化工进展 ›› 2024, Vol. 43 ›› Issue (9): 5095-5105.DOI: 10.16085/j.issn.1000-6613.2023-1342
• 材料科学与技术 • 上一篇
不同温度下斜叶桉木材吸湿、解吸等温线与热力学性质
- 南京林业大学材料科学与工程学院,江苏 南京 210037
-
收稿日期:2023-08-07修回日期:2023-10-17出版日期:2024-09-15发布日期:2024-09-30 -
通讯作者:施静波 -
作者简介:曹树扬(1998—),男,硕士,研究方向为木材与水分关系。E-mail:caosy@njfu.edu.cn。 -
基金资助:国家自然科学基金青年基金(32001253);江苏省基础研究计划(BK20200790)
Water adsorption and desorption isotherms and thermodynamic properties of Eucalyptus obliqua woods at different temperatures
CAO Shuyang( ), SHI Jingbo(
), SHI Jingbo( ), DONG Youming, LYU Jianxiong
), DONG Youming, LYU Jianxiong
- College of Materials Science and Engineering, Nanjing Forestry University, Nanjing 210037, Jiangsu, China
-
Received:2023-08-07Revised:2023-10-17Online:2024-09-15Published:2024-09-30 -
Contact:SHI Jingbo
摘要:
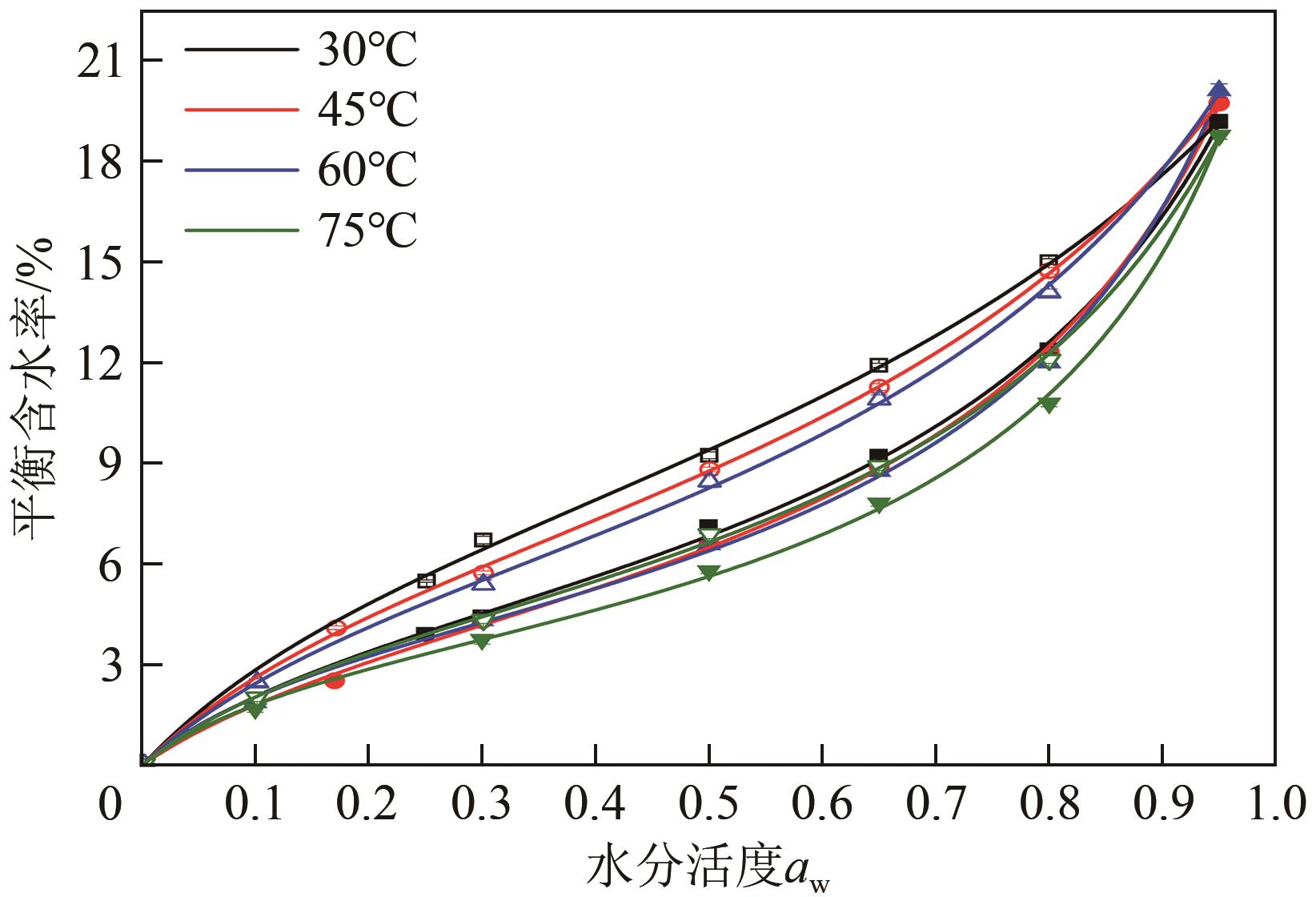
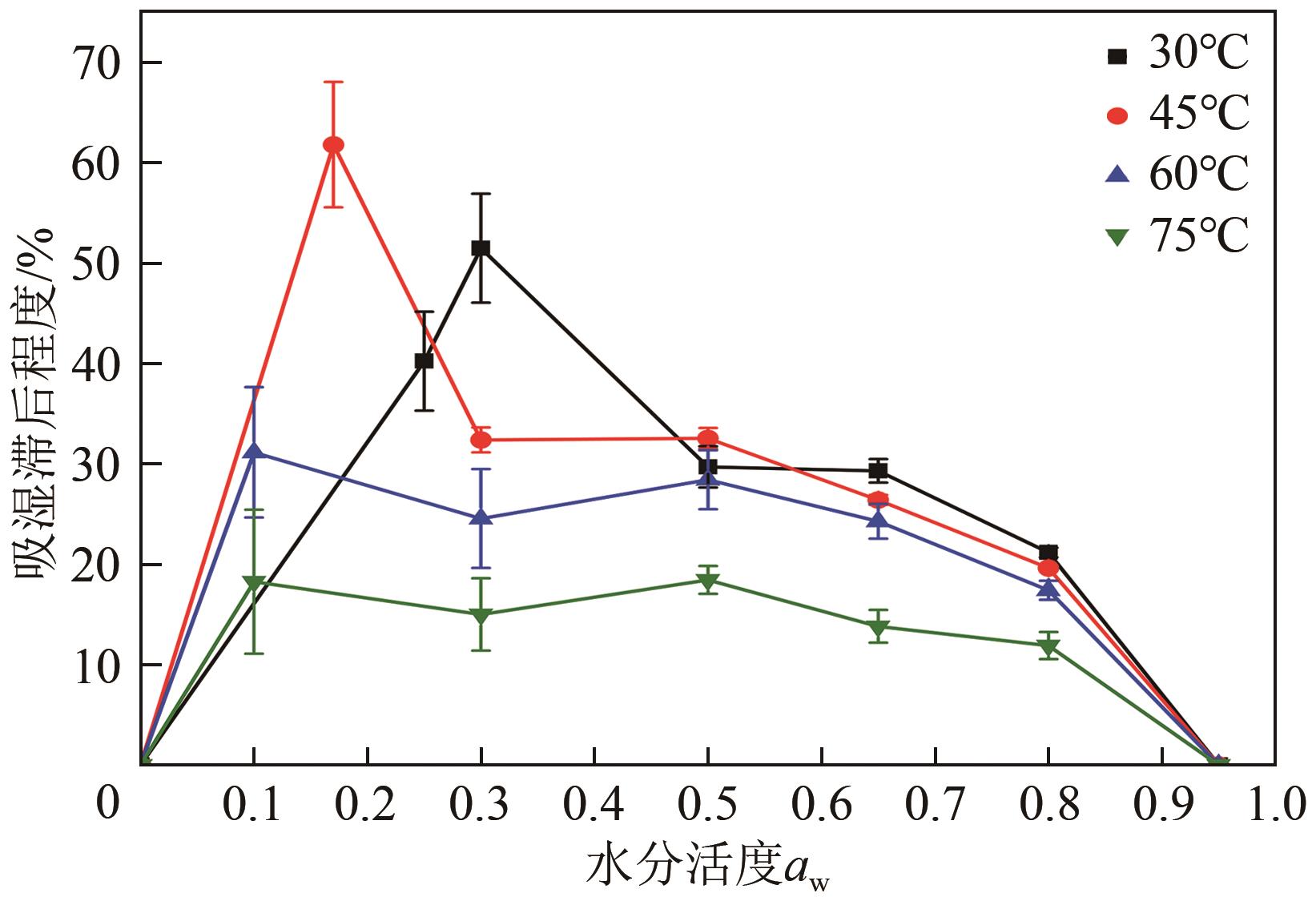
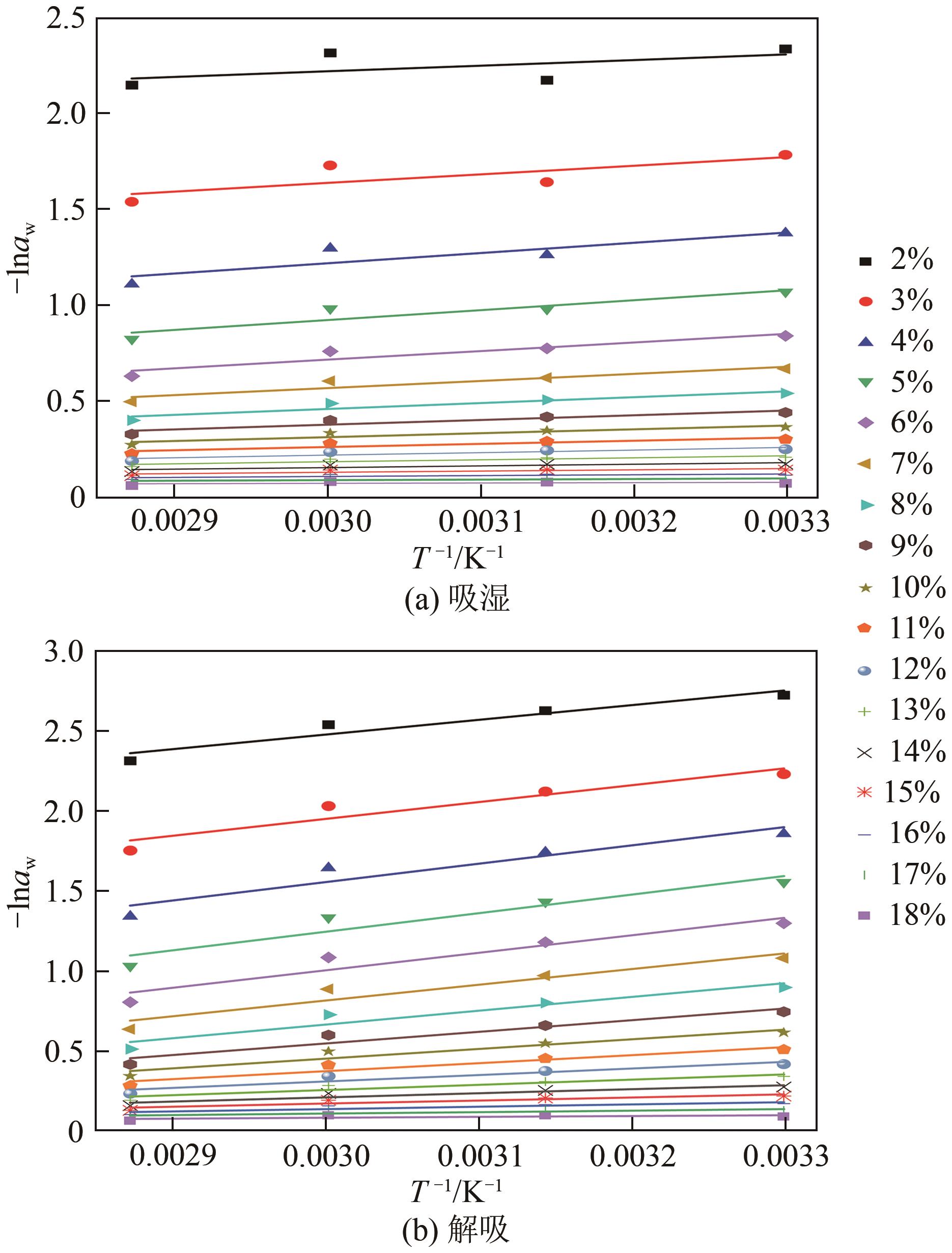
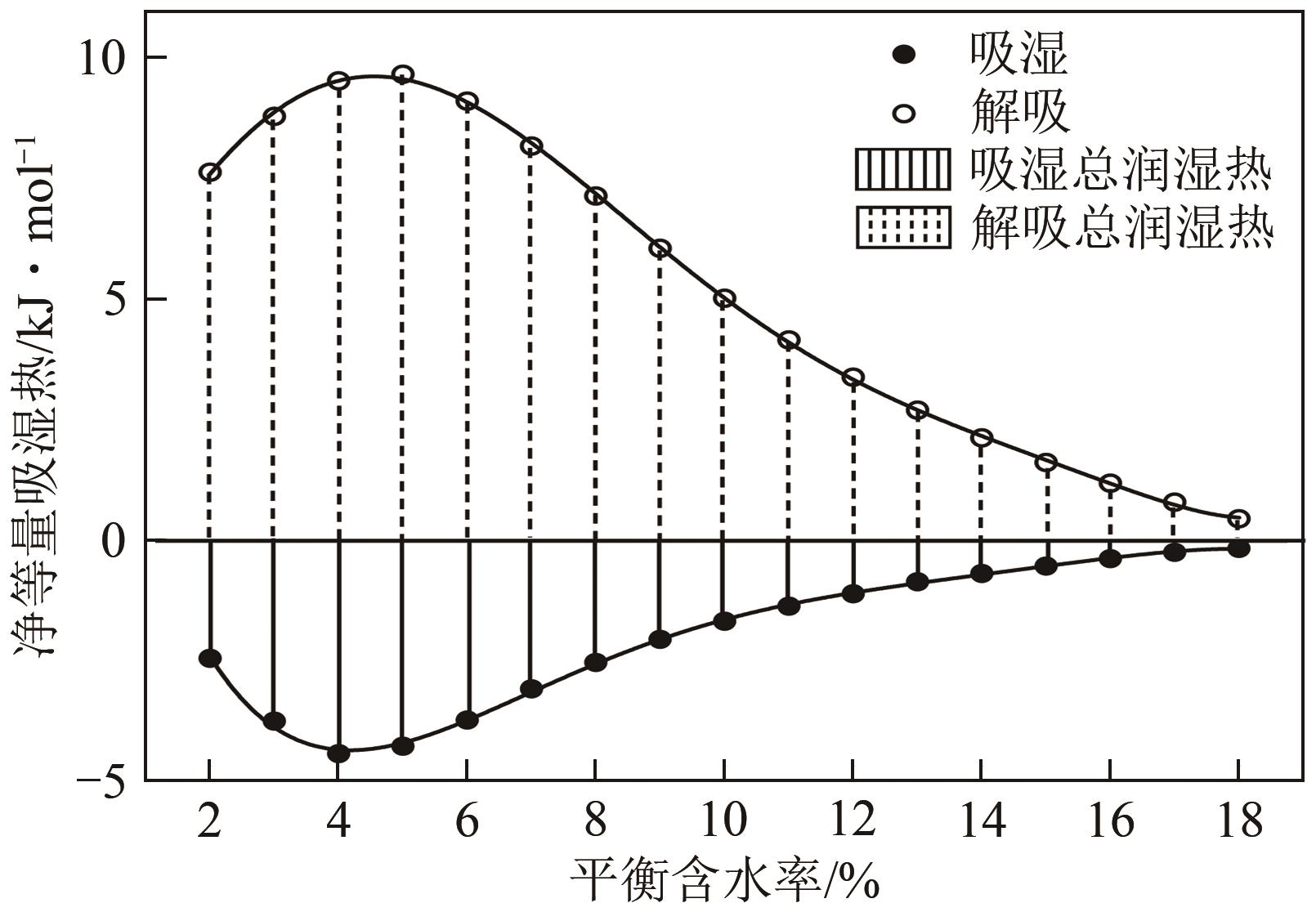
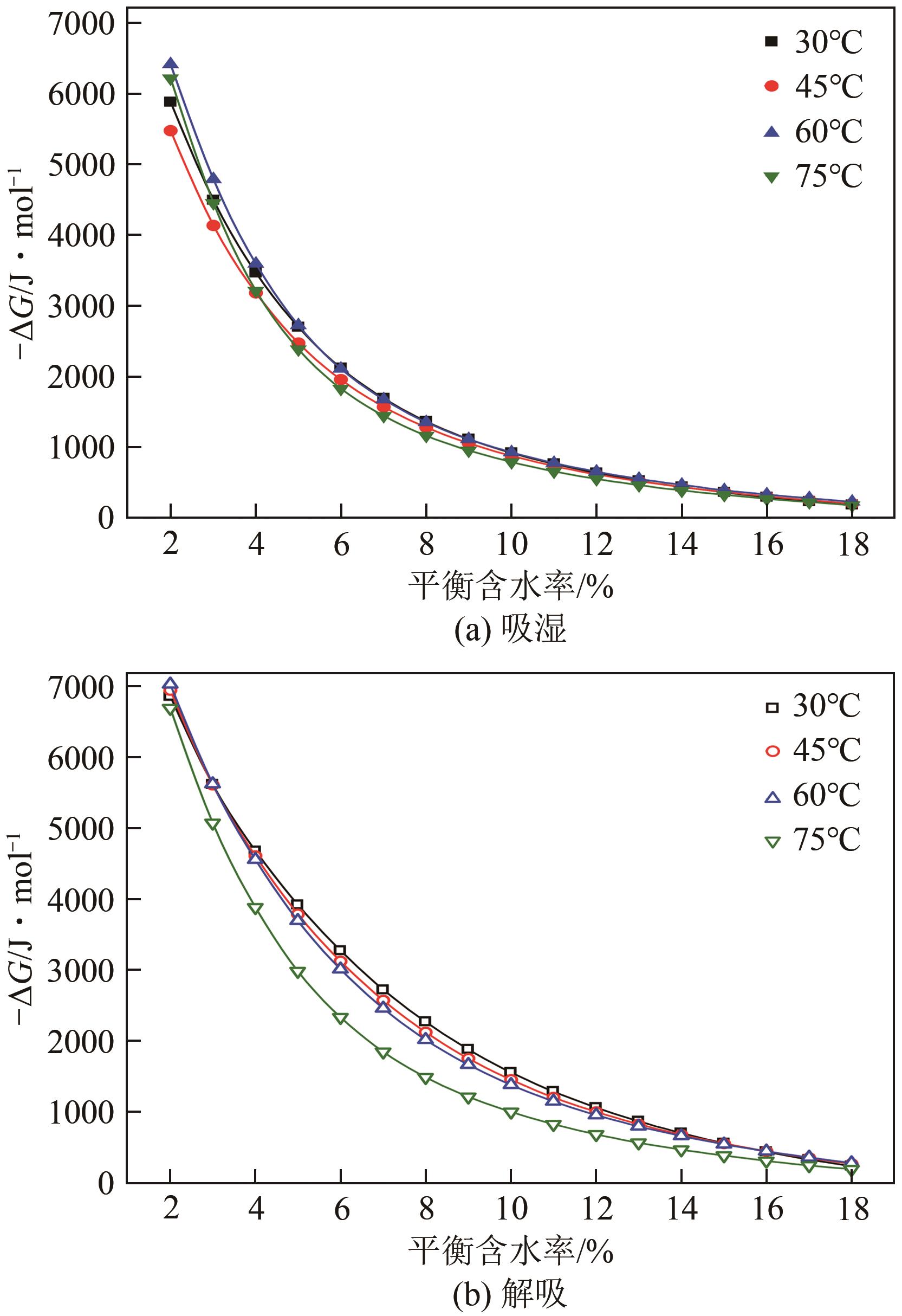
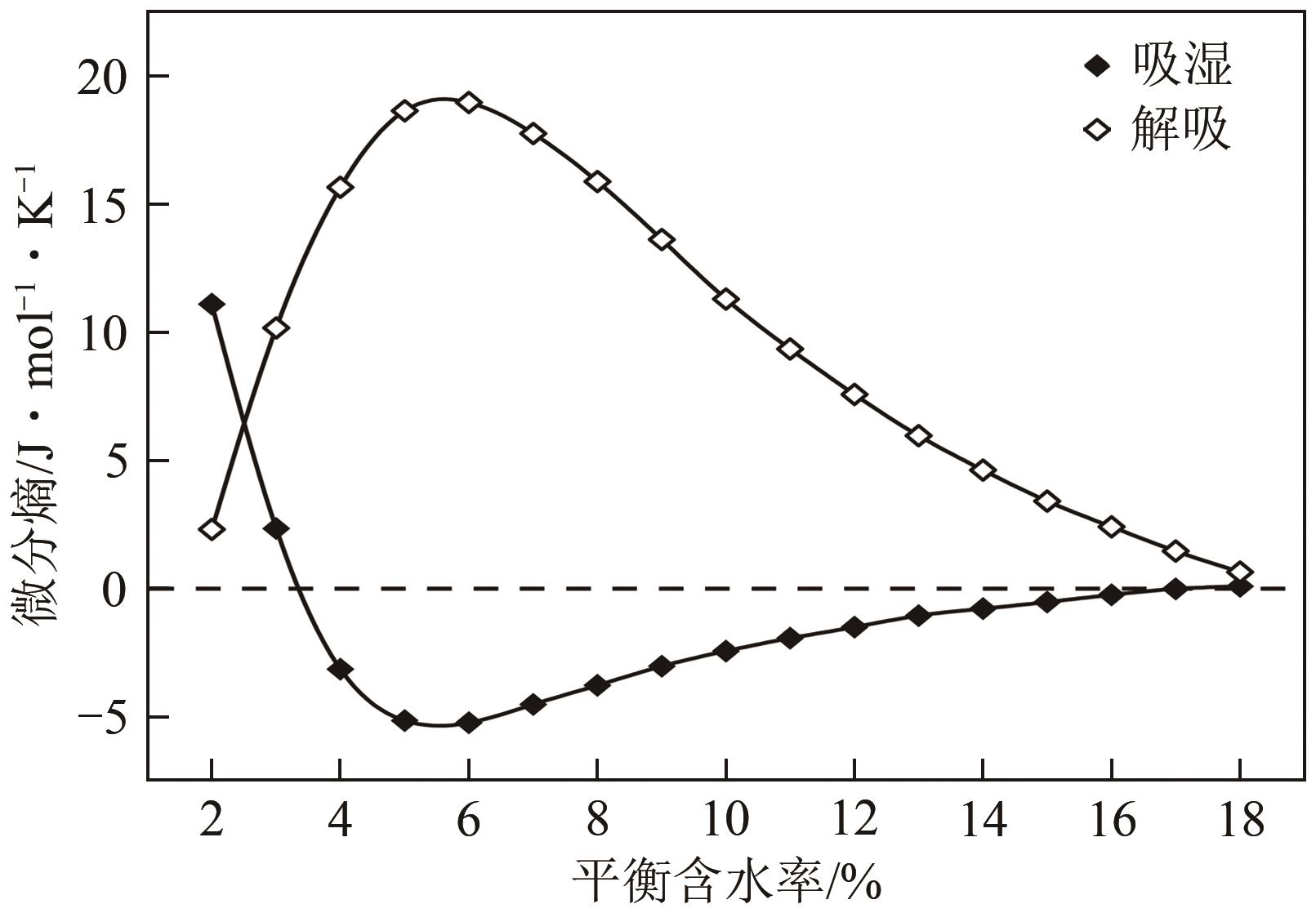
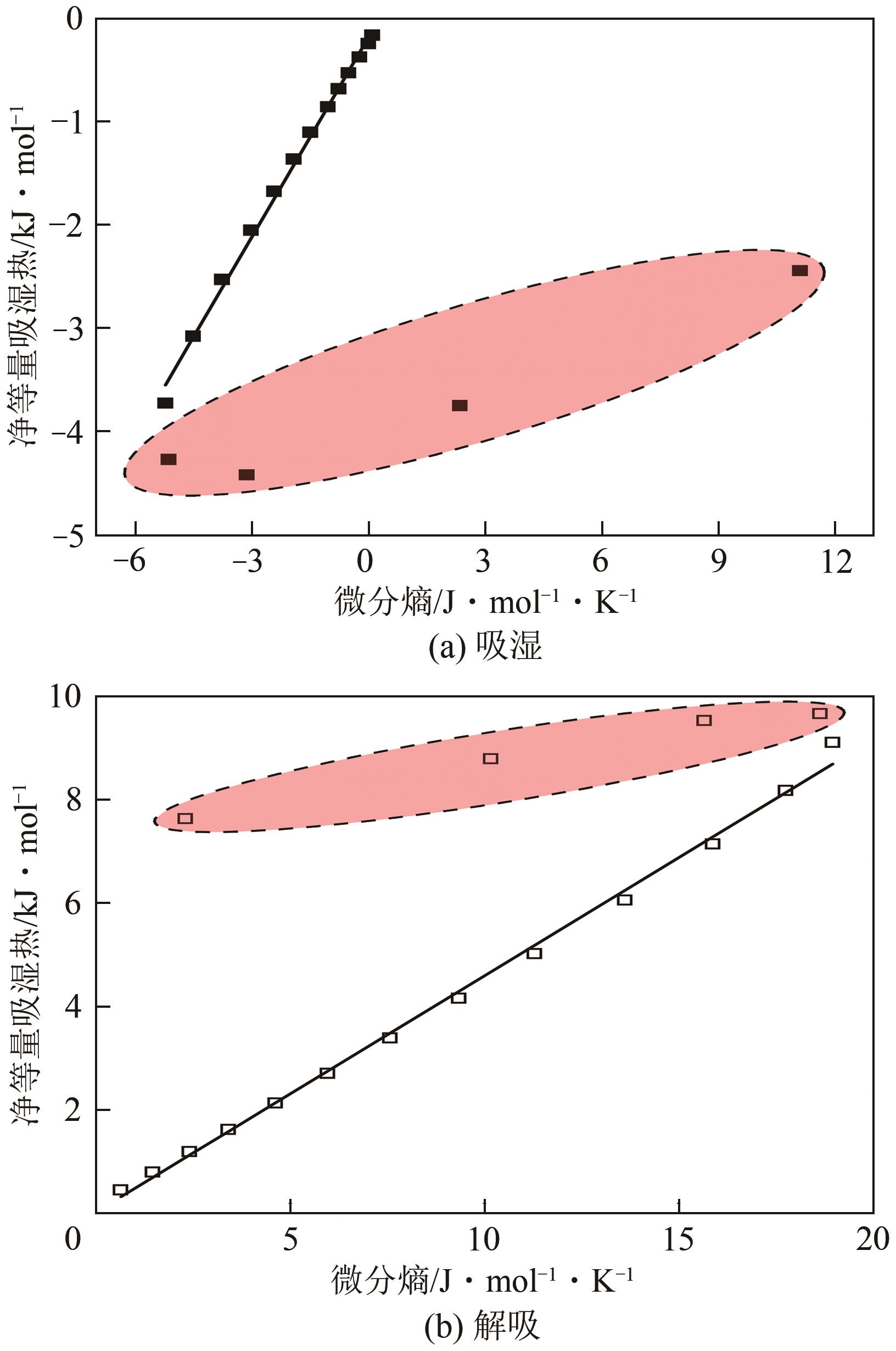
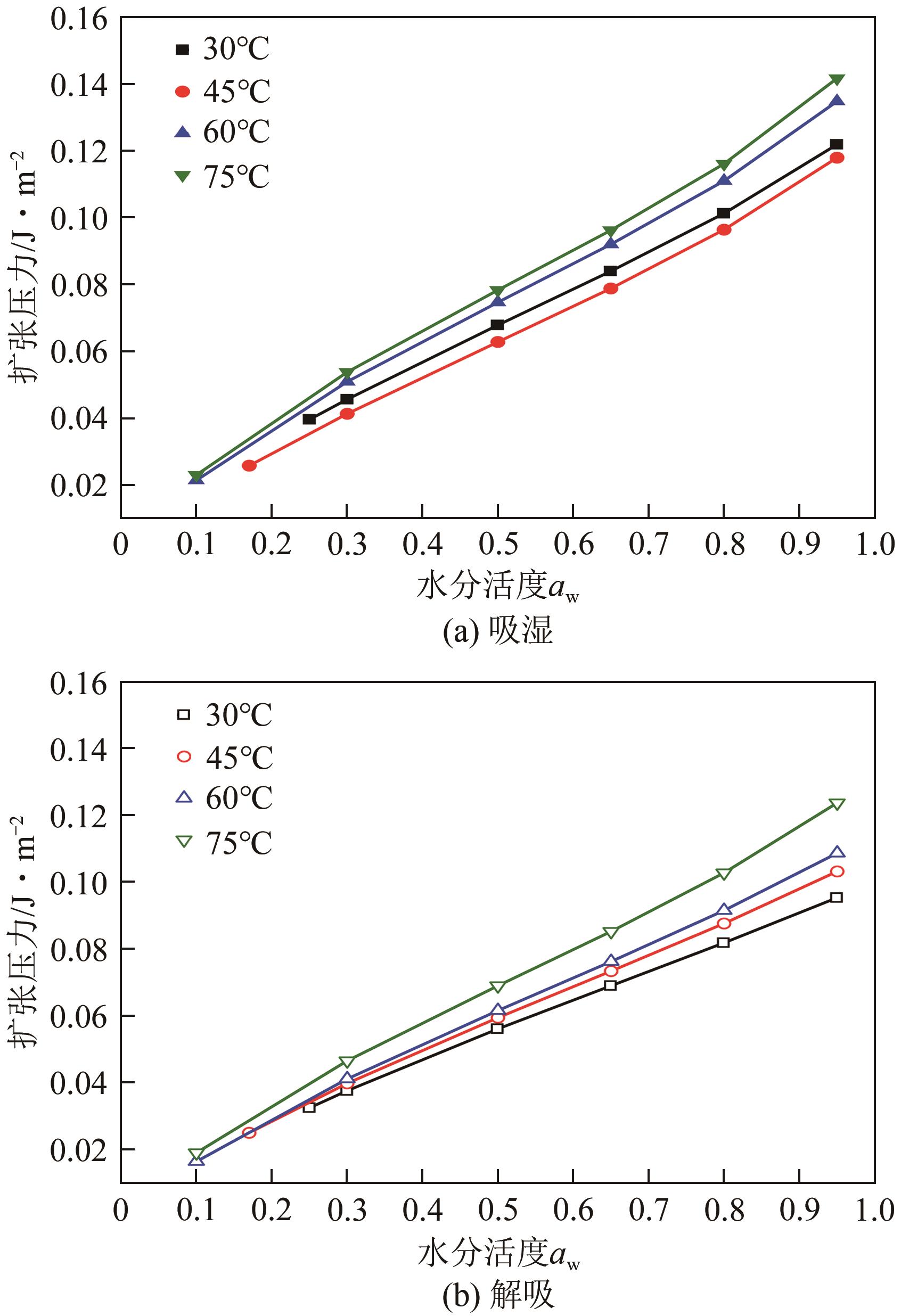
近年来斜叶桉(Eucalyptus obliqua)木材广受国内市场欢迎,需求量大,但尚无高效可行的常规干燥工艺,需对其吸湿性及热力学性质进行深入研究。本文利用恒温恒湿箱法研究了斜叶桉在不同温度下(30℃、45℃、60℃、75℃)的等温吸湿、解吸特性,并通过GAB和H-H模型对吸湿和解吸等温线进行拟合,对吸湿滞后现象,以及有效比表面积S、净等量吸湿热Qst、总润湿热W0、微分熵ΔS、吉布斯自由能变ΔG、扩张压力Φ、焓熵补偿等热力学性质进行了分析。结果表明,斜叶桉木材的吸湿和解吸等温线为Ⅱ型,且GAB和H-H模型均适用于木材-水分体系(R2大于0.999)。恒定温度下,试样平衡含水率(EMC)随水分活度的增加而增加。恒定水分活度下,EMC和吸湿滞后程度均随温度的升高而降低。有效比表面积随着温度升高而降低。总体上,吸湿过程净等量吸湿热和微分熵为负值,而解吸过程为正值,净等量吸湿热和微分熵的大小均随EMC先增大后减小,直至趋近于0。解吸过程总润湿热绝对值为83.7kJ/mol,远大于吸湿过程的32.2kJ/mol。等速温度和平均调和温度不一致,焓熵补偿理论成立。吸湿、解吸过程均为焓驱动,不同的是吸湿为自发过程,解吸为非自发过程。扩张压力会随着水分活度的升高而升高,吸湿过程中,温度对于扩张压力的影响无明显规律,而在解吸过程中,扩张压力随温度的升高而升高。
中图分类号:
引用本文
曹树扬, 施静波, 董友明, 吕建雄. 不同温度下斜叶桉木材吸湿、解吸等温线与热力学性质[J]. 化工进展, 2024, 43(9): 5095-5105.
CAO Shuyang, SHI Jingbo, DONG Youming, LYU Jianxiong. Water adsorption and desorption isotherms and thermodynamic properties of Eucalyptus obliqua woods at different temperatures[J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5095-5105.
| 阶段 | 水分活度 | 平衡时间/h | |||
|---|---|---|---|---|---|
| 30℃ | 45℃ | 60℃ | 75℃ | ||
| 吸湿 | 0.1 | — | — | 4 | 2 |
| 0.17 | — | 8 | — | — | |
| 0.25 | 66 | — | — | — | |
| 0.3 | 79 | 12 | 4 | 4 | |
| 0.5 | 72 | 12 | 6 | 4 | |
| 0.65 | 96 | 20 | 8 | 4 | |
| 0.8 | 96 | 20 | 8 | 6 | |
| 0.95 | 264 | 36 | 14 | 10 | |
| 解吸 | 0.8 | 240 | 22 | 8 | 6 |
