化工进展 ›› 2023, Vol. 42 ›› Issue (12): 6576-6588.DOI: 10.16085/j.issn.1000-6613.2023-0113
• 资源与环境化工 • 上一篇
木质素氧化解聚制备芳香醛研究进展
- 1.北京工商大学轻工科学技术学院,北京 100048
2.北京市食品风味化学重点实验室,北京 100048
-
收稿日期:2023-01-30修回日期:2023-03-24出版日期:2023-12-25发布日期:2024-01-08 -
通讯作者:白宇辰 -
作者简介:陈禹婷(1993—),女,硕士研究生,研究方向为生物质资源转化利用。E-mail:2030301018@st.btbu.edu.cn。 -
基金资助:北京市教委科技一般项目(KM202310011008);北京工商大学青年启动基金(QNJJ2021-13)
Research process of preparation of aromatic aldehyde by oxidative depolymerization of lignin
CHEN Yuting1( ), BAI Yuchen1,2(
), BAI Yuchen1,2( )
)
- 1.School of Light Industry, Beijing Technology and Business University, Beijing 100048, China
2.Beijing Key Laboratory of Flavor Chemistry, Beijing Technology and Business University, Beijing 100048, China
-
Received:2023-01-30Revised:2023-03-24Online:2023-12-25Published:2024-01-08 -
Contact:BAI Yuchen
摘要:
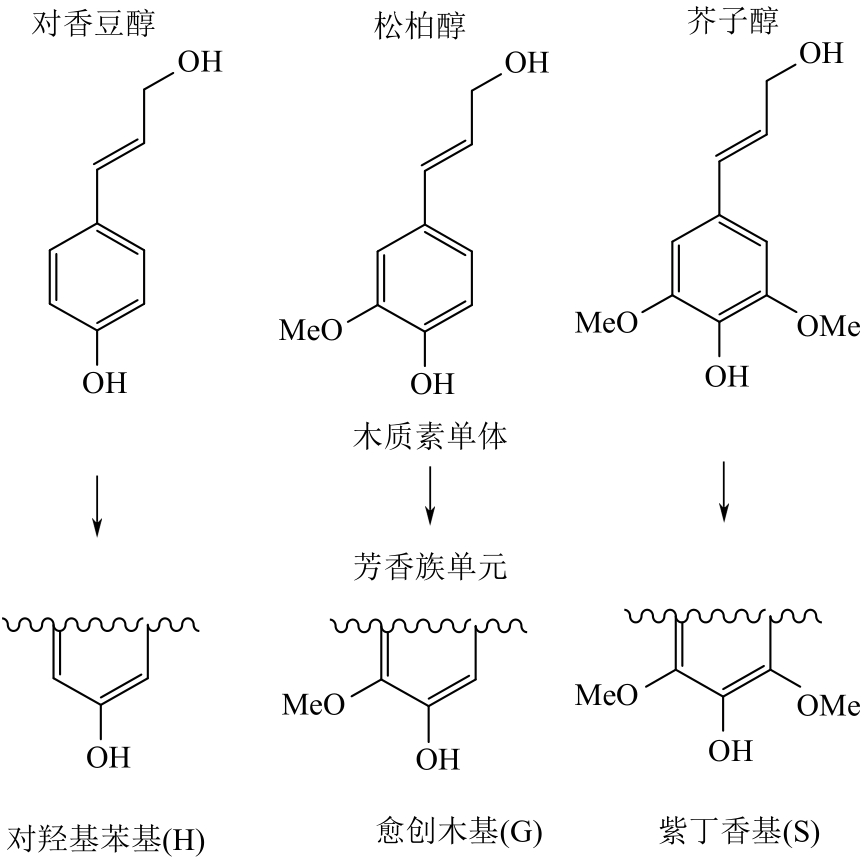
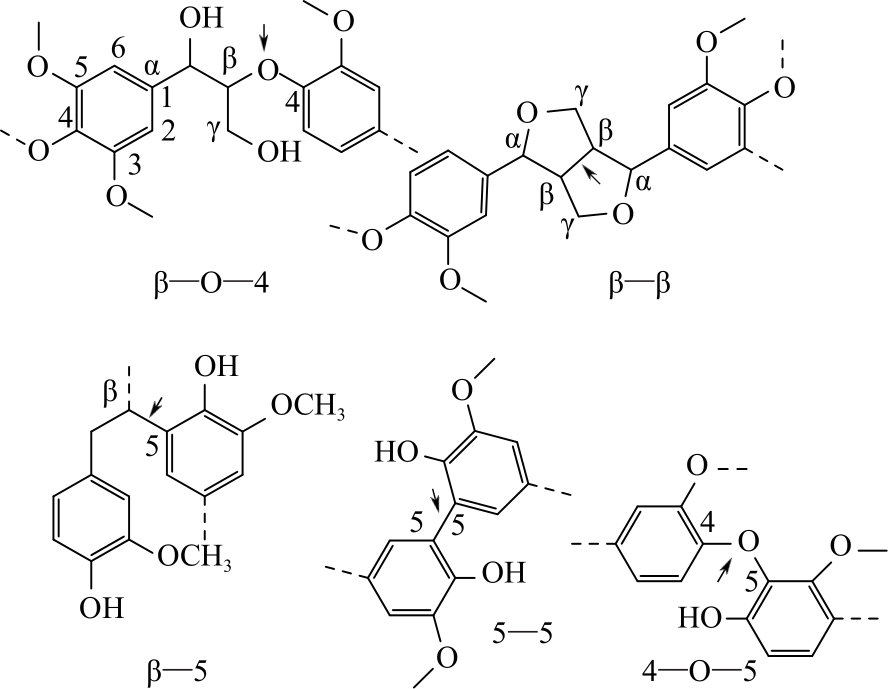
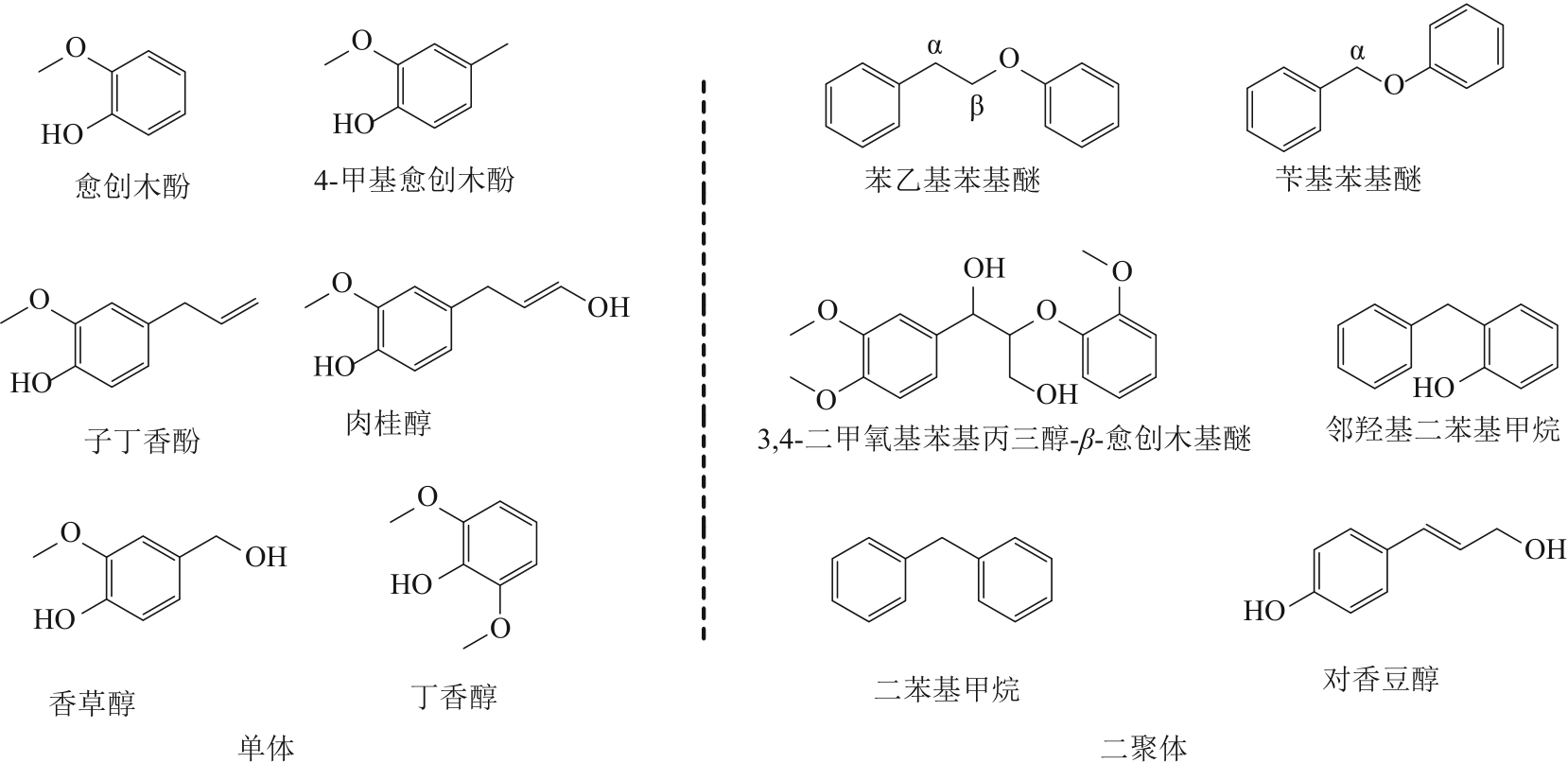
