化工进展 ›› 2024, Vol. 43 ›› Issue (10): 5890-5900.DOI: 10.16085/j.issn.1000-6613.2023-1603
• 资源与环境化工 • 上一篇
新型的BHEP-醚水溶液相变吸收二氧化硫
刁忠秀1( ), 郑宇1, 魏凤玉1(
), 郑宇1, 魏凤玉1( ), 宋小良2, 苏文国2, 李淑全2
), 宋小良2, 苏文国2, 李淑全2
- 1.合肥工业大学化学与化工学院,安徽 合肥 230009
2.双盾环境科技有限公司,江苏 无锡 214205
-
收稿日期:2023-09-11修回日期:2024-01-20出版日期:2024-10-15发布日期:2024-10-29 -
通讯作者:魏凤玉 -
作者简介:刁忠秀(1999—),女,硕士研究生,研究方向为化工传质与分离技术。E-mail:diaozhongxiu@gmail.com。
Phase-change absorption for SO2 capture by a novel aqueous BHEP/ether solution
DIAO Zhongxiu1( ), ZHENG Yu1, WEI Fengyu1(
), ZHENG Yu1, WEI Fengyu1( ), SONG Xiaoliang2, SU Wenguo2, LI Shuquan2
), SONG Xiaoliang2, SU Wenguo2, LI Shuquan2
- 1.School of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei 230009, Anhui, China
2.Shuangdun Environment Technology Company, Wuxi 214205, Jiangsu, China
-
Received:2023-09-11Revised:2024-01-20Online:2024-10-15Published:2024-10-29 -
Contact:WEI Fengyu
摘要:
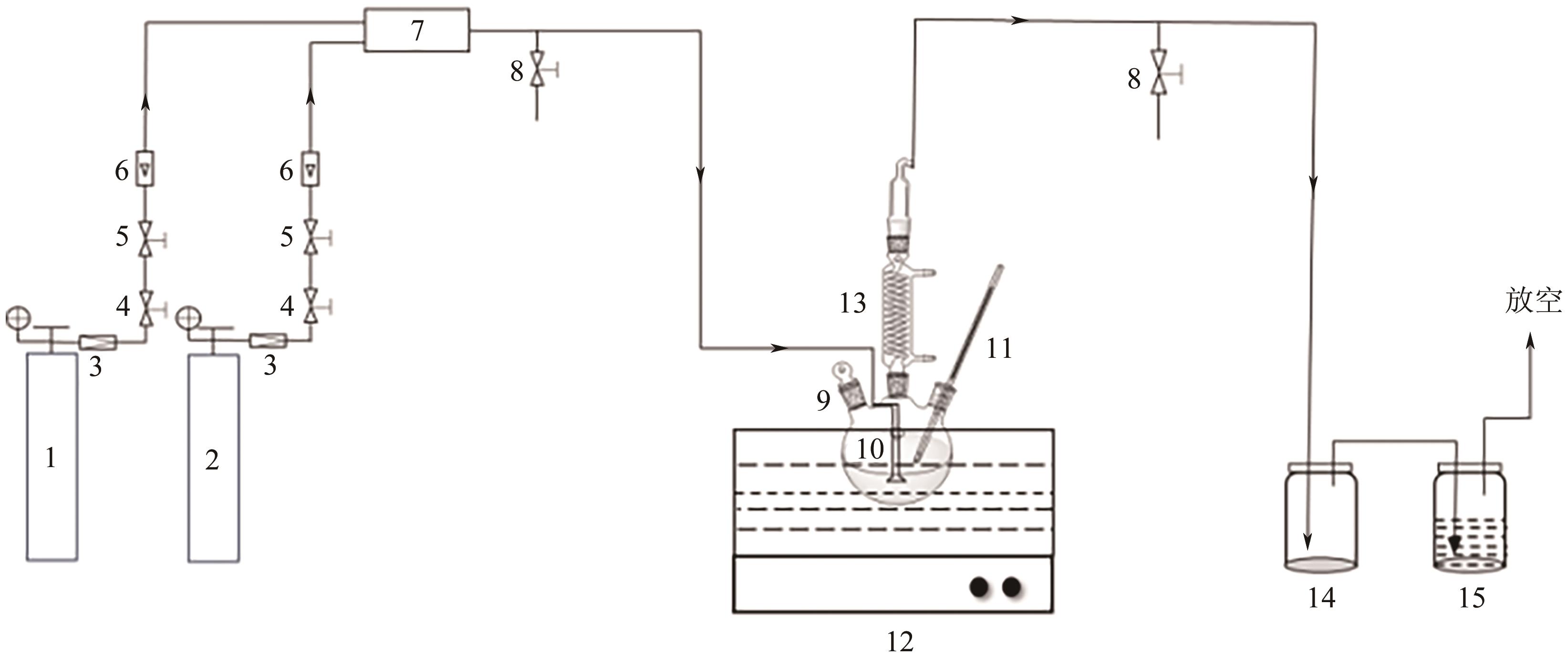
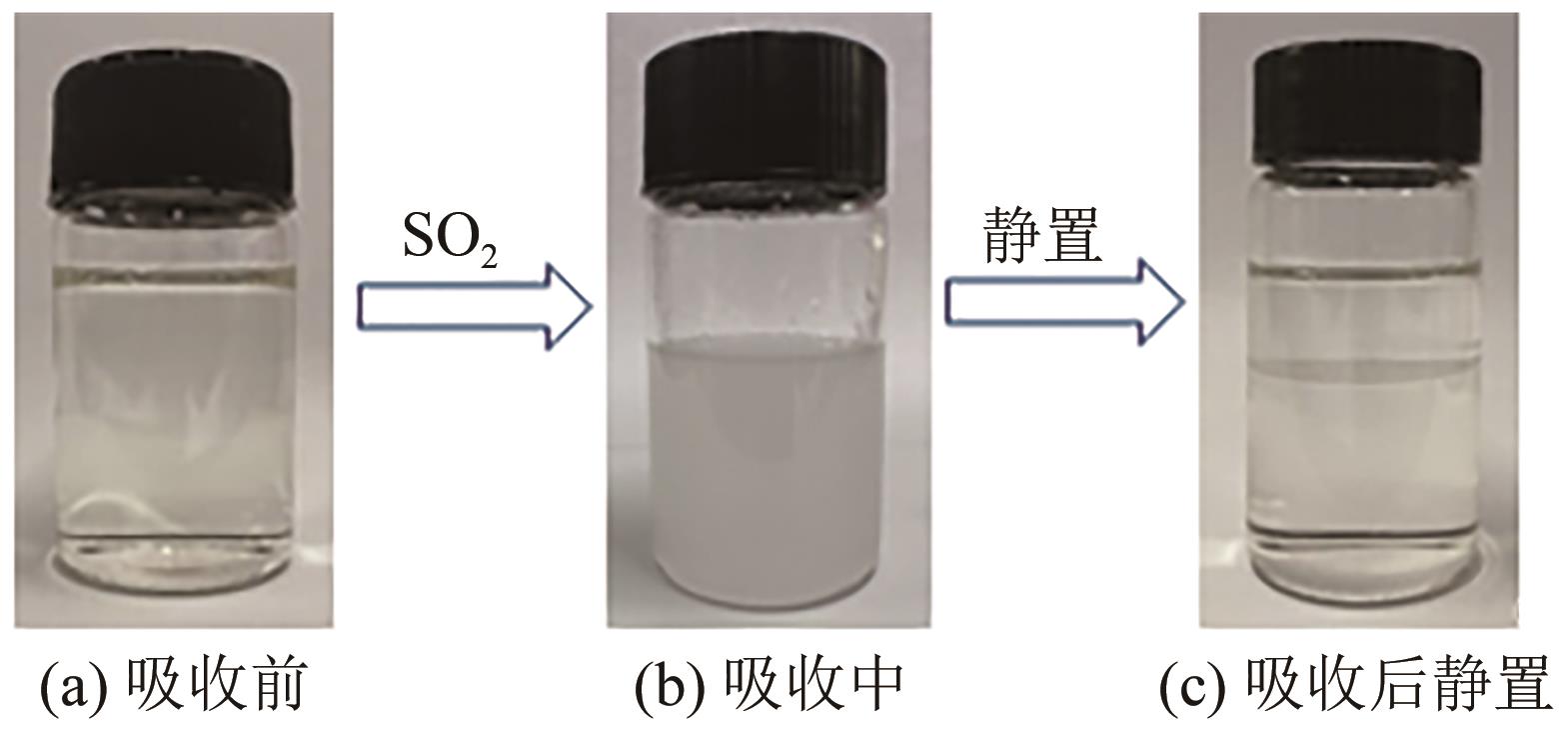
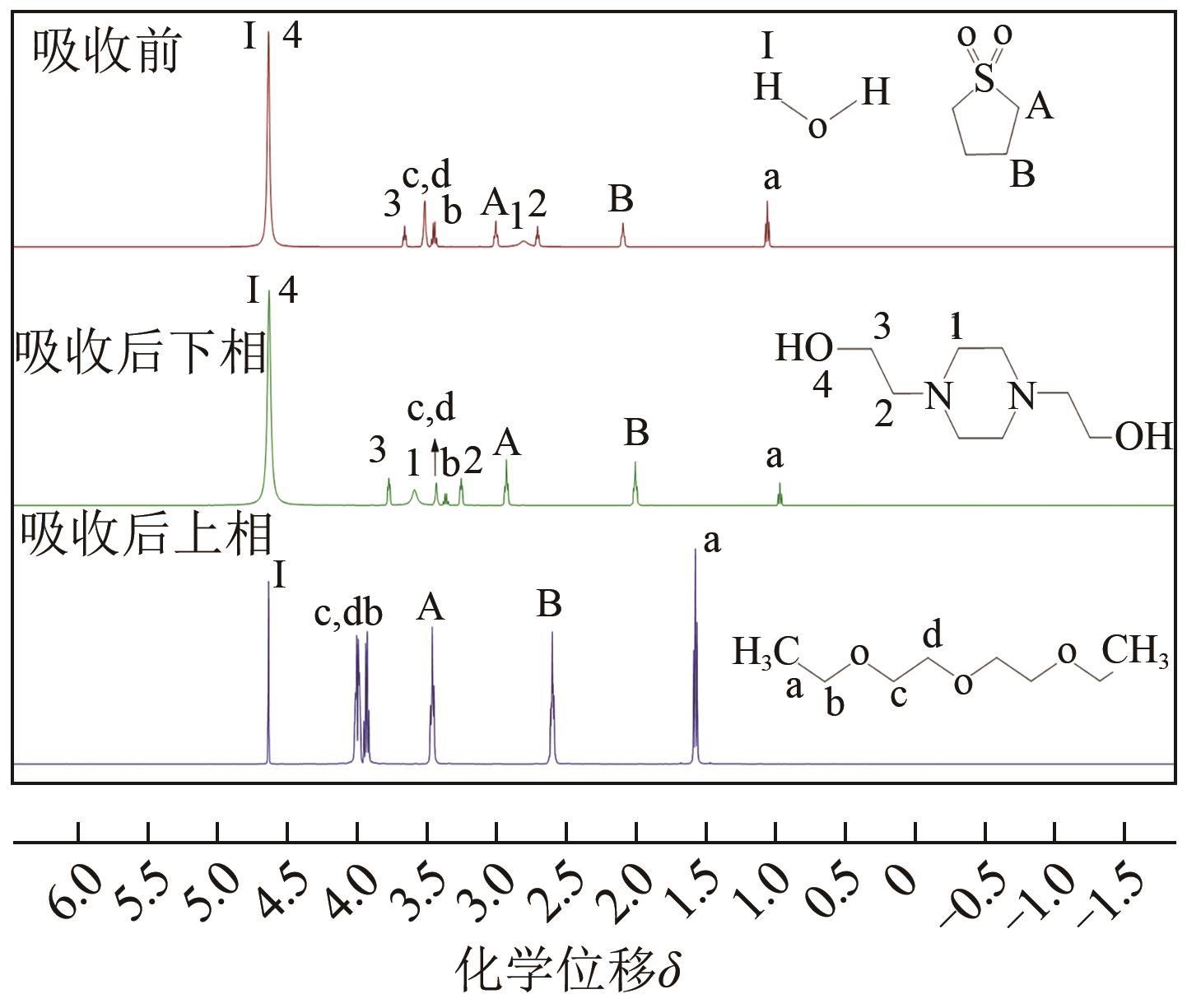
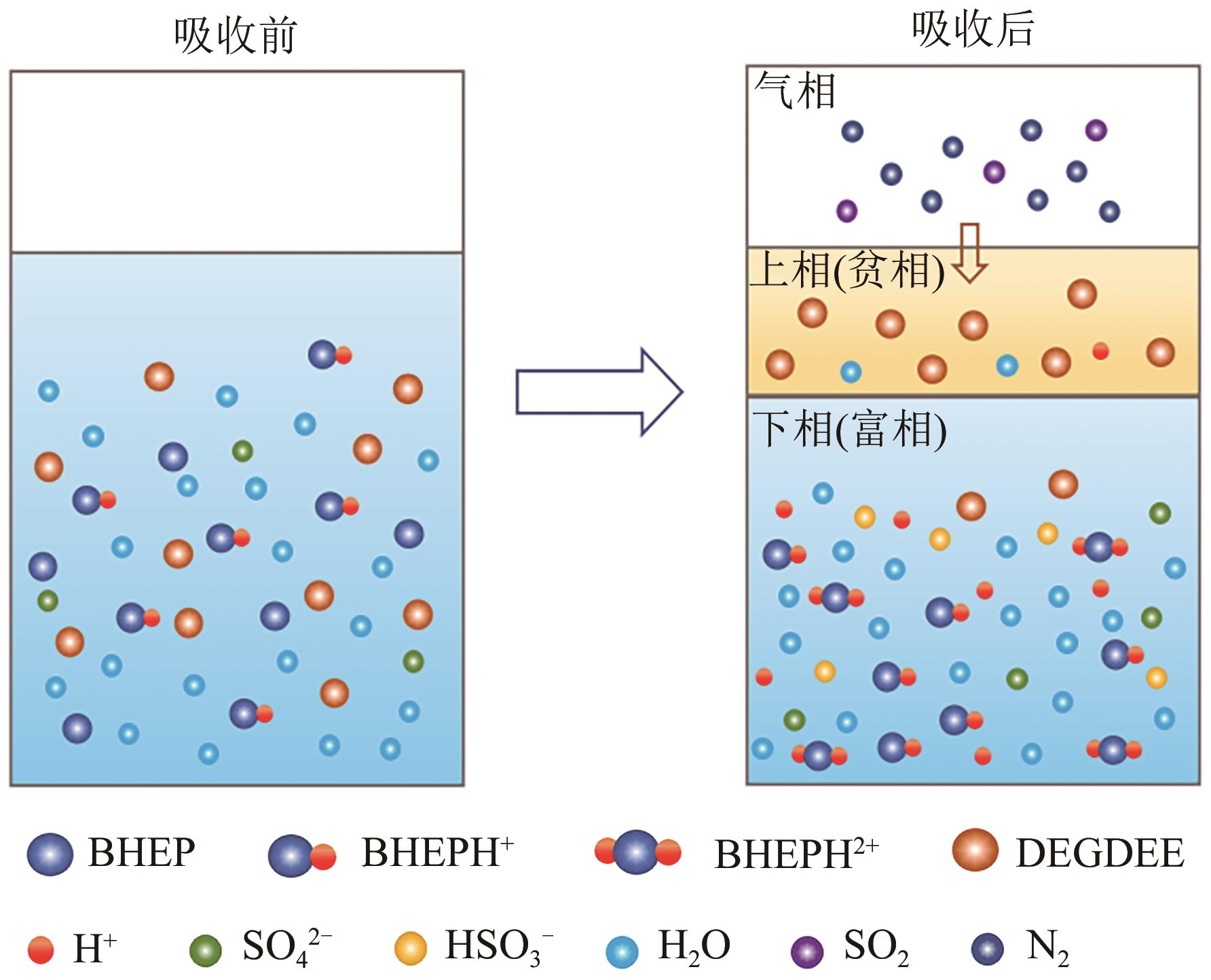
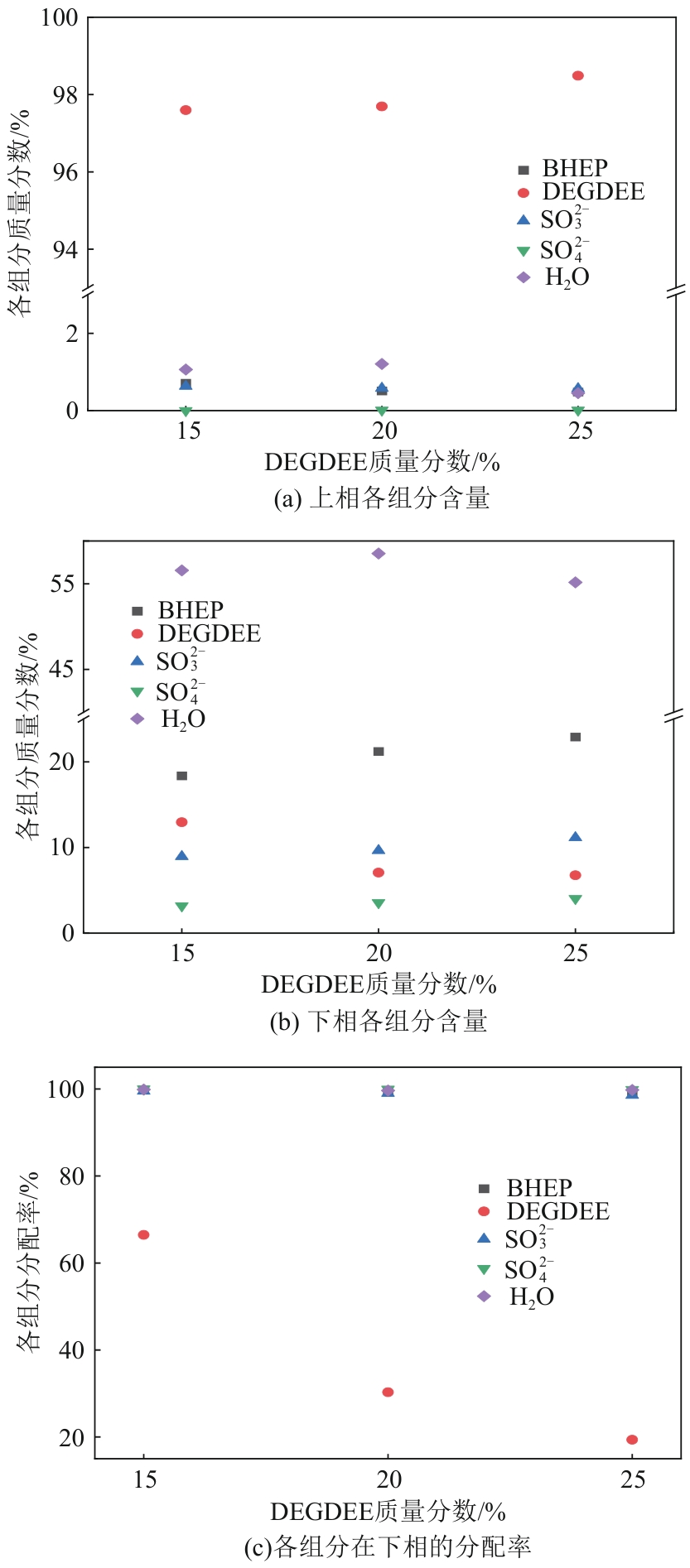
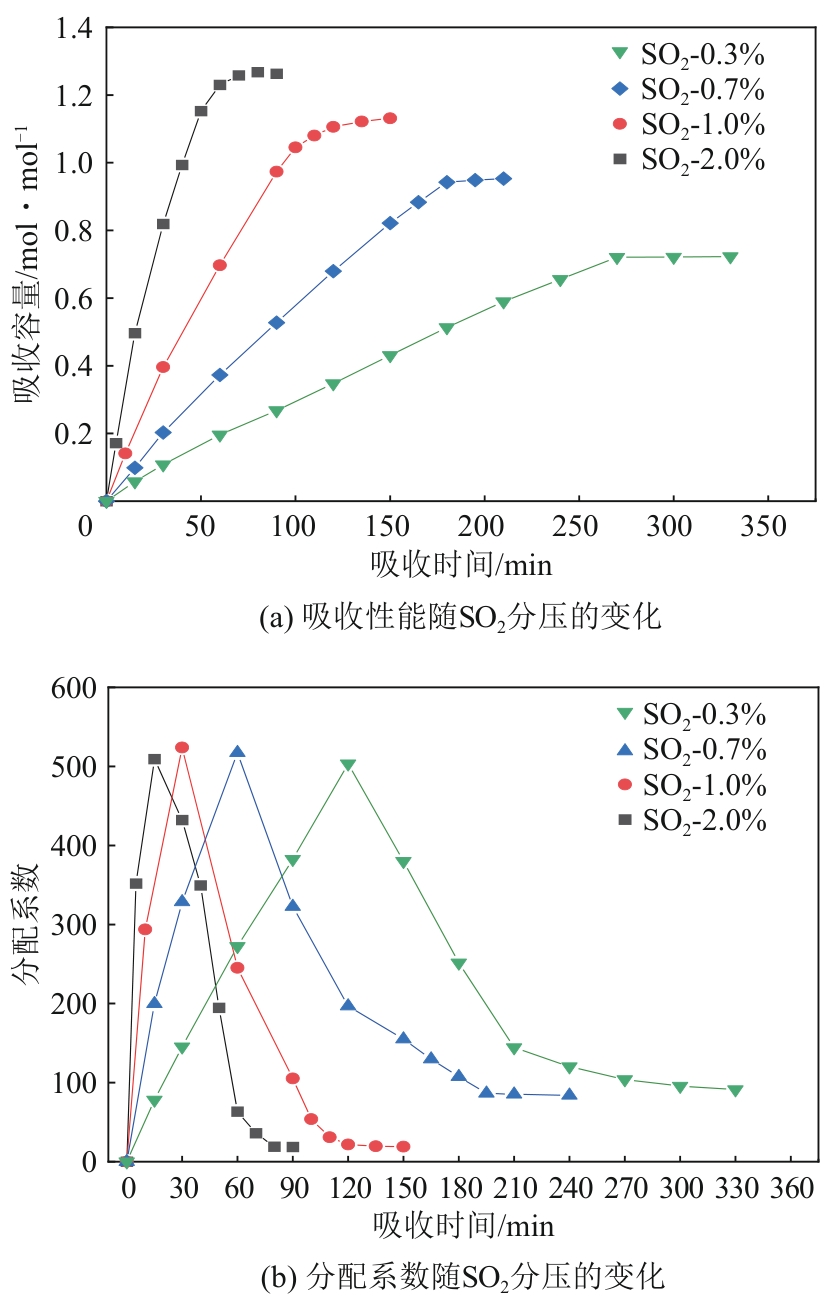
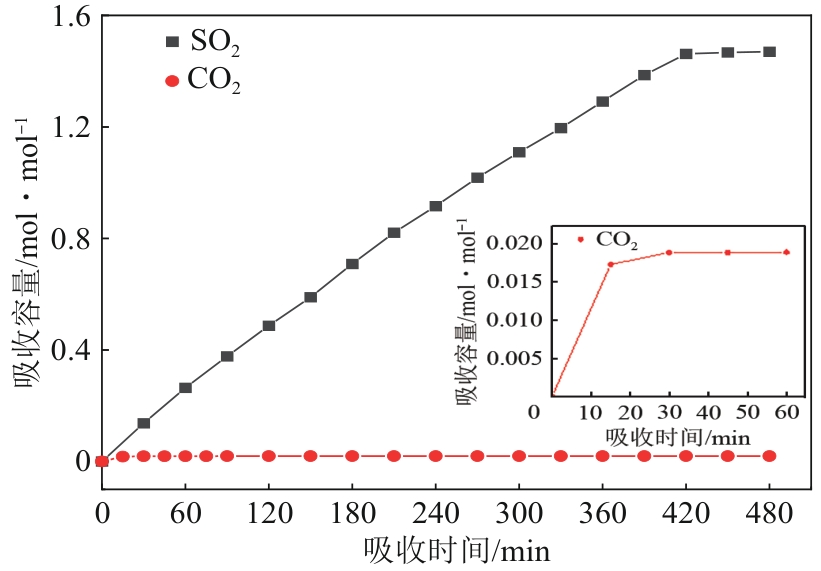
针对有机胺湿法烟气脱硫过程能耗高的问题,本文开发了一种新型的N,N′-双(2-羟乙基)哌嗪有机胺(BHEP)/二乙二醇二乙醚(DEGDEE)水系液-液相变吸收剂,并研究其吸收解吸SO2性能及相变机理。结果表明,BHEP与二氧化硫(SO2)反应生成了极性较强的铵盐,与非极性醚差异较大,产生盐析效应而导致其分相。上相中DEGDEE的浓度很高,质量分数大于97%,胺、SO2和水的质量分数低于1%,可直接循环使用;约有99%以上的胺和SO2富集在下相,只需对下相解吸,从而大大地降低了能耗。有机胺BHEP和DEGDEE的浓度越高,越易发生相变,下相体积占比和DEGDEE分配率越小,SO2和BHEP的分配率几乎不受影响;两者浓度太高时还会发生吸收前分相。DEGDEE对SO2的物理吸收使体系的吸收能力提高,在质量分数15% BHEP水溶液中加入质量分数20%的DEGDEE后,循环吸收容量和解吸率分别提高了6.43%和10.59%,能耗降低了13.69%,该相变吸收剂具有较好的应用前景。
中图分类号:
引用本文
刁忠秀, 郑宇, 魏凤玉, 宋小良, 苏文国, 李淑全. 新型的BHEP-醚水溶液相变吸收二氧化硫[J]. 化工进展, 2024, 43(10): 5890-5900.
DIAO Zhongxiu, ZHENG Yu, WEI Fengyu, SONG Xiaoliang, SU Wenguo, LI Shuquan. Phase-change absorption for SO2 capture by a novel aqueous BHEP/ether solution[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5890-5900.
