化工进展 ›› 2024, Vol. 43 ›› Issue (3): 1484-1491.DOI: 10.16085/j.issn.1000-6613.2023-1722
• 精细化工 • 上一篇
四种烷基咪唑磷酸酯离子液体的热力学性质
刘泽鹏( ), 曾纪珺, 唐晓博, 赵波, 韩升, 廖袁淏, 张伟(
), 曾纪珺, 唐晓博, 赵波, 韩升, 廖袁淏, 张伟( )
)
- 西安近代化学研究所氟氮化工资源高效开发与利用国家重点实验室,陕西 西安 710065
-
收稿日期:2023-09-28修回日期:2023-12-05出版日期:2024-03-10发布日期:2024-04-11 -
通讯作者:张伟 -
作者简介:刘泽鹏(1999—),男,硕士研究生,研究方向为催化反应工程。E-mail:245670889@qq.com。 -
基金资助:陕西省科技厅重点研发计划(2021ZDLGY13-07)
Thermodynamic properties of four alkyl imidazolium phosphate ionic liquids
LIU Zepeng( ), ZENG Jijun, TANG Xiaobo, ZHAO Bo, HAN Sheng, LIAO Yuanhao, ZHANG Wei(
), ZENG Jijun, TANG Xiaobo, ZHAO Bo, HAN Sheng, LIAO Yuanhao, ZHANG Wei( )
)
- State Key Laboratory of Fluorine & Nitrogen Chemicals, Xi’an Modern Chemistry Research Institute, Xi’an 710065, Shaanxi, China
-
Received:2023-09-28Revised:2023-12-05Online:2024-03-10Published:2024-04-11 -
Contact:ZHANG Wei
摘要:
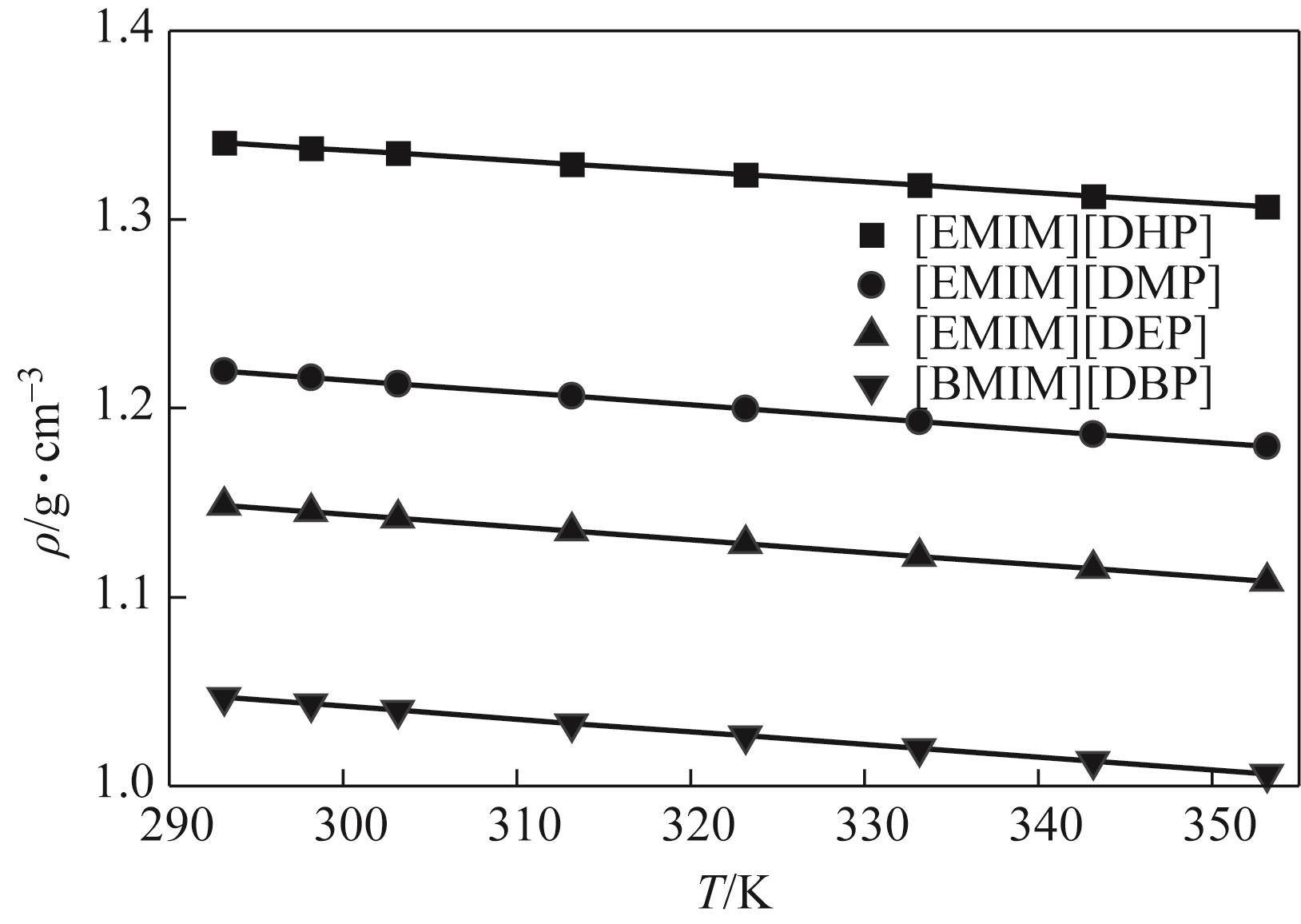
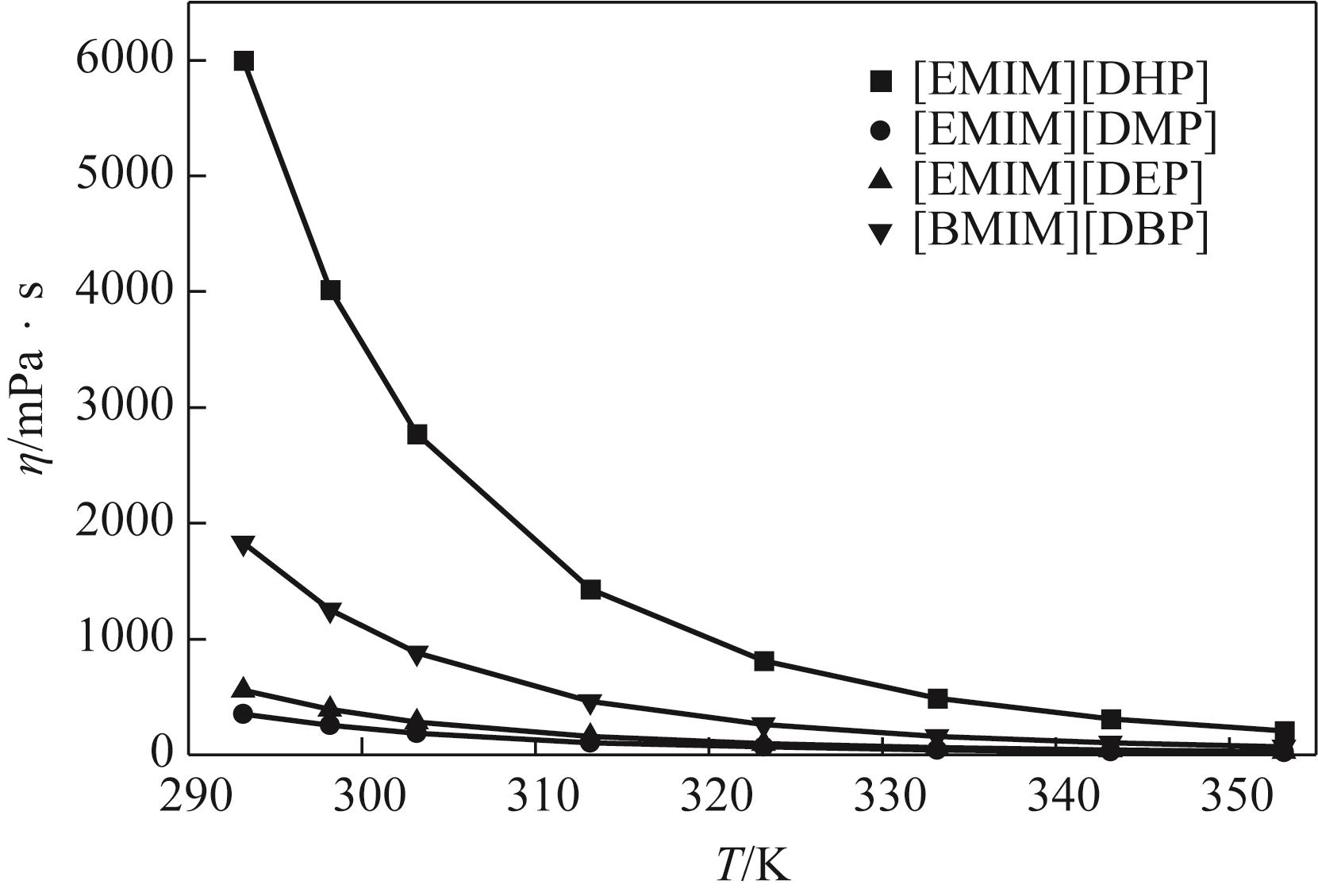
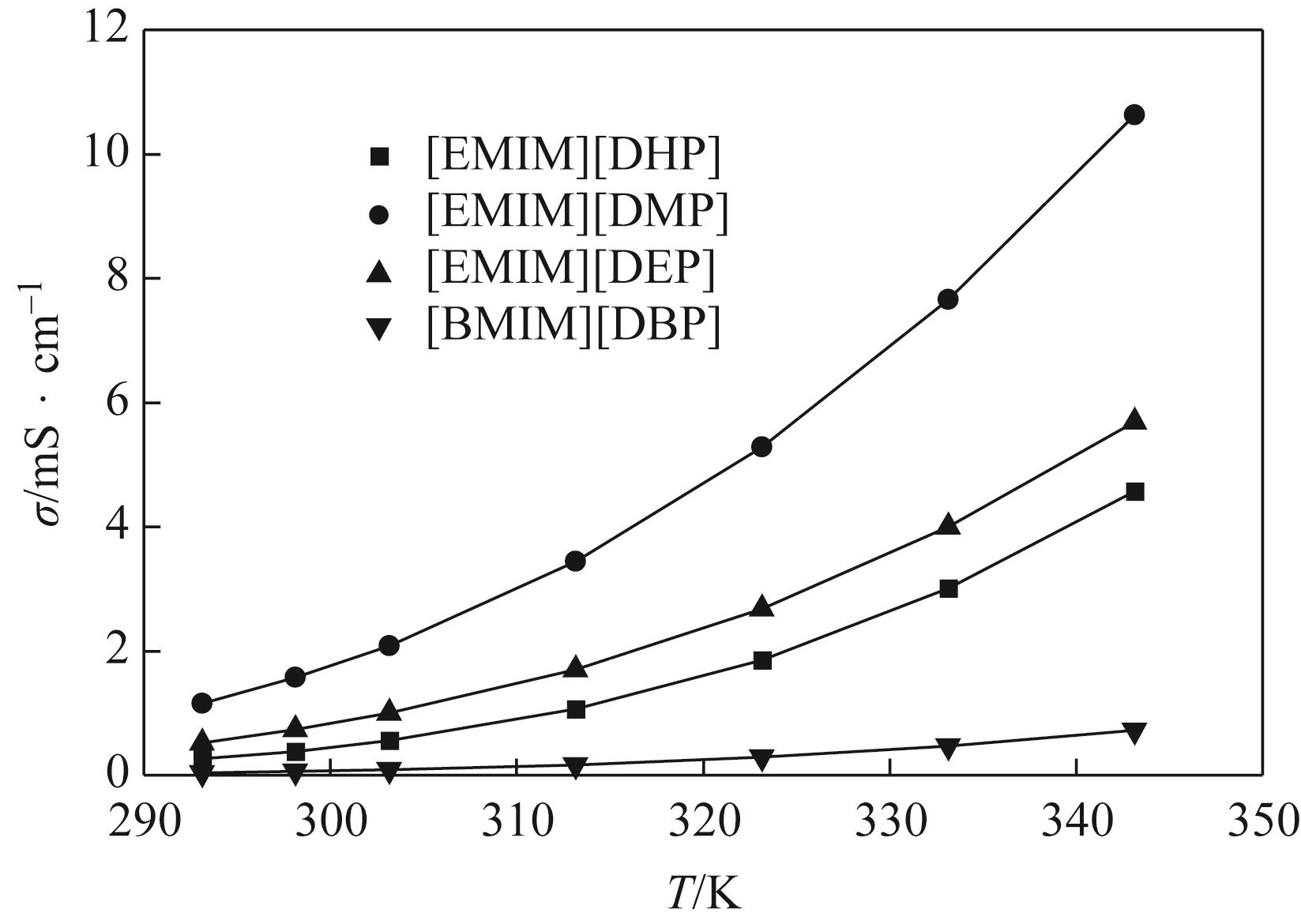
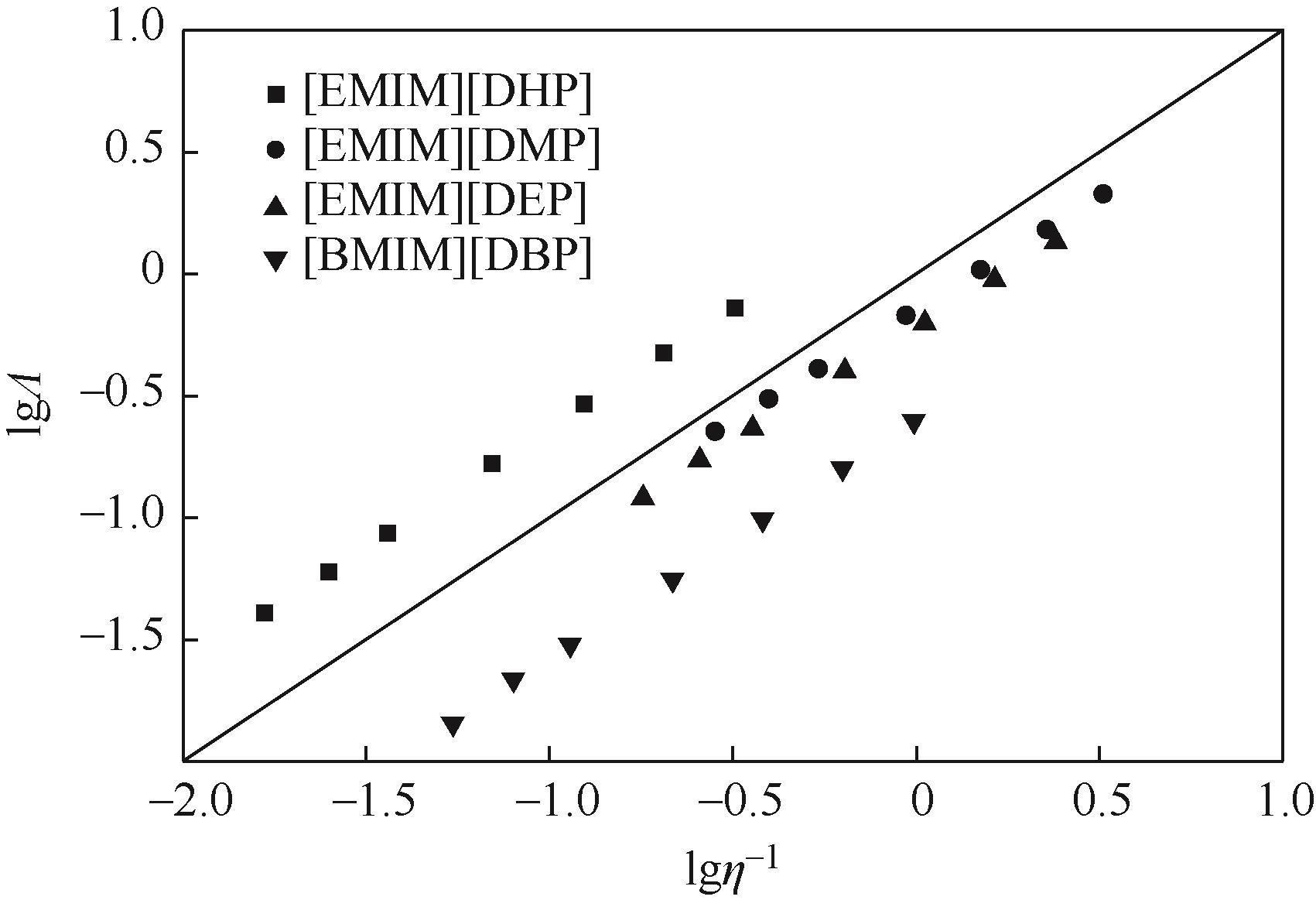
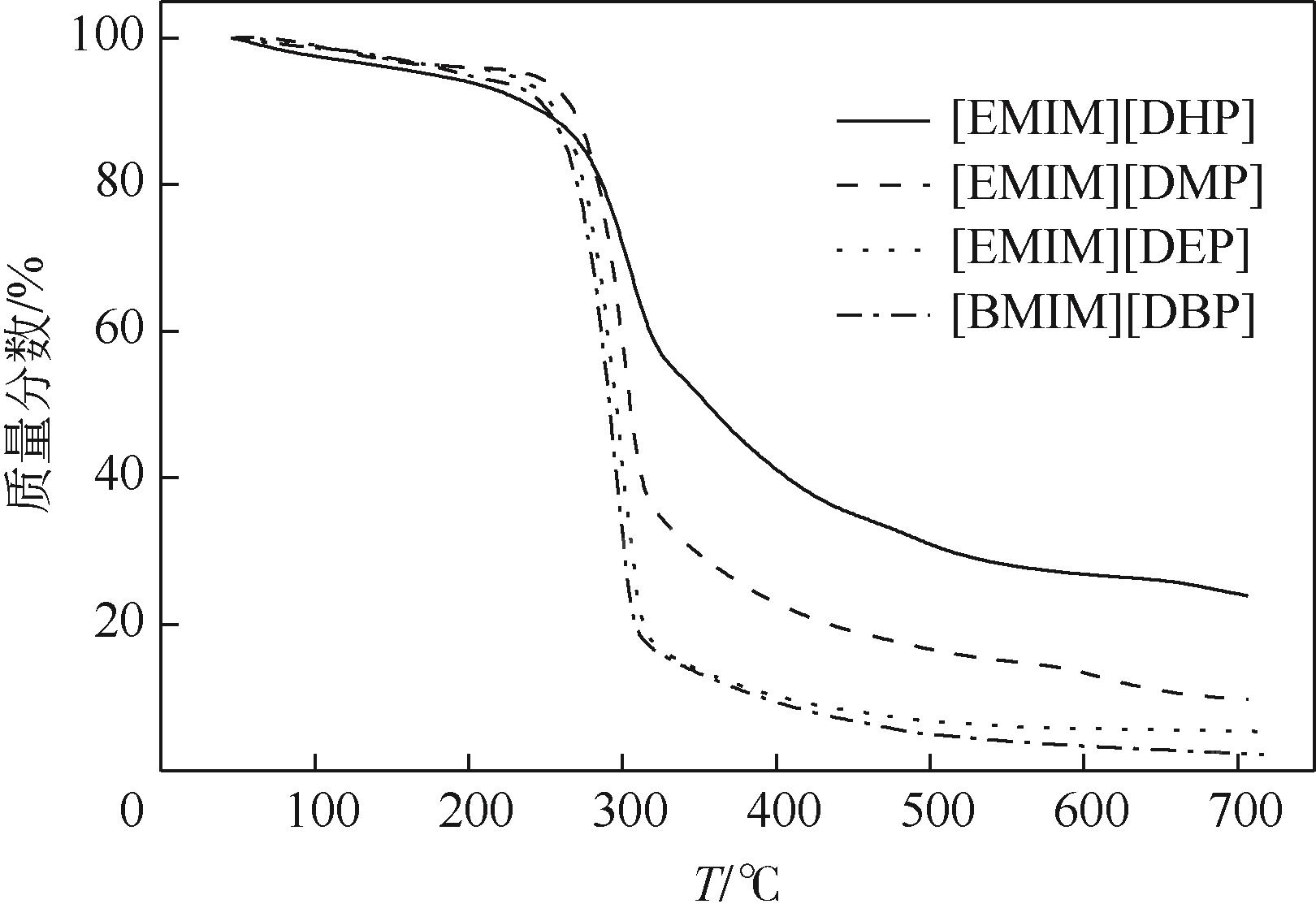
针对烷基咪唑磷酸酯离子液体的热物性数据较少的问题,本文在常压下测定了1-乙基-3-甲基咪唑磷酸二氢盐([EMIM][DHP])、1-乙基-3-甲基咪唑磷酸二甲酯盐([EMIM][DMP])、1-乙基-3-甲基咪唑磷酸二乙酯盐([EMIM][DEP])、1-丁基-3-甲基咪唑磷酸二丁酯盐([BMIM][DBP])四种烷基咪唑磷酸酯离子液体的密度、黏度(293.15~353.15K)和电导率(293.15~343.15K),并且测定了四种离子液体的热稳定性。结果表明,离子液体的密度、黏度随温度的升高而减小,而电导率随温度的升高而增大。采用自然对数方程关联四种离子液体的密度,根据实验值计算到了离子液体体积性质;采用VFT方程关联离子液体黏度和电导率,其中密度与电导率的实验值与模型相关系数R2达到0.9999,黏度相关系数R2达到0.99999,实验测定的数据与模型一致;四种离子液体的热稳定性相近,分解温度均在271.9~278.6℃范围内;瓦尔登规则分析表明,四种烷基咪唑磷酸酯离子液体符合Walden规则,而[EMIM][DMP]和[EMIM][DEP]被归类为“good ionic liquids”。
中图分类号:
引用本文
刘泽鹏, 曾纪珺, 唐晓博, 赵波, 韩升, 廖袁淏, 张伟. 四种烷基咪唑磷酸酯离子液体的热力学性质[J]. 化工进展, 2024, 43(3): 1484-1491.
LIU Zepeng, ZENG Jijun, TANG Xiaobo, ZHAO Bo, HAN Sheng, LIAO Yuanhao, ZHANG Wei. Thermodynamic properties of four alkyl imidazolium phosphate ionic liquids[J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1484-1491.
| 离子液体 | 简式 | 生产厂家 | 纯度(厂家) | 水分/μL·L-1 |
|---|---|---|---|---|
| 1-乙基-3-甲基咪唑磷酸二氢盐 | [EMIM][DHP] | 中国科学院兰州化物所 | ≥99% | 210 |
| 1-乙基-3-甲基咪唑磷酸二甲酯盐 | [EMIM[DMP] | TCI | ≥98% | 220 |
| 1-乙基-3-甲基咪唑磷酸二乙酯盐 | [EMIM][DEP] | TCI | ≥98% | 240 |
| 1-丁基-3-甲基咪唑磷酸二丁酯盐 | [BMIM][DBP] | TCI | ≥98% | 168 |
表1 实验所用离子液体的生产厂家、纯度和水分
| 离子液体 | 简式 | 生产厂家 | 纯度(厂家) | 水分/μL·L-1 |
|---|---|---|---|---|
| 1-乙基-3-甲基咪唑磷酸二氢盐 | [EMIM][DHP] | 中国科学院兰州化物所 | ≥99% | 210 |
| 1-乙基-3-甲基咪唑磷酸二甲酯盐 | [EMIM[DMP] | TCI | ≥98% | 220 |
| 1-乙基-3-甲基咪唑磷酸二乙酯盐 | [EMIM][DEP] | TCI | ≥98% | 240 |
