, JIA Feifei, et al. Novel MoS2/montmorillonite hybrid aerogel encapsulated PEG as composite phase change materials with superior solar-thermal energy harvesting and storage[J]. Journal of Colloid and Interface Science, 2024, 667: 269-281. |
| 12 | SUN Ying, YUAN Xingzhou, WEN Jiabao, et al. The surface and interlayer modification of montmorillonite and its potential application for thermal energy storage[J]. Renewable Energy, 2024, 225: 120282. |
| 13 | PAUL Abigail, MAGEE Regan, WICHERT Nathan, et al. Carbon-based porous materials for energy storage applications[J]. ECS Meeting Abstracts, 2023(24): 1621. |
| 14 | TSELEPI Marina, PROUSKAS Costas, PAPAGEORGIOU Dimitrios G, et al. Graphene-based phase change composite nano-materials for thermal storage applications[J]. Energies, 2022, 15(3): 1192. |
| 15 | MA Ying, WEI Rongrong, ZUO Hongyan, et al. N-doped EG@MOFs derived porous carbon composite phase change materials for thermal optimization of Li-ion batteries at low temperature[J]. Energy, 2024, 286: 129637. |
| 16 | YAMEEN Muhammad Zubair, NAQVI Salman Raza, Dagmar JUCHELKOVÁ, et al. Harnessing the power of functionalized biochar: Progress, challenges, and future perspectives in energy, water treatment, and environmental sustainability[J]. Biochar, 2024, 6(1): 25. |
| 17 | AGYEKUM Ephraim Bonah, NUTAKOR Christabel. Recent advancement in biochar production and utilization—A combination of traditional and bibliometric review[J]. International Journal of Hydrogen Energy, 2024, 54: 1137-1153. |
| 18 | CHAUDHARY Pankaj, BANSAL Sonia, SHARMA Bharat Bhushan, et al. Waste biomass-derived activated carbons for various energy storage device applications: A review[J]. Journal of Energy Storage, 2024, 78: 109996. |
| 19 | 李昭, 李宝让, 陈豪志, 等. 相变储热技术研究进展[J]. 化工进展, 2020, 39(12): 5066-5085. |
| LI Zhao, LI Baorang, CHEN Haozhi, et al. State of the art review on phase change thermal energy storage technology[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 5066-5085. | |
| 20 | MAGENDRAN Suhanyaa S, KHAN Fahad Saleem Ahmed, MUBARAK N M, et al. Synthesis of organic phase change materials by using carbon nanotubes as filler material[J]. Nano-Structures & Nano-Objects, 2019, 19: 100361. |
| 21 | LIU K, YUAN Z F, ZHAO H X, et al. Properties and applications of shape-stabilized phase change energy storage materials based on porous material support—A review[J]. Materials Today Sustainability, 2023, 21: 100336. |
| 22 | WARZOHA Ronald J, FLEISCHER Amy S. Effect of carbon nanotube interfacial geometry on thermal transport in solid-liquid phase change materials[J]. Applied Energy, 2015, 154: 271-276. |
| 23 | FAN Liwu, FANG Xin, WANG Xiao, et al. Effects of various carbon nanofillers on the thermal conductivity and energy storage properties of paraffin-based nanocomposite phase change materials[J]. Applied Energy, 2013, 110: 163-172. |
| 24 | KUZIEL Anna W, DZIDO Grzegorz, TURCZYN Roman, et al. Ultra-long carbon nanotube-paraffin composites of record thermal conductivity and high phase change enthalpy among paraffin-based heat storage materials[J]. Journal of Energy Storage, 2021, 36: 102396. |
| 25 | CAO Ruirui, CHEN Sai, WANG Yuzhou, et al. Functionalized carbon nanotubes as phase change materials with enhanced thermal, electrical conductivity, light-to-thermal, and electro-to-thermal performances[J]. Carbon, 2019, 149: 263-272. |