| 溶剂种类 | 分子式 | 沸点/℃ | 闪点/℃ | 水溶解性 | 吸收前 | 吸收后 |
|---|---|---|---|---|---|---|
| 三乙二醇单甲醚(TriEGME) | C7H16O4 | 233.9 | 95.30 | 互溶 | 液相 | 液相 |
| 三乙二醇二甲醚(TriEGDME) | C8H18O4 | 249.0 | 118.30 | 互溶 | 液相 | 液相① |
| 二乙二醇二乙醚(DEGDEE) | C8H18O3 | 180.0 | 71.10 | 微溶 | 液相 | 液-液 |
| 二丙二醇单丙醚(DPGPE) | C9H20O3 | 242.9 | 100.70 | 不溶 | 液-液 | 液-液 |
表1 相变吸收SO2的溶剂筛选
| 溶剂种类 | 分子式 | 沸点/℃ | 闪点/℃ | 水溶解性 | 吸收前 | 吸收后 |
|---|---|---|---|---|---|---|
| 三乙二醇单甲醚(TriEGME) | C7H16O4 | 233.9 | 95.30 | 互溶 | 液相 | 液相 |
| 三乙二醇二甲醚(TriEGDME) | C8H18O4 | 249.0 | 118.30 | 互溶 | 液相 | 液相① |
| 二乙二醇二乙醚(DEGDEE) | C8H18O3 | 180.0 | 71.10 | 微溶 | 液相 | 液-液 |
| 二丙二醇单丙醚(DPGPE) | C9H20O3 | 242.9 | 100.70 | 不溶 | 液-液 | 液-液 |
| DEGDEE用量/% | 吸收前 | 吸收后 | 分相时间/min | 黏度/mPa·s | 下相体积占比/% | 分配系数 | |
|---|---|---|---|---|---|---|---|
| 吸收前 | 吸收后下相 | ||||||
| 0 | 液相 | 液相 | 不分相 | 5.62 | 8.25 | 100 | — |
| 5 | 液相 | 液相 | 不分相 | 7.46 | 9.13 | 100 | — |
| 10 | 液相 | 液-液 | 30 | 8.03 | 10.59 | 98.70 | 14.30 |
| 15 | 液相 | 液-液 | 10 | 9.5 | 11.28 | 91.86 | 17.26 |
| 20 | 液相 | 液-液 | 3 | 10.12 | 13.03 | 83.33 | 19.40 |
| 25 | 液-液 | 液-液 | 0 | 11.33 | 16.75 | 73.68 | 21.37 |
表2 DEGDEE用量(质量分数)的影响
| DEGDEE用量/% | 吸收前 | 吸收后 | 分相时间/min | 黏度/mPa·s | 下相体积占比/% | 分配系数 | |
|---|---|---|---|---|---|---|---|
| 吸收前 | 吸收后下相 | ||||||
| 0 | 液相 | 液相 | 不分相 | 5.62 | 8.25 | 100 | — |
| 5 | 液相 | 液相 | 不分相 | 7.46 | 9.13 | 100 | — |
| 10 | 液相 | 液-液 | 30 | 8.03 | 10.59 | 98.70 | 14.30 |
| 15 | 液相 | 液-液 | 10 | 9.5 | 11.28 | 91.86 | 17.26 |
| 20 | 液相 | 液-液 | 3 | 10.12 | 13.03 | 83.33 | 19.40 |
| 25 | 液-液 | 液-液 | 0 | 11.33 | 16.75 | 73.68 | 21.37 |
| BHEP用量/% | 吸收前 | 吸收后 | 分相时间/min | 黏度/mPa·s | 下相体积占比/% | |
|---|---|---|---|---|---|---|
| 吸收前 | 吸收后下相 | |||||
| 10 | 液相 | 液-液 | 35 | 3.47 | 7.50 | 91.86 |
| 15 | 液相 | 液-液 | 10 | 5.72 | 10.41 | 84.62 |
