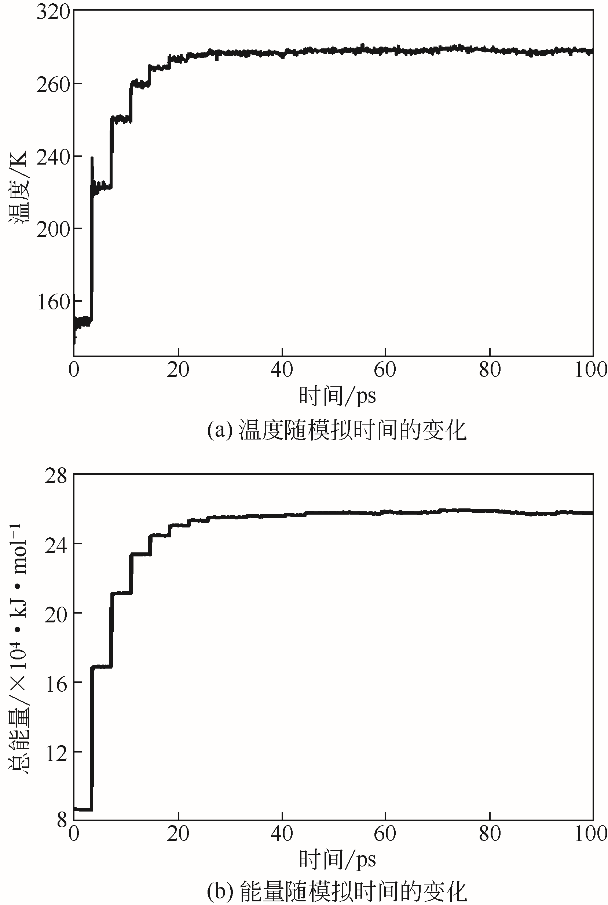
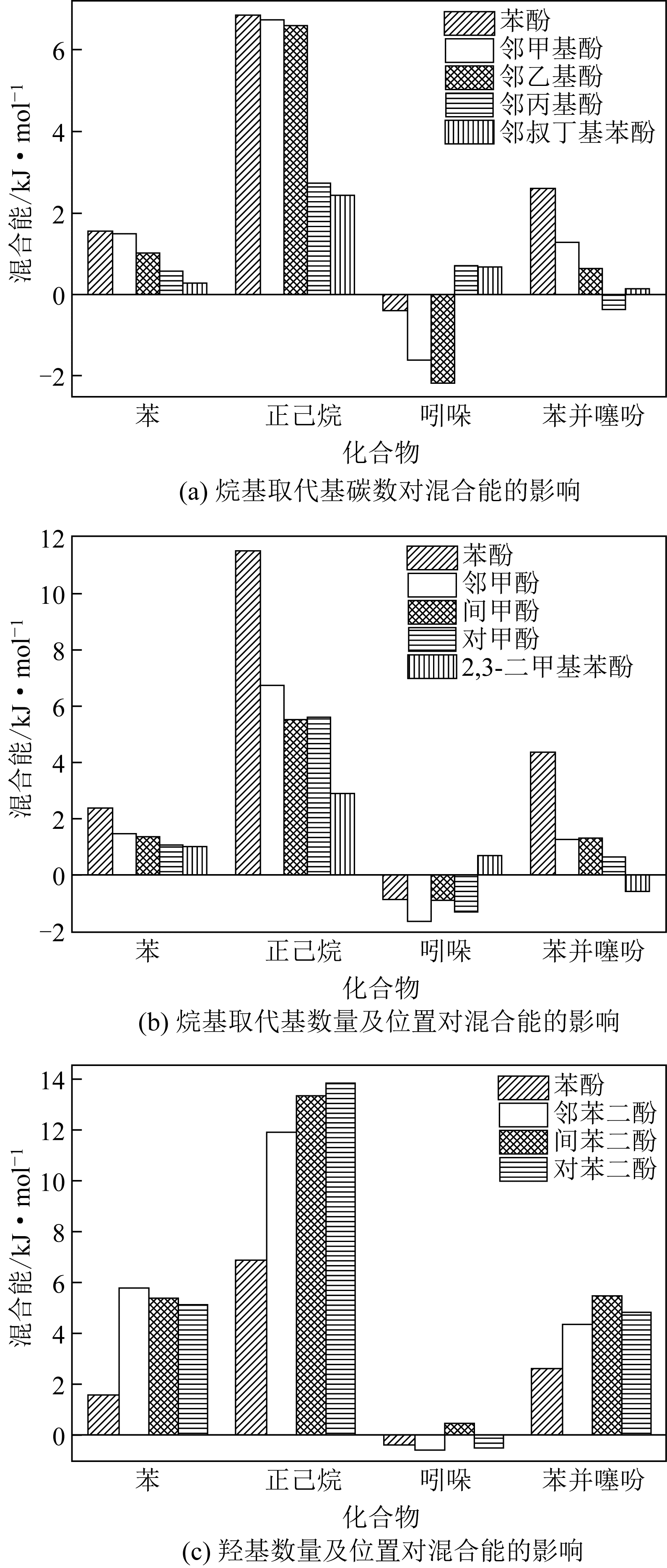
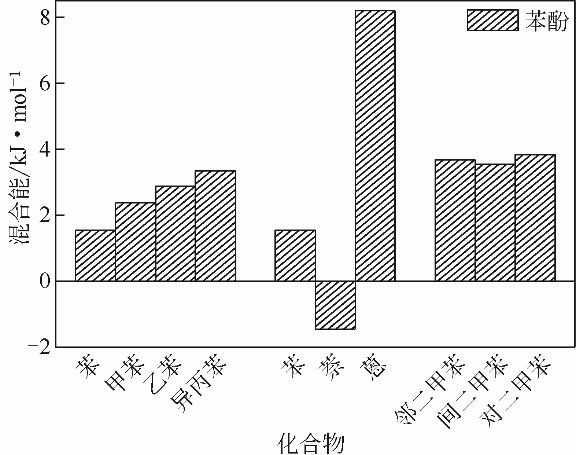
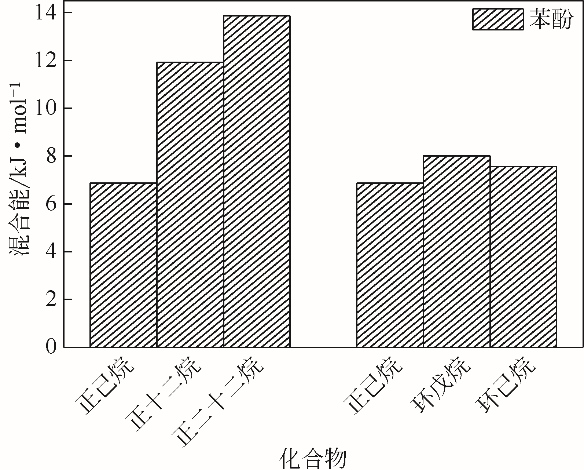
| 1 |
何璐, 解强, 梁鼎成, 等. 煤热解反应热测定方法研究进展[J]. 化工进展, 2017, 36(2): 494-501.
|
|
HE L, XIE Q, LIANG D C,et al. Measurement of reaction heat of coal pyrolysis: state-of-the-art[J]. Chemical Industry and Engineering Progress, 2017, 36(2): 494-501.
|
| 2 |
张俊杰, 徐绍平, 王光永, 等. 停留时间对低阶煤快速热解产物分布、组成及结构的影响[J]. 化工进展, 2019, 38(3): 1346-1352.
|
|
ZHANG J J, XU S P, WANG G Y, et al. Effect of residence time on distribution, composition and structure of products derived from fast coal pyrolysis[J]. Chemical Industry and Engineering Progress, 2019, 38(3): 1346-1352.
