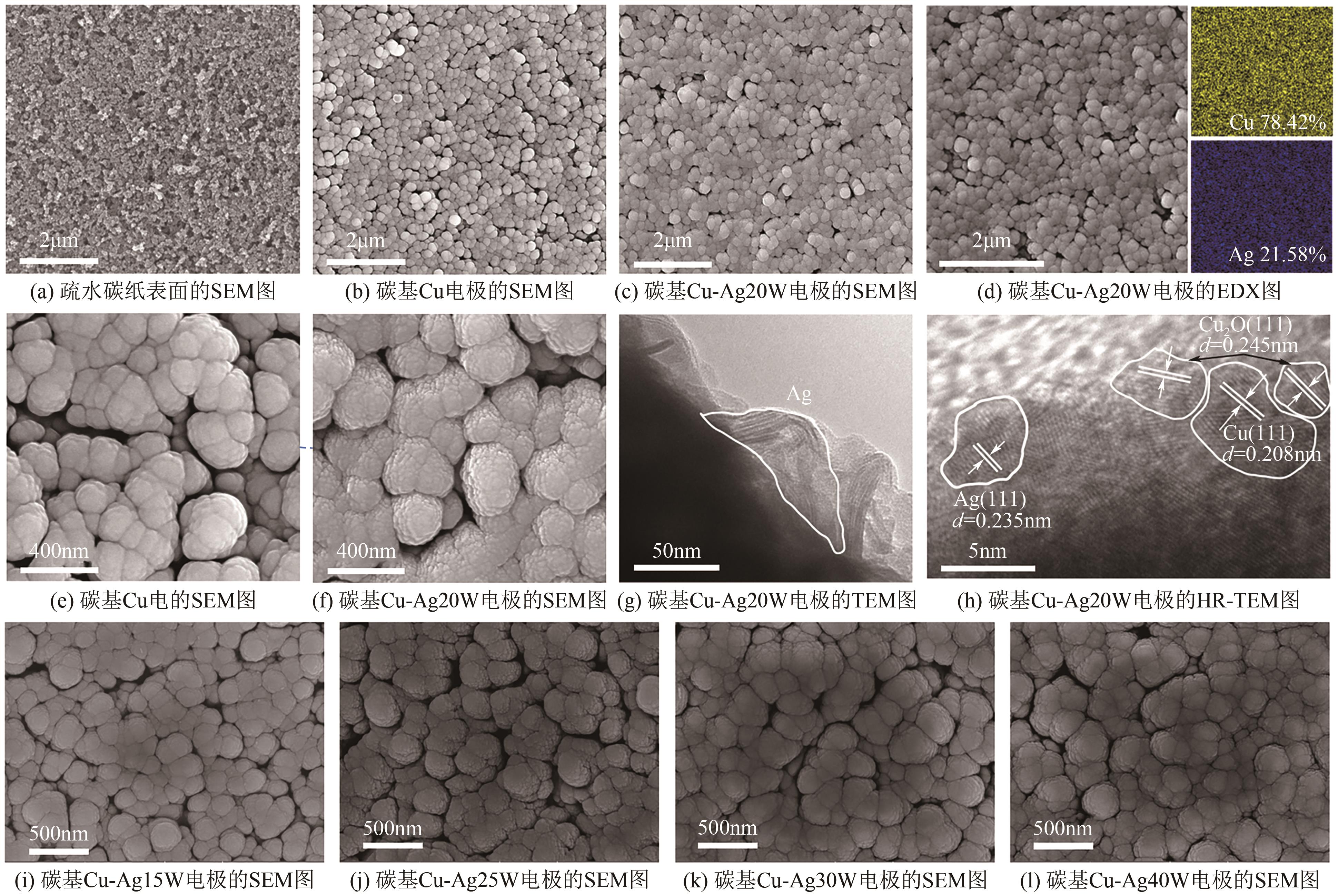
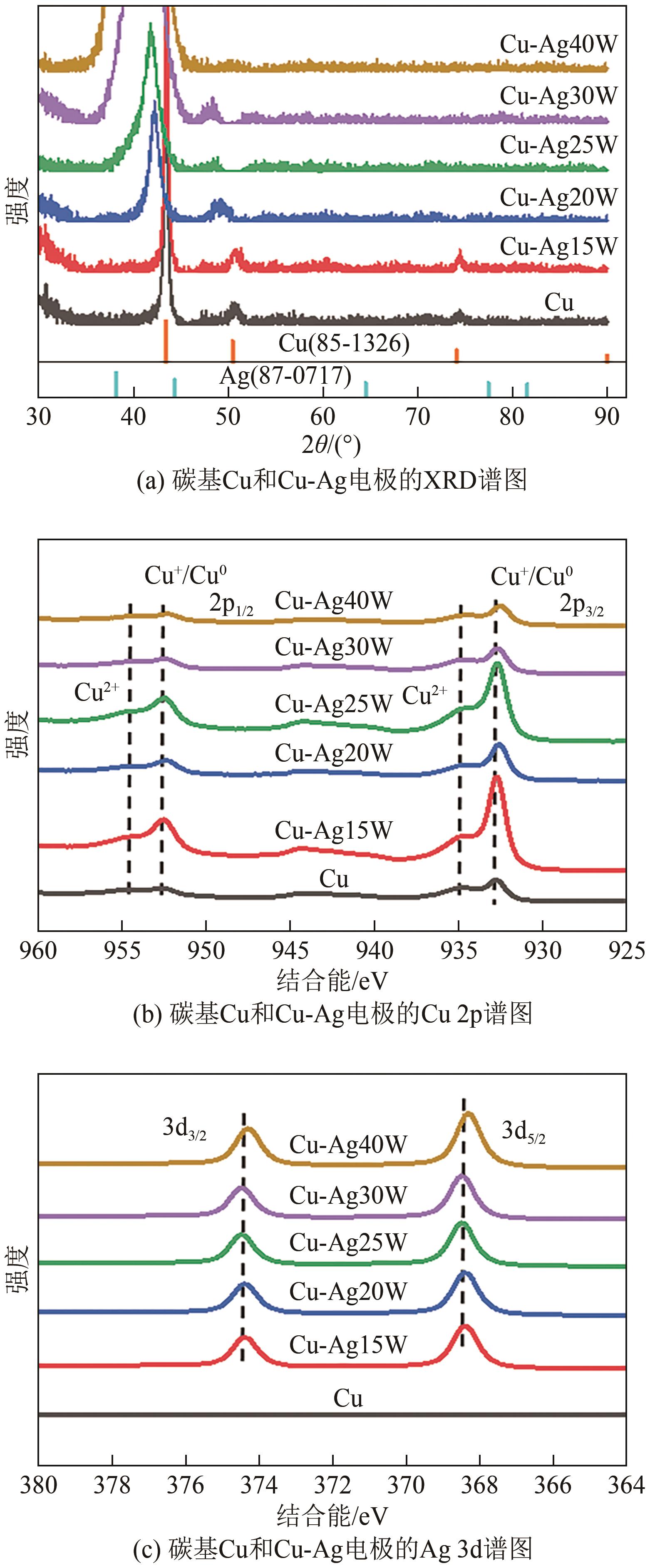