| 20 | 液相 | 液-液 | 5 | 9.56 | 11.79 | 79.22 |
| 25 | 液-液 | 液-液 | 0 | 13.28 | 18.47 | 77.92 |
表3 BHEP用量(质量分数)的影响
| BHEP用量/% | 吸收前 | 吸收后 | 分相时间/min | 黏度/mPa·s | 下相体积占比/% | |
|---|---|---|---|---|---|---|
| 吸收前 | 吸收后下相 | |||||
| 10 | 液相 | 液-液 | 35 | 3.47 | 7.50 | 91.86 |
| 15 | 液相 | 液-液 | 10 | 5.72 | 10.41 | 84.62 |
| 20 | 液相 | 液-液 | 5 | 9.56 | 11.79 | 79.22 |
| 25 | 液-液 | 液-液 | 0 | 13.28 | 18.47 | 77.92 |
| 1 | RAYNAL Ludovic, ALIX Pascal, BOUILLON Pierre-Antoine, et al. The DMX™ process: An original solution for lowering the cost of post-combustion carbon capture[J]. Energy Procedia, 2011, 4: 779-786. |
| 2 | ZHANG Shihan, SHEN Yao, WANG Lidong, et al. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges[J]. Applied Energy, 2019, 239: 876-897. |
| 3 | 张卫风, 周武, 王秋华. 相变吸收捕集烟气中CO2技术的发展现状[J]. 化工进展, 2022, 41(4): 2090-2101. |
| ZHANG Weifeng, ZHOU Wu, WANG Qiuhua. Recent developments of phase-change absorption technology for CO2 capture from flue gas[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2090-2101. | |
| 4 | 刘飞. 胺基两相吸收剂捕集二氧化碳机理研究[D]. 杭州: 浙江大学, 2020. |
| LIU Fei. Study on carbon dioxide capture mechanism of amine-based two-phase absorbent[D]. Hangzhou: Zhejiang University, 2020. | |
| 5 | 涂巍巍, 方佳伟, 李竹石, 等. 基于MEA的CO2相变化吸收剂的开发[J]. 中国科学: 化学, 2018, 48(6): 641-647. |
| TU Weiwei, FANG Jiawei, LI Zhushi, et al. Development of MEA phase change absorbent[J]. Scientia Sinica (Chimica), 2018, 48(6): 641-647. | |
| 6 | YE Qing, WANG Xinlei, LU Yongqi. Screening and evaluation of novel biphasic solvents for energy-efficient post-combustion CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 39: 205-214. |
| 7 | 沈丽, 刘凡, 沈遥, 等. 新型AEP-相变吸收剂捕集CO2研究[J]. 高校化学工程学报, 2021, 35(6): 1067-1072. |
| SHEN Li, LIU Fan, SHEN Yao, et al. Study on novel AEP-based biphasic solvents for CO2 capture[J]. Journal of Chemical Engineering of Chinese Universities, 2021, 35(6): 1067-1072. | |
