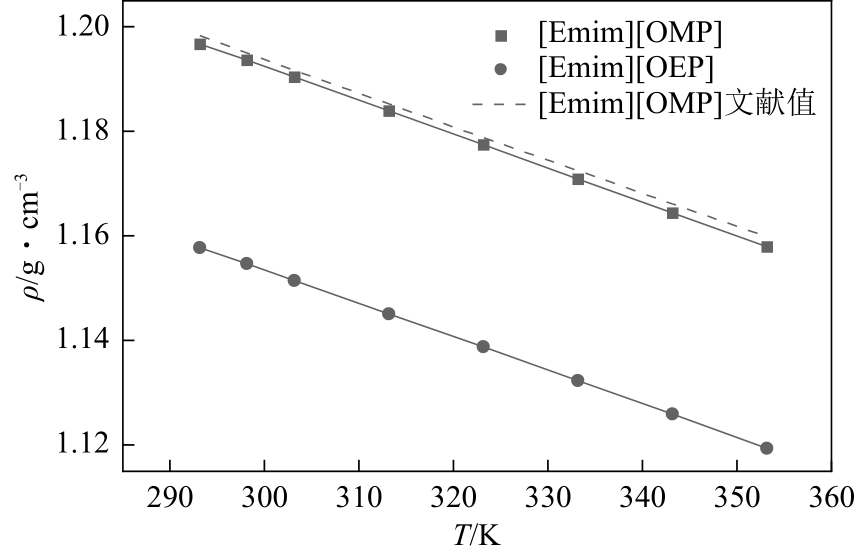
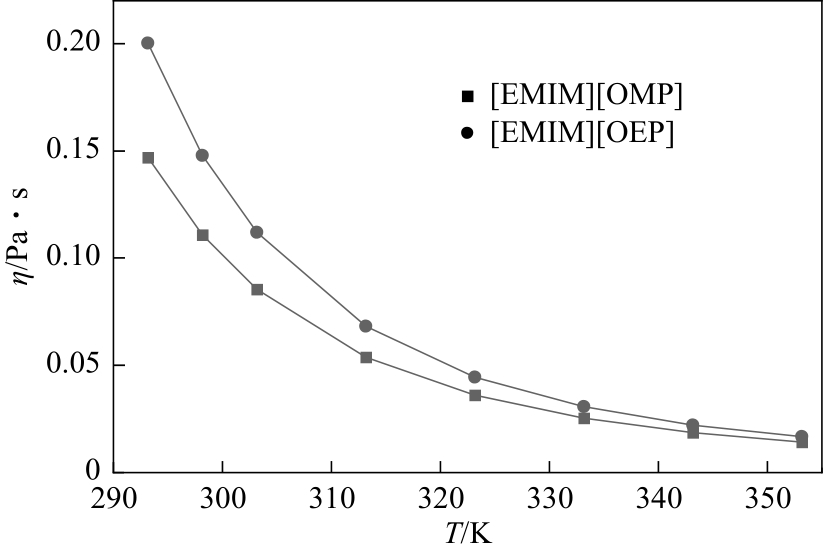
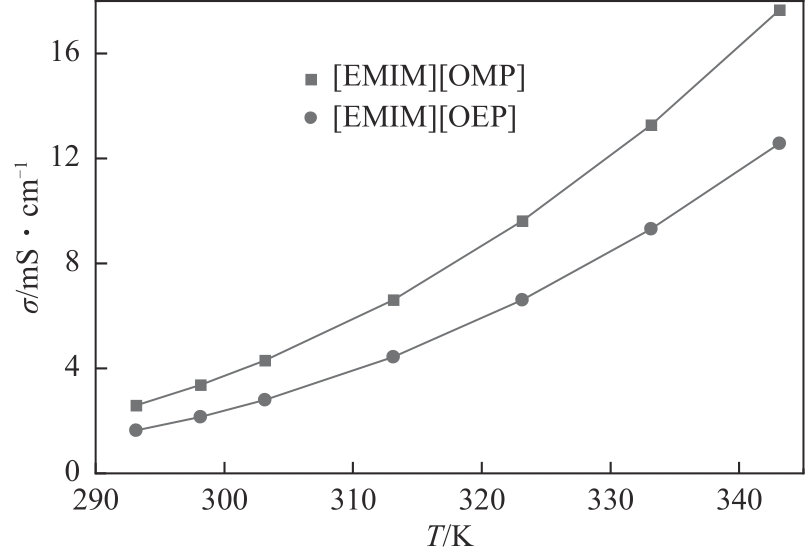
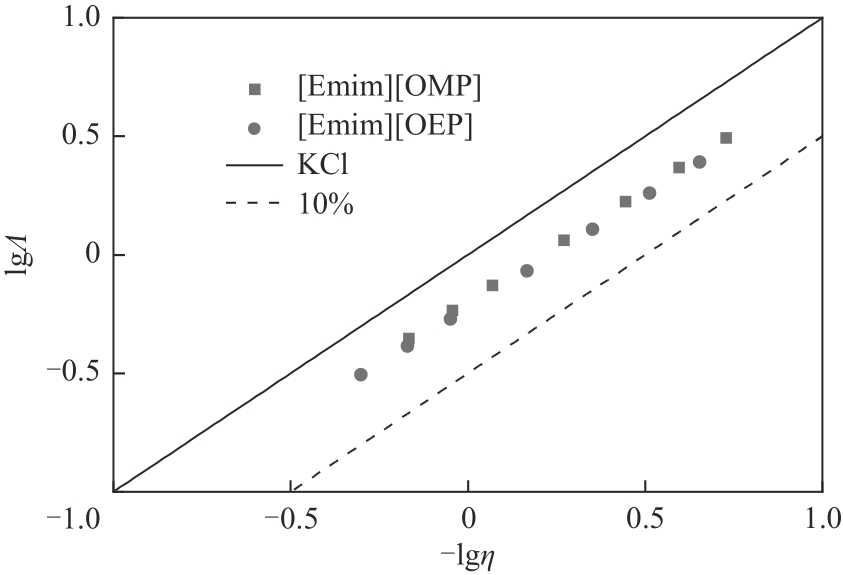
| 1 |
WILKES John S. A short history of ionic liquids—From molten salts to neoteric solvents[J]. Green Chemistry, 2002, 4(2): 73-80.
|
| 2 |
OHNO, HIROYUKI. Importance and possibility of ionic liquids [M]. New York: John Wiley & Sons, Inc. 2005.
|
| 3 |
MACFARLANE Douglas R, PRINGLE Jennifer M, JOHANSSON Katarina M, et al. Lewis base ionic liquids[J]. Chemical Communications, 2006(18): 1905-1917.
|
| 4 |
KAZUHIDE Ueno, HIROYUKI Tokuda, MASAYOSHI Watanabe. Ionicity in ionic liquids: Correlation with ionic structure and physicochemical properties[J]. Physical Chemistry Chemical Physics: PCCP, 2010, 12(8): 1649-1658.
|
| 5 |
ZHOU Zhibin, HAJIME Matsumoto, KUNIAKI Tatsumi. Structure and properties of new ionic liquids based on alkyl- and alkenyltrifluoroborates[J]. Chemphyschem: a European Journal of Chemical Physics and Physical Chemistry, 2005, 6(7): 1324-1332.
|
| 6 |
ANDERSON Jared L, DING Rongfang, ELLERN Arkady, et al. Structure and properties of high stability geminal dicationic ionic liquids[J]. Journal of the American Chemical Society, 2005, 127(2): 593-604.
|
| 7 |
APPETECCHI Giovanni B, MONTANINO Maria, ZANE Daniela, et al. Effect of the alkyl group on the synthesis and the electrochemical properties of N-alkyl-N-methyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide ionic liquids[J]. Electrochimica Acta, 2009, 54(4): 1325-1332.
|
| 8 |
DZYUBA Sergei V, BARTSCH Richard A. Influence of structural variations in 1-alkyl(aralkyl)-3-methylimidazolium hexafluorophosphates and bis(trifluoromethylsulfonyl)imides on physical properties of the ionic liquids[J]. Chemphyschem: a European Journal of Chemical Physics and Physical Chemistry, 2002, 3(2): 161-166.
|
| 9 |
KUNZE Miriam, JEONG Sangsik, PAILLARD Elie, et al. Melting behavior of pyrrolidinium-based ionic liquids and their binary mixtures[J]. The Journal of Physical Chemistry C, 2010, 114(28): 12364-12369.