| 26 | DU Xiaosheng, XU Jianing, DENG Sha, et al. Amino-functionalized single-walled carbon nanotubes-integrated polyurethane phase change composites with superior photothermal conversion efficiency and thermal conductivity[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(21): 17682-17690. |
| 27 | PUTRA Nandy, RAWI Stephanie, AMIN Muhammad, et al. Preparation of beeswax/multi-walled carbon nanotubes as novel shape-stable nanocomposite phase-change material for thermal energy storage[J]. Journal of Energy Storage, 2019, 21: 32-39. |
| 28 | ZHANG Qi, LIU Jian. Sebacic acid/CNT sponge phase change material with excellent thermal conductivity and photo-thermal performance[J]. Solar Energy Materials and Solar Cells, 2018, 179: 217-222. |
| 29 | CHEN Xiao, GAO Hongyi, Guangtong HAI, et al. Carbon nanotube bundles assembled flexible hierarchical framework based phase change material composites for thermal energy harvesting and thermotherapy[J]. Energy Storage Materials, 2020, 26: 129-137. |
| 30 | WANG Jingjing, HUANG Xiubing, GAO Hongyi, et al. Construction of CNT@Cr-MIL-101-NH2 hybrid composite for shape-stabilized phase change materials with enhanced thermal conductivity[J]. Chemical Engineering Journal, 2018, 350: 164-172. |
| 31 | AHMADI Rouhollah, MONADINIA Farhad, MALEKI Mahdi. Passive/active photovoltaic-thermal (PVT) system implementing infiltrated phase change material (PCM) in PS-CNT foam[J]. Solar Energy Materials and Solar Cells, 2021, 222: 110942. |
| 32 | CHEN Yanfeng, ZHANG Qi, WEN Xiaoyan, et al. A novel CNT encapsulated phase change material with enhanced thermal conductivity and photo-thermal conversion performance[J]. Solar Energy Materials and Solar Cells, 2018, 184: 82-90. |
| 33 | XU Bin, CHEN Chenghua, ZHOU Jing, et al. Preparation of novel microencapsulated phase change material with Cu-Cu2O/CNTs as the shell and their dispersed slurry for direct absorption solar collectors[J]. Solar Energy Materials and Solar Cells, 2019, 200: 109980. |
| 34 | MEHRALI Mohammad, LATIBARI Sara Tahan, MEHRALI Mehdi, et al. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material[J]. Applied Thermal Engineering, 2013, 61(2): 633-640. |
| 35 | AMIN Muhammad, PUTRA Nandy, KOSASIH Engkos A, et al. Thermal properties of beeswax/graphene phase change material as energy storage for building applications[J]. Applied Thermal Engineering, 2017, 112: 273-280. |
| 36 | CAO Yufeng, FAN Dongli, LIN Shaohui, et al. Phase change materials based on comb-like polynorbornenes and octadecylamine-functionalized graphene oxide nanosheets for thermal energy storage[J]. Chemical Engineering Journal, 2020, 389: 124318. |
| 37 | LIU Huanbing, PEI Dongfang, CHEN Sai, et al. Fabrication and characterization of diethylene glycol hexadecyl ether-grafted graphene oxide as a form-stable phase change material[J]. Thermochimica Acta, 2018, 661: 166-173. |
| 38 | GE Jing, WANG Yun, WANG Haixia, et al. Thermal properties and shape stabilization of epoxidized methoxy polyethylene glycol composite PCMs tailored by polydopamine-functionalized graphene oxide[J]. Solar Energy Materials and Solar Cells, 2020, 208: 110388. |
| 39 | Jing LYU, LIU Zengwei, WU Xiaohan, et al. Nanofibrous kevlar aerogel films and their phase-change composites for highly efficient infrared stealth[J]. ACS Nano, 2019, 13(2): 2236-2245. |