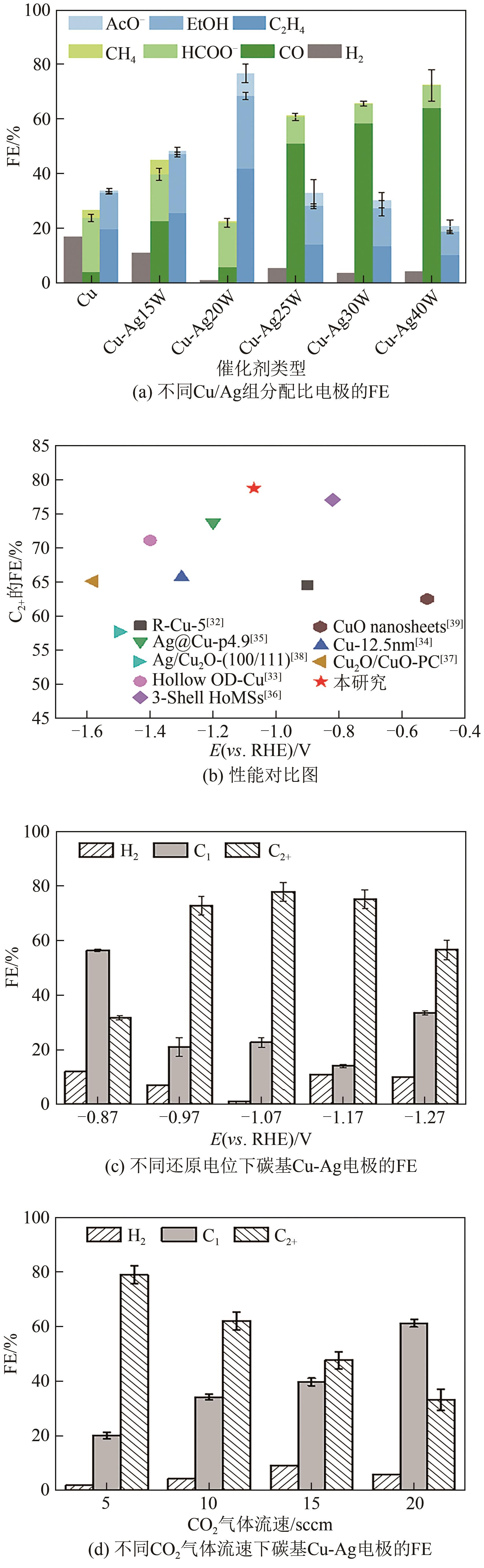
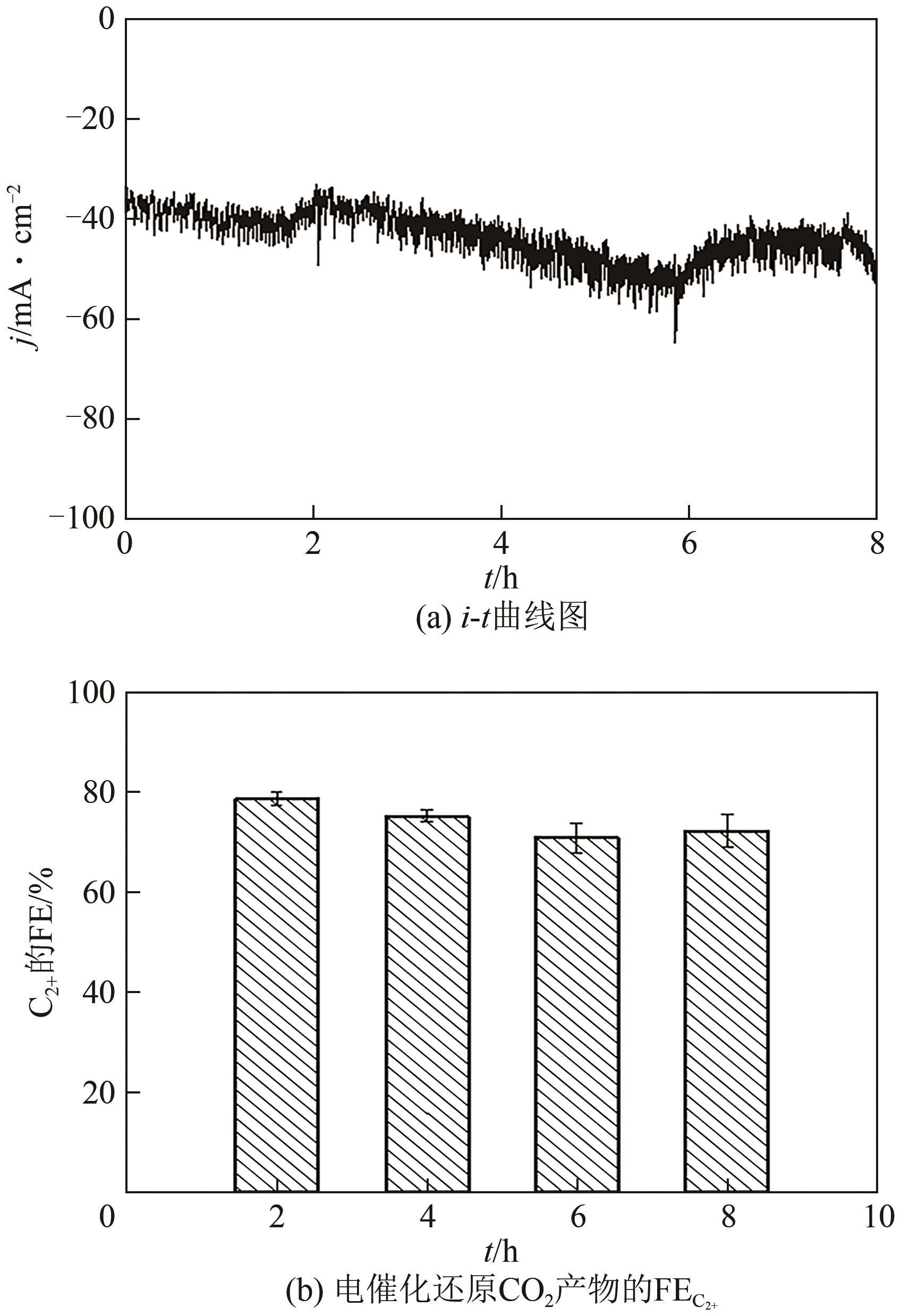