|
| 3 |
SUN M, CHEN J, DAI X M, et al. Controlled separation of low temperature coal tar based on solvent extraction-column chromatography[J]. Fuel Processing Technology, 2015, 136: 41-49.
|
| 4 |
张生娟, 高亚男, 陈刚, 等. 煤焦油中酚类化合物的分离及其组成结构鉴定研究进展[J]. 化工进展, 2018, 37(7): 2588-2596.
|
|
ZHANG S J, GAO Y N, CHEN G, et al. Progress of separation, composition and structure identification of phenolic compounds in coal tar[J]. Chemical Industry and Engineering Progress, 2018, 37(7): 2588-2596.
|
| 5 |
MENG H, GE C T, REN N N, et al. Complex extraction of phenol and cresol from model coal tar with polyols, ethanol amines, and ionic liquids thereof[J]. Industrial & Engineering Chemistry Research, 2013, 53(1): 355-362.
|
| 6 |
SUN M, MA X X, YAO Q X, et al. GC-MS and TG-FTIR study of petroleum ether extract and residue from low temperature coal tar[J]. Energy & Fuels, 2011, 25(3): 1140-1145.
|
| 7 |
SHI Q, PAN N, LONG H Y. Characterization of middle-temperature gasification coal tar. Part 3: Molecular composition of acidic compounds[J]. Energy & Fuels, 2013, 27(1): 108-117.
|
| 8 |
李文英, 慕海, 王伟, 等. 煤基粗油轻质组分定性定量分析现状与展望[J]. 化工进展, 2019, 38(1): 217-228.
|
|
LI W Y, MU H, WANG W, et al. Status quo and outlook of qualitative and quantitative analysis of light weight fractions of coal-based crude oil[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 217-228.
|
| 9 |
DAI F F, XIN K, SONG Y H, et al. Liquid-liquid equilibria for the extraction of phenols from alkane using ethylene glycol[J]. Fluid Phase Equilibria, 2016, 419: 50-56.
|
| 10 |
JIAO T T, ZHUANG X L, HE H Y, et al. Separation of phenolic compounds from coal tar via liquid-liquid extraction using amide compounds[J]. Industrial & Engineering Chemistry Research, 2015, 54(9): 2573-2579.
|
| 11 |
易兰, 李文英, 冯杰, 等. 煤基液体油分离技术研究进展[J]. 化工学报, 2017, 68(10): 3678-3692.
|
|
YI L, LI W Y, FENG J, et al. Recent progress on coal-based liquid oil separation technology[J]. CIESC Journal, 2017, 68(10): 3678-3692.
|
| 12 |
刘兴坤, 张香兰, 刘潜, 等. 正十二烷-甲苯-苯酚三元体系液液相平衡数据的测定与关联[J]. 高校化学工程学报, 2018, 32(2): 275-279.
|
|
LIU X K, ZHANG X L, LIU Q, et al. Determination and correlation of liquid equilibrium of the n-dodecane-toluene-phenol ternary system[J]. Journal of Chemical Engineering of Chinese Universities, 2018, 32(2): 275-279.
|
| 13 |
张海永, 刘潜, 刘兴坤, 等. 低温煤焦油中正十二烷-甲苯-苯酚的相平衡及分离[J]. 化工学报, 2018, 69(8): 3479-3487.
|
|
ZHANG H Y, LIU Q, LIU X K, et al. Phase equilibrium and separation of n-dodecane-toluene-phenol in low temperature coal tar[J]. CIESC Journal, 2018, 69(8): 3479-3487.
|
| 14 |
李洪, 张季, 李鑫钢, 等. 分子模拟方法计算相平衡热力学性质的研究进展[J]. 化工进展, 2017, 36(8): 2731-2741.
|
|
LI H, ZHANG J, LI X G, et al. Progress in study on thermodynamic properties of phase equilibria using molecular simulation[J]. Chemical Industry and Engineering Progress, 2017, 36(8): 2731-2741.