| 8 | SHEN Yao, JIANG Chenkai, ZHANG Shihan, et al. Biphasic solvent for CO2 capture: Amine property-performance and heat duty relationship[J]. Applied Energy, 2018, 230: 726-733. |
| 9 | 桑伟, 唐建峰, 花亦怀, 等. 物理溶剂及有机胺的性质对相变吸收性能的影响[J]. 化工进展, 2023, 42(4): 2151-2159. |
| SANG Wei, TANG Jianfeng, HUA Yihuai, et al. Effects of physical solvent and amine properties on the performance of biphasic solvent[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2151-2159. | |
| 10 | 赵文波, 李广振, 许胜超, 等. 相变吸收酸性气体的发展现状[J]. 化工进展, 2021, 40(1): 401-414. |
| ZHAO Wenbo, LI Guangzhen, XU Shengchao, et al. Recent developments of acid gas absorption by phase-change[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 401-414. | |
| 11 | XU Shengchao, ZHAO Wenbo, XIE Xuhao, et al. Dual effects of long-chain alcohols in phase change capture of SO2: Increasing mutual solubility and decreasing product viscosity[J]. Journal of Molecular Liquids, 2021, 328: 115461. |
| 12 | 李雪霏, 陈玲, 许胜超, 等. N,N-二甲基正辛胺/十六烷混合体系液液相变吸收SO2 [J]. 化学学报, 2019, 77(12): 1287-1293. |
| LI Xuefei, CHEN Ling, XU Shengchao, et al. Liquid-liquid phase-change absorption of SO2 using N,N-dimethyl-n-octylamine mixed with hexadecane[J]. Acta Chimica Sinica, 2019, 77(12): 1287-1293. | |
| 13 | CHAI Muyuan, ZHAO Wenbo, LI Genming, et al. Novel SO2 phase-change absorbent: Mixture of N,N-dimethylaniline and liquid paraffin[J]. Industrial & Engineering Chemistry Research, 2018, 57(37): 12502-12510. |
| 14 | LI Genming, ZHAO Wenbo, CHAI Muyuan, et al. Liquid-liquid phase-change absorption of SO2 using N,N-dimethylcyclohexylamine as absorbent and liquid paraffin as solvent[J]. Journal of Hazardous Materials, 2018, 360: 89-96. |
| 15 | HELDEBRANT David J, KOECH Phillip K, YONKER Clement R. A reversible zwitterionic SO2-binding organic liquid[J]. Energy & Environmental Science, 2010, 3(1): 111-113. |