木质素作为自然界最丰富的芳香族化合物资源,通过可控的氧化解聚方式得到小分子芳香醛类化合物是实现木质素高附加值利用的重要途径。芳香醛作为重要的食用及日化香料、医药中间体、大宗化学品,目前主要通过石油化工产业链生产,以木质素生产芳香醛是具有重要研究意义和应用潜力的可再生资源利用途径。本文综述了国内外有关木质素制备芳香醛的最新研究进展,包括化学氧化解聚、电化学解聚、光催化解聚和生物催化解聚;对不同方法制备芳香醛的转化率和产率进行了对比,基于不同的解聚工艺与催化体系的特点进行评述与分析,展望未来木质素生产芳香醛的研究方向,以实现木质素的高值化利用。得出多方法耦合催化是可能进一步提高木质素生产芳香醛转化率与选择性的有效策略。
中图分类号:
引用本文
陈禹婷, 白宇辰. 木质素氧化解聚制备芳香醛研究进展[J]. 化工进展, 2023, 42(12): 6576-6588.
CHEN Yuting, BAI Yuchen. Research process of preparation of aromatic aldehyde by oxidative depolymerization of lignin[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6576-6588.
| 底物 | 氧化剂 | 溶剂 | 反应条件 | 芳香醛主要产物 | 参考文献 |
|---|---|---|---|---|---|
| 异子丁香酚 | H2O2 | 乙腈 | 现制0.5Fe/Al-SBA-15, 90℃ | 香草醛 | [ |
| 有机溶剂木质素 | O2 | 甲醇 | Pd/CeO2 | 香草醛,对羟基苯甲醛 | [ |
| 碱木质素 | 空气 | 水 | 150~200℃ | 香草醛,对羟基苯甲醛 | [ |
| 有机溶剂木质素 | 硝基苯 | 水 | NaOH,170℃ | 香草醛,丁香醛 | [ |
| 有机溶剂木质素 | — | 丙酮 | 300℃,40min | 对羟基苯甲醛 | [ |
| 有机溶剂木质素 | — | 乙醇 | 300℃,41min | 丁香醛 | [ |
表1 木质素氧化解聚中使用的几种典型的溶剂及氧化剂
| 底物 | 氧化剂 | 溶剂 | 反应条件 | 芳香醛主要产物 | 参考文献 |
|---|---|---|---|---|---|
| 异子丁香酚 | H2O2 | 乙腈 | 现制0.5Fe/Al-SBA-15, 90℃ | 香草醛 | [ |