| 40 | DING Jie, WU Xiaodong, SHEN Xiaodong, et al. Form-stable phase change material embedded in three-dimensional reduced graphene aerogel with large latent heat for thermal energy management[J]. Applied Surface Science, 2020, 534: 147612. |
| 41 | MU Boyuan, LI Min. Synthesis of novel form-stable composite phase change materials with modified graphene aerogel for solar energy conversion and storage[J]. Solar Energy Materials and Solar Cells, 2019, 191: 466-475. |
| 42 | ZOU Deqiu, MA Xianfeng, LIU Xiaoshi, et al. Thermal performance enhancement of composite phase change materials (PCM) using graphene and carbon nanotubes as additives for the potential application in lithium-ion power battery[J]. International Journal of Heat and Mass Transfer, 2018, 120: 33-41. |
| 43 | CAO Qianyun, HE Fangfang, XIE Changqiong, et al. Paraffin-based shape-stable phase change materials with graphene/carbon nanotube three-dimensional network structure[J]. Fullerenes, Nanotubes and Carbon Nanostructures, 2019, 27(6): 492-497. |
| 44 | SHCHUKINA E M, GRAHAM M, ZHENG Z, et al. Nanoencapsulation of phase change materials for advanced thermal energy storage systems[J]. Chemical Society Reviews, 2018, 47(11): 4156-4175. |
| 45 | KANT Karunesh, SHUKLA A, SHARMA Atul, et al. Heat transfer study of phase change materials with graphene nano particle for thermal energy storage[J]. Solar Energy, 2017, 146: 453-463. |
| 46 | MAITI Tushar Kanti, DIXIT Prakhar, SUHAG Amit, et al. Advancements in organic and inorganic shell materials for the preparation of microencapsulated phase change materials for thermal energy storage applications[J]. RSC Sustainability, 2023, 1(4): 665-697. |
| 47 | ZHANG Yuang, WANG Jiasheng, QIU Jinjing, et al. Ag-graphene/PEG composite phase change materials for enhancing solar-thermal energy conversion and storage capacity[J]. Applied Energy, 2019, 237: 83-90. |
| 48 | LIU Zhifang, CHEN Zhonghua, YU Fei. Microencapsulated phase change material modified by graphene oxide with different degrees of oxidation for solar energy storage[J]. Solar Energy Materials and Solar Cells, 2018, 174: 453-459. |
| 49 | JEONG Su-Gwang, Jeong Eun HEO, CHOI Sang-Yeon, et al. Heating efficiency enhanced by combination of phase change materials and activated carbon for dry floor heating system[J]. Journal of Energy Storage, 2023, 70: 108027. |
| 50 | FENG Lili, ZHENG Jie, YANG Huazhe, et al. Preparation and characterization of polyethylene glycol/active carbon composites as shape-stabilized phase change materials[J]. Solar Energy Materials and Solar Cells, 2011, 95(2): 644-650. |
| 51 | Imran HUSSAIN S, DINESH R, Ameelia ROSELINE A, et al. Enhanced thermal performance and study the influence of sub cooling on activated carbon dispersed eutectic PCM for cold storage applications[J]. Energy and Buildings, 2017, 143: 17-24. |
| 52 | CHEN Yanghua, GE Minrong, ZHAO Feng, et al. Synthesis of lauric-myristic acid/activated carbon composite as a new shape-stabilized energy storage material[J]. Materials Science, 2022, 28(1): 68-74. |
| 53 | CHEN Yanghua, WANG Zhaohe, GE Minrong, et al. Preparation and thermal properties of hexadecanol-myristic acid eutectics/activated carbon composites as shape-stabilized phase change materials in thermal energy storage[J]. Materials Science, 2021, 27(4): 437-443. |
