化工进展 ›› 2023, Vol. 42 ›› Issue (11): 6015-6030.DOI: 10.16085/j.issn.1000-6613.2022-2319
• 资源与环境化工 • 上一篇
多元胺-TFSA型质子化离子液体吸收CO2的理论分析
- 1.北方民族大学化学与化学工程学院,银川 宁夏 750021
2.国家民委化工技术基础重点实验室
1.宁夏 银川 750021,宁夏太阳能化学转化技术重点实验室,宁夏 银川 750021
-
收稿日期:2022-12-15修回日期:2023-03-07出版日期:2023-11-20发布日期:2023-12-15 -
通讯作者:花儿 -
作者简介:米泽豪(1998—),男,硕士研究生,研究方向为离子液体。E-mail:875501689@qq.com。 -
基金资助:宁夏高等学校研究项目(NGY2020063)
Theoretical analysis of CO2 absorption by polyamines-TFSA type protic ionic liquids
- 1.Chemical Science and Engineering College, North Minzu University, Yinchuan 750021, Ningxia, China
2.Key Laboratory of Chemical Engineering and Technology, State Ethnic Affairs Commission, Yinchuan 750021, Ningxia, China
3.Ningxia Key Labaratory of Solar Chemical Conversion Technology, Yinchuan 750021, Ningxia, China
-
Received:2022-12-15Revised:2023-03-07Online:2023-11-20Published:2023-12-15 -
Contact:HUA Er
摘要:
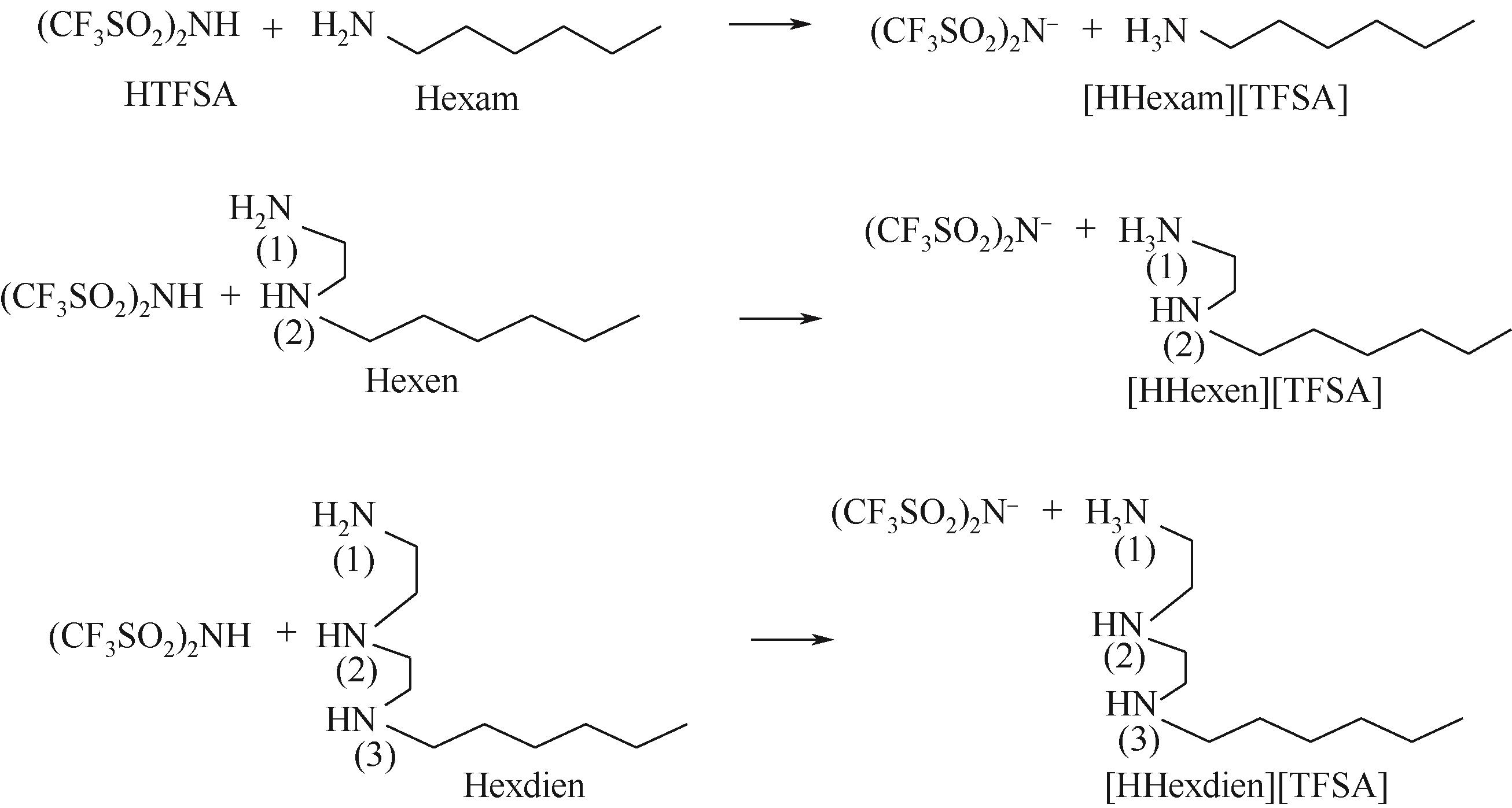
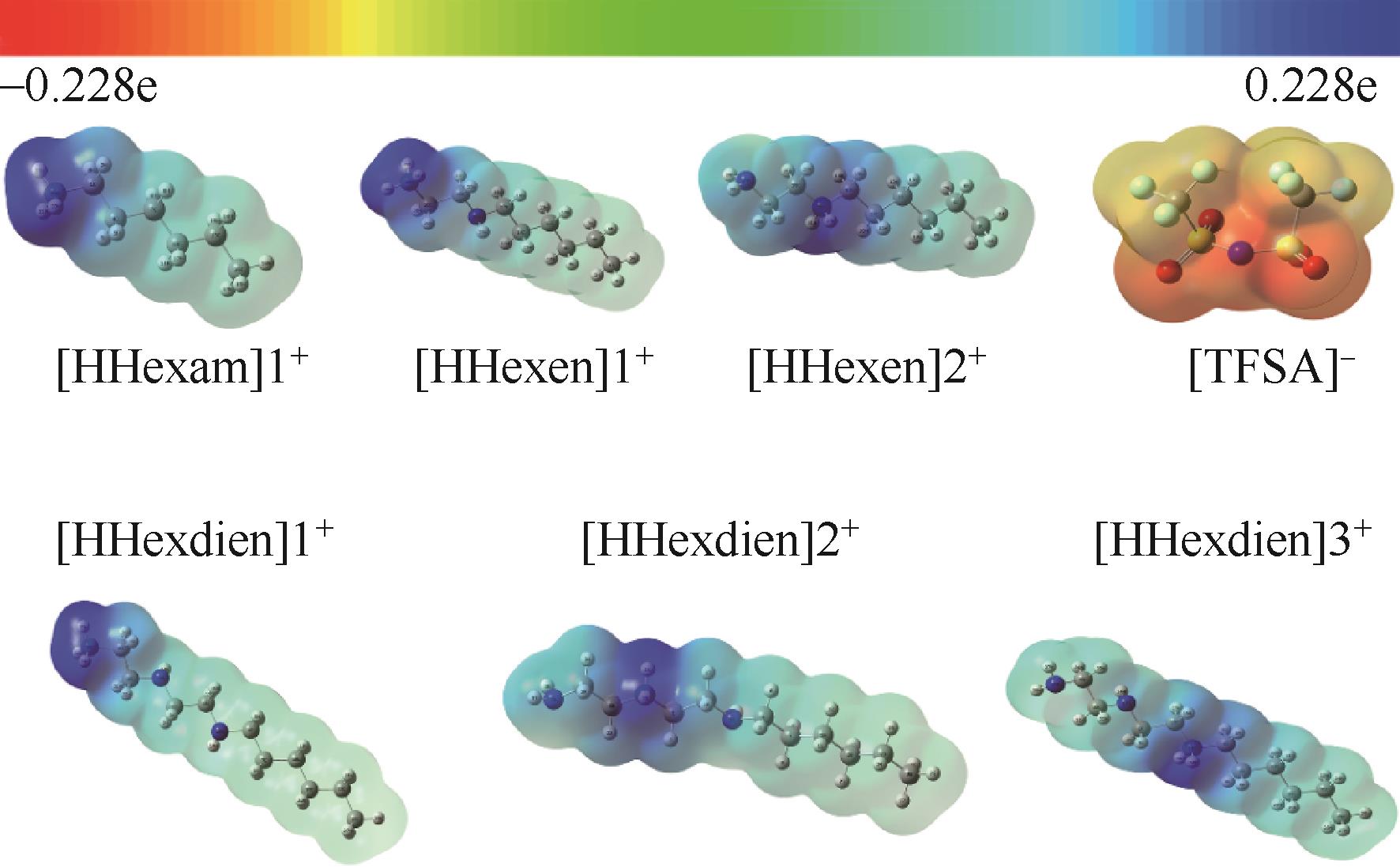
