化工进展 ›› 2023, Vol. 42 ›› Issue (12): 6589-6599.DOI: 10.16085/j.issn.1000-6613.2023-0123
• 资源与环境化工 • 上一篇
氨基功能化双醛淀粉吸附剂的制备及其对Pb(Ⅱ)的吸附行为
- 辽宁工业大学化学与环境工程学院,辽宁 锦州 121001
-
收稿日期:2023-02-01修回日期:2023-03-12出版日期:2023-12-25发布日期:2024-01-08 -
通讯作者:郭宇 -
作者简介:郭宇(1981—),男,教授,博士生导师,研究方向为化工新材料。E-mail:guoyulnut@163.com。 -
基金资助:国家自然科学基金(21601075);辽宁省“兴辽英才计划”(XLYC2007171);辽宁省自然科学基金面上项目(2021-MS-321)
Preparation of amino-functionalized dialdehyde starch adsorbent for adsorption of Pb(Ⅱ) ions
GUO Yu( ), TONG Minxin, WU Hongmei
), TONG Minxin, WU Hongmei
- School of Chemical and Environmental Engineering, Liaoning University of Technology, Jinzhou 121001, Liaoning, China
-
Received:2023-02-01Revised:2023-03-12Online:2023-12-25Published:2024-01-08 -
Contact:GUO Yu
摘要:
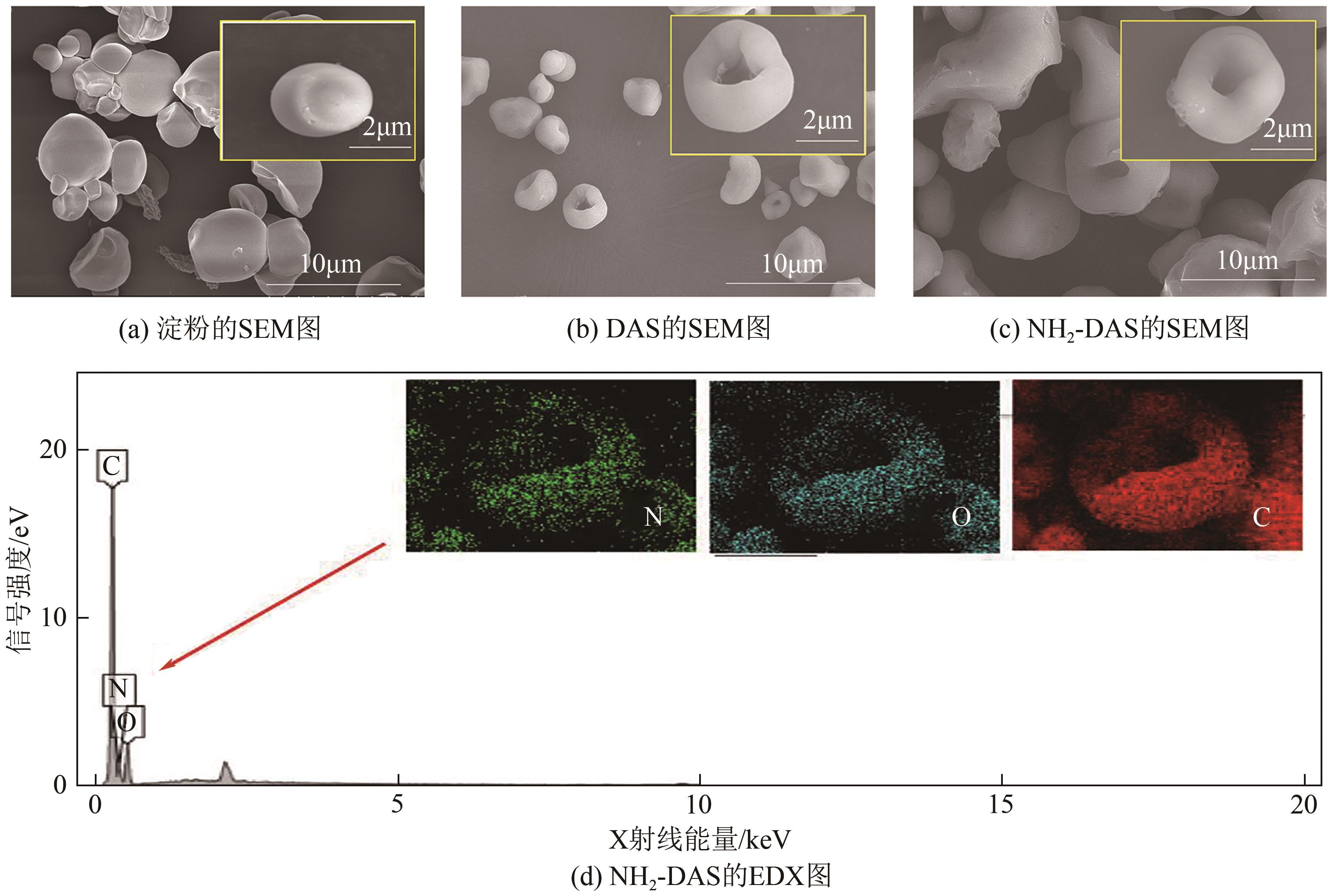
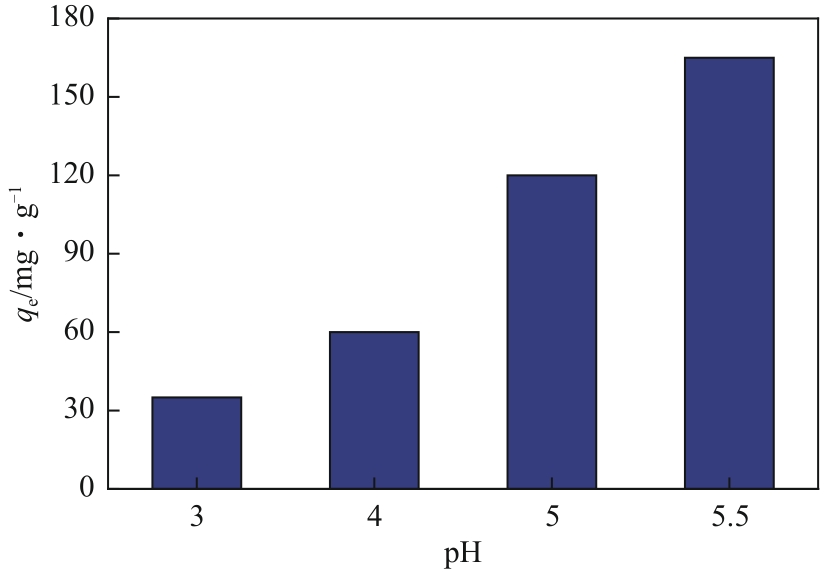
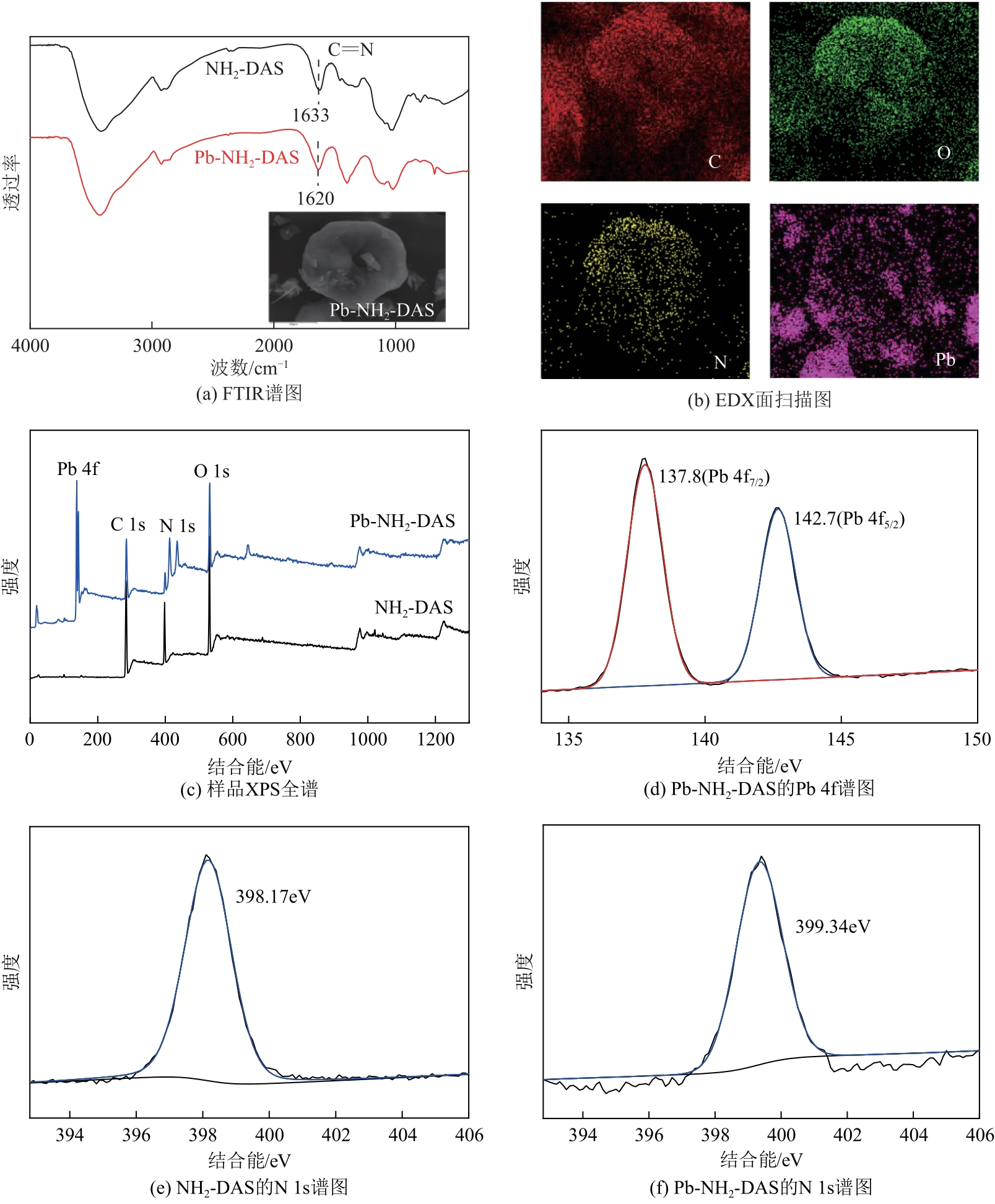
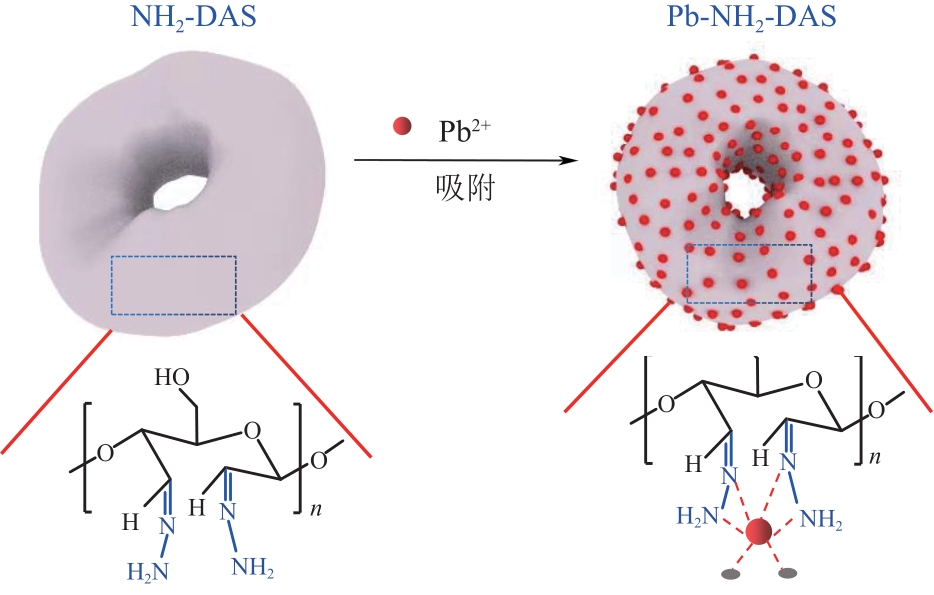
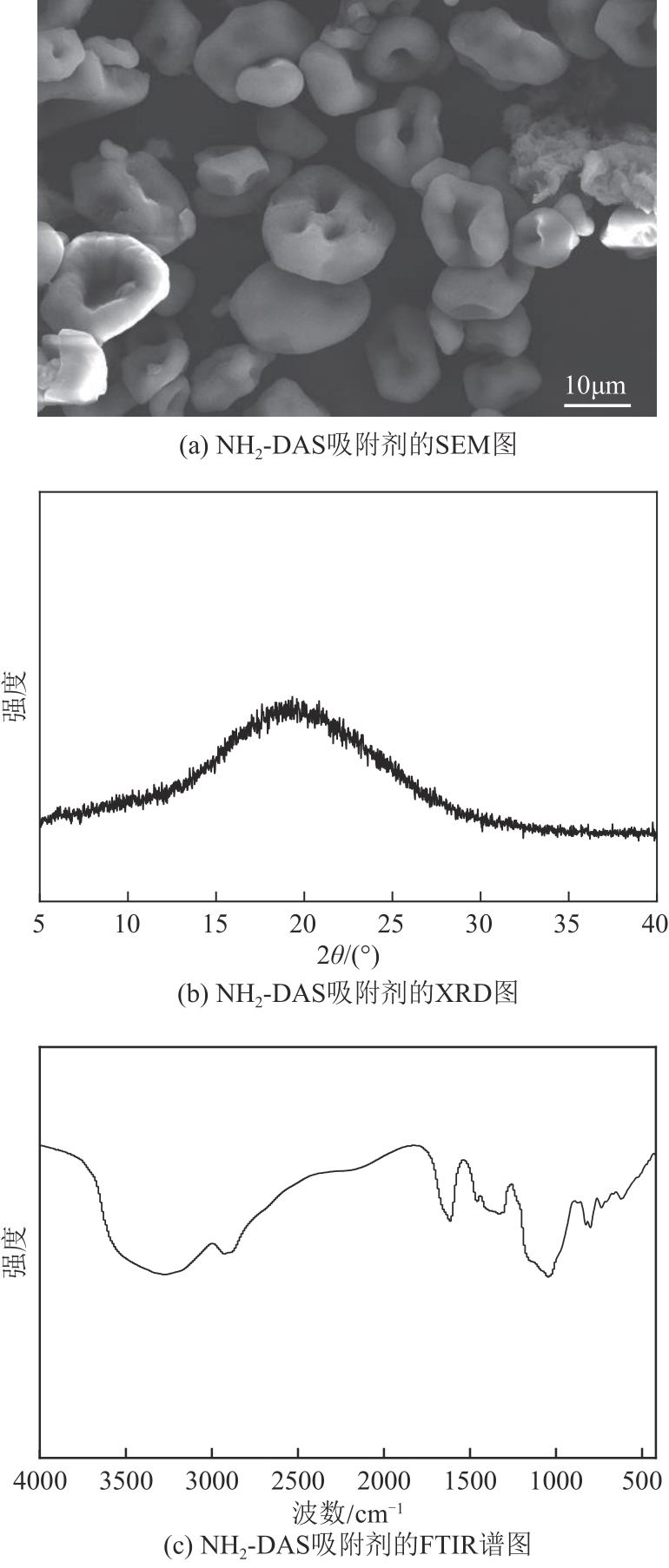
以淀粉为原料,高碘酸钠为氧化剂,制备出双醛淀粉(DAS),然后与水合肼反应制备了氨基功能化双醛淀粉吸附剂(NH2-DAS)。采用扫描电子显微镜、能量色散X射线光谱仪、X射线衍射仪、热重分析仪和X射线光电子能谱等表征手段对NH2-DAS的形貌、元素组成、热稳定性和表面化学性质进行了分析。详细研究了NH2-DAS吸附剂对水溶液中Pb(Ⅱ)离子的吸附行为和吸附机理。结果表明,NH2-DAS对Pb(Ⅱ)的吸附符合Langmuir等温吸附模型和准二级吸附动力学模型。当吸附温度为45℃、pH为5.5、吸附时间为240min时,NH2-DAS对Pb(Ⅱ)的吸附容量达到165mg/g。NH2-DAS对Pb(Ⅱ)的吸附作用主要是利用其表面—C
中图分类号:
引用本文
郭宇, 佟民心, 吴红梅. 氨基功能化双醛淀粉吸附剂的制备及其对Pb(Ⅱ)的吸附行为[J]. 化工进展, 2023, 42(12): 6589-6599.
GUO Yu, TONG Minxin, WU Hongmei. Preparation of amino-functionalized dialdehyde starch adsorbent for adsorption of Pb(Ⅱ) ions[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6589-6599.
| 温度/℃ | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm/mg·g-1 | KL | R2 | KF | n | R2 | |
| 25 | 139.86 | 0.029 | 0.982 | 19.55 | 2.77 | 0.816 |
| 35 | 162.07 | 0.0.38 | 0.991 | 31.68 | 3.29 | 0.814 |