|
| 10 |
SEKI Shiro, KOBAYASHI Takeshi, KOBAYASHI Yo, et al. Effects of cation and anion on physical properties of room-temperature ionic liquids[J]. Journal of Molecular Liquids, 2010, 152(1/2/3): 9-13.
|
| 11 |
HIROYUKI Tokuda, KUNIKAZU Ishii, Abu Bin Hasan Susan Md, et al. Physicochemical properties and structures of room-temperature ionic liquids. 3. Variation of cationic structures[J]. The Journal of Physical Chemistry B, 2006, 110(6): 2833-2839.
|
| 12 |
RAMAJO B, BLANCO D, RIVERA N, et al. Long-term thermal stability of fatty acid anion-based ionic liquids[J]. Journal of Molecular Liquids, 2021, 328: 115492.
|
| 13 |
LIU Kexin, WANG Zhuyi, SHI Liyi, et al. Ionic liquids for high performance lithium metal batteries[J]. Journal of Energy Chemistry, 2021, 59: 320-333.
|
| 14 |
REN Tianlin, MA Xiwen, WU Xiaoqiong, et al. Degradation of imidazolium ionic liquids in a thermally activated persulfate system[J]. Chemical Engineering Journal, 2021, 412: 128624.
|
| 15 |
HIROSAWA Kazu, FUJII Kenta, HASHIMOTO Kei, et al. Solvated structure of cellulose in a phosphonate-based ionic liquid[J]. Macromolecules, 2017, 50(17): 6509-6517.
|
| 16 |
HAN Yunyan, QIAO Dan, GUO Yuexia, et al. Influence of competitive adsorption on lubricating property of phosphonate ionic liquid additives in PEG[J]. Tribology Letters, 2016, 64(2): 22.
|
| 17 |
HAN Yunyan, QIAO Dan, ZHANG Lin, et al. Study of tribological performance and mechanism of phosphonate ionic liquids for steel/aluminum contact[J]. Tribology International, 2015, 84: 71-80.
|
| 18 |
HIRAGA Yuya, KATO Aya, SATO Yoshiyuki, et al. Densities at pressures up to 200 MPa and atmospheric pressure viscosities of ionic liquids 1-ethyl-3-methylimidazolium methylphosphate, 1-ethyl-3-methylimidazolium diethylphosphate, 1-butyl-3-methylimidazolium acetate, and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide[J]. Journal of Chemical & Engineering Data, 2015, 60(3): 876-885.
|
| 19 |
HASSE Benjamin, LEHMANN Julia, ASSENBAUM Daniel, et al. Viscosity, interfacial tension, density, and refractive index of ionic liquids[EMIM][MeSO3], [EMIM][MeOHPO2], [EMIM][OcSO4], and[BBIM][NTf2]in dependence on temperature at atmospheric pressure[J]. Journal of Chemical & Engineering Data, 2009, 54(9): 2576-2583.
|
| 20 |
ALMEIDA Hugo F D, PASSOS Helena, LOPES-DA-SILVA José A, et al. Thermophysical properties of five acetate-based ionic liquids[J]. Journal of Chemical & Engineering Data, 2012, 57(11): 3005-3013.
|
| 21 |
CAO Yuanyuan, MU Tiancheng. Comprehensive investigation on the thermal stability of 66 ionic liquids by thermogravimetric analysis[J]. Industrial & Engineering Chemistry Research, 2014, 53(20): 8651-8664.
|
| 22 |
Pierre BONHÔTE, DIAS Ana Paula, PAPAGEORGIOU Nicholas, et al. Hydrophobic, highly conductive ambient-temperature molten salts[J]. Inorganic Chemistry, 1996, 35(5): 1168-1178.
|
| 23 |
ALMEIDA Hugo F D, TELES Ana Rita R, LOPES-DA-SILVA José A, et al. Influence of the anion on the surface tension of 1-ethyl-3-methylimidazolium-based ionic liquids[J]. The Journal of Chemical Thermodynamics, 2012, 54: 49-54.
|
| 24 |
王义闹, 吴利丰. 基于平均相对误差绝对值最小的GM(1, 1)建模[J]. 华中科技大学学报(自然科学版), 2009, 37(10): 29-31.