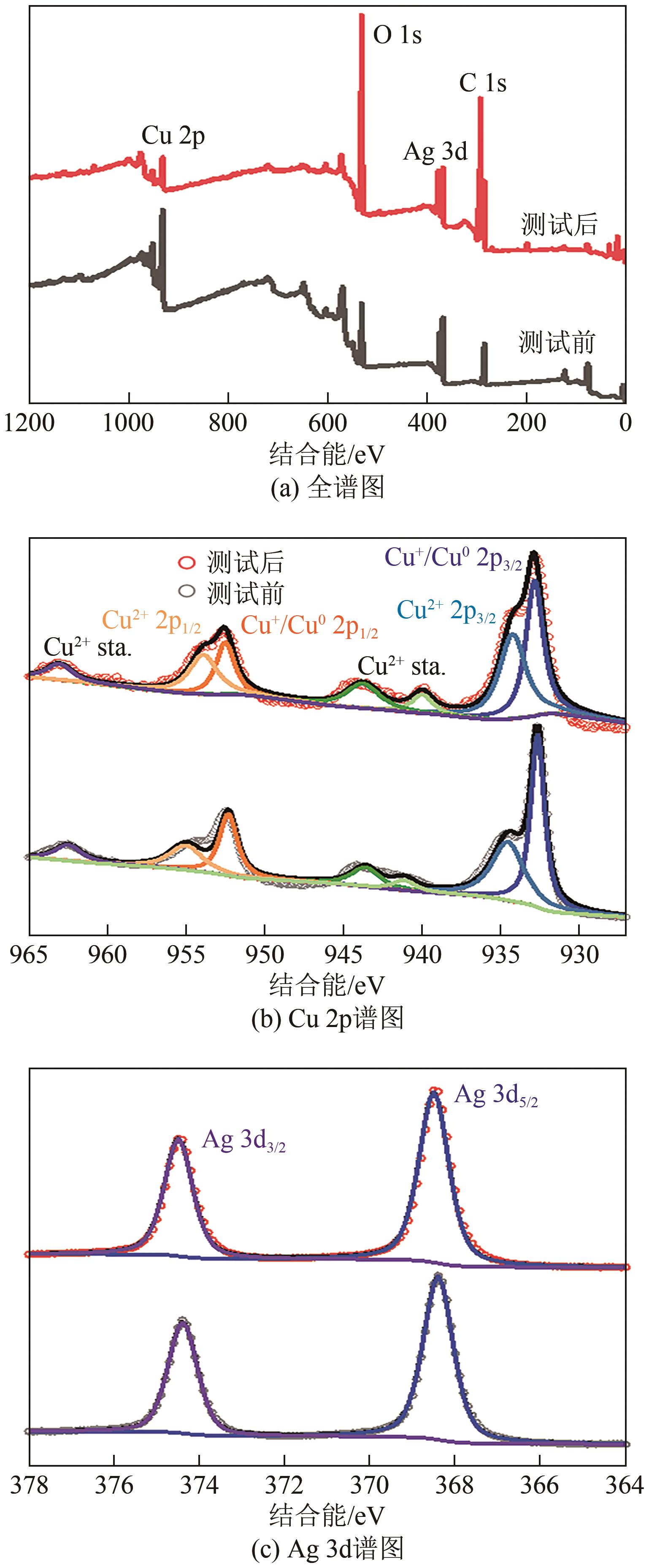
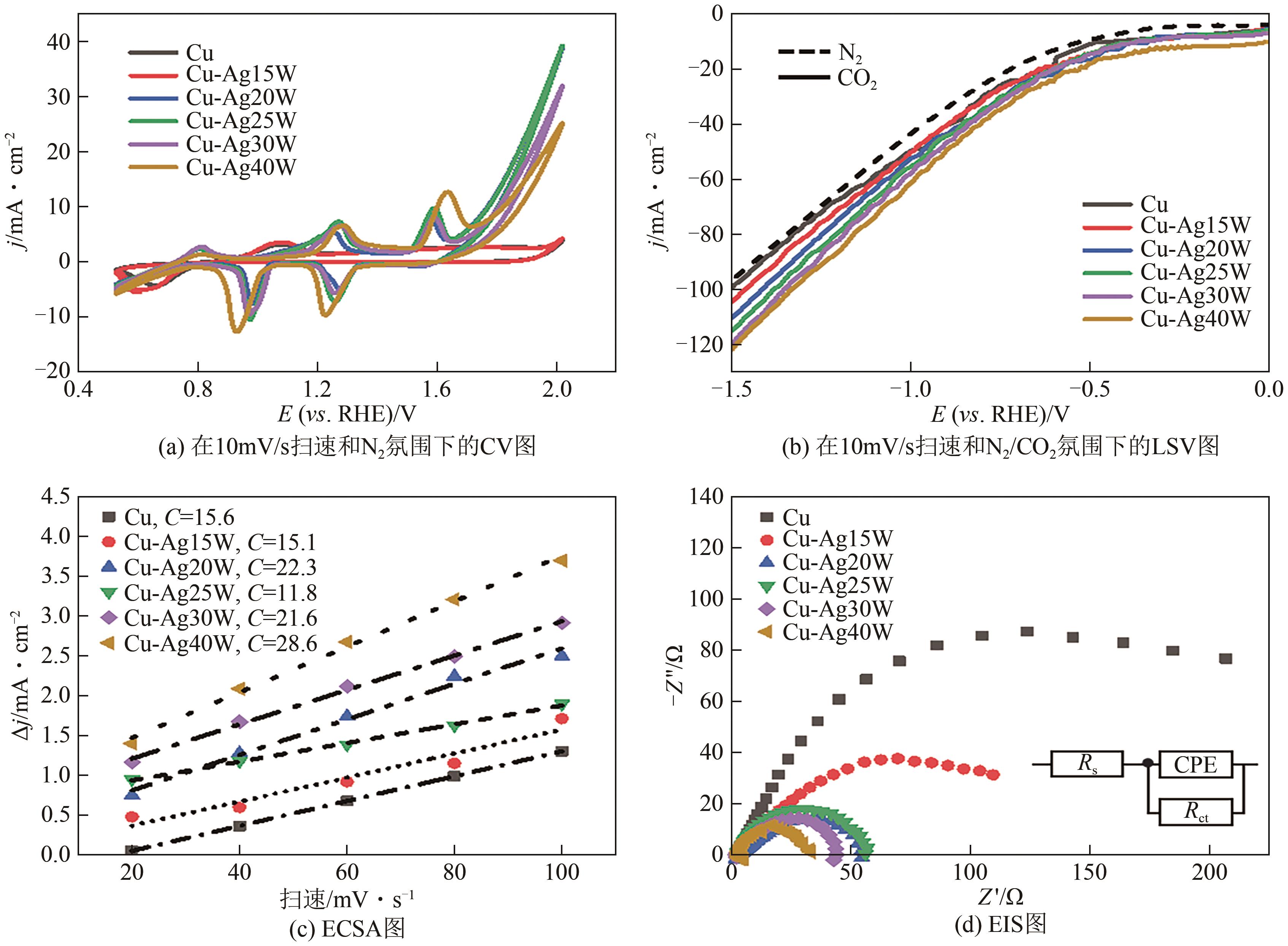
| 1 |
JORDAAN Sarah M, WANG Chao. Electrocatalytic conversion of carbon dioxide for the Paris goals[J]. Nature Catalysis, 2021, 4(11): 915-920.