| 45 | 177.93 | 0.122 | 0.998 | 78.63 | 6.19 | 0.555 |
表1 不同温度下NH2-DAS对Pb(Ⅱ)的吸附等温线拟合常数
| 温度/℃ | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm/mg·g-1 | KL | R2 | KF | n | R2 | |
| 25 | 139.86 | 0.029 | 0.982 | 19.55 | 2.77 | 0.816 |
| 35 | 162.07 | 0.0.38 | 0.991 | 31.68 | 3.29 | 0.814 |
| 45 | 177.93 | 0.122 | 0.998 | 78.63 | 6.19 | 0.555 |
| 温度/K | Kc | ΔG/kJ·mol-1 | ΔH/kJ·mol-1 | ΔS/kJ·mol·K-1 | R2 |
|---|---|---|---|---|---|
| 298 | 1.289 | -0.629 | 25.28 | 0.087 | 0.979 |
| 303 | 1.494 | -1.011 | |||
| 308 | 1.667 | -1.308 | |||
| 313 | 2.042 | -1.858 | |||
| 318 | 2.463 | -2.583 |
表2 NH2-DAS吸附Pb(Ⅱ)的热力学方程拟合参数
| 温度/K | Kc | ΔG/kJ·mol-1 | ΔH/kJ·mol-1 | ΔS/kJ·mol·K-1 | R2 |
|---|---|---|---|---|---|
| 298 | 1.289 | -0.629 | 25.28 | 0.087 | 0.979 |
| 303 | 1.494 | -1.011 | |||
| 308 | 1.667 | -1.308 | |||
| 313 | 2.042 | -1.858 | |||
| 318 | 2.463 | -2.583 |
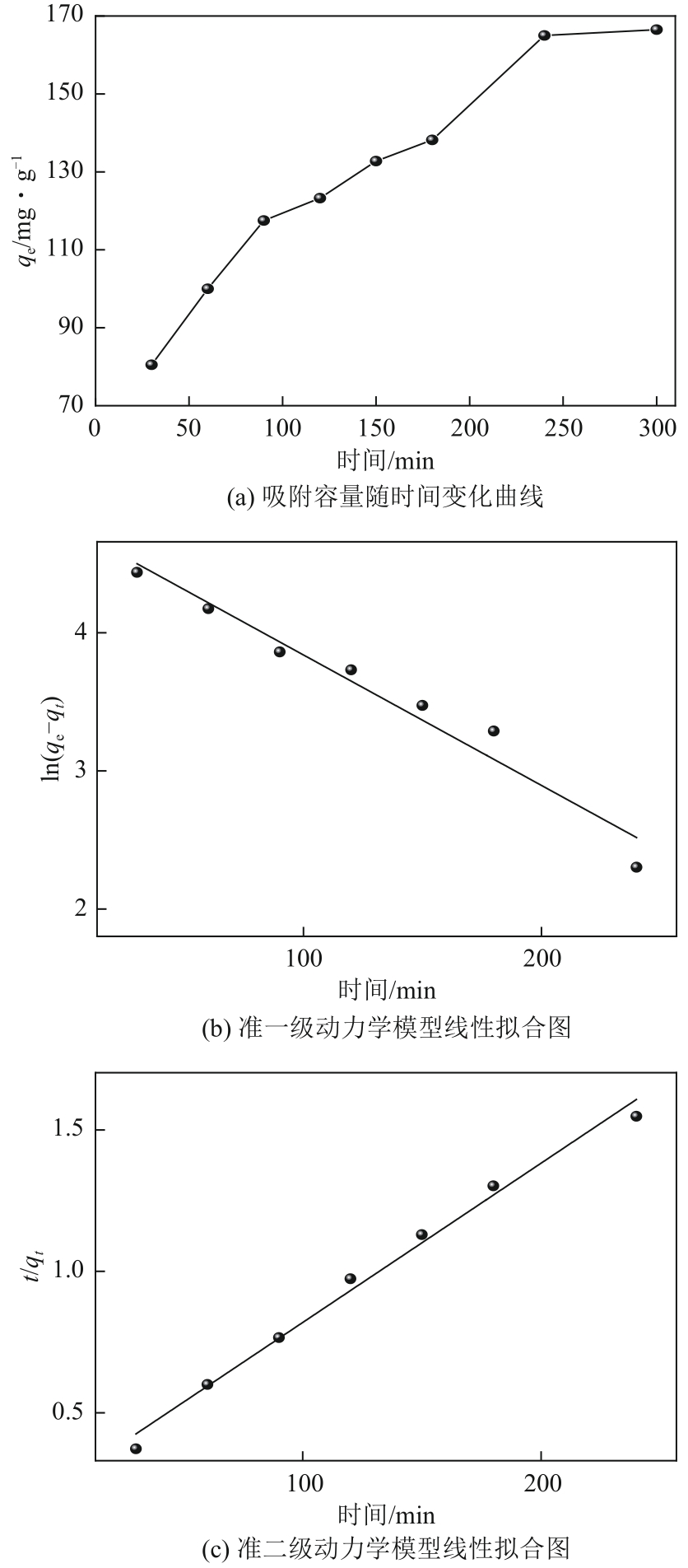
| 吸附剂 | qe(实验)/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | qe(计算)/mg·g-1 | R2 | k2/g·mg-1·min-1 | qe(计算)/mg·g-1 | R2 | ||
| NH2-SBA-15 | 165 | 0.009 | 119.42 | 0.952 | 1.24×10-5 | 177.62 | 0.988 |
表3 NH2-DAS吸附铅(Ⅱ)动力学方程拟合参数
| 吸附剂 | qe(实验)/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | qe(计算)/mg·g-1 | R2 | k2/g·mg-1·min-1 | qe(计算)/mg·g-1 | R2 | ||
| NH2-SBA-15 | 165 | 0.009 | 119.42 | 0.952 | 1.24×10-5 | 177.62 | 0.988 |
| 吸附剂 | qm/mg·g-1 | 参考文献 |
|---|---|---|
| 氧化淀粉 | 48.27 | [ |
| 淀粉-FeS@PSB | 90.15 | [ |
| MMT/淀粉 | 21.5 | [ |
| 交联两性淀粉 | 152.74 | [ |
| 氧化淀粉纳米颗粒 | 110.9 | [ |
| 双醛淀粉 | 38.25 | 本工作 |
| NH2-DAS | 165 | 本工作 |
表4 各种吸附剂对Pb(Ⅱ)的吸附性能
| 吸附剂 | qm/mg·g-1 | 参考文献 |
|---|---|---|
| 氧化淀粉 | 48.27 | [ |
| 淀粉-FeS@PSB | 90.15 | [ |
| MMT/淀粉 | 21.5 | [ |
| 交联两性淀粉 | 152.74 | [ |
| 氧化淀粉纳米颗粒 | 110.9 | [ |
| 双醛淀粉 | 38.25 | 本工作 |
| NH2-DAS | 165 | 本工作 |