| 0.65 | 144 | 22 | 8 | 4 | |
| 0.5 | 144 | 16 | 8 | 4 | |
| 0.3 | 96 | 16 | 4 | 4 | |
| 0.25 | 96 | — | — | — | |
| 0.17 | — | 10 | — | — | |
| 0.1 | — | — | 4 | 2 | |
表1 斜叶桉在不同温度和水分活度下平衡所需时间
| 阶段 | 水分活度 | 平衡时间/h | |||
|---|---|---|---|---|---|
| 30℃ | 45℃ | 60℃ | 75℃ | ||
| 吸湿 | 0.1 | — | — | 4 | 2 |
| 0.17 | — | 8 | — | — | |
| 0.25 | 66 | — | — | — | |
| 0.3 | 79 | 12 | 4 | 4 | |
| 0.5 | 72 | 12 | 6 | 4 | |
| 0.65 | 96 | 20 | 8 | 4 | |
| 0.8 | 96 | 20 | 8 | 6 | |
| 0.95 | 264 | 36 | 14 | 10 | |
| 解吸 | 0.8 | 240 | 22 | 8 | 6 |
| 0.65 | 144 | 22 | 8 | 4 | |
| 0.5 | 144 | 16 | 8 | 4 | |
| 0.3 | 96 | 16 | 4 | 4 | |
| 0.25 | 96 | — | — | — | |
| 0.17 | — | 10 | — | — | |
| 0.1 | — | — | 4 | 2 | |
| 模型 | 参数 | 吸湿 | 解吸 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30℃ | 45℃ | 60℃ | 75℃ | 30℃ | 45℃ | 60℃ | 75℃ | ||
| GAB | Vm/cm3·g-1 | 0.05042 | 0.04876 | 0.0441 | 0.03755 | 0.08473 | 0.07372 | 0.0665 | 0.04842 |
| C | 7.0358 | 5.80023 | 7.9611 | 8.5223 | 6.83001 | 6.67331 | 6.5971 | 7.22827 | |
| K | 0.788 | 0.805 | 0.8285 | 0.8475 | 0.63 | 0.689 | 0.7259 | 0.79167 | |
| R2 | 0.99936 | 0.99958 | 0.9994 | 0.9994 | 0.99942 | 0.99974 | 0.9996 | 0.9996 | |
| SSE | 1.55×10-5 | 1.11×10-5 | 1.70×10-5 | 1.46×10-5 | 1.41×10-5 | 6.98×10-6 | 1.21×10-5 | 1.03×10-5 | |
| H-H | W1/g·mol-1 | 35398.91 | 37120.47 | 40954.94 | 47646.97 | 21181.73 | 24301.4 | 26947.43 | 37082.32 |
| K1 | 5.85426 | 4.88979 | 7.0538 | 7.3357 | 5.79242 | 5.60737 | 5.529 | 6.2284 | |
| K2 | 0.78594 | 0.80625 | 0.8293 | 0.8462 | 0.629 | 0.68753 | 0.7246 | 0.7917 | |
| R2 | 0.99936 | 0.99959 | 0.9994 | 0.9994 | 0.99942 | 0.99974 | 0.9996 | 0.9996 | |
| SSE | 1.53×10-5 | 1.10×10-5 | 1.70×10-5 | 1.45×10-5 | 1.41×10-5 | 6.95×10-6 | 1.21×10-5 | 1.03×10-5 | |
表2 利用GAB与H-H模型计算的水分吸附等温线参数
| 模型 | 参数 | 吸湿 | 解吸 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30℃ | 45℃ | 60℃ | 75℃ | 30℃ | 45℃ | 60℃ | 75℃ | ||
| GAB | Vm/cm3·g-1 | 0.05042 | 0.04876 | 0.0441 | 0.03755 | 0.08473 | 0.07372 | 0.0665 | 0.04842 |
| C | 7.0358 | 5.80023 | 7.9611 | 8.5223 | 6.83001 | 6.67331 | 6.5971 | 7.22827 | |
| K | 0.788 | 0.805 | 0.8285 | 0.8475 | 0.63 | 0.689 | 0.7259 | 0.79167 | |
| R2 | 0.99936 | 0.99958 | 0.9994 | 0.9994 | 0.99942 | 0.99974 | 0.9996 | 0.9996 | |
| SSE | 1.55×10-5 | 1.11×10-5 | 1.70×10-5 | 1.46×10-5 | 1.41×10-5 | 6.98×10-6 | 1.21×10-5 | 1.03×10-5 | |