| 16 | 沈紫薇, 常栋渊, 郭本帅, 等. 水对无水相变吸收剂捕集CO2性能的影响[J]. 低碳化学与化工, 2023(4): 107-113. |
| SHEN Ziwei, CHANG Dongyuan, GUO Benshuai, et al. Effect of water on CO2 capture performance of anhydrous phase change absorbent[J]. Low-Carbon Chemistry and Chemical Engineering, 2023(4): 107-113. | |
| 17 | DUAN Erhong, GUO Bin, ZHANG Miaomiao, et al. Efficient capture of SO2 by a binary mixture of caprolactam tetrabutyl ammonium bromide ionic liquid and water[J]. Journal of Hazardous Materials, 2011, 194: 48-52. |
| 18 | 杨福绅. 燃煤烟气碳捕集相变化吸收剂开发及杂质脱除技术研究[D]. 北京: 北京化工大学, 2019. |
| YANG Fushen. Development of phase change absorbent for carbon capture in coal-fired flue gas and study on impurity removal technology[D]. Beijing: Beijing University of Chemical Technology, 2019. | |
| 19 | WEI Fengyu, HE Yuan, XUE Pan, et al. Mass transfer performance for low SO2 absorption into aqueous N,N′-bis(2-hydroxypropyl) piperazine solution in a θ-ring packed column[J]. Industrial & Engineering Chemistry Research, 2014, 53(11): 4462-4468. |
| 20 | 刘建芳. 无机酸和Fe2+/Fe3+对有机胺吸收/解吸SO2气体的影响[D]. 合肥: 合肥工业大学, 2012. |
| LIU Jianfang. Effects of inorganic acids and Fe2+/Fe3+ on absorption/desorption of SO2 gas by organic amines[D]. Hefei: Hefei University of Technology, 2012. | |
| 21 | 魏凤玉. 一种吸收捕集SO2的水系液-液相变吸收剂及其制备方法及其应用: CN116196728A[P]. 2023-06-02. |
| WEI Fengyu. The invention relates to an aqueous liquid-liquid variable absorber for absorbing and trapping SO, a preparation method and application thereof: CN116196728A[P]. 2023-06-02. | |
| 22 | 魏凤玉, 童晨. 哌嗪类二胺PA-A-硫酸动态吸收模拟烟气中的二氧化硫[J]. 化工进展, 2010, 29(S2): 330-333. |
| WEI Fengyu, TONG Chen. Dynamic absorption of SO2 in simulated flue gas with piperazines diamine(PA-A)-sulfuric acid solution[J]. Chemical Industry and Engineering Progress, 2010, 29(S2): 330-333. | |