| 54 | KHADIRAN Tumirah, HUSSEIN Mohd Zobir, ZAINAL Zulkarnain, et al. Activated carbon derived from peat soil as a framework for the preparation of shape-stabilized phase change material[J]. Energy, 2015, 82: 468-478. |
| 55 | NICHOLAS Ahmad Fariz, HUSSEIN Mohd Zobir, ZAINAL Zulkarnain, et al. Palm kernel shell activated carbon as an inorganic framework for shape-stabilized phase change material[J]. Nanomaterials, 2018, 8(9): 689. |
| 56 | KHADIRAN Tumirah, HUSSEIN Mohd Zobir, ZAINAL Zulkarnain, et al. Shape-stabilised n-octadecane/activated carbon nanocomposite phase change material for thermal energy storage[J]. Journal of the Taiwan Institute of Chemical Engineers, 2015, 55: 189-197. |
| 57 | XU Jianuo, SUN Jingmeng, ZHAO Junqi, et al. Eco-friendly wood plastic composites with biomass-activated carbon-based form-stable phase change material for building energy conversion[J]. Industrial Crops and Products, 2023, 197: 116573. |
| 58 | CHIN Chun On, YANG Xu, PAUL Suvash Chandra, et al. Development of thermal energy storage lightweight concrete using paraffin-oil palm kernel shell-activated carbon composite[J]. Journal of Cleaner Production, 2020, 261: 121227. |
| 59 | LEE Jongki, Seunghwan WI, YUN Beom Yeol, et al. Thermal and characteristic analysis of shape-stabilization phase change materials by advanced vacuum impregnation method using carbon-based materials[J]. Journal of Industrial and Engineering Chemistry, 2019, 70: 281-289. |
| 60 | LIU Yuan, CHEN Yanghua. Preparation and properties of lauryl alcohol-caprylic acid eutectics/activated charcoal composites as shape-stabilized phase change materials for cold energy storage[J]. Materials Science, 2020, 26(3): 300-307. |
| 61 | Jiyeol BAE, KIM Suho, KIM Kwangsoo, et al. Impregnation of activated carbon with organic phase-change material[J]. Materials, 2023, 17(1): 67. |
| 62 | MERT Hatice Hande, ESLEK Ali, MERT Emine Hilal, et al. Preparation and characterization of shape-stable bio-based composite phase change materials for thermal energy storage: Coconut oil/activated carbon from cherry stones doped composites[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2022, 44(2): 5381-5397. |
| 63 | ZOROUFCHI BENIS Khaled, SOLTAN Jafar, MCPHEDRAN Kerry N. Biochar: A potent adsorbent[M]//Biochar and Its Composites. Singapore: Springer Nature Singapore, 2023: 49-72. |
| 64 | ZHUANG Zitong, LIU Yanbing, WEI Wenwen, et al. Preparation of biochar adsorption material from walnut shell by supercritical CO2 pretreatment[J]. Biochar, 2024, 6(1): 11. |
| 65 | WAN Yechao, CHEN Yan, CUI Zhixing, et al. A promising form-stable phase change material prepared using cost effective pinecone biochar as the matrix of palmitic acid for thermal energy storage[J]. Scientific Reports, 2019, 9(1): 11535. |
| 66 | WU Xijie, LI Junying, MA Feng. Preparation and properties of hexadecanol-stearic acid/bamboo charcoal composite[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2018, 40(9): 1044-1050. |
| 67 | ATINAFU Dimberu G, CHANG Seong Jin, KIM Sumin. Infiltration properties of n-alkanes in mesoporous biochar: The capacity of smokeless support for stability and energy storage[J]. Journal of Hazardous Materials, 2020, 399: 123041. |
| 68 | TAN Bo, HUANG Zhaohui, YIN Zhaoyu, et al. Preparation and thermal properties of shape-stabilized composite phase change materials based on polyethylene glycol and porous carbon prepared from potato[J]. RSC Advances, 2016, 6(19): 15821-15830. |