| H-H | W1/g·mol-1 | 35398.91 | 37120.47 | 40954.94 | 47646.97 | 21181.73 | 24301.4 | 26947.43 | 37082.32 |
| K1 | 5.85426 | 4.88979 | 7.0538 | 7.3357 | 5.79242 | 5.60737 | 5.529 | 6.2284 | |
| K2 | 0.78594 | 0.80625 | 0.8293 | 0.8462 | 0.629 | 0.68753 | 0.7246 | 0.7917 | |
| R2 | 0.99936 | 0.99959 | 0.9994 | 0.9994 | 0.99942 | 0.99974 | 0.9996 | 0.9996 | |
| SSE | 1.53×10-5 | 1.10×10-5 | 1.70×10-5 | 1.45×10-5 | 1.41×10-5 | 6.95×10-6 | 1.21×10-5 | 1.03×10-5 | |
| 参数 | 吸湿 | 解吸 | ||||||
|---|---|---|---|---|---|---|---|---|
| 30℃ | 45℃ | 60℃ | 75℃ | 30℃ | 45℃ | 60℃ | 75℃ | |
| S/m2∙g-1 | 192 | 186 | 168 | 143 | 323 | 281 | 254 | 185 |
表3 不同温度下吸湿、解吸的内比表面积
| 参数 | 吸湿 | 解吸 | ||||||
|---|---|---|---|---|---|---|---|---|
| 30℃ | 45℃ | 60℃ | 75℃ | 30℃ | 45℃ | 60℃ | 75℃ | |
| S/m2∙g-1 | 192 | 186 | 168 | 143 | 323 | 281 | 254 | 185 |
| 过程 | 等速温度Tβ/K | 决定系数R2 |
|---|---|---|
| 吸湿 | 644.0 | 0.996 |
| 解吸 | 457.4 | 0.997 |
表4 吸湿和解吸阶段等速温度与决定系数
| 过程 | 等速温度Tβ/K | 决定系数R2 |
|---|---|---|
| 吸湿 | 644.0 | 0.996 |
| 解吸 | 457.4 | 0.997 |
| 35 | LEFFLER John E. The enthalpy-entropy relationship and its implications for organic chemistry[J]. The Journal of Organic Chemistry, 1955, 20(9): 1202-1231. |
| 36 | KRUG R R, HUNTER W G, GRIEGER R A. Enthalpy-entropy compensation. 1. Some fundamental statistical problems associated with the analysis of van’t Hoff and Arrhenius data[J]. The Journal of Physical Chemistry, 1976, 80(21): 2335-2341. |
| 37 | KRUG R R, HUNTER W G, GRIEGER R A. Enthalpy-entropy compensation. 2. Separation of the chemical from the statistical effect[J]. The Journal of Physical Chemistry, 1976, 80(21): 2341-2351. |
| 38 | BRUNAUER Stephen, DEMING Lola S, Edwards DEMING W, et al. On a theory of the van der Waals adsorption of gases[J]. Journal of the American Chemical Society, 1940, 62(7): 1723-1732. |
| 39 | DONOHUE M D, ARANOVICH G L. Classification of Gibbs adsorption isotherms[J]. Advances in Colloid and Interface Science, 1998, 76/77: 137-152. |
| 40 | SING Kenneth S W. Physisorption of gases by carbon blacks[J]. Carbon, 1994, 32(7): 1311-1317. |
| 41 | GONELI André Luis Duarte, CORRÊA Paulo Cesar, DE OLIVEIRA Gabriel Henrique Horta, et al. Water sorption isotherms and thermodynamic properties of pearl millet grain[J]. International Journal of Food Science & Technology, 2010, 45(4): 828-838. |
| 42 | FREDRIKSSON Maria, THYBRING Emil Engelund. Scanning or desorption isotherms? Characterising sorption hysteresis of wood[J]. Cellulose, 2018, 25(8): 4477-4485. |