| 1 | CHAPLYGIN Victor A, RAJPUT Vishnu D, MANDZHIEVA Saglara S, et al. Comparison of heavy metal content in Artemisia austriaca in various impact zones[J]. ACS Omega, 2020, 5(36): 23393-23400. |
| 2 | MAO Changping, SONG Yinxian, CHEN Lingxiao, et al. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice[J]. CATENA, 2019, 175: 339-348. |
| 3 | NARANJO Valeria I, HENDRICKS Michael, JONES Kimberly S. Lead toxicity in children: An unremitting public health problem[J]. Pediatric Neurology, 2020, 113: 51-55. |
| 4 | BANSOD BabanKumar, KUMAR Tejinder, THAKUR Ritula, et al. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms[J]. Biosensors and Bioelectronics, 2017, 94: 443-455. |
| 5 | Rahul RAM, MORRISROE Liam, ETSCHMANN Barbara, et al. Lead (Pb) sorption and co-precipitation on natural sulfide, sulfate and oxide minerals under environmental conditions[J]. Minerals Engineering, 2021, 163: 106801. |
| 6 | TAVAKOLI Omid, GOODARZI Vahabodin, SAEB Mohammad Reza, et al. Competitive removal of heavy metal ions from squid oil under isothermal condition by CR11 chelate ion exchanger[J]. Journal of Hazardous Materials, 2017, 334: 256-266. |
| 7 | CUI Lin, WU Jie, JU Huangxian. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials[J]. Biosensors and Bioelectronics, 2015, 63: 276-286. |
| 8 | MONDAL Somen, KUMAR MAJUMDER Subrata. Fabrication of the polysulfone-based composite ultrafiltration membranes for the adsorptive removal of heavy metal ions from their contaminated aqueous solutions[J]. Chemical Engineering Journal, 2020, 401: 126036. |
| 9 | WU Hongmei, XIAO Yu, GUO Yu, et al. Functionalization of SBA-15 mesoporous materials with 2-acetylthiophene for adsorption of Cr(Ⅲ) ions[J]. Microporous and Mesoporous Materials, 2020, 292: 109754. |