|
| 15 |
ZHAO Y, ZHANG X H, ZHANG W, et al. Simulation and experimental on the solvation interaction between the GAP matrix and insensitive energetic plasticizers in solid propellants[J]. The Journal of Physical Chemistry A, 2015, 120(5): 765-770.
|
| 16 |
FU X L, FAN X Z, JU X H, et al. Molecular dynamic simulations on the interaction between an HTPE polymer and energetic plasticizers in a solid propellant[J]. RSC Advances, 2015, 65(5): 52844-52851.
|
| 17 |
ZENG F L, SUN Y, ZHOU Y, et al. Molecular simulations of the miscibility in binary mixtures of PVDF and POSS compounds[J]. Modelling and Simulation in Materials Science and Engineering, 2009, 17(7): 75002.
|
| 18 |
于共奇. 重质油组分及其溶解机理的分子动力学研究[D]. 青岛: 中国石油大学(华东), 2013.
|
|
YU G Q. Molecular dynamics study of the composition and the dissolution theory of heavy oil[D]. Qingdao: China University of Petroleum (East China), 2013.
|
| 19 |
FAN C F, OLAFSON B D, BLANCO M, et al. Application of molecular simulation to derive phase diagrams of binary mixtures[J]. Macromolecules, 1992, 25(14): 3667-3676.
|
| 20 |
BLANCO M. Molecular silverware. Ⅰ. General solutions to excluded volume constrained problems[J]. Journal of Computational Chemistry, 1991, 12(2): 237-247.
|
| 21 |
FLORY. Principles of polymer chemistry[M]. New York: Cornell University Press, 1953: 672.
|
| 22 |
周公度. 结构化学基础[M]. 北京: 北京大学出版社, 2008: 363.
|
|
ZHOU G D. Structural chemistry foundation[M]. Beijing: Peking University Press, 2008: 363.
|
| 23 |
邓蕾, 张炜, 鲍桐, 等. PBT与含能增塑剂相互作用的分子动力学模拟[J]. 含能材料, 2017, 25(1): 32-38.
|
|
DENG L, ZHANG W, BAO T, et al. Molecular dynamics simulation of interaction between PBT and energetic plasticizer[J]. Chinese Journal of Energetic Materials, 2017, 25(1): 32-38.
|
| 24 |
STEINER T, DESIRAJU G R. Distinction between the weak hydrogen bond and the van der Waals interaction[J]. Chemical Communications, 1998(8): 891-892.
|
| 25 |
PAN N, CUI D C, LI R L, et al. Characterization of middle-temperature gasification coal tar. Part 1: bulk properties and molecular compositions of distillates and basic fractions[J]. Energy & Fuels, 2012, 26(9): 5719-5728.
|
| 26 |
LONG H Y, SHI Q, PAN N, et al. Characterization of middle-temperature gasification coal tar. Part 2: Neutral fraction by extrography followed by gas chromatography-mass spectrometry and electrospray ionization coupled with fourier transform ion cyclotron resonance mass spectrometry[J]. Energy & Fuels, 2012, 26(6): 3424-3431.
|
| 27 |
SUN H. COMPASS: an ab initio force-field optimized for condensed-phase applications-overview with details on alkane and benzene compounds[J]. The Journal of Physical Chemistry B, 1988, 102(38): 7338-7364.
|
| 28 |
肖瑞华. 煤焦油化工学[M]. 2版. 北京: 冶金工业出版社, 2009: 375-442.
|
|
XIAO R H. Coal tar chemical engineering[M]. 2nd ed. Beijing: Metallurgical Industry Press, 2009: 375-442.
|
| 29 |
ZHANG L Z, ZHANG M, GAO J, et al. Efficient extraction of neutral heterocyclic nitrogen compounds from coal tar via ionic liquids and its mechanism analysis[J]. Energy & Fuels, 2018, 32(9): 9358-9370.
|
 ),Qiang XIE(
),Qiang XIE( ),Xianglan ZHANG,Haiyong ZHANG
),Xianglan ZHANG,Haiyong ZHANG