| 1-丁基-3-甲基咪唑磷酸二丁酯盐 | [BMIM][DBP] | TCI | ≥98% | 168 |
| T/K | 密度/g | |||
|---|---|---|---|---|
| [EMIM][DHP] | [EMIM][DMP] | [EMIM][DEP] | [BMIM][DBP] | |
| 293.15 | 1.3406 | 1.2198 | 1.1485 | 1.0468 |
| 298.15 | 1.3379 | 1.2164 | 1.1450 | 1.0435 |
| 303.15 | 1.3350 | 1.2131 | 1.1416 | 1.0401 |
| 313.15 | 1.3295 | 1.2064 | 1.1349 | 1.0332 |
| 323.15 | 1.3238 | 1.1998 | 1.1283 | 1.0265 |
| 333.15 | 1.3181 | 1.1929 | 1.1215 | 1.0198 |
| 343.15 | 1.3125 | 1.1863 | 1.1149 | 1.0131 |
| 353.15 | 1.3070 | 1.1798 | 1.1083 | 1.0064 |
文献值 (298.15) | — | 1.2161[ | 1.1442[ | 1.045[ |
| — | 1.220[ | 1.145[ | 1.045[ | |
| — | 1.2178[ | 1.1449[ | 1.045[ | |
表2 四种离子液体在293.15~353.15K范围内的密度
| T/K | 密度/g | |||
|---|---|---|---|---|
| [EMIM][DHP] | [EMIM][DMP] | [EMIM][DEP] | [BMIM][DBP] | |
| 293.15 | 1.3406 | 1.2198 | 1.1485 | 1.0468 |
| 298.15 | 1.3379 | 1.2164 | 1.1450 | 1.0435 |
| 303.15 | 1.3350 | 1.2131 | 1.1416 | 1.0401 |
| 313.15 | 1.3295 | 1.2064 | 1.1349 | 1.0332 |
| 323.15 | 1.3238 | 1.1998 | 1.1283 | 1.0265 |
| 333.15 | 1.3181 | 1.1929 | 1.1215 | 1.0198 |
| 343.15 | 1.3125 | 1.1863 | 1.1149 | 1.0131 |
| 353.15 | 1.3070 | 1.1798 | 1.1083 | 1.0064 |
文献值 (298.15) | — | 1.2161[ | 1.1442[ | 1.045[ |
| — | 1.220[ | 1.145[ | 1.045[ | |
| — | 1.2178[ | 1.1449[ | 1.045[ | |
| 离子液体 | ρ0/g·cm-3 | R2 | ||
|---|---|---|---|---|
| [EMIM][DHP] | 4.24×10-4 | 1.5184 | 0.99996 | 0.0032% |
| [EMIM][DMP] | 5.56×10-4 | 1.4358 | 0.99998 | 0.0034% |
| [EMIM][DEP] | 5.92×10-4 | 1.3662 | 0.99998 | 0.0033% |
| [BMIM][DBP] | 6.57×10-4 | 1.2691 | 0.99998 | 0.0043% |
表3 离子液体密度的自然对数方程拟合参数、相关系数及平均相对误差
| 离子液体 | ρ0/g·cm-3 | R2 | ||
|---|---|---|---|---|
| [EMIM][DHP] | 4.24×10-4 | 1.5184 | 0.99996 | 0.0032% |
| [EMIM][DMP] | 5.56×10-4 | 1.4358 | 0.99998 | 0.0034% |
| [EMIM][DEP] | 5.92×10-4 | 1.3662 | 0.99998 | 0.0033% |
| [BMIM][DBP] | 6.57×10-4 | 1.2691 | 0.99998 | 0.0043% |
| 离子液体 | M/g∙mol-3 | Vm/nm3 | S0/J∙mol-1∙K-1 | UPOT/kJ∙mol-1 |
|---|---|---|---|---|
| [EMIM][DHP] | 208.15 | 0.2584 | 351.42 | 472.19 |
| [EMIM][DMP] | 236.26 | 0.3225 | 431.40 | 445.93 |
| [EMIM][DEP] | 264.26 | 0.3833 | 507.06 | 426.81 |
| [BMIM][DBP] | 348.42 | 0.5545 | 720.41 | 389.40 |
表4 四种离子液体在298.15K下的分子体积、标准熵及晶格能
| 离子液体 | M/g∙mol-3 | Vm/nm3 | S0/J∙mol-1∙K-1 | UPOT/kJ∙mol-1 |
|---|---|---|---|---|
| [EMIM][DHP] | 208.15 | 0.2584 | 351.42 | 472.19 |
| [EMIM][DMP] | 236.26 | 0.3225 | 431.40 | 445.93 |
| [EMIM][DEP] | 264.26 | 0.3833 | 507.06 | 426.81 |
| [BMIM][DBP] | 348.42 | 0.5545 | 720.41 | 389.40 |