| 43 | MERAKEB Seddik, DUBOIS Frédéric, PETIT Christophe. Modeling of the sorption hysteresis for wood[J]. Wood Science and Technology, 2009, 43(7): 575-589. |
| 44 | SHI Jingbo, AVRAMIDIS Stavros. Water sorption hysteresis in wood: Ⅰ Review and experimental patterns-geometric characteristics of scanning curves[J]. Holzforschung, 2017, 71(4): 307-316. |
| 45 | PATERA Alessandra, DERLUYN Hannelore, DEROME Dominique, et al. Influence of sorption hysteresis on moisture transport in wood[J]. Wood Science and Technology, 2016, 50(2): 259-283. |
| 46 | SALIN Jarl-Gunnar. Inclusion of the sorption hysteresis phenomenon in future drying models. Some basic considerations[J]. Maderas Ciencia y Tecnología, 2011, 13(2): 173-182. |
| 47 | 黄彦快, 王喜明. 木材吸湿机理及其应用[J]. 世界林业研究, 2014, 27(3): 35-40. |
| HUANG Yankuai, WANG Ximing. Wood hygroscopic mechanism and its application[J]. World Forestry Research, 2014, 27(3): 35-40. | |
| 48 | 王舒, 魏洪斌, 伊松林, 等. 浸渍杉木吸湿滞后研究[J]. 安徽农业科学, 2010, 38(21): 11591-11593. |
| WANG Shu, WEI Hongbin, YI Songlin, et al. Comparative research on sorption hysteresis of resin-impregnated Chinese fir[J]. Journal of Anhui Agricultural Sciences, 2010, 38(21): 11591-11593. | |
| 49 | PATERA Alessandra, DEROME Dominique, GRIFFA Michele, et al. Hysteresis in swelling and in sorption of wood tissue[J]. Journal of Structural Biology, 2013, 182(3): 226-234. |
| 50 | 杨昭, 李想, 陶志超. 豌豆种子吸附等温线与热力学性质研究[J]. 农业机械学报, 2017, 48(10): 323-329. |
| YANG Zhao, LI Xiang, TAO Zhichao. Sorption isotherms and thermodynamic properties of pea seed[J]. Transactions of the Chinese Society for Agricultural Machinery, 2017, 48(10): 323-329. | |
| 51 | TSAMI E. Net isosteric heat of sorption in dried fruits[J]. Journal of Food Engineering, 1991, 14(4): 327-335. |
| 52 | BOUGAYR El Houssayne, LAKHAL El Khadir, IDLIMAM Ali, et al. Experimental study of hygroscopic equilibrium and thermodynamic properties of sewage sludge[J]. Applied Thermal Engineering, 2018, 143: 521-531. |
| 53 | RAWAT S P S, KHALI D P. Enthalpy-entropy compensation during sorption of water in wood[J]. Journal of Applied Polymer Science, 1996, 60(5): 787-790. |
| 54 | 李莞璐, 李京予, 郭娟, 等. 古代与现代柏木的水分吸附热力学比较研究[J]. 北京林业大学学报, 2023, 45(4): 126-135. |