| 10 | GHAHREMANI Parastoo, VAKILI Mohammad Hassan, Alireza NEZAMZADEH-EJHIEH. Optimization of Pb(Ⅱ) removal by a novel modified silica aerogel using Quince seed mucilage with response surface methodology[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106648. |
| 11 | 姜晓庆, 郭宇, 吴红梅. 2-吡啶甲醛功能化SBA-15介孔材料的制备及其对Cr(Ⅲ)离子的吸附[J]. 化工进展, 2022, 41(7): 3915-3924. |
| JIANG Xiaoqing, GUO Yu, WU Hongmei. Synthesis of 2-pyridinecarboxaldehyde functionalized SBA-15 mesoporous material for the adsorption of Cr(Ⅲ) ions from aqueous solution[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3915-3924. | |
| 12 | GUO Y, WU D F, WU H M, et al. Efficient removal of Pb(Ⅱ) ions by using 2-acetylthiophene-modified graphene oxide from aqueous solution[J]. Materials Today Sustainability, 2022, 20: 100212. |
| 13 | WANG Wenyong, WU Guohao, ZHU Tao, et al. Synthesis of-thiazole Schiff base modified SBA-15 mesoporous silica for selective Pb(Ⅱ) adsorption[J]. Journal of the Taiwan Institute of Chemical Engineers, 2021, 125: 349-359. |
| 14 | 周丽莎, 李若男, 卞雨洁, 等. TOCNF与磁性羧甲基壳聚糖纳米粒子复合物的制备及吸附Pb2+的特性[J]. 化工进展, 2022, 41(2): 901-910. |
| ZHOU Lisha, LI Ruonan, BIAN Yujie, et al. Preparation of TOCNF and magnetic carboxymethyl chitosan nanoparticles composite and adsorption properties of Pb2+ [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 901-910. | |
| 15 | WANG Anqi, ZHENG Zhikeng, LI Ruiqi, et al. Biomass-derived porous carbon highly efficient for removal of Pb(Ⅱ) and Cd(Ⅱ)[J]. Green Energy & Environment, 2019, 4(4): 414-423. |
| 16 | AWOKOYA Kehinde N, ONINLA Vincent O, BELLO Dolapo J. Synthesis of oxidized Dioscorea dumentorum starch nanoparticles for the adsorption of lead(Ⅱ) and cadmium(Ⅱ) ions from wastewater[J]. Environmental Nanotechnology, Monitoring & Management, 2021, 15: 100440. |