| T/K | 黏度/mPa | |||
|---|---|---|---|---|
| [EMIM][DHP] | [EMIM][DMP] | [EMIM][DEP] | [BMIM][DBP] | |
| 293.15 | 5996.100 | 353.387 | 555.920 | 1829.600 |
| 298.15 | 4014.100 | 251.860 | 388.783 | 1253.267 |
| 303.15 | 2774.167 | 184.733 | 279.847 | 879.270 |
| 313.15 | 1433.633 | 106.600 | 156.193 | 462.327 |
| 323.15 | 808.673 | 66.478 | 94.413 | 262.087 |
| 333.15 | 488.687 | 44.148 | 61.017 | 158.613 |
| 343.15 | 312.090 | 30.875 | 41.605 | 101.400 |
| 353.15 | 208.983 | 22.512 | 29.666 | 67.920 |
| 文献值(303.15) | — | 192.7[ | 285.0[ | — |
| — | 186.0[ | 274.0[ | — | |
| — | 194.5[ | 284.0[ | — | |
表5 四种烷基咪唑磷酸酯离子液体在293.15~353.15K范围内的黏度
| T/K | 黏度/mPa | |||
|---|---|---|---|---|
| [EMIM][DHP] | [EMIM][DMP] | [EMIM][DEP] | [BMIM][DBP] | |
| 293.15 | 5996.100 | 353.387 | 555.920 | 1829.600 |
| 298.15 | 4014.100 | 251.860 | 388.783 | 1253.267 |
| 303.15 | 2774.167 | 184.733 | 279.847 | 879.270 |
| 313.15 | 1433.633 | 106.600 | 156.193 | 462.327 |
| 323.15 | 808.673 | 66.478 | 94.413 | 262.087 |
| 333.15 | 488.687 | 44.148 | 61.017 | 158.613 |
| 343.15 | 312.090 | 30.875 | 41.605 | 101.400 |
| 353.15 | 208.983 | 22.512 | 29.666 | 67.920 |
| 文献值(303.15) | — | 192.7[ | 285.0[ | — |
| — | 186.0[ | 274.0[ | — | |
| — | 194.5[ | 284.0[ | — | |
| 离子液体 | R2 | ||||
|---|---|---|---|---|---|
| [EMIM][DHP] | 0.2500 | 1219.0 | 172.28 | >0.99999 | 0.25% |
| [EMIM][DMP] | 0.1380 | 870.1 | 182.24 | >0.99999 | 0.03% |
| [EMIM][DEP] | 0.1150 | 963.3 | 179.58 | >0.99999 | 0.04% |
| [BMIM][DBP] | 0.0287 | 1559.6 | 152.19 | >0.99999 | 0.21% |
表6 离子液体黏度的VFT方程拟合参数、相关系数及平均相对误差
| 离子液体 | R2 | ||||
|---|---|---|---|---|---|
| [EMIM][DHP] | 0.2500 | 1219.0 | 172.28 | >0.99999 | 0.25% |
| [EMIM][DMP] | 0.1380 | 870.1 | 182.24 | >0.99999 | 0.03% |
| [EMIM][DEP] | 0.1150 | 963.3 | 179.58 | >0.99999 | 0.04% |
| [BMIM][DBP] | 0.0287 | 1559.6 | 152.19 | >0.99999 | 0.21% |
| T/K | 电导率/mS | |||
|---|---|---|---|---|
| [EMIM][DHP] | [EMIM][DMP] | [EMIM][DEP] | [BMIM][DBP] | |
| 293.15 | 0.262 | 1.161 | 0.521 | 0.043 |
| 298.15 | 0.384 | 1.574 | 0.742 | 0.065 |
| 303.15 | 0.556 | 2.088 | 1.002 | 0.090 |
| 313.15 | 1.069 | 3.437 | 1.706 | 0.165 |
| 323.15 | 1.859 | 5.277 | 2.681 | 0.291 |
| 333.15 | 3.005 | 7.658 | 4.003 | 0.468 |
| 343.15 | 4.575 | 10.63 | 5.686 | 0.724 |
表7 四种离子液体在293.15~343.15 K范围内的电导率
| T/K | 电导率/mS | |||
|---|---|---|---|---|
| [EMIM][DHP] | [EMIM][DMP] | [EMIM][DEP] | [BMIM][DBP] | |
| 293.15 | 0.262 | 1.161 | 0.521 | 0.043 |