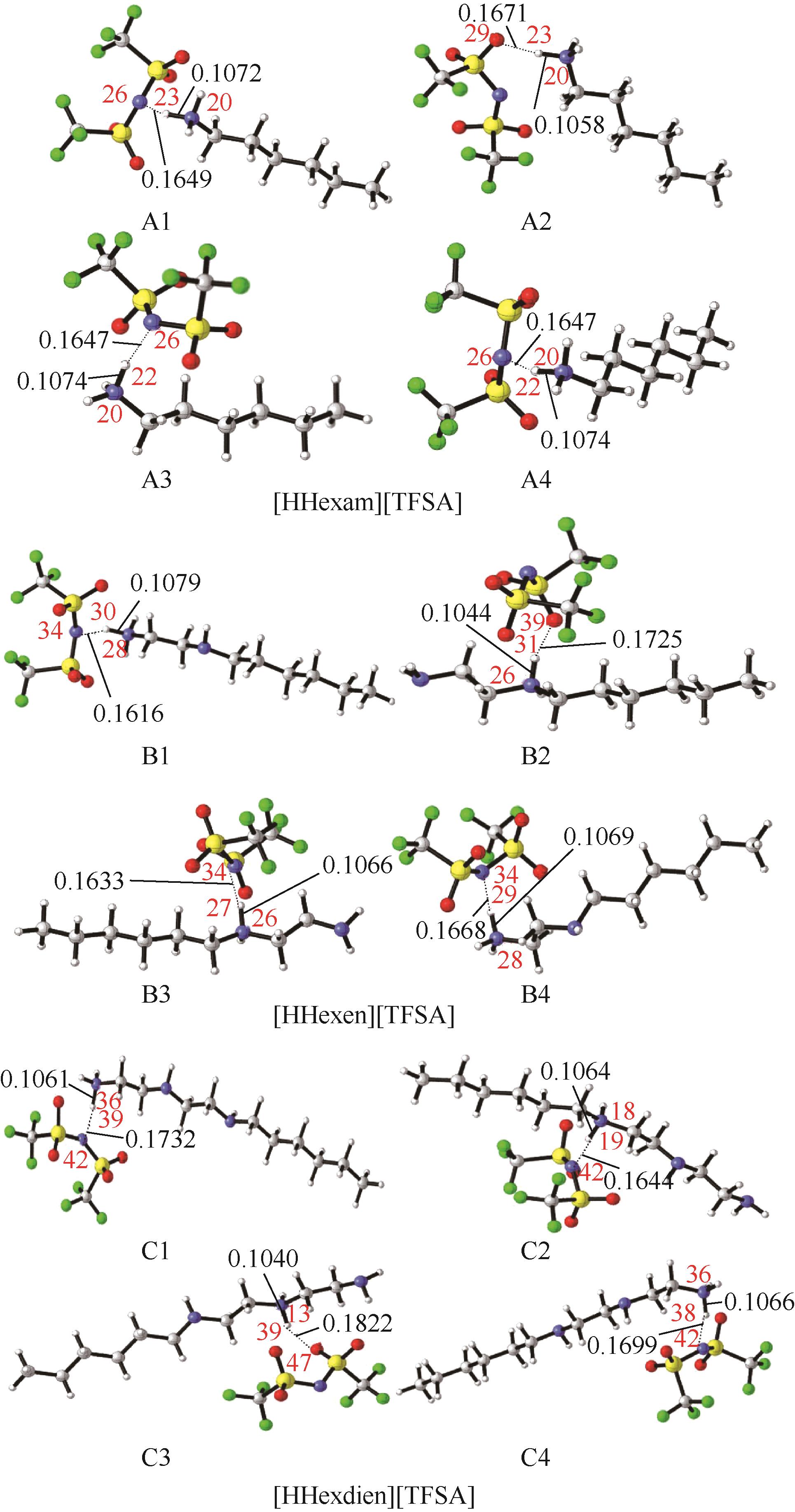
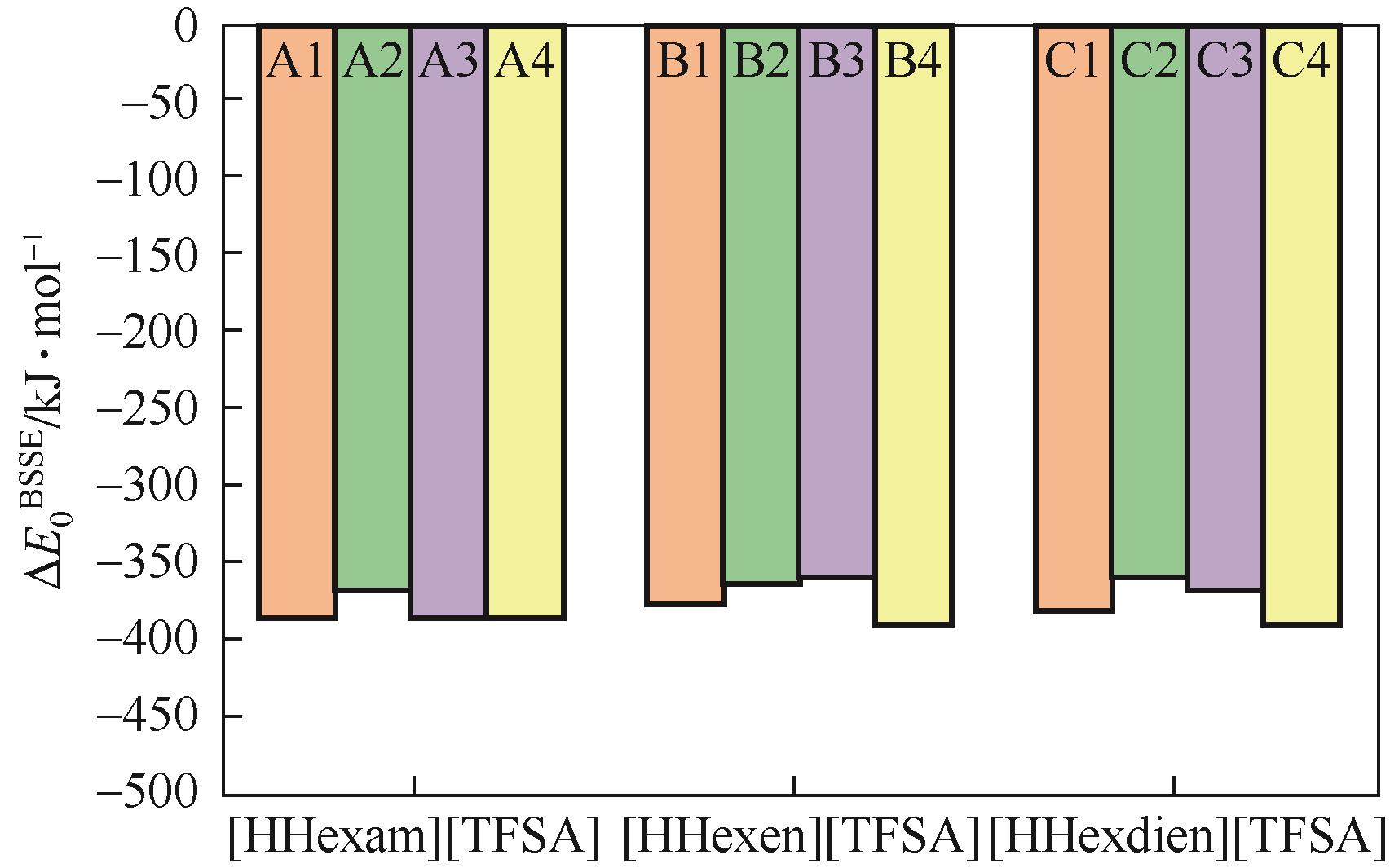
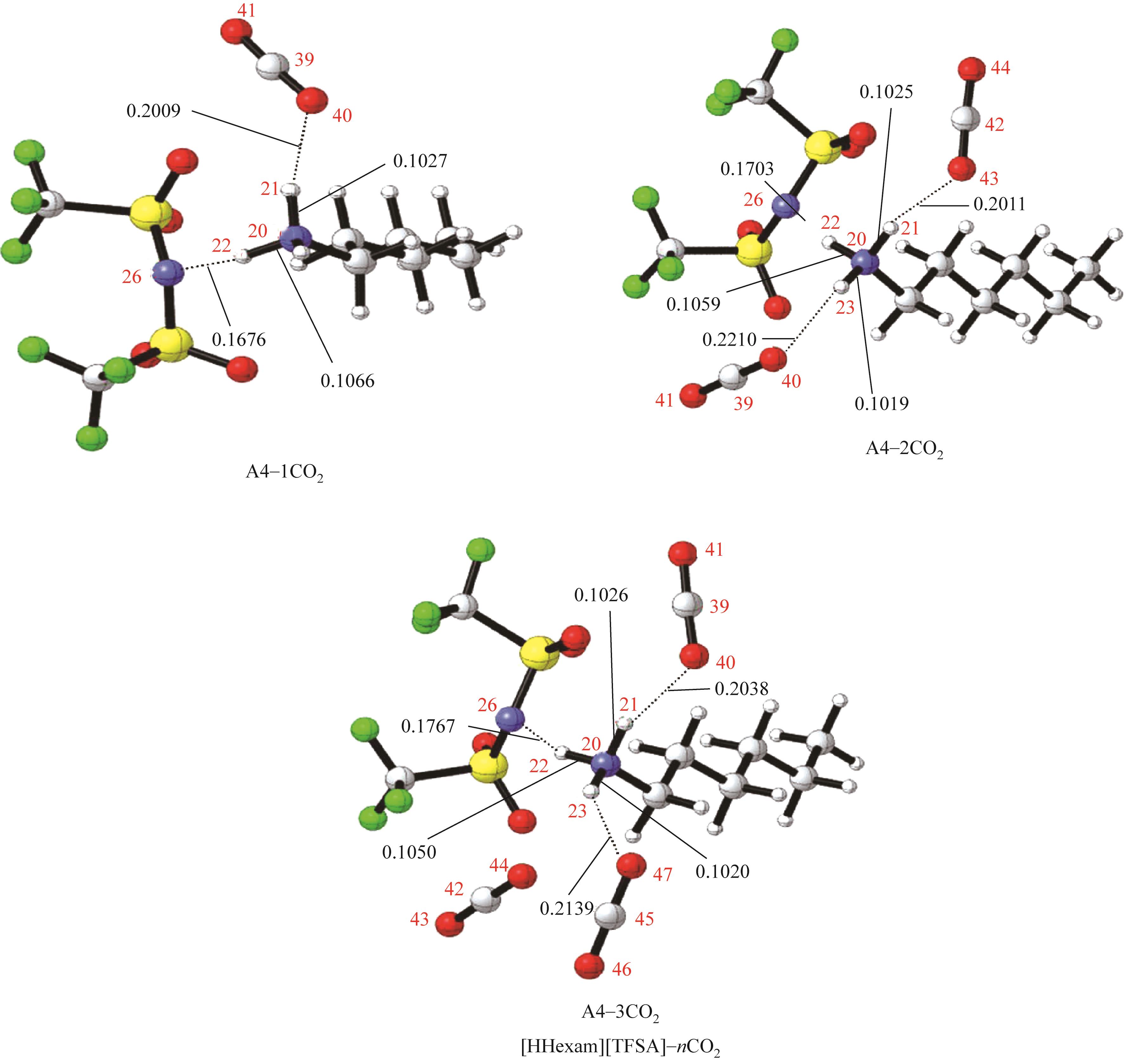
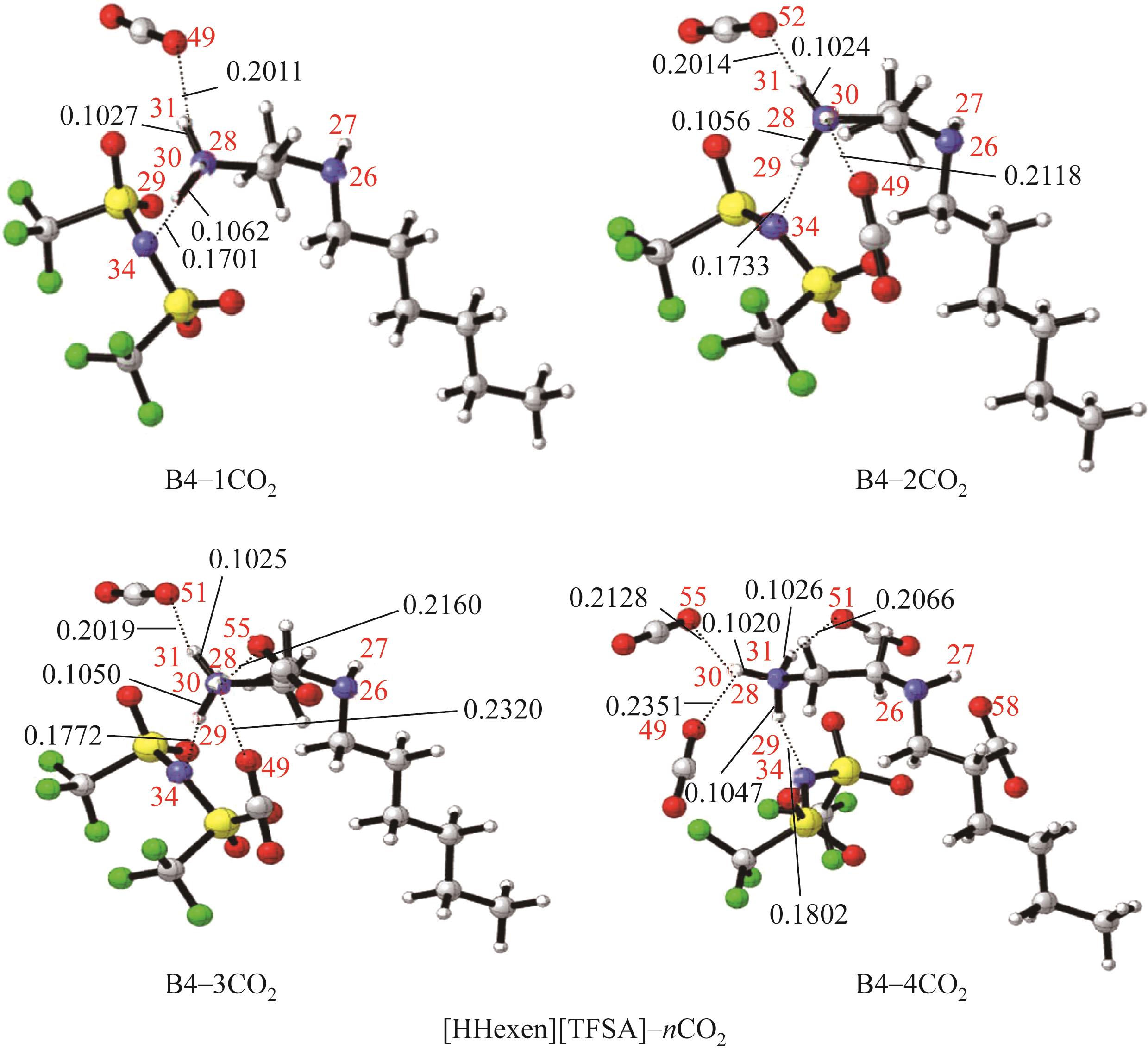
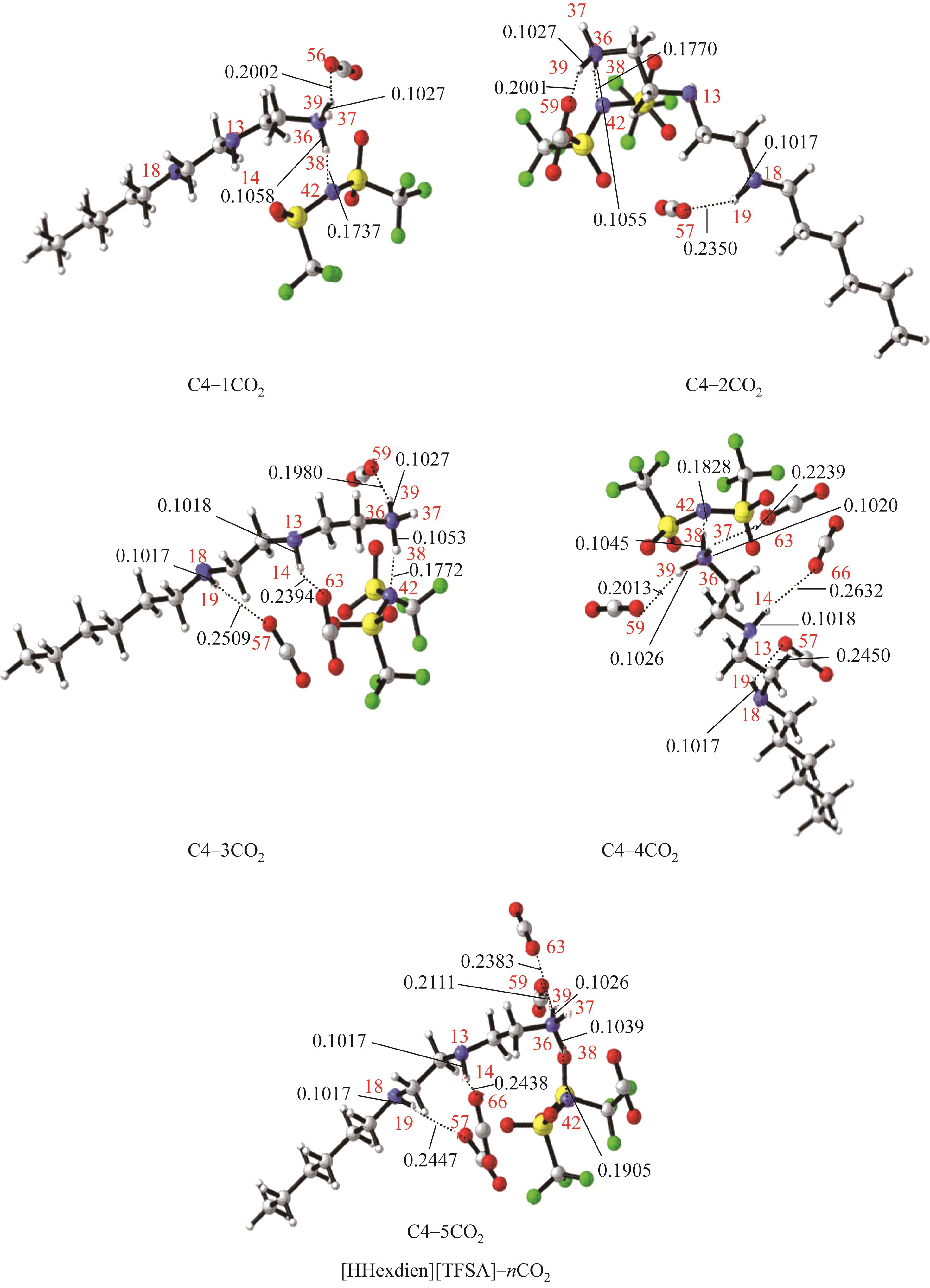
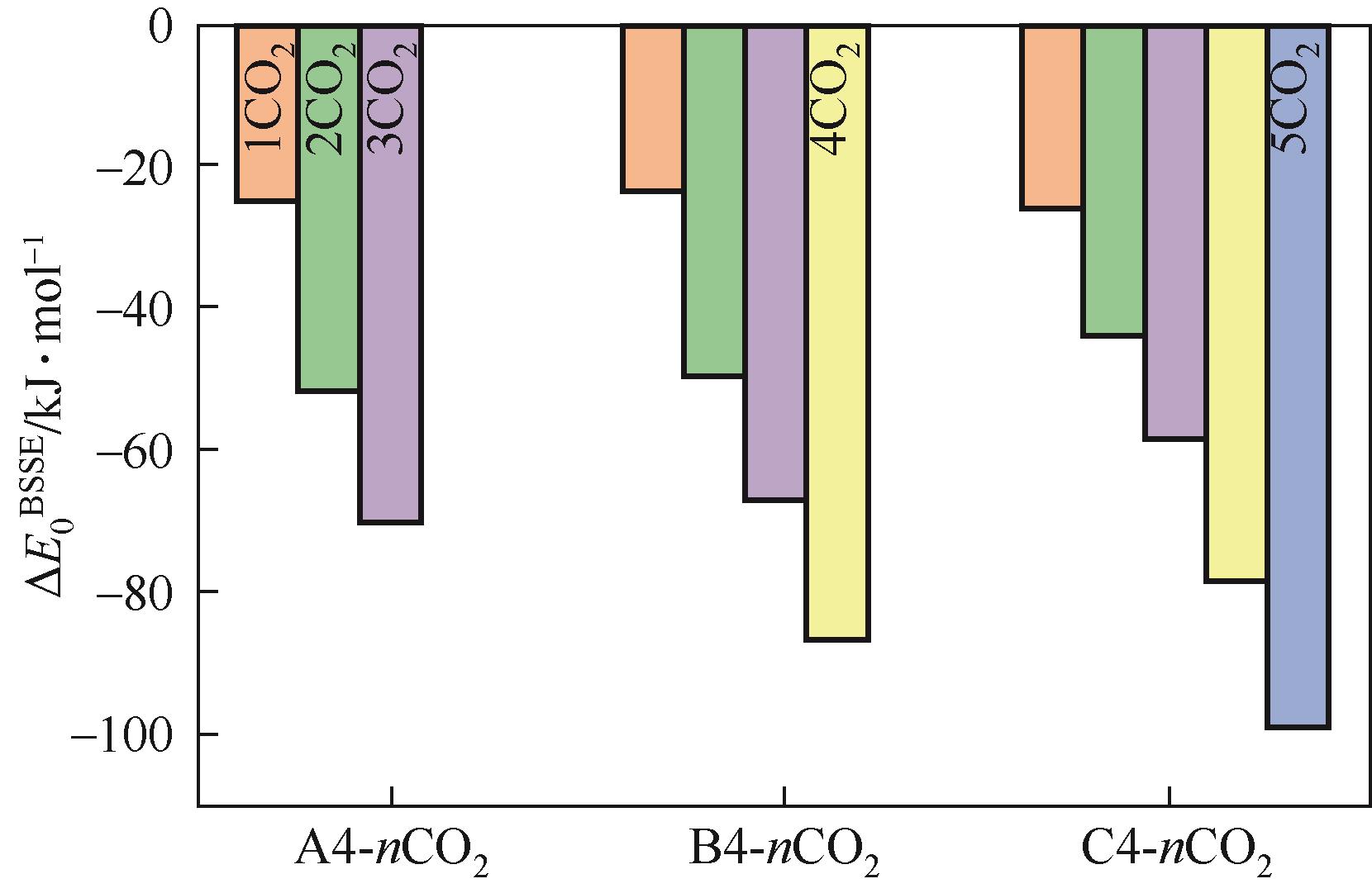
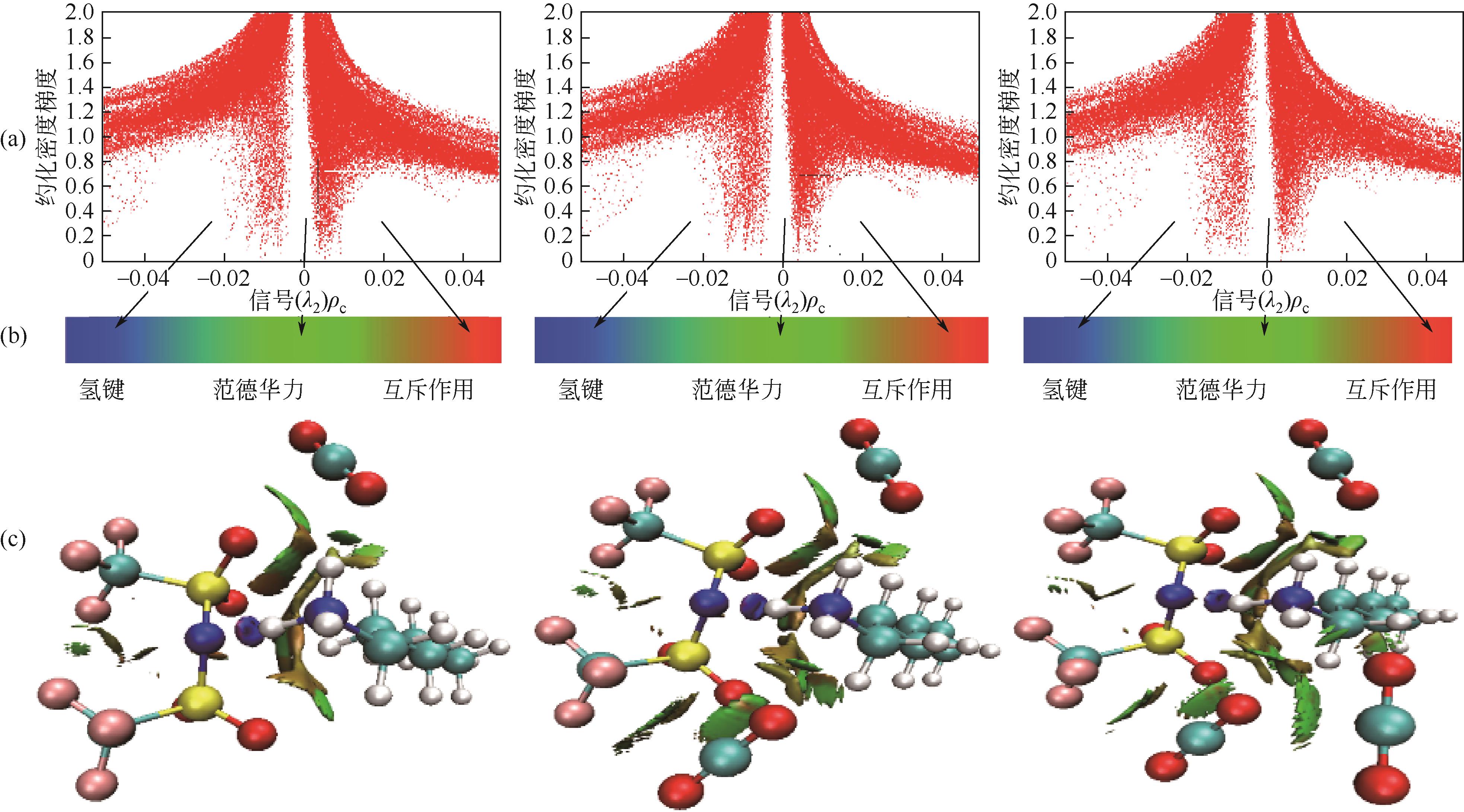
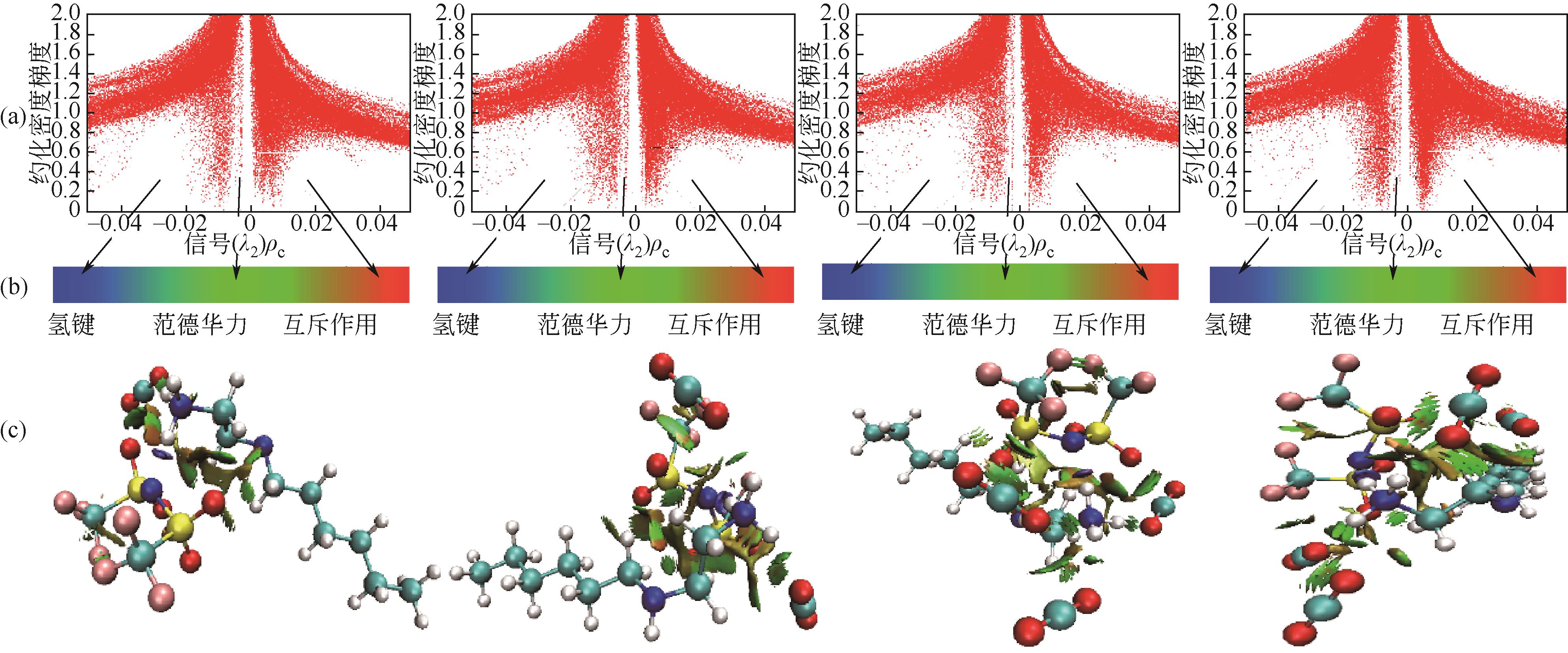
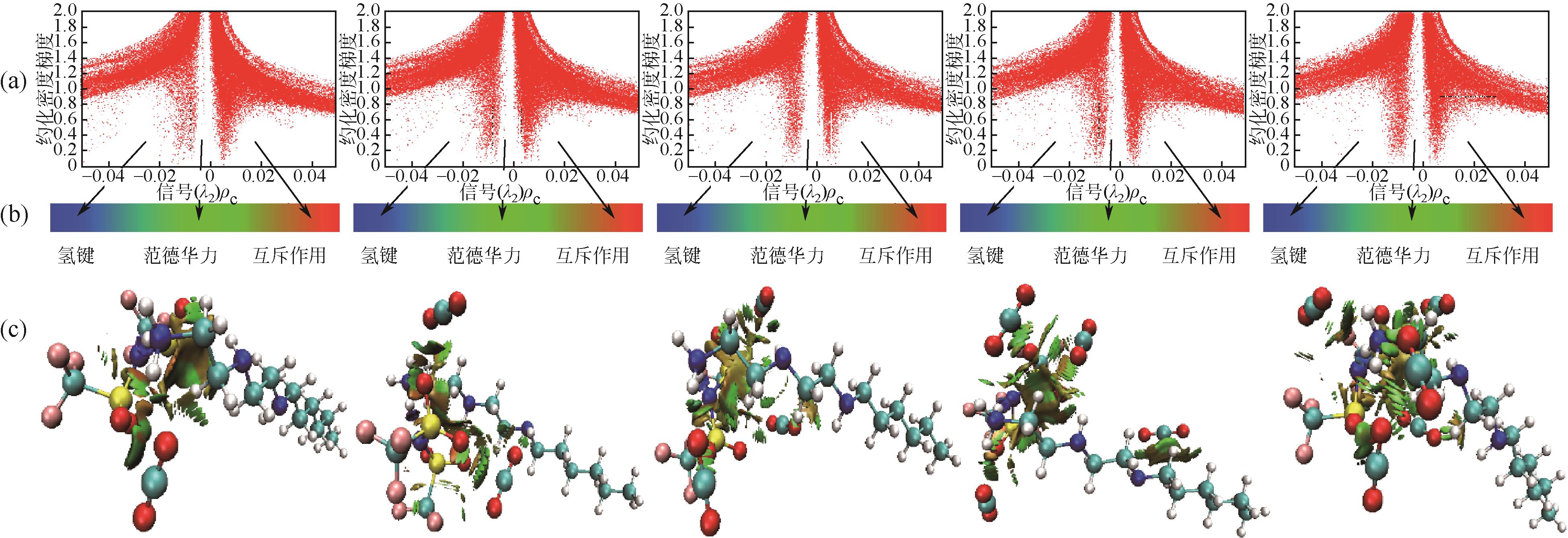
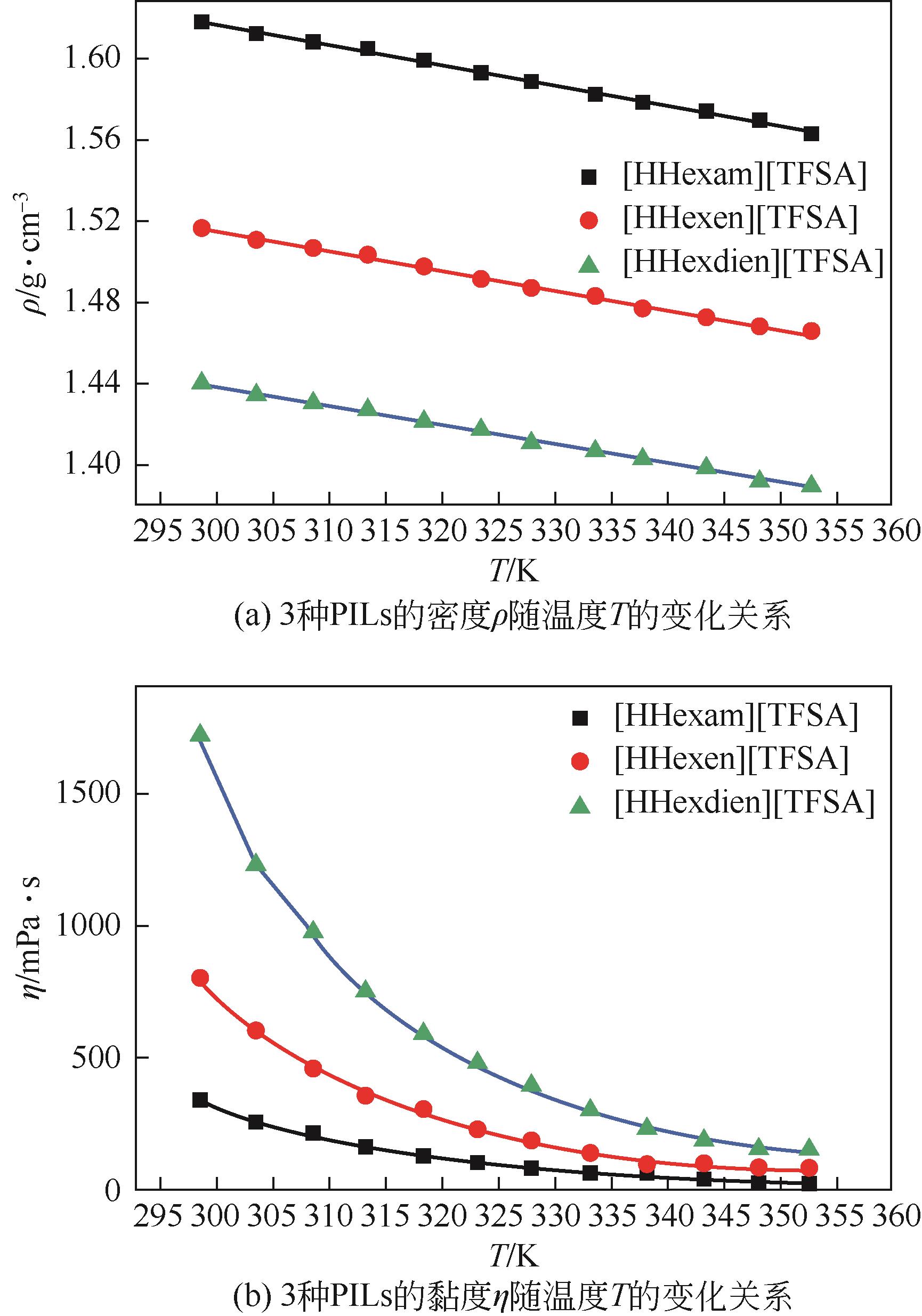
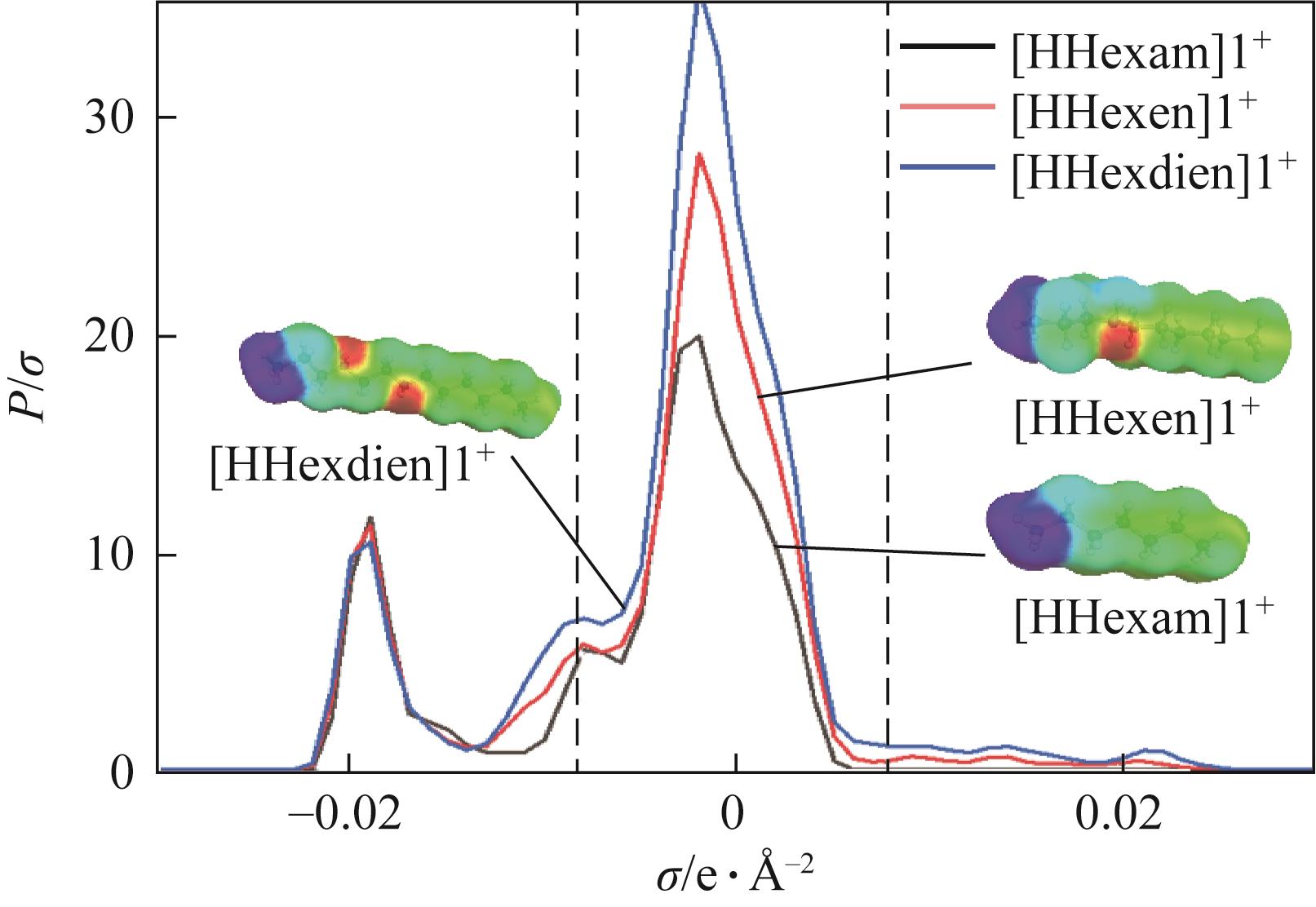
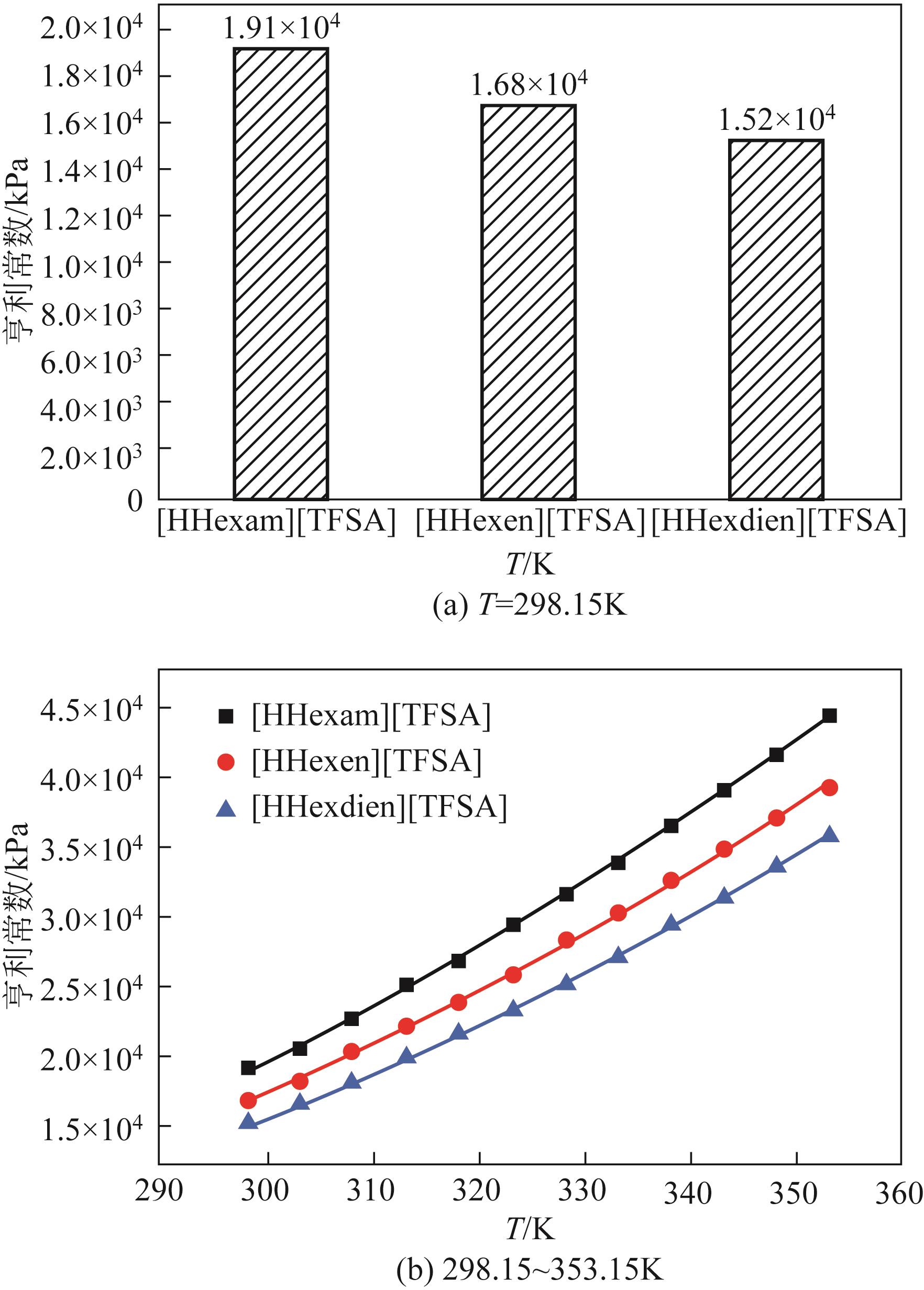