| 23 | 孙志豪, 郭子东, 陈俊, 等. 哌嗪类有机胺脱除二氧化硫性能及机理探讨[J]. 化工进展, 2019, 38(S1): 46-51. |
| SUN Zhihao, GUO Zidong, CHEN Jun, et al. Performances and mechanism of piperazine-based organic amines removal of SO2 [J]. Chemical Industry and Engineering Progress, 2019, 38(S1): 46-51. | |
| 24 | 华东理工大学, 四川大学. 分析化学[M]. 7版. 北京: 高等教育出版社, 2018: 43-192. |
| East China University of Science and Technology, Sichuan University. Analytical chemistry[M]. 7th ed. Beijing: Higher Education Press, 2018: 43-192. | |
| 25 | 袁存光, 祝优珍, 田晶, 等. 现代仪器分析[M]. 北京: 化学工业出版社, 2012. |
| YUAN Cunguang, ZHU Youzhen, TIAN Jing, et al. Modern instrumental analysis[M]. Beijing: Chemical Industry Press, 2012. | |
| 26 | ZHANG J, MISCH R, TAN Y, et al. Novel thermomorphic biphasic amine solvents for CO2 absorption and low-temperature extractive regeneration[J]. Chemical Engineering & Technology, 2011, 34(9): 1481-1489. |
| 27 | 刘彪. 离去基团法制备端氨基聚醚及其吸收解吸二氧化碳的研究[D]. 昆明: 昆明理工大学, 2015. |
| LIU Biao. Study on preparation of amino-terminated polyether by leaving group method and its absorption and desorption of carbon dioxide[D]. Kunming: Kunming University of Science and Technology, 2015. | |
| 28 | MACHIDA Hiroshi, Kazuki OBA, TOMIKAWA Takashi, et al. Development of phase separation solvent for CO2 capture by aqueous (amine + ether) solution[J]. The Journal of Chemical Thermodynamics, 2017, 113: 64-70. |
| 29 | 唐思扬, 李星宇, 鲁厚芳, 等. 低能耗化学吸收碳捕集技术展望[J]. 化工进展, 2022, 41(3): 1102-1106. |
| TANG Siyang, LI Xingyu, LU Houfang, et al. Perspective on low-energy chemical absorption for CO2 capture[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1102-1106. | |
| 30 | 翁诗甫, 徐怡庄. 傅里叶变换红外光谱分析[M]. 3版. 北京: 化学工业出版社, 2016: 490-507. |
| WENG Shifu, XU Yizhuang. Fourier transform infrared spectrum analysis[M]. 3rd ed. Beijing: Chemical Industry Press, 2016: 490-507. | |