| 69 | CHEN Yan, CUI Zhixing, DING Han, et al. Cost-effective biochar produced from agricultural residues and its application for preparation of high performance form-stable phase change material via simple method[J]. International Journal of Molecular Sciences, 2018, 19(10): 3055. |
| 70 | ATINAFU Dimberu G, YUN Beom Yeol, KIM Young Uk, et al. Introduction of eicosane into biochar derived from softwood and wheat straw: Influence of porous structure and surface chemistry[J]. Chemical Engineering Journal, 2021, 415: 128887. |
| 71 | ZHAO Yajing, MIN Xin, HUANG Zhaohui, et al. Honeycomb-like structured biological porous carbon encapsulating PEG: A shape-stable phase change material with enhanced thermal conductivity for thermal energy storage[J]. Energy and Buildings, 2018, 158: 1049-1062. |
| 72 | WANG Jiawei, JIA Xilai, ATINAFU Dimberu G, et al. Synthesis of “graphene-like” mesoporous carbons for shape-stabilized phase change materials with high loading capacity and improved latent heat[J]. Journal of Materials Chemistry A, 2017, 5(46): 24321-24328. |
| 73 | ATINAFU Dimberu G, CHANG Seong Jin, KIM Ki-Hyun, et al. Tuning surface functionality of standard biochars and the resulting uplift capacity of loading/energy storage for organic phase change materials[J]. Chemical Engineering Journal, 2020, 394: 125049. |
| 74 | YANG Zhiwei, DENG Yong, LI Jinhong. Preparation of porous carbonized woods impregnated with lauric acid as shape-stable composite phase change materials[J]. Applied Thermal Engineering, 2019, 150: 967-976. |
| 75 | CHEN Yan, DING Han, GAO Junkai, et al. A novel strategy for enhancing the thermal conductivity of shape-stable phase change materials via carbon-based in situ reduction of metal ions[J]. Journal of Cleaner Production, 2020, 243: 118627. |
| 76 | Dudul DAS, BORDOLOI Urbashi, MUIGAI Harrison Hihu, et al. A novel form stable PCM based bio composite material for solar thermal energy storage applications[J]. Journal of Energy Storage, 2020, 30: 101403. |
| 77 | ATINAFU Dimberu G, Seunghwan WI, YUN Beom Yeol, et al. Engineering biochar with multiwalled carbon nanotube for efficient phase change material encapsulation and thermal energy storage[J]. Energy, 2021, 216: 119294. |
| 78 | CHAO Weixiang, YANG Haiyue, CAO Guoliang, et al. Carbonized wood flour matrix with functional phase change material composite for magnetocaloric-assisted photothermal conversion and storage[J]. Energy, 2020, 202: 117636. |
| 79 | JEON Jisoo, PARK Ji Hun, Seunghwan WI, et al. Characterization of biocomposite using coconut oil impregnated biochar as latent heat storage insulation[J]. Chemosphere, 2019, 236: 124269. |
| 80 | JEON Jisoo, PARK Ji Hun, Seunghwan WI, et al. Latent heat storage biocomposites of phase change material-biochar as feasible eco-friendly building materials[J]. Environmental Research, 2019, 172: 637-648. |
| 81 | Amir AL-AHMED, SARI Ahmet, JAFAR MAZUMDER Mohammad ABU, et al. Thermal energy storage and thermal conductivity properties of fatty acid/fatty acid-grafted-CNTs and fatty acid/CNTs as novel composite phase change materials[J]. Scientific Reports, 2020, 10(1): 15388. |