| LI Wanlu, LI Jingyu, GUO Juan, et al. A comparative study on moisture sorption thermodynamics of ancient and recent cypress[J]. Journal of Beijing Forestry University, 2023, 45(4): 126-135. | |
| 55 | MIHOUBI D, BELLAGI A. Thermodynamic analysis of sorption isotherms of bentonite[J]. The Journal of Chemical Thermodynamics, 2006, 38(9): 1105-1110. |
| 1 | 刘文静, 张玉君. 细胞壁空隙对木材性能及加工利用的影响[J]. 世界林业研究, 2021, 34(2): 44-48. |
| LIU Wenjing, ZHANG Yujun. Effects of pore structure in cell wall on wood properties and processing utilization[J]. World Forestry Research, 2021, 34(2): 44-48. | |
| 2 | 刘一星, 赵广杰. 木材学[M]. 2版. 北京: 中国林业出版社, 2012. |
| LIU Yixing, ZHAO Guangjie. Wood science[M]. 2nd ed. Beijing: China Forestry Publishing House, 2012. | |
| 3 | 仲翔, 张少军, 马尔妮. 不同含水率状态下木材细胞壁孔径分布变化[J]. 北京林业大学学报, 2021, 43(11): 128-136. |
| ZHONG Xiang, ZHANG Shaojun, MA Erni. Variation in pore size distribution of wood cell wall under different moisture states[J]. Journal of Beijing Forestry University, 2021, 43(11): 128-136. | |
| 4 | 施静波, 程帅, 董会军, 等. 30mm厚斜叶桉锯材常规间歇干燥基准[J]. 北京林业大学学报, 2022, 44(11): 132-139. |
| SHI Jingbo, CHENG Shuai, DONG Huijun, et al. An intermittent conventional drying schedule for 30mm-thick Eucalyptus obliqua lumbers[J]. Journal of Beijing Forestry University, 2022, 44(11): 132-139. | |
| 5 | 程新峰, 潘玲, 徐保国, 等. 菊花粉水分吸附等温线及热力学特性[J]. 食品科学, 2022, 43(3): 62-69. |
| CHENG Xinfeng, PAN Ling, XU Baoguo, et al. Moisture adsorption isotherms and thermodynamic properties of chrysanthemum powder[J]. Food Science, 2022, 43(3): 62-69. | |
| 6 | BUXTON Patrick A. The measurement and control of atmospheric humidity in relation to entomological problems[J]. Bulletin of Entomological Research, 1931, 22(3): 431-447. |
| 7 | CLOUTIER A, FORTIN Y. Moisture content-water potential relationship of wood from saturated to dry conditions[J]. Wood Science and Technology, 1991, 25(4): 263-280. |
| 8 | AVRAMIDIS S. Enthalpy-entropy compensation and thermodynamic considerations in sorption phenomena[J]. Wood Science and Technology, 1992, 26(5): 329-333. |
| 9 | JALALUDIN Zaihan, HILL Callum A S, XIE Yanjun, et al. Analysis of the water vapour sorption isotherms of thermally modified acacia and sesendok[J]. Wood Material Science and Engineering, 2010, 5(3/4): 194-203. |
| 10 | XIE Yanjun, HILL Callum A S, XIAO Zefang, et al. Water vapor sorption kinetics of wood modified with glutaraldehyde[J]. Journal of Applied Polymer Science, 2010, 117(3): 1674-1682. |