| 298.15 | 0.384 | 1.574 | 0.742 | 0.065 |
| 303.15 | 0.556 | 2.088 | 1.002 | 0.090 |
| 313.15 | 1.069 | 3.437 | 1.706 | 0.165 |
| 323.15 | 1.859 | 5.277 | 2.681 | 0.291 |
| 333.15 | 3.005 | 7.658 | 4.003 | 0.468 |
| 343.15 | 4.575 | 10.63 | 5.686 | 0.724 |
| 离子液体 | C/K | R2 | |||
|---|---|---|---|---|---|
| [EMIM][DHP] | 2382.9 | 994.1 | 184.23 | >0.99999 | 0.34% |
| [EMIM][DMP] | 1617.5 | 821.5 | 179.67 | >0.99999 | 0.05% |
| [EMIM][DEP] | 1233.4 | 880.2 | 179.54 | 0.99998 | 0.57% |
| [BMIM][DBP] | 1550.0 | 1433.6 | 156.22 | 0.99995 | 1.01% |
表8 离子液体电导率的VFT方程拟合参数、相关系数及平均相对误差
| 离子液体 | C/K | R2 | |||
|---|---|---|---|---|---|
| [EMIM][DHP] | 2382.9 | 994.1 | 184.23 | >0.99999 | 0.34% |
| [EMIM][DMP] | 1617.5 | 821.5 | 179.67 | >0.99999 | 0.05% |
| [EMIM][DEP] | 1233.4 | 880.2 | 179.54 | 0.99998 | 0.57% |
| [BMIM][DBP] | 1550.0 | 1433.6 | 156.22 | 0.99995 | 1.01% |
| 参数 | [EMIM][DHP] | [EMIM][DMP] | [EMIM][DEP] | [BMIM][DBP] |
|---|---|---|---|---|
| Tstart/℃ | 184.8 | 198.0 | 190.9 | 151.4 |
| Tonset/℃ | 275.8 | 278.6 | 274.5 | 271.9 |
表9 四种离子液体的Tstart和Tonset
| 参数 | [EMIM][DHP] | [EMIM][DMP] | [EMIM][DEP] | [BMIM][DBP] |
|---|---|---|---|---|
| Tstart/℃ | 184.8 | 198.0 | 190.9 | 151.4 |
| Tonset/℃ | 275.8 | 278.6 | 274.5 | 271.9 |
| 1 | 刘洁, 牟浩文, 李文深. 1-丁基-3-甲基咪唑硫酸氢盐离子液体水溶液的密度、黏度与温度、组成的关系[J]. 石油学报(石油加工), 2019, 35(5): 995-1000. |
| LIU Jie, MOU Haowen, LI Wenshen. Temperature and composition dependence on the density and viscosity of aqueous solutions of 1-butyl-3-methylimidazolium hydrosulphate ionic liquid[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2019, 35(5): 995-1000. | |
| 2 | 何丽娟, 王飒, 吴夏梦, 等. 离子液体[BMP][Tf2N]基本物性的实验研究[J]. 工程热物理学报, 2021, 42(6): 1378-1383. |
| HE Lijuan, WANG Sa, WU Xiameng, et al. Experimental study on basic physical properties of ionic liquid[BMP][Tf2N[J]. Journal of Engineering Thermophysics, 2021, 42(6): 1378-1383. | |
| 3 | 厉刚, 孙甜甜. 四种叠氮铵类离子液体的合成及其物性研究[J]. 含能材料, 2014, 22(2): 136-140. |
| LI Gang, SUN Tiantian. Synthesis and physicochemical properties of four azidoammonium-based ionic liquids[J]. Chinese Journal of Energetic Materials, 2014, 22(2): 136-140. | |
| 4 | 王西亚, 陈俊, 左家家, 等. 离子液体在电催化领域的研究进展[J]. 当代化工研究, 2022(16): 163-165. |
| WANG Xiya, CHEN Jun, ZUO Jiajia, et al. Research progress of ionic liquids in electrocatalysis[J]. Modern Chemical Research, 2022(16): 163-165. | |
| 5 | 张芳芳, 郑飞飞, 吴学红, 等. 离子液体[EMIm]Ac比热容、导热系数及黏度的研究[J]. 工程热物理学报, 2018, 39(8): 1809-1813. |