| 82 | Amir AL-AHMED, SARI Ahmet, JAFAR MAZUMDER Mohammad ABU, et al. Thermal energy storage and thermal conductivity properties of octadecanol-MWCNT composite PCMs as promising organic heat storage materials[J]. Scientific Reports, 2020, 10(1): 9168. |
| 83 | XIAO X, ZHANG P, LI M. Preparation and thermal characterization of paraffin/metal foam composite phase change material[J]. Applied Energy, 2013, 112: 1357-1366. |
| 84 | SHI Jianan, Ming-Der GER, LIU Yih-Ming, et al. Improving the thermal conductivity and shape-stabilization of phase change materials using nanographite additives[J]. Carbon, 2013, 51: 365-372. |
| 85 | NOMURA Takahiro, TABUCHI Kazuki, ZHU Chunyu, et al. High thermal conductivity phase change composite with percolating carbon fiber network[J]. Applied Energy, 2015, 154: 678-685. |
| 86 | FEI Hua, DU Wenqing, GU Qingjun, et al. The phase change characteristics of capric acid-based binary low eutectic mixtures adsorbed in expanded graphite[J]. Energy & Fuels, 2020, 34(11): 14893-14901. |
| 87 | ZHOU Sunxi, ZHANG Xuelai, LIU Sheng, et al. Performance study on expand graphite/organic composite phase change material for cold thermal energy storage[J]. Energy Procedia, 2019, 158: 5305-5310. |
| 88 | YANG Xiaojiao, YUAN Yanping, ZHANG Nan, et al. Preparation and properties of myristic-palmitic-stearic acid/expanded graphite composites as phase change materials for energy storage[J]. Solar Energy, 2014, 99: 259-266. |
| 89 | HUANG Yifan, ZOU Minming, CHEN Wenjing, et al. A novel room-temperature flexible phase change material for solar energy photothermal conversion and battery thermal management[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(11): 4662-4675. |
| 90 | XU Xiaoying, LIU Jiahua, OUYANG Xing, et al. In-situ temperature regulation of flexible supercapacitors by designing intelligent electrode with microencapsulated phase change materials[J]. Electrochimica Acta, 2020, 334: 135551. |
| 91 | PAPADIMITRATOS Alexios, SOBHANSARBANDI Sarvenaz, POZDIN Vladimir, et al. Evacuated tube solar collectors integrated with phase change materials[J]. Solar Energy, 2016, 129: 10-19. |
| 92 | MURATORE C, AOUADI S M, VOEVODIN A A. Embedded phase change material microinclusions for thermal control of surfaces[J]. Surface and Coatings Technology, 2012, 206(23): 4828-4832. |
| 93 | CUI Hongzhi, WANG Pizhuang, YANG Haibin, et al. Enhancing the heat transfer and photothermal conversion of salt hydrate phase change material for efficient solar energy utilization[J]. Journal of Energy Storage, 2022, 49: 104130. |
| 94 | TOMIZAWA Yusuke, SASAKI Katsuhiko, KURODA Akiyoshi, et al. Experimental and numerical study on phase change material (PCM) for thermal management of mobile devices[J]. Applied Thermal Engineering, 2016, 98: 320-329. |
| 95 | YAN Zixiang. Application of energy saving and environmental protection materials in architectural design[J]. Advances in Materials Science and Engineering, 2023, 2023: 9823035. |
| 96 | YANG Huizhi, YU Xiaohan, GE Chunhua, et al. Hydrothermal carbon-doped polyethylene glycol as phase-change materials with good thermal conductivity and shape-stability[J]. ChemistrySelect, 2020, 5(2): 480-487. |
| 97 | HUANG M J, EAMES P C, NORTON B. Thermal regulation of building-integrated photovoltaics using phase change materials[J]. International Journal of Heat and Mass Transfer, 2004, 47(12/13): 2715-2733. |