| 17 | GUO Yu, XIAO Yu, WU Hongmei, et al. Efficient removal of Pb(Ⅱ) ions from aqueous solution by using a novel functionalized bio-material with two tridentate coordinated units[J]. Journal of Chemical Technology & Biotechnology, 2021, 96(6): 1709-1719. |
| 18 | WANG Rou, DONG Zhengping, WANG Renqi, et al. Efficient removal of Cd2+ by dialdehyde phenylhydrazine starch from aqueous solution[J]. RSC Advances, 2013, 3(43): 20480. |
| 19 | WANG Yang, ZHANG Yun, HOU Chen, et al. Facile synthesis of monodisperse functional magnetic dialdehyde starch nano-composite and used for highly effective recovery of Hg(Ⅱ)[J]. Chemosphere, 2015, 141: 26-33. |
| 20 | ZHANG Yaoyao, MAGAGNIN Luca, YUAN Kangze, et al. Highly-efficient removal of Pb (Ⅱ) from water by mesoporous amino functionalized silica aerogels: Experimental, DFT investigations and life cycle assessment[J]. Microporous and Mesoporous Materials, 2022, 345: 112280. |
| 21 | TANG Ni, LIU Xue, JIA Mengru, et al. Amine- and thiol-bifunctionalized mesoporous silica material for immobilization of Pb and Cd: Characterization, efficiency, and mechanism[J]. Chemosphere, 2022, 291: 132771. |
| 22 | HERNÁNDEZ-MORALES V, NAVA R, ACOSTA-SILVA Y J, et al. Adsorption of lead (Ⅱ) on SBA-15 mesoporous molecular sieve functionalized with-NH2 groups[J]. Microporous and Mesoporous Materials, 2012, 160: 133-142. |
| 23 | YIN Qiangfeng, JU Benzhi, ZHANG Shufen, et al. Preparation and characteristics of novel dialdehyde aminothiazole starch and its adsorption properties for Cu (Ⅱ) ions from aqueous solution[J]. Carbohydrate Polymers, 2008, 72(2): 326-333. |
| 24 | HOFREITER B T, ALEXANDER B H, WOLFF I A. Rapid estimation of dialdehyde content of periodate oxystarch through quantitative alkali consumption[J]. Analytical Chemistry, 1955, 27(12): 1930-1931. |