| ZHANG Fangfang, ZHENG Feifei, WU Xuehong, et al. Research of ionic liquid[EMIm]Ac specific heat capacity, thermal conductivity and viscosity[J]. Journal of Engineering Thermophysics, 2018, 39(8): 1809-1813. | |
| 6 | SENG Leong Kok, MASDAR Mohd Shahbudin, SHYUAN Loh Kee. Ionic liquid in phosphoric acid-doped polybenzimidazole (PA-PBI) as electrolyte membranes for PEM fuel cells: A review[J]. Membranes, 2021, 11(10): 728. |
| 7 | MOHAMMED Sulafa Abdalmageed Saadaldeen, YAHYA Wan Zaireen Nisa, BUSTAM Mohamad Azmi, et al. Elucidation of the roles of ionic liquid in CO2 electrochemical reduction to value-added chemicals and fuels[J]. Molecules, 2021, 26(22): 6962. |
| 8 | 王吉林, 王璐璐. 离子液体1-丁基-3-甲基咪唑磷酸二丁酯的制备及其催化酯化反应的性能[J]. 石油化工, 2011, 40(6): 635-639. |
| WANG Jilin, WANG Lulu. Synthesis of 1-butyl-3-methyl imidazolium dibutyl phosphate ionic liquid and its catalytic activity in esterification[J]. Petrochemical Technology, 2011, 40(6): 635-639. | |
| 9 | XING Lu, MA Xifei, HU Kaibo, et al. Selective separation of Nd from La/Ce/Pr using phosphate-based ionic liquids: Solvent extraction studies and density functional theory[J]. Minerals Engineering, 2023, 191: 107967. |
| 10 | THOMAS Marie F, LI Luen-Luen, HANDLEY-PENDLETON Jocelyn M, et al. Enzyme activity in dialkyl phosphate ionic liquids[J]. Bioresource Technology, 2011, 102(24): 11200-11203. |
| 11 | 卢向军, 窦辉, 戴耀东, 等. 磷酸酯盐离子液体的微波辅助合成及其在Knoevenagel反应中的应用[J]. 南京航空航天大学学报, 2009, 41(3): 414-417. |
| LU Xiangjun, DOU Hui, DAI Yaodong, et al. Microwave-assisted synthesis of ionic liquids of phosphate and application in Knoevenagel reaction[J]. Journal of Nanjing University of Aeronautics & Astronautics, 2009, 41(3): 414-417. | |
| 12 | 冯婕, 李春喜, 孟洪, 等. 磷酸酯类离子液体在燃油深度脱硫中的应用[J]. 石油化工, 2006, 35(3): 272-276. |
| FENG Jie, LI Chunxi, MENG Hong, et al. Application of phosphate ionic liquids in deep desulfurization of fuel[J]. Petrochemical Technology, 2006, 35(3): 272-276. | |
| 13 | 蒋小川, 于春影, 冯婕, 等. 离子液体1-丁基-3-甲基咪唑磷酸二丁酯的制备与应用[J]. 北京化工大学学报(自然科学版), 2006, 33(1): 5-7. |
| JIANG Xiaochuan, YU Chunying, FENG Jie, et al. Synthesis and application of ionic liquid 1-butyl-3-methyl imidazolium dibutyl phosphate[J]. Journal of Beijing University of Chemical Technology (Natural Science Edition), 2006, 33(1): 5-7. | |
| 14 | 李静, 王克良, 吴红, 等. [DMIM]DMP萃取精馏分离丙酮和甲醇共沸体系的研究[J]. 天然气化工(C1化学与化工), 2017, 42(4): 46-50, 61. |
| LI Jing, WANG Keliang, WU Hong, et al. Extractive distillation of acetone and methanol azeotrope using[DMIM]DMP as solvent[J]. Natural Gas Chemical Industry, 2017, 42(4): 46-50, 61. | |