开展了以质子化的正己胺(HHexam+)、己基乙二胺(HHexen+)及己基二亚乙基三胺(HHexdien+)为阳离子的TFSA [== (CF3SO2)2N-]型质子化离子液体(PILs),即[HHexam][TFSA]、[HHexen][TFSA]及[HHexdien][TFSA]型PILs吸收CO2的研究。首先,选择密度泛函理论,在M06-2X/6-311G(d, p)水平下,对上述3种PILs的构型进行优化,分别得到了其较稳定构象,结果显示,PILs的阳离子中N—H和阴离子中N原子间主要形成N—H···N型较强氢键。然后,分别利用其中最稳定构象,创建并优化PILs-nCO2构型,PILs和nCO2分子间主要形成N—H···O型弱或中等强度氢键。主要氢键部位N—H···O中N—H键的振动频率的变化值、电子密度值及二阶微扰能的计算结果显示,[HHexam][TFSA]、[HHexen][TFSA]及[HHexdien][TFSA]分别与2、3、4分子CO2结合时将不再形成氢键网络。采用COSMOtherm软件计算的CO2在3种PILs中的亨利常数(kPa)大小为[HHexam][TFSA] (1.91×104) > [HHexen][TFSA] (1.68×104) > [HHexdien][TFSA] (1.51×104),即3种PILs对CO2的溶解能力大小为极性头部具有3个氨基的[HHexdien][TFSA] > 2个氨基的[HHexen][TFSA] > 1个氨基的[HHexam][TFSA]。以上结果中可以看出,PILs结构中氨基数目的多少对其吸收CO2的能力有较显著影响,即随着PILs结构中氨基数目的增多,其对CO2的溶解能力随之增大。
中图分类号:
引用本文
米泽豪, 花儿. 多元胺-TFSA型质子化离子液体吸收CO2的理论分析[J]. 化工进展, 2023, 42(11): 6015-6030.
MI Zehao, HUA Er. Theoretical analysis of CO2 absorption by polyamines-TFSA type protic ionic liquids[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6015-6030.