|
| 2 |
KONDRATENKO Evgenii V, Guido MUL, BALTRUSAITIS Jonas, et al. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes[J]. Energy & Environmental Science, 2013, 6(11): 3112-3135.
|
| 3 |
MA Wenchao, HE Xiaoyang, WANG Wei, et al. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts[J]. Chemical Society Reviews, 2021, 50(23): 12897-12914.
|
| 4 |
KONG Qingquan, AN Xuguang, LIU Qian, et al. Copper-based catalysts for the electrochemical reduction of carbon dioxide: Progress and future prospects[J]. Materials Horizons, 2023, 10(3): 698-721.
|
| 5 |
Ana SOMOZA-TORNOS, GUERRA Omar J, CROW Allison M, et al. Process modeling, techno-economic assessment, and life cycle assessment of the electrochemical reduction of CO2: A review[J]. iScience, 2021, 24(7): 102813.
|
| 6 |
GATTRELL M, GUPTA N, CO A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper[J]. Journal of Electroanalytical Chemistry, 2006, 594(1): 1-19.
|
| 7 |
MA Wenchao, XIE Shunji, LIU Tongtong, et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C—C coupling over fluorine-modified copper[J]. Nature Catalysis, 2020, 3(6): 478-487.
|
| 8 |
KUANG Siyu, SU Yaqiong, LI Minglu, et al. Asymmetrical electrohydrogenation of CO2 to ethanol with copper-gold heterojunctions[J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(4): e2214175120.
|
| 9 |
VASILEFF Anthony, XU Chaochen, JIAO Yan, et al. Surface and interface engineering in copper-based bimetallic materials for selective CO2 electroreduction[J]. Chem, 2018, 4(8): 1809-1831.
|
| 10 |
ZHENG Yiqun, ZHANG Jiawei, MA Zesong, et al. Seeded growth of gold-copper Janus nanostructures as a tandem catalyst for efficient electroreduction of CO2 to C2+ products[J]. Small, 2022, 18(19): 2201695.
|
| 11 |
CAI Zhizhou, CAO Ning, ZHANG Fanxing, et al. Hierarchical Ag-Cu interfaces promote C—C coupling in tandem CO2 electroreduction[J]. Applied Catalysis B: Environmental, 2022, 1325: 122310.
|
| 12 |
XIE Yi, Pengfei OU, WANG Xue, et al. High carbon utilization in CO2 reduction to multi-carbon products in acidic media[J]. Nature Catalysis, 2022, 5(6): 564-570.
|
| 13 |
ZI Xin, ZHOU Yajiao, ZHU Li, et al. Breaking K+ concentration limit on Cu nanoneedles for acidic electrocatalytic CO2 reduction to multi-carbon products[J]. Angewandte Chemie International Edition, 2023, 62(42): e202309351.
|
| 14 |
WANG Pengtang, YANG Hao, TANG Cheng, et al. Boosting electrocatalytic CO2-to-ethanol production via asymmetric C-C coupling[J]. Nature Communications, 2022, 13(1): 3754.
|
| 15 |
ZHANG Tingting, YUAN Bowen, WANG Wenlong, et al. Tailoring *H intermediate coverage on the CuAl2 O4/CuO catalyst for enhanced electrocatalytic CO2 reduction to ethanol[J]. Angewandte Chemie International Edition, 2023, 62(29): e202302096.