| 15 | HAN Yunyan, QIAO Dan, GUO Yuexia, et al. Influence of competitive adsorption on lubricating property of phosphonate ionic liquid additives in PEG[J]. Tribology Letters, 2016, 64(2): 22. |
| 16 | HAN Yunyan, QIAO Dan, ZHANG Lin, et al. Study of tribological performance and mechanism of phosphonate ionic liquids for steel/aluminum contact[J]. Tribology International, 2015, 84: 71-80. |
| 17 | HAN Yunyan, QIAO Dan, ZHANG Songwei, et al. Influence of phosphate and phosphonate ionic liquid structures on lubrication for different alloys (Mg, Al, Cu)[J]. Tribology International, 2017, 114: 469-477. |
| 18 | Erika VATAŠČIN, DOHNAL Vladimír. Phase equilibria and energetics of binary mixtures of water with highly hydrophilic[EMIM]-based ionic liquids: Methanesulfonate, methylsulfate, and dimethylphosphate[J]. Fluid Phase Equilibria, 2020, 521: 112659. |
| 19 | WANG Junfeng, LI Chunxi, SHEN Chong, et al. Towards understanding the effect of electrostatic interactions on the density of ionic liquids[J]. Fluid Phase Equilibria, 2009, 279(2): 87-91. |
| 20 | SKONIECZNY Michał, Marta KRÓLIKOWSKA. Thermodynamic properties of{diethyl phosphate-based ionic liquid (1) + ethanol (2)} systems, experimental data and correlation[J]. Journal of Chemical & Engineering Data, 2022, 67(4): 869-885. |
| 21 | Mac DOWELL N, LLOVELL F, SUN N, et al. New experimental density data and soft-SAFT models of alkylimidazolium ([C n C1im]+) chloride (Cl-), methylsulfate ([MeSO4]-), and dimethylphosphate ([Me2PO4]-) based ionic liquids[J]. The Journal of Physical Chemistry B, 2014, 118(23): 6206-6221. |
| 22 | Vojtěch ŠTEJFA, Jan ROHLÍČEK, Ctirad ČERVINKA. Phase behaviour and heat capacities of selected 1-ethyl-3-methylimidazolium-based ionic liquids[J]. The Journal of Chemical Thermodynamics, 2020, 142: 106020. |
| 23 | Vojtěch ŠTEJFA, Jan ROHLÍČEK, Ctirad ČERVINKA. Phase behaviour and heat capacities of selected 1-ethyl-3-methylimidazolium-based ionic liquids II[J]. The Journal of Chemical Thermodynamics, 2021, 160: 106392. |
| 24 | GONG Yinhui, SHEN Chong, LU Yingzhou, et al. Viscosity and density measurements for six binary mixtures of water (methanol or ethanol) with an ionic liquid ([BMIM][DMP]or[EMIM][DMP]) at atmospheric pressure in the temperature range of (293.15 to 333.15) K[J]. Journal of Chemical & Engineering Data, 2012, 57(1): 33-39. |