| 构型 | 化学键 | 振动频率ν/cm-1 | 频率变化值Δν/cm-1 |
|---|---|---|---|
| CO2 | 1C—2O | 1422 | — |
| 1C—3O | 1422 | — | |
| [HHexam][TFSA] | 20N—21H | 3450 | — |
| 20N—22H | 2591 | — | |
| 20N—23H | 3563 | — | |
| A4-1CO2 | 20N—21H | 3410 | 40 |
| 20N—22H | 2709 | -118 | |
| A4-2CO2 | 20N—21H | 3430 | 20 |
| 20N—22H | 2825 | -234 | |
| 20N—23H | 3532 | 31 | |
| A4-3CO2 | 20N—21H | 3421 | 29 |
| 20N—22H | 2985 | -394 | |
| 20N—23H | 3522 | 41 | |
| [HHexen][TFSA] | 26N—27H | 3561 | — |
| 28N—30H | 3555 | — | |
| 28N—31H | 3429 | — | |
| 28N—29H | 2652 | — | |
| B4-1CO2 | 28N—31H | 3424 | 5 |
| 28N—29H | 2760 | -108 | |
| B4-2CO2 | 28N—30H | 3533 | 22 |
| 28N—29H | 2870 | -218 | |
| B4-3CO2 | 28N—30H | 3534 | 21 |
| 28N—29H | 2980 | -328 | |
| B4-4CO2 | 28N—30H | 3530 | 25 |
| 28N—29H | 3035 | -383 | |
| [HHexdien][TFSA] | 36N—37H | 3450 | — |
| 18N—19H | 3527 | — | |
| 13N—14H | 3511 | — | |
| 36N—39H | 3450 | — | |
| 36N—38H | 2706 | — | |
| C4-1CO2 | 36N—39H | 3414 | 36 |
| 36N—38H | 2852 | -147 | |
| C4-2CO2 | 36N—39H | 3417 | 33 |
| 36N—38H | 2916 | -211 | |
| C4-3CO2 | 36N—39H | 3421 | 29 |
| 36N—38H | 2932 | -226 | |
| C4-4CO2 | 36N—39H | 3432 | 18 |
| 36N—38H | 3060 | -354 | |
| C4-5CO2 | 36N—39H | 3436 | 14 |
| 36N—38H | 3206 | -500 |
表1 CO2、PILs及PILs-nCO2的较稳定构象的振动频率v及其变化值Δν
| 构型 | 化学键 | 振动频率ν/cm-1 | 频率变化值Δν/cm-1 |
|---|---|---|---|
| CO2 | 1C—2O | 1422 | — |
| 1C—3O | 1422 | — | |
| [HHexam][TFSA] | 20N—21H | 3450 | — |
| 20N—22H | 2591 | — | |
| 20N—23H | 3563 | — | |
| A4-1CO2 | 20N—21H | 3410 | 40 |
| 20N—22H | 2709 | -118 | |
| A4-2CO2 | 20N—21H | 3430 | 20 |
| 20N—22H | 2825 | -234 | |
| 20N—23H | 3532 | 31 | |
| A4-3CO2 | 20N—21H | 3421 | 29 |
| 20N—22H | 2985 | -394 | |
| 20N—23H | 3522 | 41 | |
| [HHexen][TFSA] | 26N—27H | 3561 | — |
| 28N—30H | 3555 | — | |
| 28N—31H | 3429 | — | |
| 28N—29H | 2652 | — | |
| B4-1CO2 | 28N—31H | 3424 | 5 |
| 28N—29H | 2760 | -108 | |
| B4-2CO2 | 28N—30H | 3533 | 22 |
| 28N—29H | 2870 | -218 | |
| B4-3CO2 | 28N—30H | 3534 | 21 |
| 28N—29H | 2980 | -328 | |
| B4-4CO2 | 28N—30H | 3530 | 25 |
| 28N—29H | 3035 | -383 | |
| [HHexdien][TFSA] | 36N—37H | 3450 | — |
| 18N—19H | 3527 | — | |
| 13N—14H | 3511 | — | |
| 36N—39H | 3450 | — | |
| 36N—38H | 2706 | — | |
| C4-1CO2 | 36N—39H | 3414 | 36 |
| 36N—38H | 2852 | -147 | |
| C4-2CO2 | 36N—39H | 3417 | 33 |
| 36N—38H | 2916 | -211 | |
| C4-3CO2 | 36N—39H | 3421 | 29 |
| 36N—38H | 2932 | -226 | |
| C4-4CO2 | 36N—39H | 3432 | 18 |
| 36N—38H | 3060 | -354 | |
| C4-5CO2 | 36N—39H | 3436 | 14 |
| 36N—38H | 3206 | -500 |
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | ||||
|---|---|---|---|---|---|---|
| 20N | 21H | 22H | 23H | O | ||
| CO2 | C—O | -0.518 | ||||
| A4 | 20N—21H | -0.737 | 0.441 | |||
| 20N—22H | 0.485 | |||||
| 20N—23H | 0.413 | |||||
| A4-1CO2 | 20N—21H···40O | -0.741 (-0.004) | 0.450 (+0.009) | -0.598 (-0.080) | ||
| A4-2CO2 | 20N—21H···43O | -0.743 (-0.006) | 0.445 (+0.004) | -0.596 (-0.078) | ||
| 20N—23H···40O | 0.430 (+0.017) | -0.593 (-0.075) | ||||
| A4-3CO2 | 20N—21H···40O | -0.741 (-0.004) | 0.449 (+0.008) | -0.592 (-0.074) | ||
| 20N—23H···47O | 0.436 (+0.023) | -0.571 (-0.053) | ||||
表2 [HHexam][TFSA]-nCO2主要氢键部位原子上的NPA电荷分布值(括号中为结合CO2后的电荷变化值)
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | ||||
|---|---|---|---|---|---|---|
| 20N | 21H | 22H | 23H | O | ||
| CO2 | C—O | -0.518 | ||||
| A4 | 20N—21H | -0.737 | 0.441 | |||
| 20N—22H | 0.485 | |||||
| 20N—23H | 0.413 | |||||
| A4-1CO2 | 20N—21H···40O | -0.741 (-0.004) | 0.450 (+0.009) | -0.598 (-0.080) | ||
| A4-2CO2 | 20N—21H···43O | -0.743 (-0.006) | 0.445 (+0.004) | -0.596 (-0.078) | ||
| 20N—23H···40O | 0.430 (+0.017) | -0.593 (-0.075) | ||||
| A4-3CO2 | 20N—21H···40O | -0.741 (-0.004) | 0.449 (+0.008) | -0.592 (-0.074) | ||
| 20N—23H···47O | 0.436 (+0.023) | -0.571 (-0.053) | ||||
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | |||||
|---|---|---|---|---|---|---|---|
| 26N | 28N | 27H | 30H | 31H | O | ||
| CO2 | C—O | -0.518 | |||||
| B4 | 26N—27H | -0.689 | 0.357 | ||||
| 28N—29H | -0.740 | ||||||
| 28N—30H | 0.415 | ||||||
| 28N—31H | 0.441 | ||||||
| B4-1CO2 | 28N—31H···49O | -0.744 (-0.004) | 0.449 (+0.008) | -0.599 (-0.081) | |||
| B4-2CO2 | 28N—30H···49O | -0.746 (-0.006) | 0.436 (+0.021) | -0.596 (-0.078) | |||
| 28N—31H···52O | -0.746 (-0.006) | 0.441 | -0.600 (-0.082) | ||||
| B4-3CO2 | 28N—30H···49O | -0.745 (-0.005) | 0.440 (+0.025) | -0.608 (-0.090) | |||
| 28N—30H···55O | -0.745 (-0.005) | 0.440 (+0.025) | -0.570 (-0.052) | ||||
| 28N—31H···51O | -0.745 (-0.005) | 0.443 (+0.028) | -0.596 (-0.078) | ||||
| B4-4CO2 | 28N—30H···49O | -0.743 (-0.003) | 0.440 (+0.025) | -0.607 -0.089) | |||
| 28N—30H···55O | -0.743 (-0.003) | 0.440 (+0.025) | -0.572 (-0.054) | ||||
| 28N—31H···51O | -0.743 (-0.003) | 0.444 (+0.003) | -0.595 (-0.077) | ||||
表3 [HHexen][TFSA]-nCO2主要氢键部位原子上的NPA电荷分布值(括号中为结合CO2后的电荷变化值)
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | |||||
|---|---|---|---|---|---|---|---|
| 26N | 28N | 27H | 30H | 31H | O | ||
| CO2 | C—O | -0.518 | |||||
| B4 | 26N—27H | -0.689 | 0.357 | ||||
| 28N—29H | -0.740 | ||||||
| 28N—30H | 0.415 | ||||||
| 28N—31H | 0.441 | ||||||
| B4-1CO2 | 28N—31H···49O | -0.744 (-0.004) | 0.449 (+0.008) | -0.599 (-0.081) | |||
| B4-2CO2 | 28N—30H···49O | -0.746 (-0.006) | 0.436 (+0.021) | -0.596 (-0.078) | |||
| 28N—31H···52O | -0.746 (-0.006) | 0.441 | -0.600 (-0.082) | ||||
| B4-3CO2 | 28N—30H···49O | -0.745 (-0.005) | 0.440 (+0.025) | -0.608 (-0.090) | |||
| 28N—30H···55O | -0.745 (-0.005) | 0.440 (+0.025) | -0.570 (-0.052) | ||||
| 28N—31H···51O | -0.745 (-0.005) | 0.443 (+0.028) | -0.596 (-0.078) | ||||
| B4-4CO2 | 28N—30H···49O | -0.743 (-0.003) | 0.440 (+0.025) | -0.607 -0.089) | |||
| 28N—30H···55O | -0.743 (-0.003) | 0.440 (+0.025) | -0.572 (-0.054) | ||||
| 28N—31H···51O | -0.743 (-0.003) | 0.444 (+0.003) | -0.595 (-0.077) | ||||
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 13N | 18N | 36N | 14H | 19H | 37H | 39H | O | ||
| CO2 | C—O | -0.518 | |||||||
| C4 | 13N—14H | -0.689 | 0.364 | ||||||
| 18N—19H | -0.682 | 0.342 | |||||||
| 36N—37H | -0.738 | 0.415 | |||||||
| 36N—39H | 0.444 | ||||||||
| C4-1CO2 | 36N—39H···56O | -0.742 (-0.004) | 0.455 (+0.011) | -0.600 (-0.082) | |||||
| C4-2CO2 | 18N—19H···57O | -0.688 (-0.006) | 0.358 (+0.016) | -0.557 (-0.039) | |||||
| 36N—39H···59O | -0.739 (-0.001) | 0.454 (+0.010) | -0.601 (-0.083) | ||||||
| C4-3CO2 | 18N—19H···57O | -0.683 (-0.001) | 0.351 (+0.009) | -0.559 (-0.041) | |||||
| 13N—14H···63O | -0.686 | 0.369 (+0.005) | -0.549 (-0.031) | ||||||
| 36N—39H···59O | -0.738 | 0.450 (+0.006) | -0.601 (-0.083) | ||||||
| C4-4CO2 | 18N—19H···57O | -0.683 (-0.001) | 0.350 (+0.008) | -0.526 (-0.008) | |||||
| 36N—39H···59O | -0.735 (+0.003) | 0.450 (+0.006) | -0.597 (-0.079) | ||||||
| 36N—37H···63O | -0.735 (+0.003) | 0.432 (+0.017) | -0.590 (-0.072) | ||||||
| 13N—14H···66O | -0.688 (+0.001) | 0.365 (+0.001) | -0.568 (-0.050) | ||||||
| C4-5CO2 | 18N—19H···57O | -0.682 | 0.351 (+0.009) | -0.557 (-0.039) | |||||
| 36N—39H···59O | -0.737 (+0.001) | 0.450 (+0.006) | -0.612 (-0.094) | ||||||
| 13N—14H···66O | -0.687 (+0.002) | 0.370 (+0.006) | -0.547 (-0.029) | ||||||
| 36N—39H···63O | -0.737 (+0.001) | 0.450 (+0.006) | -0.573 (-0.055) | ||||||
表4 [HHexdien][TFSA]-nCO2主要氢键部位原子上的NPA电荷分布值(括号中为结合CO2后的电荷变化值)
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 13N | 18N | 36N | 14H | 19H | 37H | 39H | O | ||
| CO2 | C—O | -0.518 | |||||||
| C4 | 13N—14H | -0.689 | 0.364 | ||||||
| 18N—19H | -0.682 | 0.342 | |||||||
| 36N—37H | -0.738 | 0.415 | |||||||
| 36N—39H | 0.444 | ||||||||
| C4-1CO2 | 36N—39H···56O | -0.742 (-0.004) | 0.455 (+0.011) | -0.600 (-0.082) | |||||
| C4-2CO2 | 18N—19H···57O | -0.688 (-0.006) | 0.358 (+0.016) | -0.557 (-0.039) | |||||
| 36N—39H···59O | -0.739 (-0.001) | 0.454 (+0.010) | -0.601 (-0.083) | ||||||
| C4-3CO2 | 18N—19H···57O | -0.683 (-0.001) | 0.351 (+0.009) | -0.559 (-0.041) | |||||