| 11 | HILL Callum A S, NORTON Andrew J, NEWMAN Gary. The water vapour sorption properties of Sitka spruce determined using a dynamic vapour sorption apparatus[J]. Wood Science and Technology, 2010, 44(3): 497-514. |
| 12 | POPESCU Carmen-Mihaela, HILL Callum A S, CURLING Simon, et al. The water vapour sorption behaviour of acetylated birch wood: How acetylation affects the sorption isotherm and accessible hydroxyl content[J]. Journal of Materials Science, 2014, 49(5): 2362-2371. |
| 13 | HIMMEL Sarah, Carsten MAI. Effects of acetylation and formalization on the dynamic water vapor sorption behavior of wood[J]. Holzforschung, 2015, 69(5): 633-643. |
| 14 | OUERTANI Sahbi, AZZOUZ Soufien, HASSINI Lamine, et al. Moisture sorption isotherms and thermodynamic properties of Jack pine and palm wood: Comparative study[J]. Industrial Crops and Products, 2014, 56: 200-210. |
| 15 | BRATASZ Ł, KOZŁOWSKA A, KOZŁOWSKI R. Analysis of water adsorption by wood using the Guggenheim-Anderson-de Boer equation[J]. European Journal of Wood and Wood Products, 2012, 70(4): 445-451. |
| 16 | 谷志攀, 阳季春, 张叶, 等. 市政污泥吸附等温线模型和热力学性质[J]. 化工进展, 2022, 41(2): 998-1008. |
| GU Zhipan, YANG Jichun, ZHANG Ye, et al. Mathematical modelling of water sorption isotherms and thermodynamic properties of municipal sewage sludge[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 998-1008. | |
| 17 | 赵亚, 张平平, 石启龙. 花生壳/仁的吸附等温线与热力学特性[J]. 食品科学, 2017, 38(7): 55-62. |
| ZHAO Ya, ZHANG Pingping, SHI Qilong. Moisture adsorption isotherms and thermodynamic properties of peanut shell and kernel[J]. Food Science, 2017, 38(7): 55-62. | |
| 18 | OSWIN C R. The kinetics of package life. Ⅲ. The isotherm[J]. Journal of the Society of Chemical Industry, 1946, 65(12): 419-421. |
| 19 | DENT R W. A multilayer theory for gas sorption: Part Ⅰ: Sorption of a single gas [J]. Textile Research Journal, 1977, 47(3): 188-198. |
| 20 | BRUNAUER Stephen, EMMETT P H, TELLER Edward. Adsorption of gases in multimolecular layers[J]. Journal of the American Chemical Society, 1938, 60(2): 309-319. |
| 21 | HAILWOOD A J, HORROBIN S. Absorption of water by polymers: Analysis in terms of a simple model[J]. Transactions of the Faraday Society, 1946, 42(0): B084-B092. |
| 22 | PELEG Micha. Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms1[J]. Journal of Food Process Engineering, 1993, 16(1): 21-37. |