|
| 16 |
ALEXEEVA O K, FATEEV V N. Application of the magnetron sputtering for nanostructured electrocatalysts synthesis[J]. International Journal of Hydrogen Energy, 2016, 41(5): 3373-3386.
|
| 17 |
WANG Hongzhi, QIN Ning, LI Yingzhi, et al. Nafion as a facile binder additive stabilizes solid electrolyte interphase on graphite anode[J]. Carbon, 2023, 205: 435-443.
|
| 18 |
SANDHYA Athira Lekshmi Mohandas, PLESKUNOV P, BOGAR Marco, et al. Tuning the morphology of sputter-deposited platinum catalyst: From compact layers to dispersed nanoparticles[J]. Surfaces and Interfaces, 2023, 40: 103079.
|
| 19 |
ZHAO Yu, ZHANG Jie, ZHANG Wusheng, et al. Growth of Ni/Mo/Cu on carbon fiber paper: An efficient electrocatalyst for hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2021, 46(72): 35550-35558.
|
| 20 |
LEE Hyunju, KIM Junhyeong, CHOI Insoo, et al. Nanostructured Ag/In/Cu foam catalyst for electrochemical reduction of CO2 to CO[J]. Electrochimica Acta, 2019, 323: 133102.
|
| 21 |
ZHANG Shuaishuai, ZHAO Shulin, QU Dongxue, et al. Electrochemical reduction of CO2 toward C2 valuables on Cu@Ag core-shell tandem catalyst with tunable shell thickness[J]. Small, 2021, 17(37): 2102293.
|
| 22 |
WANG Zhiqiang, DENG Chenghua, LI Bo, et al. Hierarchical surface-modification of nano-Cu toward one pot H-transfer-coupling-cyclization-CO2 fixation tandem reactions[J]. Materials Horizons, 2024, 11(8): 1957-1963.
|
| 23 |
SUN Min, ZHANG Luxiao, TIAN Fuli, et al. Mechanistic investigation on Ag-Cu2O in electrocatalytic CO2 to CH4 by in situ/operando spectroscopic and theoretical analysis[J]. Journal of Energy Chemistry, 2024, 88: 521-531.
|
| 24 |
ZHANG Tao, LIU Yan, YANG Chuncheng, et al. Monotonically increasing relationship between conversion selectivity from CO2 to CO and the interface area of Cu-Ag biphasic electrochemical catalyst[J]. Journal of Alloys and Compounds, 2023, 947: 169638.
|
| 25 |
ZHANG Yong, CHEN Feifei, HAO Xiaoya, et al. Enhanced interfacial effect-induced asymmetric coupling boost electroreduction of CO2 to ethylene[J]. Applied Catalysis B: Environmental and Energy, 2024, 344: 123666.
|
| 26 |
SHAO Ping, ZHANG Haixia, HONG Qinlong, et al. Enhancing CO2 electroreduction to ethylene via copper-silver tandem catalyst in boron-imidazolate framework nanosheet[J]. Advanced Energy Materials, 2023, 13(19): 2300088.
|
| 27 |
CHATTERJEE Swarnendu, GRIEGO Charles, HART James L, et al. Free standing nanoporous palladium alloys as CO poisoning tolerant electrocatalysts for the electrochemical reduction of CO2 to formate[J]. ACS Catalysis, 2019, 9(6): 5290-5301.
|
| 28 |
KLINKOVA Anna, DE LUNA Phil, DINH Cao-Thang, et al. Rational design of efficient palladium catalysts for electroreduction of carbon dioxide to formate[J]. ACS Catalysis, 2016, 6(12): 8115-8120.
|
| 29 |
LEE Seunghwa, PARK Gibeom, LEE Jaeyoung. Importance of Ag-Cu biphasic boundaries for selective electrochemical reduction of CO2 to ethanol[J]. ACS Catalysis, 2017, 7(12): 8594-8604.