| 25 | YU Jiugao, CHANG Peter R, MA Xiaofei. The preparation and properties of dialdehyde starch and thermoplastic dialdehyde starch[J]. Carbohydrate Polymers, 2010, 79(2): 296-300. |
| 26 | WANG Shujun, YU Jinglin, GAO Wenyuan, et al. Granule structural changes in native Chinese Yam (Dioscorea opposita Thunb var. Anguo) starch during acid hydrolysis[J]. Carbohydrate Polymers, 2007, 69(2): 286-292. |
| 27 | FIEDOROWICZ Maciej, PARA Andrzej. Structural and molecular properties of dialdehyde starch[J]. Carbohydrate Polymers, 2006, 63(3): 360-366. |
| 28 | DING Wen, ZHAI Shenyong, LIU Juntao, et al. Preparation and adsorption properties of dialdehyde 8-aminoquinoline starch[J]. Water Science and Technology, 2013, 67(2): 306-310. |
| 29 | JIANG Yijun, GAO Qiuming, YU Huaguang, et al. Intensively competitive adsorption for heavy metal ions by PAMAM-SBA-15 and EDTA-PAMAM-SBA-15 inorganic-organic hybrid materials[J]. Microporous and Mesoporous Materials, 2007, 103(1/2/3): 316-324. |
| 30 | RAHMAN Noor Hidayah ABD, JAAFAR Nardiah Rizwana, SHAMSUL ANNUAR Nur Arbainah, et al. Efficient substrate accessibility of cross-linked levanase aggregates using dialdehyde starch as a macromolecular cross-linker[J]. Carbohydrate Polymers, 2021, 267: 118159. |
| 31 | ZHANG Liming, YAN Pengchao, LI Yan, et al. Preparation and antibacterial activity of a cellulose-based Schiff base derived from dialdehyde cellulose and L-lysine[J]. Industrial Crops and Products, 2020, 145: 112126. |
| 32 | HE Panyang, ZHANG Yaojun, ZHANG Xiaomin, et al. Diverse zeolites derived from a circulating fluidized bed fly ash based geopolymer for the adsorption of lead ions from wastewater[J]. Journal of Cleaner Production, 2021, 312: 127769. |