| 31 | FINNEY J L, BOWRON D T. Anion bridges and salting out[J]. Current Opinion in Colloid & Interface Science, 2004, 9(1/2): 59-63. |
| 32 | 金显杭. 面向CO2捕集的相变吸收剂开发及应用研究[D]. 北京: 北京化工大学, 2017. |
| JIN Xianhang. The development and application of phase change absorbents for CO2 capture[D]. Beijing: Beijing University of Chemical Technology, 2017. | |
| 33 | 马婷. 几种类型有机溶剂吸收SO2的研究[D]. 太原: 太原理工大学, 2019. |
| MA Ting. Research on absorbing SO2 by several different type organic solvents[D]. Taiyuan: Taiyuan University of Technology, 2019. | |
| 34 | 王蓉. 物理溶剂对N-甲基二乙醇胺体系相变化吸收剂液液相平衡及吸收性能的影响[D]. 北京: 北京化工大学, 2021. |
| WANG Rong. Effect of physical solvents on liquid-liquid equilibrium and absorption performance of phase change absorbent in N-methyldiethanolamine system[D]. Beijing: Beijing University of Chemical Technology, 2021. | |
| 35 | SHAMIRI A, SHAFEEYAN M S, TEE H C, et al. Absorption of CO2 into aqueous mixtures of glycerol and monoethanolamine[J]. Journal of Natural Gas Science and Engineering, 2016, 35: 605-613. |
| 36 | 何园. 有机二胺吸收SO2的传质及其动力学研究[D]. 合肥: 合肥工业大学, 2013. |
| HE Yuan. Absorption of SO2 into aqueous organic diamine: Mass transfer and reaction kinetics[D]. Hefei: Hefei University of Technology, 2013. | |
| 37 | CHEN Kaihong, LIN Wenjun, YU Xini, et al. Designing of anion-functionalized ionic liquids for efficient capture of SO2 from flue gas[J]. AIChE Journal, 2015, 61(6): 2028-2034. |
| 38 | 张建斌, 李强, 刘占英, 等. 聚乙二醇及其水溶液吸收SO2机理研究[J]. 化学工程, 2010, 38(12): 76-79. |
| ZHANG Jianbin, LI Qiang, LIU Zhanying, et al. Absorption mechanism of SO2 in polyethylene glycol and its aqueous solution[J]. Chemical Engineering (China), 2010, 38(12): 76-79. | |
| 39 | UYANGA Itoro J, IDEM Raphael O. Studies of SO2- and O2-induced degradation of aqueous MEA during CO2 capture from power plant flue gas streams[J]. Industrial & Engineering Chemistry Research, 2007, 46(8): 2558-2566. |