|
| 30 |
CHEN Chunjun, SUN Xiaofu, YAN Xupeng, et al. A strategy to control the grain boundary density and Cu+/Cu0 ratio of Cu-based catalysts for efficient electroreduction of CO2 to C2 products[J]. Green Chemistry, 2020, 22(5): 1572-1576.
|
| 31 |
CUI Wengang, LI Yanting, YU Lei, et al. Zeolite-encapsulated ultrasmall Cu/ZnO x nanoparticles for the hydrogenation of CO2 to methanol[J]. ACS Applied Materials & Interfaces, 2021, 13(16): 18693-18703.
|
| 32 |
CHEN Zhao, SONG Yao, ZHANG Zhenyu, et al. Mechanically induced Cu active sites for selective C-C coupling in CO2 electroreduction[J]. Journal of Energy Chemistry, 2022, 74: 198-202.
|
| 33 |
ZHONG Yongzhi, KONG Xiangdong, SONG Zhimin, et al. Adjusting local CO confinement in porous-shell Ag@Cu catalysts for enhancing C-C coupling toward CO2 eletroreduction[J]. Nano Letters, 2022, 22(6): 2554-2560.
|
| 34 |
LIU Chunxiao, ZHANG Menglu, LI Jiawei, et al. Nanoconfinement engineering over hollow multi-shell structured copper towards efficient electrocatalytical C-C coupling[J]. Angewandte Chemie International Edition, 2022, 61(3): e202113498.
|
| 35 |
JIAO Jiqing, LIN Rui, LIU Shoujie, et al. Copper atom-pair catalyst anchored on alloy nanowires for selective and efficient electrochemical reduction of CO2 [J]. Nature Chemistry, 2019, 11(3): 222-228.
|
| 36 |
ZHANG Jianfang, WANG Yan, LI Zhengyuan, et al. Grain boundary-derived Cu+/Cu0 interfaces in CuO nanosheets for low overpotential carbon dioxide electroreduction to ethylene[J]. Advanced Science, 2022, 9(21): 2200454.
|
| 37 |
刘毅, 房强, 钟达忠, 等. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142.
|
|
LIU Yi, FANG Qiang, ZHONG Dazhong, et al. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142.
|
| 38 |
YUAN Lei, ZENG Shaojuan, ZHANG Xiangping, et al. Advances and challenges of electrolyzers for large-scale CO2 electroreduction[J]. Materials Reports: Energy, 2023, 3(1): 100177.
|
| 39 |
CAO Yufei, CHEN Zhu, LI Peihao, et al. Surface hydroxide promotes CO2 electrolysis to ethylene in acidic conditions[J]. Nature Communications, 2023, 14(1): 2387.
|
| 40 |
CIGNONI Paolo, HOSSEINI Pouya, KAISER Christoph, et al. Validating electrochemical active surface area determination of nanostructured electrodes: Surface oxide reduction on AuPd nanoparticles[J]. Journal of the Electrochemical Society, 2023, 170(11): 116505.
|
| 41 |
JIANG Yong, ZHONG Dazhong, WANG Lei, et al. Roughness effect of Cu on electrocatalytic CO2 reduction towards C2H4 [J]. Chemistry—An Asian Journal, 2022, 17(14): e202200380.
|
| 42 |
ANANTHARAJ Sengeni, NODA Suguru. Appropriate use of electrochemical impedance spectroscopy in water splitting electrocatalysis[J]. ChemElectroChem, 2020, 7(10): 2297-2308.
|
| 43 |
MA Jiamin, LIU Chunmei, BAI Meng, et al. Recent advances in application of tandem catalyst for electrocatalytic CO2 reduction[J]. Molecular Catalysis, 2023, 551: 113632.
|
 ), 万芬2, 伏炫羽1, 范雨婷1, 陈令修3, 李鹏1(
), 万芬2, 伏炫羽1, 范雨婷1, 陈令修3, 李鹏1( )
)
 ), WAN Fen2, FU Xuanyu1, FAN Yuting1, CHEN Lingxiu3, LI Peng1(
), WAN Fen2, FU Xuanyu1, FAN Yuting1, CHEN Lingxiu3, LI Peng1( )
)