| 33 | TOUIHRI Manel, GUESMI Fatma, HANNACHI Chiraz, et al. Single and simultaneous adsorption of Cr(Ⅵ) and Cu(Ⅱ) on a novel Fe3O4/pine cones gel beads nanocomposite: Experiments, characterization and isotherms modeling[J]. Chemical Engineering Journal, 2021, 416: 129101. |
| 34 | XU Hao, HU Xinjiang, CHEN Yonghua, et al. Cd(Ⅱ) and Pb(Ⅱ) absorbed on humic acid-iron-pillared bentonite: Kinetics, thermodynamics and mechanism of adsorption[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 612: 126005. |
| 35 | MOHAN Sweta, KUMAR Vijay, SINGH Devendra Kumar, et al. Effective removal of lead ions using graphene oxide-MgO nanohybrid from aqueous solution: Isotherm, kinetic and thermodynamic modeling of adsorption[J]. Journal of Environmental Chemical Engineering, 2017, 5(3): 2259-2273. |
| 36 | D-K KWEON, J-K CHOI, KIM E-K, et al. Adsorption of divalent metal ions by succinylated and oxidized corn starches[J]. Carbohydrate Polymers, 2001, 46(2): 171-177. |
| 37 | WANG Hai, LIU Renrong, CHEN Qian, et al. Biochar-supported starch/chitosan-stabilized nano-iron sulfide composites for the removal of lead ions and nitrogen from aqueous solutions[J]. Bioresource Technology, 2022, 347: 126700. |
| 38 | Tam Hoang LUU, VAN NGUYEN Hung, Nhan Thuc Chi HA, et al. Synthesis of starch modified montmorillonite as an effective adsorbent for Pb(Ⅱ) removal from water[J]. Vietnam Journal of Science and Technology, 2019, 57(3A): 94-102. |
| 39 | XU Shimei, FENG Shun, PENG Gui, et al. Removal of Pb(Ⅱ) by crosslinked amphoteric starch containing the carboxymethyl group[J]. Carbohydrate Polymers, 2005, 60(3): 301-305. |
| 40 | SHI Tianzhu, XIE Zhengfeng, MO Xinliang, et al. Adsorption behaviors of heavy metal ions by different hydrazone-modified sodium alginate in aqueous medium: Experimental and DFT studies[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 659: 130754. |