| 有机溶剂木质素 | O2 | 甲醇 | Pd/CeO2 | 香草醛,对羟基苯甲醛 | [ |
| 碱木质素 | 空气 | 水 | 150~200℃ | 香草醛,对羟基苯甲醛 | [ |
| 有机溶剂木质素 | 硝基苯 | 水 | NaOH,170℃ | 香草醛,丁香醛 | [ |
| 有机溶剂木质素 | — | 丙酮 | 300℃,40min | 对羟基苯甲醛 | [ |
| 有机溶剂木质素 | — | 乙醇 | 300℃,41min | 丁香醛 | [ |
| 原料 | 催化剂 | 溶剂 | 氧化剂 | 反应条件 | 芳香醛主要产物 | 转化率/% | 产率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 酶水解蒸汽爆破玉米秆 | LaCoO3 | 氢氧化钠水溶液 | O2 | 120℃,2MPa,0~3h | 香草醛 | 8~60 | 3.9~4.55 | [ |
| 对羟基苯甲醛 | 0.85~2.23 | |||||||
| 丁香醛 | 2.1~9.99 | |||||||
| 酶水解蒸汽爆破玉米秆 | LaFe1-x Cu x O3 (x=0、0.1、0.2) | 氢氧化钠水溶液 | O2 | 120℃,2MPa,0~3h | 香草醛 | 0~70 | 2.4~4.6 | [ |
| 对羟基苯甲醛 | 0.4~2.5 | |||||||
| 丁香醛 | 0~11.5 | |||||||
| 有机溶剂木质素 | Pd/CeO2 | 甲醇 | O2 | 185℃,0.1MPa | 香草醛 | — | 5.2 | [ |
| 对羟基苯甲醛 | 2.4 | |||||||
| 有机溶剂木质素 | La/SBA-15 | 氢氧化钠水溶液 | H2O2 | 60℃,5~1440min | 香草醛 | — | 0.38~9.94 | [ |
| 丁香醛 | 0.52~15.66 | |||||||
| 香草醇 | Co3O4 | H2O | O2 | 140℃,0.689~4MPa | 香草醛 | 86 | — | [ |
| 香草醇 | MnCo-MO | 乙腈 | 空气 | 140℃,2.1MPa,2h | 香草醛 | 62 | 51 | [ |