| 40 | 谢江鹏. 二氧化硫捕集过程胺基吸收剂筛选与工艺流程匹配研究[D]. 兰州: 兰州理工大学, 2022. |
| XIE Jiangpeng. Study on screening of amino absorbent and matching of process flow in sulfur dioxide capture process[D]. Lanzhou: Lanzhou University of Technology, 2022. | |
| 41 | 张宇, 薛攀, 魏凤玉. 哌嗪类有机胺对二氧化硫的吸收及解吸性能研究[J]. 应用化工, 2014, 43(7): 1237-1239, 1242. |
| ZHANG Yu, XUE Pan, WEI Fengyu. Study on the properties of absorption and desorption of SO2 by piperazines organic amines in aqueous solution[J]. Applied Chemical Industry, 2014, 43(7): 1237-1239, 1242. |
| [1] | 耿秀梅, 张逢, 张翔, 单美霞, 张亚涛. 用于CO2分离的Pebax基混合基质膜稳定性研究进展[J]. 化工进展, 2024, 43(9): 4996-5012. |
| [2] | 曹树扬, 施静波, 董友明, 吕建雄. 不同温度下斜叶桉木材吸湿、解吸等温线与热力学性质[J]. 化工进展, 2024, 43(9): 5095-5105. |
| [3] | 陆诗建, 张娟娟, 杨菲, 刘玲, 陈思铭, 康国俊, 房芹芹. 化学吸收法胺液逃逸控制技术研究进展[J]. 化工进展, 2024, 43(8): 4562-4570. |
| [4] | 顾颂琦, 孙凡飞, 韦尧, 宋兴飞, 南兵, 李丽娜, 黄宇营. 时间分辨热化学原位XAFS方法[J]. 化工进展, 2024, 43(7): 3747-3755. |
| [5] | 刘克峰, 刘陶然, 蔡勇, 胡雪生, 董卫刚, 周华群, 高飞. 二氧化碳捕集技术研究和工程示范进展[J]. 化工进展, 2024, 43(6): 2901-2914. |
| [6] | 廖昌建, 张可伟, 王晶, 曾翔宇, 金平, 刘志禹. 直接空气捕集二氧化碳技术研究进展[J]. 化工进展, 2024, 43(4): 2031-2048. |
| [7] | 苗丰, 许传龙, 李健, 张彪, 韩少鹏, 汤光华. 基于SO2吸收谱线的光谱仪波长在线校准方法[J]. 化工进展, 2024, 43(2): 818-822. |
| [8] | 苏辉辉, 王恩禄, 徐逸飞. 液体吸收剂捕集燃烧后CO2的研究进展[J]. 化工进展, 2024, 43(10): 5734-5747. |
| [9] | 杜翠花, 张茜, 王晓东, 黄伟, 周明. 用于C3H6/N2分离的PDA@PEBA2533膜的制备[J]. 化工进展, 2024, 43(1): 437-446. |
| [10] | 李季桐, 王刚, 熊亚选, 徐钱. 不同工质单效吸收式制冷系统的能量和㶲分析[J]. 化工进展, 2023, 42(S1): 104-112. |
| [11] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [12] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [13] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [14] | 张凤岐, 崔成东, 鲍学伟, 朱炜玄, 董宏光. 胺液吸收-分步解吸脱硫工艺的设计与评价[J]. 化工进展, 2023, 42(S1): 518-528. |
| [15] | 谈继淮, 余敏, 张彤彤, 黄能坤, 王梓雯, 朱新宝. 新型栲胶聚丙氧基醚酯的合成及增塑PVC性能[J]. 化工进展, 2023, 42(9): 4847-4855. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||