| 13N—14H···63O | -0.686 | 0.369 (+0.005) | -0.549 (-0.031) | ||||||
| 36N—39H···59O | -0.738 | 0.450 (+0.006) | -0.601 (-0.083) | ||||||
| C4-4CO2 | 18N—19H···57O | -0.683 (-0.001) | 0.350 (+0.008) | -0.526 (-0.008) | |||||
| 36N—39H···59O | -0.735 (+0.003) | 0.450 (+0.006) | -0.597 (-0.079) | ||||||
| 36N—37H···63O | -0.735 (+0.003) | 0.432 (+0.017) | -0.590 (-0.072) | ||||||
| 13N—14H···66O | -0.688 (+0.001) | 0.365 (+0.001) | -0.568 (-0.050) | ||||||
| C4-5CO2 | 18N—19H···57O | -0.682 | 0.351 (+0.009) | -0.557 (-0.039) | |||||
| 36N—39H···59O | -0.737 (+0.001) | 0.450 (+0.006) | -0.612 (-0.094) | ||||||
| 13N—14H···66O | -0.687 (+0.002) | 0.370 (+0.006) | -0.547 (-0.029) | ||||||
| 36N—39H···63O | -0.737 (+0.001) | 0.450 (+0.006) | -0.573 (-0.055) | ||||||
| 构型 | CO2数量n | 电荷转移 | 二阶微扰能E(2)/kJ·mol-1 | 二阶微扰能总值E(2) sum/kJ·mol-1 |
|---|---|---|---|---|
| A4 | 1CO2 | LP(O40)→BD*(N20-H21) | 29 | 29 |
| 2CO2 | LP(O43)→BD*(N20-H21) | 28 | 34 | |
| LP(O40)→BD*(N20-H23) | 4 | |||
| LP(O40)→BD*(N20-H21) | 1 | |||
| 3CO2 | LP(O40)→BD*(N20-H21) | 26 | 43 | |
| LP(O47)→BD*(N20-H23) | 13 | |||
| B4 | 1CO2 | LP(O49)→BD*(N28-H31) | 29 | 29 |
| 2CO2 | LP(O49)→BD*(N28-H30) | 9 | 35 | |
| LP(O52)→BD*(N28-H31) | 25 | |||
| 3CO2 | LP(O49)→BD*(N28-H30) | 2 | 42 | |
| LP(O51)→BD*(N28-H31) | 27 | |||
| LP(O55)→BD*(N28-H30) | 13 | |||
| 4CO2 | LP(O51)→BD*(N28-H31) | 22 | 40 | |
| LP(O55)→BD*(N28-H30) | 15 | |||
| C4 | 1CO2 | LP(O56)→BD*(N36-H39) | 27 | 27 |
| 2CO2 | LP(O59)→BD*(N36-H39) | 29 | 33 | |
| 3CO2 | LP(O59)→BD*(N36-H39) | 31 | 37 | |
| LP(O63)→BD*(N13-H14) | 4 | |||
| 4CO2 | LP(O59)→BD*(N36-H39) | 28 | 36 | |
| LP(O57)→BD*(N18-H19) | 3 | |||
| LP(O63)→BD*(N36-H37) | 2 | |||
| 5CO2 | LP(O59)→BD*(N36-H39) | 20 | 29 | |
| LP(O63)→BD*(N36-H39) | 2 | |||
| LP(O66)→BD*(N13-H14) | 4 |
表5 PILs-nCO2主要氢键部位BCPs的二阶微扰能E(2)sum
| 构型 | CO2数量n | 电荷转移 | 二阶微扰能E(2)/kJ·mol-1 | 二阶微扰能总值E(2) sum/kJ·mol-1 |
|---|---|---|---|---|
| A4 | 1CO2 | LP(O40)→BD*(N20-H21) | 29 | 29 |
| 2CO2 | LP(O43)→BD*(N20-H21) | 28 | 34 | |
| LP(O40)→BD*(N20-H23) | 4 | |||
| LP(O40)→BD*(N20-H21) | 1 | |||
| 3CO2 | LP(O40)→BD*(N20-H21) | 26 | 43 | |
| LP(O47)→BD*(N20-H23) | 13 | |||
| B4 | 1CO2 | LP(O49)→BD*(N28-H31) | 29 | 29 |
| 2CO2 | LP(O49)→BD*(N28-H30) | 9 | 35 | |
| LP(O52)→BD*(N28-H31) | 25 | |||
| 3CO2 | LP(O49)→BD*(N28-H30) | 2 | 42 | |
| LP(O51)→BD*(N28-H31) | 27 | |||
| LP(O55)→BD*(N28-H30) | 13 | |||
| 4CO2 | LP(O51)→BD*(N28-H31) | 22 | 40 | |
| LP(O55)→BD*(N28-H30) | 15 | |||
| C4 | 1CO2 | LP(O56)→BD*(N36-H39) | 27 | 27 |
| 2CO2 | LP(O59)→BD*(N36-H39) | 29 | 33 | |
| 3CO2 | LP(O59)→BD*(N36-H39) | 31 | 37 | |
| LP(O63)→BD*(N13-H14) | 4 | |||
| 4CO2 | LP(O59)→BD*(N36-H39) | 28 | 36 | |
| LP(O57)→BD*(N18-H19) | 3 | |||
| LP(O63)→BD*(N36-H37) | 2 | |||
| 5CO2 | LP(O59)→BD*(N36-H39) | 20 | 29 | |
| LP(O63)→BD*(N36-H39) | 2 | |||
| LP(O66)→BD*(N13-H14) | 4 |
| 构型 | CO2数量n | 键临界点BCPs | 拉普拉斯值∇2ρc | 电子密度ρc | 电子密度总值ρc(sum) | 氢键能EHB/kJ·mol-1 | 氢键能总值EHB(sum)/kJ·mol-1 |
|---|---|---|---|---|---|---|---|
| A4 | 1CO2 | 40O···20N—21H | 0.0809 | 0.0190 | 0.0190 | 18 | 18 |
| 2CO2 | 43O···20N—21H | 0.0807 | 0.0189 | 0.0327 | 18 | 31 | |
| 40O···20N—23H | 0.0593 | 0.0138 | 13 | ||||
| 3CO2 | 40O···20N—21H | 0.0756 | 0.0179 | 0.0331 | 17 | 31 | |
| 47O···20N—23H | 0.0626 | 0.0152 | 14 | ||||
| B4 | 1CO2 | 49O···28N—31H | 0.0806 | 0.0189 | 0.0189 | 18 | 18 |
| 2CO2 | 49O···28N—30H | 0.0695 | 0.0159 | 0.0347 | 15 | 33 | |
| 52O···28N—31H | 0.0813 | 0.0188 | 18 | ||||
| 3CO2 | 49O···28N—30H | 0.0468 | 0.0112 | 0.0443 | 10 | 41 | |
| 55O···28N—30H | 0.0597 | 0.0146 | 13 | ||||
| 51O···28N—31H | 0.0794 | 0.0185 | 18 | ||||
| 4CO2 | 49O···28N—30H | 0.0459 | 0.0108 | 0.0430 | 10 | 39 | |
| 51O···28N—31H | 0.0704 | 0.0168 | 15 | ||||
| 55O···28N—30H | 0.0635 | 0.0154 | 14 | ||||
| C4 | 1CO2 | 56O···36N—39H | 0.0831 | 0.0195 | 0.0195 | 19 | 19 |
| 2CO2 | 59O···36N—39H | 0.0837 | 0.0196 | 0.0297 | 19 | 28 | |
| 57O···18N—19H | 0.0373 | 0.0100 | 9 | ||||
| 3CO2 | 57O···18N—19H | 0.0292 | 0.0077 | 0.0375 | 7 | 35 | |
| 63O···13N—14H | 0.0350 | 0.0097 | 8 | ||||
| 59O···36N—39H | 0.0867 | 0.0201 | 20 | ||||
| 4CO2 | 57O···18N—19H | 0.0341 | 0.0096 | 0.0476 | 9 | 44 | |
| 59O···36N—39H | 0.0799 | 0.0187 | 18 | ||||
| 63O···36N—37H | 0.0570 | 0.0132 | 12 | ||||
| 66O···13N—14H | 0.0210 | 0.0060 | 5 | ||||
| 5CO2 | 57O···18N—19H | 0.0333 | 0.0088 | 0.0442 | 8 | 41 | |
| 59O···36N—39H | 0.0625 | 0.0150 | 14 | ||||
| 66O···13N—14H | 0.0316 | 0.0089 | 8 | ||||
| 63O···36N—39H | 0.0475 | 0.0115 | 11 |
表6 PILs-nCO2构型BCP处的电子密度值ρc、拉普拉斯值∇2ρc和氢键能EHB
| 构型 | CO2数量n | 键临界点BCPs | 拉普拉斯值∇2ρc | 电子密度ρc | 电子密度总值ρc(sum) | 氢键能EHB/kJ·mol-1 | 氢键能总值EHB(sum)/kJ·mol-1 |
|---|---|---|---|---|---|---|---|
| A4 | 1CO2 | 40O···20N—21H | 0.0809 | 0.0190 | 0.0190 | 18 | 18 |
| 2CO2 | 43O···20N—21H | 0.0807 | 0.0189 | 0.0327 | 18 | 31 | |
| 40O···20N—23H | 0.0593 | 0.0138 | 13 | ||||
| 3CO2 | 40O···20N—21H | 0.0756 | 0.0179 | 0.0331 | 17 | 31 | |
| 47O···20N—23H | 0.0626 | 0.0152 | 14 | ||||
| B4 | 1CO2 | 49O···28N—31H | 0.0806 | 0.0189 | 0.0189 | 18 | 18 |
| 2CO2 | 49O···28N—30H | 0.0695 | 0.0159 | 0.0347 | 15 | 33 | |
| 52O···28N—31H | 0.0813 | 0.0188 | 18 | ||||
| 3CO2 | 49O···28N—30H | 0.0468 | 0.0112 | 0.0443 | 10 | 41 | |
| 55O···28N—30H | 0.0597 | 0.0146 | 13 | ||||
| 51O···28N—31H | 0.0794 | 0.0185 | 18 | ||||
| 4CO2 | 49O···28N—30H | 0.0459 | 0.0108 | 0.0430 | 10 | 39 | |
| 51O···28N—31H | 0.0704 | 0.0168 | 15 | ||||
| 55O···28N—30H | 0.0635 | 0.0154 | 14 | ||||
| C4 | 1CO2 | 56O···36N—39H | 0.0831 | 0.0195 | 0.0195 | 19 | 19 |
| 2CO2 | 59O···36N—39H | 0.0837 | 0.0196 | 0.0297 | 19 | 28 | |
| 57O···18N—19H | 0.0373 | 0.0100 | 9 | ||||
| 3CO2 | 57O···18N—19H | 0.0292 | 0.0077 | 0.0375 | 7 | 35 | |
| 63O···13N—14H | 0.0350 | 0.0097 | 8 | ||||
| 59O···36N—39H | 0.0867 | 0.0201 | 20 | ||||
| 4CO2 | 57O···18N—19H | 0.0341 | 0.0096 | 0.0476 | 9 | 44 | |
| 59O···36N—39H | 0.0799 | 0.0187 | 18 | ||||
| 63O···36N—37H | 0.0570 | 0.0132 | 12 | ||||
| 66O···13N—14H | 0.0210 | 0.0060 | 5 | ||||
| 5CO2 | 57O···18N—19H | 0.0333 | 0.0088 | 0.0442 | 8 | 41 | |
| 59O···36N—39H | 0.0625 | 0.0150 | 14 | ||||
| 66O···13N—14H | 0.0316 | 0.0089 | 8 | ||||
| 63O···36N—39H | 0.0475 | 0.0115 | 11 |
| 1 | 雷婷, 喻树楠, 周昶安, 等. 吸附法碳捕集固体胺吸附剂成型技术研究进展[J]. 化工进展, 2022, 41(12): 6213-6225. |
| LEI Ting, YU Shunan, ZHOU Chang'an, et al. Research progress on the shaping technology of solid amine adsorbents for CO2 capture by adsorption method[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6213-6225. | |
| 2 | 周红军, 周颖, 徐春明. 中国碳中和目标下CO2转化的思考与实践[J]. 化工进展, 2022, 41(6): 3381-3385. |
| ZHOU Hongjun, ZHOU Ying, XU Chunming. Exploration of the CO2 conversion under China’s carbon neutrality goal[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3381-3385. | |
| 3 | 黄晟, 王静宇, 郭沛, 等. 碳中和目标下能源结构优化的近期策略与远期展望[J]. 化工进展, 2022, 41(11): 5695-5708. |
| HUANG Sheng, WANG Jingyu, GUO Pei, et al. Short-term strategy and long-term prospect of energy structure optimization under carbon neutrality target[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5695-5708. | |
| 4 | EGOROVA Ksenia S, GORDEEV Evgeniy G, ANANIKOV Valentine P. Biological activity of ionic liquids and their application in pharmaceutics and medicine[J]. Chemical Reviews, 2017, 117(10): 7132-7189. |
| 5 | KALIKIN Nikolai N, KOLESNIKOV Andrei L, BUDKOV Yury A. Polymerized ionic liquids on charged electrodes: New prospects for electrochemistry[J]. Current Opinion in Electrochemistry, 2022, 36: 101134. |
| 6 | SOMMER Julia, BROMBERGER Birgit, ROBBEN Christian, et al. Liquid-liquid extraction of viral particles with ionic liquids[J]. Separation and Purification Technology, 2021, 254: 117591. |
| 7 | ZENG Shaojuan, ZHANG Xiangping, BAI Lu, et al. Ionic-liquid-based CO2 capture systems: structure, interaction and process[J]. Chemical Reviews, 2017, 117(14): 9625-9673. |