| 41 | NAUSHAD Mu, AHAMAD Tansir, SHARMA Gaurav, et al. Synthesis and characterization of a new starch/SnO2 nanocomposite for efficient adsorption of toxic Hg2+ metal ion[J]. Chemical Engineering Journal, 2016, 300: 306-316. |
| 42 | ZHANG Wei, WANG Hongyu, HU Xiaoling, et al. Multicavity triethylenetetramine-chitosan/alginate composite beads for enhanced Cr(Ⅵ) removal[J]. Journal of Cleaner Production, 2019, 231: 733-745. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [3] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [4] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [5] | 赵重阳, 赵磊, 石详文, 黄俊, 李治尧, 沈凯, 张亚平. O2/H2O/SO2 对改性富铁凹凸棒石高温吸附PbCl2 的影响[J]. 化工进展, 2023, 42(4): 2190-2200. |
| [6] | 郭帅帅, 陈锦路, 金梁程龙, 陶醉, 陈小丽, 彭国文. 基于海水提铀的多孔芳香框架材料研究进展[J]. 化工进展, 2023, 42(3): 1426-1436. |
| [7] | 叶沁辉, 陈红, 于鑫, 王凯, 于露滢, 曾可佳. 沼渣生物炭的制备及资源化利用研究进展[J]. 化工进展, 2023, 42(12): 6554-6566. |
| [8] | 柯玉鑫, 朱晓丽, 司绍诚, 张婷, 王军强, 张子夜. 废白土衍生吸附剂协同去除水中的四环素和铜[J]. 化工进展, 2023, 42(11): 5981-5992. |
| [9] | 伍岳, 李晓宇, 陶春珲, 张莹, 李印辉, 张文祥, 杨伯伦, 马和平. 离子改性的CON材料用于吸附分离NF3[J]. 化工进展, 2023, 42(11): 6076-6085. |
| [10] | 王胜楠, 郑旭. 空气取水用活性炭纤维复合吸附剂的研究[J]. 化工进展, 2023, 42(10): 5567-5573. |
| [11] | 王子航, 梁瑞升, 邓超和, 王佳韵. 离子凝胶复合吸附剂的制备及空气取水性能[J]. 化工进展, 2022, 41(S1): 389-396. |
| [12] | 曹正凯, 米晓斌, 吴子明, 孙士可, 曹均丰, 彭德强, 梁相程. 煤合成气净化除尘装置压降问题分析及应用优化[J]. 化工进展, 2022, 41(S1): 15-21. |
| [13] | 王一茹, 宋小三, 水博阳, 王三反. 胺功能化介孔二氧化硅捕集CO2的研究进展[J]. 化工进展, 2022, 41(S1): 536-544. |
| [14] | 李兴, 黄宏宇, 大坂侑吾, 呼和涛力, 肖林发, 李军. 碳材料吸附脱除二氧化硫性能的影响因素[J]. 化工进展, 2022, 41(9): 4963-4972. |
| [15] | 张雨珂, 刘倩, 段媛媛, 赵英杰, 崔阳, 史利娟, 李向远, 李剑川, 范海明, 易群. 基于MOFs材料的低碳烃(C1~C3)分离研究进展[J]. 化工进展, 2022, 41(8): 4288-4302. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||