| 23 | 万婕, 夏雪, 周国辉, 等. 方便米粉的水分吸附和热力学特性[J]. 食品科学, 2019, 40(15): 8-14. |
| WAN Jie, XIA Xue, ZHOU Guohui, et al. Moisture sorption isotherms and thermodynamic properties of instant rice noodles[J]. Food Science, 2019, 40(15): 8-14. | |
| 24 | HILL Callum A S. The reduction in the fibre saturation point of wood due to chemical modification using anhydride reagents: A reappraisal[J]. Holzforschung, 2008, 62(4): 423-428. |
| 25 | Cristina SIMÓN, ESTEBAN Luis García, PALACIOS Paloma De, et al. Thermodynamic properties of the water sorption isotherms of wood of limba (Terminalia superba Engl. & Diels), obeche (Triplochiton scleroxylon K. Schum. ), radiata pine (Pinus radiata D. Don) and chestnut (Castanea sativa Mill.)[J]. Industrial Crops and Products, 2016, 94: 122-131. |
| 26 | 姚晴, 蔡家斌. 热处理辐射松吸湿解吸等温线的测定与分析[J]. 林业工程学报, 2018, 3(3): 35-41. |
| YAO Qing, CAI Jiabin. Determination and analysis of moisture adsorption and desorption isotherms of heat-treated radiata pine[J]. Journal of Forestry Engineering, 2018, 3(3): 35-41. | |
| 27 | SHI Jingbo, KAWAI Yasuo, AVRAMIDIS Stavros, et al. Water sorption hysteresis in wood near 100℃[J]. Holzforschung, 2021, 75(1): 13-21. |
| 28 | 李珠, 殷方宇, 蒋佳荔, 等. 杉木应压木和对应木的水分吸附特性比较研究[J]. 木材科学与技术, 2022, 36(5): 37-42. |
| LI Zhu, YIN Fangyu, JIANG Jiali, et al. Comparative studies on water vapor sorption characteristics between compression wood and opposite wood of Chinese fir[J]. Chinese Journal of Wood Science and Technology, 2022, 36(5): 37-42. | |
| 29 | SPALT Howard A. The fundamentals of water vapor sorption by wood[J]. Forest Products Journal, 1958, 8(10): 288-295. |
| 30 | 高鑫, 周凡, 付宗营, 等. 高温热处理对欧洲云杉和花旗松吸湿特性的影响[J]. 林业工程学报, 2018, 3(4): 25-29. |
| GAO Xin, ZHOU Fan, FU Zongying, et al. Sorption isotherms characteristics of high temperature heat-treated Picea abies and Pseudotsuga menziesii [J]. Journal of Forestry Engineering, 2018, 3(4): 25-29. | |
| 31 | FAKHFAKH Rihab, MIHOUBI Daoued, KECHAOU Nabil. Moisture sorption isotherms and thermodynamic properties of bovine leather[J]. Heat and Mass Transfer, 2018, 54(4): 1163-1176. |
| 32 | AVIARA N A, AJIBOLA O O. Thermodynamics of moisture sorption in melon seed and cassava[J]. Journal of Food Engineering, 2002, 55(2): 107-113. |
| 33 | MASKAN Medeni, Fahrettin GÖĞÜŞ. Sorption isotherms and drying characteristics of mulberry (Morus alba)[J]. Journal of Food Engineering, 1998, 37(4): 437-449. |