| 8 | NOORANI Narmin, MEHRDAD Abbas, ZAREI DIZNAB Rana. Thermodynamic study on carbon dioxide absorption in vinyl imidazolium-amino acid ionic liquids[J]. Fluid Phase Equilibria, 2022, 557: 113433. |
| 9 | TU Zhuoheng, SHI Mingzhen, ZHANG Xiaomin, et al. Selective membrane separation of CO2 using novel epichlorohydrin-amine-based crosslinked protic ionic liquids: Crosslinking mechanism and enhanced salting-out effect[J]. Journal of CO2 Utilization, 2021, 46: 101473. |
| 10 | SHUKLA Shashi Kant, KHOKARALE Santosh G, Thai Q BUI, et al. Ionic liquids: Potential materials for carbon dioxide capture and utilization[J]. Frontiers in Materials, 2019, 6: 42. |
| 11 | KUMAR Pradeep, VARYANI Manish, KHATRI Praveen K, et al. Post combustion capture and conversion of carbon dioxide using histidine derived ionic liquid at ambient conditions[J]. Journal of Industrial and Engineering Chemistry, 2017, 49: 152-157. |
| 12 | MA Jing, ZHU Mingxuan, YANG Xueqing, et al. Different cation-anion interaction mechanisms of diamino protic ionic liquids: A density functional theory study[J]. Chemical Physics Letters, 2021, 774: 138615. |
| 13 | PATIL Kunal R, BARGE Seema S, BHOSALE Babasaheb D, et al. Influence of protic ionic liquids on hydration of glycine based peptides[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2022, 265: 120378. |
| 14 | KONDRATENKO Yulia A, ANTUGANOV Dmitrii O, KADNIKOVA Olga Yu, et al. Synthesis, crystal structure and properties of tris(2-hydroxypropyl)ammonium based protic ionic liquids and protic molten salts[J]. Journal of Molecular Liquids, 2021, 324: 114717. |
| 15 | CAO Bobo, DU Jiuyao, LIU Shuangyue, et al. Carbon dioxide capture by amino-functionalized ionic liquids: DFT based theoretical analysis substantiated by FT-IR investigation[J]. RSC Advances, 2016, 6(13): 10462-10470. |
| 16 | NOORANI Narmin, MEHRDAD Abbas. CO2 solubility in some amino acid-based ionic liquids: Measurement, correlation and DFT studies[J]. Fluid Phase Equilibria, 2020, 517: 112591. |
| 17 | ZHANG Jinrui, LV Naixia, CHAO Yanhong, et al. The interaction nature between hollow silica-based porous ionic liquids and CO2: A DFT study[J]. Journal of Molecular Graphics and Modelling, 2020, 100: 107694. |
| 18 | KUMAR Madhu Deepan, SUNNY Shilpa, JACCOB Madhavan. Conversion of CO2 to cyclic carbonates by metal-ethylenediamine complexes in ionic liquid: A DFT mechanistic study[J]. Journal of CO2 Utilization, 2022, 57: 101872. |
| 19 | MA Jing, WANG Yutong, YANG Xueqing, et al. DFT study on the chemical absorption mechanism of CO2 in diamino protic ionic liquids[J]. The Journal of Physical Chemistry B, 2021, 125(5): 1416-1428. |
| 20 | Hua ER, XU Yu, ZHAO Hong. Properties of mono-protic ionic liquids composed of hexylammonium and hexylethylenediaminium cations with trifluoroacetate and bis (trifluoromethylsulfonyl) imide anions[J]. Journal of Molecular Liquids, 2019, 276: 379-384. |
| 21 | HUA Er, MASAYASU Iida. Protonation properties, Applications and effects: Properties of protic ionic liquids comprising N-alkyl polyamines (Chapter 3) [M]. Nova Science Publishers, 2019, 237-247. |
| 22 | 徐宇, 花儿. 烷基乙二胺-CF3CO2型质子化离子液体的分子间氢键作用[J]. 高等学校化学学报, 2018, 39(9):1954-1960. |
| XU Yu, HUA Er. Hydrogen bonding study on protic ionic liquids composed of N-alkylethylenediaminum cations with trifluoroacetic anion[J]. Chemical Journal of Chinese Universities, 2018, 39(9): 1954-1960. | |
| 23 | XU Yu, HUA Er, HAGHANI Abdolhossein. Structure and hydrogen bonding of mono-protic ionic liquids composed of N-alkylethylenediaminium cations and trifluoromethanesulfonate anion[J]. Materials Today Communications, 2021, 28: 102633. |
| 24 | TORKZADEH Mehrangiz, MOOSAVI Majid. DFT and COSMO-RS studies on dicationic ionic liquids (DILs) as potential candidates for CO2 capture: the effects of alkyl side chain length and symmetry in cations[J]. RSC Advances, 2022, 12(54): 35418-35435. |
| 25 | PARR Robert G. Density functional theory of atoms and molecules[C]//FUKUI K, PULLMAN B. Horizons of Quantum Chemistry: Proceedings of the Third International Congress of Quantum Chemistry Held at Kyoto. Japan: Springer Netherlands, 1980: 5-15. |
| 26 | ZHAO Yan, TRUHLAR Donald G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1): 215-241. |
| 27 | BOYS S F, BERNARDI F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors[J]. Molecular Physics, 2002, 100(1): 65-73. |
| 28 | Renqing LÜ, WU Chongchong, LIN Jin, et al. Theoretical study on interactions between trifluoromethanesulfonate(triflate) based ionic liquid and thiophene[J]. Journal of Molecular Liquids, 2017, 237: 289-294. |
| 29 | ANBU V, VIJAYALAKSHMI K A, KARUNATHAN R, et al. Explosives properties of high energetic trinitrophenyl nitramide molecules: A DFT and AIM analysis[J]. Arabian Journal of Chemistry, 2019, 12(5): 621-632. |
| 30 | HUNT Patricia A, ASHWORTH Claire R, MATTHEWS Richard P. Hydrogen bonding in ionic liquids[J]. Chemical Society Reviews, 2015, 44(5): 1257-1288. |
| 31 | DOMAGALA Małgorzata, JABLONSKI Mirosław, DUBIS Alina T, et al. Testing of exchange-correlation functionals of DFT for a reliable description of the electron density distribution in organic molecules[J]. International Journal of Molecular Sciences, 2022, 23(23): 14719. |
| 32 | Julia CONTRERAS-GARCÍA, JOHNSON Erin R, KEINAN Shahar, et al. NCIPLOT: A program for plotting non-covalent interaction regions[J]. Journal of Chemical Theory and Computation, 2011, 7(3): 625-632. |
| 33 | 姜焱龙, 张妮, 李淡然, 等. 基于COSMO-RS方法筛选离子液体用于焦油脱除[J]. 化工学报, 2022, 73(4): 1704-1713. |
| JIANG Yanlong, ZHANG Ni, LI Danran, et al. Selected ionic liquids by COSMO-RS method for tar removal[J]. CIESC Journal, 2022, 73(4): 1704-1713. | |
| 34 | 刘向阳. 气体在离子液体中溶解度的实验测量与理论计算[D]. 西安: 西安交通大学, 2017. |
| LIU Xiangyang. Measurement and calculation of gas solubility in ionic liquid[D]. Xi'an: XI'an Jiaotong University, 2017. | |
| 35 | HUSSAIN Shahid, DONG Haifeng, ZENG Shaojuan, et al. Investigation uncovered the impact of anions on CO2 absorption by low viscous ether functionalized pyridinium ionic liquids[J]. Journal of Molecular Liquids, 2021, 336: 116362. |
| 36 | PINTO J, FABRE E, MURSHED S S. Evaluation of stability, viscosity and electrical conductivity of [C2mim][DCA] based ionanocolloids[J]. Heat and Mass Transfer, 2022: 1-7. |
| 37 | YANG Fuxin, WANG Bangju, JIAO Yanhao, et al. Density and viscosity of three ionic liquids with 2,2,2-trifluoroethanol[J]. The Journal of Chemical Thermodynamics, 2023, 181: 107038. |
| 38 | ZHANG Xiaosong, PAN Jiawei, WANG Liang, et al. COSMO-based solvent selection and Aspen plus process simulation for tar absorptive removal[J]. Applied Energy, 2019, 251: 113314. |
| 39 | 吴进, 张承龙, 王瑞雪, 等. 离子液体吸收废印刷线路板热拆解过程中挥发性有机物——基于COSMO-RS模型[J]. 中国环境科学, 2020, 40(5): 1946-1952. |
| WU Jin, ZHANG Chenglong, WANG Ruixue, et al. Absorbing volatile organic compounds discharged during thermal dismantling of waste printed circuit boards using ionic liquids—Based on COSMO-RS model[J]. China Environmental Science, 2020, 40(5): 1946-1952. | |
| 40 | LIU Yanrong, YU Hang, SUN Yunhao, et al. Screening deep eutectic solvents for CO2 capture with COSMO-RS[J]. Frontiers in Chemistry, 2020, 8: 82. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [4] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [5] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [6] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [7] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [8] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [9] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [10] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [11] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [12] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [13] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [14] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| [15] | 马源, 肖晴月, 岳君容, 崔彦斌, 刘姣, 许光文. CeO2-Al2O3复合载体负载Ni基催化剂催化CO x 共甲烷化性能[J]. 化工进展, 2023, 42(5): 2421-2428. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||