| 98 | PARK Jungwoo, KIM Taeyeon, LEIGH Seung-Bok. Application of a phase-change material to improve the electrical performance of vertical-building-added photovoltaics considering the annual weather conditions[J]. Solar Energy, 2014, 105: 561-574. |
| 99 | HASAN A, MCCORMACK S J, HUANG M J, et al. Increased photovoltaic performance through temperature regulation by phase change materials: Materials comparison in different climates[J]. Solar Energy, 2015, 115: 264-276. |
| 100 | SU Yun, ZHU Wen, TIAN Miao, et al. Intelligent bidirectional thermal regulation of phase change material incorporated in thermal protective clothing[J]. Applied Thermal Engineering, 2020, 174: 115340. |
| 101 | LI Hua, XIAO Xiangyu, WANG Yanan, et al. Performance investigation of a battery thermal management system with microencapsulated phase change material suspension[J]. Applied Thermal Engineering, 2020, 180: 115795. |
| 102 | FOSS Carl Erik Lie, SVENSSON Ann Mari, Øystein GULLBREKKEN, et al. Temperature effects on performance of graphite anodes in carbonate based electrolytes for lithium ion batteries[J]. Journal of Energy Storage, 2018, 17: 395-402. |
| 103 | WEI Xiao, XUE Fei, QI Xiaodong, et al. Photo- and electro-responsive phase change materials based on highly anisotropic microcrystalline cellulose/graphene nanoplatelet structure[J]. Applied Energy, 2019, 236: 70-80. |
| 104 | FAROUK Naeim, ALOTAIBI Abdullah Alhumaidi, ALSHAHRI Abdullah H, et al. Challenges in incorporating phase change materials into thermal control units for lithium-ion battery cooling[J]. Journal of Energy Storage, 2022, 49: 104094. |
| 105 | WENG Jingwen, HE Yaping, OUYANG Dongxu, et al. Thermal performance of PCM and branch-structured fins for cylindrical power battery in a high-temperature environment[J]. Energy Conversion and Management, 2019, 200: 112106. |
| [1] | 张舒茜, 陈佩婷, 蒲建波, 王宇作, 阮殿波, 乔志军. 进风量对硅/碳负极材料二次颗粒尺寸及电化学性能的影响[J]. 化工进展, 2025, 44(4): 2196-2201. |
| [2] | 朱方龙, 冯倩倩. 红外透过型尼龙多孔膜的制备及光热性能[J]. 化工进展, 2025, 44(4): 2215-2224. |
| [3] | 冯琬淇, 杨翠平, 郝俊尧, 倪红梅, 赵俭波. 棉浆黑液提取物基木塑复合材料的制备及性能[J]. 化工进展, 2025, 44(3): 1768-1775. |
| [4] | 张琪, 王涛, 张雪冰, 李为真, 程萌, 张魁, 吕毅军, 门卓武. 合成气/CO2转化制高级醇Fe基催化剂研究进展[J]. 化工进展, 2025, 44(3): 1323-1337. |
| [5] | 单雪影, 李玲玉, 张濛, 张家傅, 李锦春. 阻燃环氧树脂/低分子聚苯醚材料的制备及性能[J]. 化工进展, 2025, 44(3): 1533-1541. |
| [6] | 雪冰峰, 张烨, 张世元, 付鹏, 崔喆, 张袁铖, 李鑫, 庞新厂, 赵蔚, 张晓朦, 刘民英. 直接固相聚合法制备聚酰胺PA12T及性能表征[J]. 化工进展, 2025, 44(3): 1559-1569. |
| [7] | 赖俊华, 潘大伟, 巨晓洁, 刘壮, 谢锐, 汪伟, 褚良银. 光栅结构对智能凝胶光栅传感器检测性能的影响规律[J]. 化工进展, 2025, 44(3): 1578-1587. |
| [8] | 马晓宇, 张岩, 周阿武, 李涵冰, 杨飞华, 李建荣. MOF-on-MOF复合材料制备与光催化性能的研究进展[J]. 化工进展, 2025, 44(3): 1417-1431. |
| [9] | 杨帆, 赵溢涛, 朱学栋, 王达锐. 三元尖晶石与孪晶ZSM-5分子筛在苯与二氧化碳甲基化中的应用[J]. 化工进展, 2025, 44(2): 856-866. |
| [10] | 闫鹏程, 高卓凡, 周志辉, 吴红丹, 陈霞, 周显, 范泽宇, 邓闪闪, 鲁麒, 向媛. 聚酰胺/聚醚醚酮复合膜的制备及其有机溶剂纳滤性能[J]. 化工进展, 2025, 44(2): 1147-1156. |
| [11] | 张喆, 纪献兵, 杨聿昊, 刘家璇, 姚泊丞. 多尺度结构烧结沟槽表面沸腾传热性能[J]. 化工进展, 2025, 44(2): 669-676. |
| [12] | 赵珂, 张恒, 翟倩, 甄琪, 苏天阳, 崔景强. PLA/PEG@SDS超细纤维水蒸发器的复合结构设计及其液体传输行为[J]. 化工进展, 2025, 44(2): 1014-1024. |
| [13] | 张琪, 王涛, 张雪冰, 李为真, 冯波, 蒋智慧, 吕毅军, 门卓武. 合成气制高级醇Co基催化剂研究进展[J]. 化工进展, 2025, 44(2): 773-787. |
| [14] | 李章良, 杨月珠, 伍传田, 吕源财. 活性炭纤维毡负载N-TiO2/MoS2/N-TiO2固定化漆酶降解双酚A[J]. 化工进展, 2025, 44(2): 887-898. |
| [15] | 庄柯, 陈宏, 许芸, 仲兆平, 周峻伍, 周凯, 董月红. SiO2改性Ce-V-W/Ti催化剂载体的抗碱(土)金属中毒性能[J]. 化工进展, 2025, 44(1): 266-276. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||