| 34 | MADAMBA P S, DRISCOLL R H, BUCKLE K A. Enthalpy-entropy compensation models for sorption and browning of garlic[J]. Journal of Food Engineering, 1996, 28(2): 109-119. |
| 56 | Y N Nkolo MEZE’E, Noah NGAMVENG J, BARDET Sandrine. Effect of enthalpy-entropy compensation during sorption of water vapour in tropical woods: The case of Bubinga (Guibourtia Tessmanii J. Léonard; G. Pellegriniana J. L.)[J]. Thermochimica Acta, 2008, 468(1/2): 1-5. |
| 57 | ARSLAN Nurhan, Hasan TOGˇRUL. Modelling of water sorption isotherms of macaroni stored in a chamber under controlled humidity and thermodynamic approach[J]. Journal of Food Engineering, 2005, 69(2): 133-145. |
| 58 | KAYA Sevim, KAHYAOGLU Talip. Influence of dehulling and roasting process on the thermodynamics of moisture adsorption in sesame seed[J]. Journal of Food Engineering, 2006, 76(2): 139-147. |
| [1] | 杨许召, 李庆, 袁康康, 张盈盈, 韩敬莉, 吴诗德. 含Gemini离子液体低共熔溶剂热力学性质[J]. 化工进展, 2023, 42(6): 3123-3129. |
| [2] | 陈磊, 闫兴清, 胡延伟, 于帅, 杨凯, 陈绍云, 关辉, 喻健良, HMAHGEREFTE Haroun, MARTYNOV Sergey. 二氧化碳管道意外泄漏减压过程的断裂控制研究进展[J]. 化工进展, 2022, 41(3): 1241-1255. |
| [3] | 谷志攀, 阳季春, 张叶, 陶乐仁, 刘泛函. 市政污泥吸附等温线模型和热力学性质[J]. 化工进展, 2022, 41(2): 998-1008. |
| [4] | 张豪, 叶国华, 陈子杨, 谢禹, 左琪. 黏土钒矿直接常压活化酸浸提钒热力学分析[J]. 化工进展, 2021, 40(10): 5360-5369. |
| [5] | 张凯,武多多,刘强,彭越,杨震,段远源. 高密度流体声速测量中脉冲回波传播时间的测定[J]. 化工进展, 2020, 39(4): 1219-1226. |
| [6] | 邓建红, 费华, 王林雅, 顾庆军. 癸酸-石蜡/膨胀石墨定形相变材料的制备及性能[J]. 化工进展, 2020, 39(11): 4537-4543. |
| [7] | 李光升,解强,张香兰,张海永. 基于分子模拟的低温煤焦油中酚类化合物的溶解特性[J]. 化工进展, 2020, 39(1): 137-144. |
| [8] | 王珺瑶, 张月, 邓帅, 赵军, 孙太尉, 李恺翔, 徐耀锋. CO2 混合物热物性在CCS研究中的作用:实验数据、理论模型和典型应用[J]. 化工进展, 2019, 38(03): 1244-1258. |
| [9] | 杨梦,张华,秦延斌,孟照峰. 混合制冷剂R134a/R1234yf(R513A)与R134a热力学性能对比及实验[J]. 化工进展, 2019, 38(03): 1182-1189. |
| [10] | 纪珺, 陈跃, 章学来, 徐笑锋, 李玉洋, 陈启杨. 甘露醇水溶液低温储能相变材料的制备及热物性[J]. 化工进展, 2018, 37(03): 1111-1117. |
| [11] | 李玉洋, 章学来, 徐笑锋, MUNYALO Jotham Muthoka, 陈跃, 陈启杨. 正辛酸-肉豆蔻酸低温相变材料的制备和循环性能[J]. 化工进展, 2018, 37(02): 689-693. |
| [12] | 赵玉清, 吕冰. 新型低全球变暖潜能值混合制冷剂替代R22的试验研究[J]. 化工进展, 2017, 36(08): 2866-2873. |
| [13] | 贾艳萍, 姜修平, 张兰河, 张海丰, 王嵬, 陈子成. HCl/H2SO4改性粉煤灰的制备及其吸附性能[J]. 化工进展, 2017, 36(06): 2331-2336. |
| [14] | 樊铁林, 陈蜜蜜, 檀星, 赵风清. 脂肪酸/废加气块定形相变储能集料制备及性能[J]. 化工进展, 2017, 36(03): 996-1002. |
| [15] | 李颖, 周丹, 徐琴琴, 银建中. 超临界二氧化碳微乳液系统的分子模拟研究进展[J]. 化工进展, 2017, 36(03): 774-782. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||