|
|
WANG Yinao, WU Lifeng. Modeling GM(1, 1) based on the minimum of mean absolute percentage error[J]. Journal of Huazhong University of Science and Technology (Nature Science Edition), 2009, 37(10): 29-31.
|
| 25 |
NEVES Catarina M S S, KIKI Adi Kurnia, COUTINHO João A P, et al. Systematic study of the thermophysical properties of imidazolium-based ionic liquids with cyano-functionalized anions[J]. The Journal of Physical Chemistry B, 2013, 117(35): 10271-10283.
|
| 26 |
王晓玲, 王建英, 李小云, 等. 离子液体[C2mim]NO3与[C2mim][MetSO4]的热力学性能研究[J]. 河北科技大学学报, 2011, 32(2): 103-106.
|
|
WANG Xiaoling, WANG Jianying, LI Xiaoyun, et al. Study on thermophysical properties of ionic liquids of 1-ethyl-3-methylimid azolium nitrate and 1-ethyl-3-methylimidazolium methylsulfate[J]. Journal of Hebei University of Science and Technology, 2011, 32(2): 103-106.
|
| 27 |
MACFARLANE Douglas R, KAR Mega, PRINGLE Jennifer M. Fundamentals of ionic liquids: From chemistry to applications [M/OB]. New York:John Wiley & Sons, 2017. DOI: 10.1002/9783527340033 .
|
| 28 |
ZHOU Zhibin, HAJIME Matsumoto, KUNIAKI Tatsumi. Cyclic quaternary ammonium ionic liquids with perfluoroalkyltrifluoroborates: Synthesis, characterization, and properties[J]. Chemistry, 2006, 12(8): 2196-2212.
|
| 29 |
TAO Duanjian, HU Wenjing, CHEN Fengfeng, et al. Low-viscosity tetramethylguanidinum-based ionic liquids with different phenolate anions: Synthesis, characterization, and physical properties[J]. Journal of Chemical & Engineering Data, 2014, 59(12): 4031-4038.
|
| 30 |
BITTNER Bożena, WROBEL Rafal J, MILCHERT Eugeniusz. Physical properties of pyridinium ionic liquids[J]. The Journal of Chemical Thermodynamics, 2012, 55: 159-165.
|
| 31 |
BRAUER Ulises G, DE LA HOZ Andreah T, MILLER Kevin M. The effect of counteranion on the physicochemical and thermal properties of 4-methyl-1-propyl-1,2,4-triazolium ionic liquids[J]. Journal of Molecular Liquids, 2015, 210: 286-292.
|
| 32 |
PLECHKOVA Natalia V, SEDDON Kenneth R. Applications of ionic liquids in the chemical industry[J]. Chemical Society Reviews, 2008, 37(1): 123-150.
|
| 33 |
Anouti MÉRIÈM, MAGALY Caillon-Caravanier, YOSRA Dridi, et al. Synthesis and characterization of new pyrrolidinium based protic ionic liquids. Good and superionic liquids[J]. The Journal of Physical Chemistry B, 2008, 112(42): 13335-13343.
|
| 34 |
MANN Sarah K, BROWN Steven P, MACFARLANE Douglas R. Structure effects on the ionicity of protic ionic liquids[J]. Chemphyschem: A European Journal of Chemical Physics and Physical Chemistry, 2020, 21(13): 1444-1454.
|
| 35 |
MOHAMMAD Tariq, FREIRE Mara G, Saramago Benilde, et al. Surface tension of ionic liquids and ionic liquid solutions[J]. Chemical Society Reviews, 2012, 41(2): 829-868.
|
| 36 |
KOLBECK C, LEHMANN J, LOVELOCK K R J, et al. Density and surface tension of ionic liquids[J]. The Journal of Physical Chemistry B, 2010, 114(51): 17025-17036.
|
 ), 曾纪珺, 廖袁淏, 唐晓博, 赵波, 韩升, 张伟(
), 曾纪珺, 廖袁淏, 唐晓博, 赵波, 韩升, 张伟( )
)
 ), ZENG Jijun, LIAO Yuanhao, TANG Xiaobo, ZHAO Bo, HAN Sheng, ZHANG Wei(
), ZENG Jijun, LIAO Yuanhao, TANG Xiaobo, ZHAO Bo, HAN Sheng, ZHANG Wei( )
)