| 25 | Edward ZORĘBSKI, Małgorzata MUSIAŁ, Karolina BAŁUSZYŃSKA, et al. Isobaric and isochoric heat capacities as well as isentropic and isothermal compressibilities of di- and trisubstituted imidazolium-based ionic liquids as a function of temperature[J]. Industrial & Engineering Chemistry Research, 2018, 57(14): 5161-5172. |
| 26 | CHENG S, MUSIAŁ M, WOJNAROWSKA Z, et al. Universal scaling behavior of entropy and conductivity in ionic liquids[J]. Journal of Molecular Liquids, 2020, 316: 113824. |
| 27 | LIU Qingshan, LI Peipei, Urs WELZ-BIERMANN, et al. Density, electrical conductivity, and dynamic viscosity of N-alkyl-4-methylpyridinium bis(trifluoromethylsulfonyl)imide[J]. Journal of Chemical & Engineering Data, 2012, 57(11): 2999-3004. |
| 28 | ZHANG Qingguo, WEI Ying, SUN Sisi, et al. Study on thermodynamic properties of ionic liquid N-butyl-3-methylpyridinium bis(trifluoromethylsulfonyl)imide[J]. Journal of Chemical & Engineering Data, 2012, 57(8): 2185-2190. |
| 29 | 王义闹, 吴利丰. 基于平均相对误差绝对值最小的GM(1, 1)建模[J]. 华中科技大学学报(自然科学版), 2009, 37(10): 29-31. |
| WANG Yinao, WU Lifeng. Modeling GM(1, 1) based on the minimum of mean absolute percentage error[J]. Journal of Huazhong University of Science and Technology (Nature Science Edition), 2009, 37(10): 29-31. | |
| 30 | GLASSER Leslie. Lattice and phase transition thermodynamics of ionic liquids[J]. Thermochimica Acta, 2004, 421(1/2): 87-93. |
| 31 | FREIRE Mara G, TELES Ana Rita R, ROCHA Marisa A A, et al. Thermophysical characterization of ionic liquids able to dissolve biomass[J]. Journal of Chemical & Engineering Data, 2011, 56(12): 4813-4822. |
| 32 | HIRAGA Yuya, KATO Aya, SATO Yoshiyuki, et al. Densities at pressures up to 200 MPa and atmospheric pressure viscosities of ionic liquids 1-ethyl-3-methylimidazolium methylphosphate, 1-ethyl-3-methylimidazolium diethylphosphate, 1-butyl-3-methylimidazolium acetate, and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide[J]. Journal of Chemical & Engineering Data, 2015, 60(3): 876-885. |
| 33 | DE PABLO Laura, SEGOVIA PURAS José Juan, Carmen MARTÍN, et al. Determination of density and viscosity of binary mixtures of water and dimethyl sulfoxide with 1-ethyl-3-methylimidazolium diethylphosphate[EtMeIm]+[Et2PO4]– at atmospheric pressure[J]. Journal of Chemical & Engineering Data, 2018, 63(4): 1053-1064. |
| 34 | WAN NORMAZLAN Wan Melissa Diyana, SAIRI Nor Asrina, ALIAS Yatimah, et al. Composition and temperature dependence of density, surface tension, and viscosity of emim dep/mmim dmp + water + 1-propanol/2-propanol ternary mixtures and their mathematical representation using the jouyban-acree model[J]. Journal of Chemical & Engineering Data, 2014, 59(8): 2337-2348. |
| 35 | BITTNER Bożena, WROBEL Rafal J, MILCHERT Eugeniusz. Physical properties of pyridinium ionic liquids[J]. The Journal of Chemical Thermodynamics, 2012, 55: 159-165. |
| 36 | LIU Qingshan, LIU Jing, LIU Xiaoxia, et al. Density, dynamic viscosity, and electrical conductivity of two hydrophobic functionalized ionic liquids[J]. The Journal of Chemical Thermodynamics, 2015, 90: 39-45. |
| 37 | ANOUTI Mérièm, Magaly CAILLON-CARAVANIER, DRIDI Yosra, et al. Synthesis and characterization of new pyrrolidinium based protic ionic liquids. good and superionic liquids[J]. The Journal of Physical Chemistry B, 2008, 112(42): 13335-13343. |
| 38 | HEYM F, KORTH W, ETZOLD B J M, et al. Determination of vapor pressure and thermal decomposition using thermogravimetrical analysis[J]. Thermochimica Acta, 2015, 622: 9-17. |
| [1] | 丁丽华, 徐洪涛, 张晨宇. 基于圆台波浪形换热管的潜热储热单元性能分析[J]. 化工进展, 2024, 43(3): 1214-1223. |
| [2] | 刘泽鹏, 曾纪珺, 廖袁淏, 唐晓博, 赵波, 韩升, 张伟. 离子液体1-乙基-3-甲基咪唑亚磷酸甲酯盐与1-乙基-3-甲基咪唑亚磷酸乙酯盐的热物性[J]. 化工进展, 2024, 43(2): 1054-1062. |
| [3] | 王一笑, 张丹, 涂茂萍, 周文博, 赵冰超. 双膜量子点表面热流密度场测量技术[J]. 化工进展, 2024, 43(2): 872-881. |
| [4] | 叶振东, 刘涵, 吕静, 张亚宁, 刘洪芝. 基于钙镁二元盐的热化学储能反应器的性能优化[J]. 化工进展, 2023, 42(8): 4307-4314. |
| [5] | 陈蔚阳, 宋欣, 殷亚然, 张先明, 朱春英, 付涛涛, 马友光. 矩形微通道内液相黏度对气泡界面的作用机制[J]. 化工进展, 2023, 42(7): 3468-3477. |
| [6] | 谢志伟, 吴张永, 朱启晨, 蒋佳骏, 梁天祥, 刘振阳. 植物油基Ni0.5Zn0.5Fe2O4磁流体的黏度特性及磁黏特性[J]. 化工进展, 2023, 42(7): 3623-3633. |
| [7] | 孙征楠, 李洪晶, 荆国林, 张福宁, 颜飚, 刘晓燕. EVA及其改性聚合物在原油降凝剂领域的应用[J]. 化工进展, 2023, 42(6): 2987-2998. |
| [8] | 赵毅, 杨臻, 张新为, 王刚, 杨旋. 不同裂缝损伤和愈合温度条件下沥青自愈合行为的分子模拟[J]. 化工进展, 2023, 42(6): 3147-3156. |
| [9] | 李云闯, 谢方明, 席亚男, 万新月, 孙玉虎, 赵永峰, 李根, 刘宏海, 高雄厚, 刘洪涛. 高水热稳定性介孔分子筛的低成本合成研究进展[J]. 化工进展, 2023, 42(4): 1877-1884. |
| [10] | 王钰琢, 李刚. 硫、氮共掺杂三维石墨烯的全固态超级电容器[J]. 化工进展, 2023, 42(4): 1974-1982. |
| [11] | 李光文, 华渠成, 黄作鑫, 达志坚. 聚甲基丙烯酸酯类黏度指数改进剂的研究进展[J]. 化工进展, 2023, 42(3): 1562-1571. |
| [12] | 张晨光, 封硕, 邢玉烨, 沈伯雄, 苏立超. 柴油车用NH3-SCR铜基分子筛催化剂孤立态Cu2+研究进展[J]. 化工进展, 2023, 42(3): 1321-1331. |
| [13] | 蒋佳骏, 吴张永, 朱启晨, 蔡昌礼, 朱家军, 王志强. In-Bi-Sn基Si3N4/GNFs混合纳米流体的流变性和润滑性[J]. 化工进展, 2023, 42(12): 6197-6206. |
| [14] | 崔腾达, 文华, 赵颖. 改性液滴撞击荷叶表面沉积特性对比[J]. 化工进展, 2023, 42(11): 5882-5890. |
| [15] | 米泽豪, 花儿. 多元胺-TFSA型质子化离子液体吸收CO2的理论分析[J]. 化工进展, 2023, 42(11): 6015-6030. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||