| 藜芦醇 | Co-ZIF-9 | 甲苯/氢氧化钠 | O2 | 150℃,0.5MPa,0~4h | 藜芦醛 | 0~45 | 46 | [ |
| 香草醇 | 香草醛 | 90 | [ | |||||
| 异子丁香酚 | CH3ReO3 | 叔丁醇 | H2O2 | 70℃,4h | 香草醛 | 90.3 | [ |
表2 非均相催化剂在木质素/木质素模型化合物中的使用
| 原料 | 催化剂 | 溶剂 | 氧化剂 | 反应条件 | 芳香醛主要产物 | 转化率/% | 产率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 酶水解蒸汽爆破玉米秆 | LaCoO3 | 氢氧化钠水溶液 | O2 | 120℃,2MPa,0~3h | 香草醛 | 8~60 | 3.9~4.55 | [ |
| 对羟基苯甲醛 | 0.85~2.23 | |||||||
| 丁香醛 | 2.1~9.99 | |||||||
| 酶水解蒸汽爆破玉米秆 | LaFe1-x Cu x O3 (x=0、0.1、0.2) | 氢氧化钠水溶液 | O2 | 120℃,2MPa,0~3h | 香草醛 | 0~70 | 2.4~4.6 | [ |
| 对羟基苯甲醛 | 0.4~2.5 | |||||||
| 丁香醛 | 0~11.5 | |||||||
| 有机溶剂木质素 | Pd/CeO2 | 甲醇 | O2 | 185℃,0.1MPa | 香草醛 | — | 5.2 | [ |
| 对羟基苯甲醛 | 2.4 | |||||||
| 有机溶剂木质素 | La/SBA-15 | 氢氧化钠水溶液 | H2O2 | 60℃,5~1440min | 香草醛 | — | 0.38~9.94 | [ |
| 丁香醛 | 0.52~15.66 | |||||||
| 香草醇 | Co3O4 | H2O | O2 | 140℃,0.689~4MPa | 香草醛 | 86 | — | [ |
| 香草醇 | MnCo-MO | 乙腈 | 空气 | 140℃,2.1MPa,2h | 香草醛 | 62 | 51 | [ |
| 藜芦醇 | Co-ZIF-9 | 甲苯/氢氧化钠 | O2 | 150℃,0.5MPa,0~4h | 藜芦醛 | 0~45 | 46 | [ |
| 香草醇 | 香草醛 | 90 | [ | |||||
| 异子丁香酚 | CH3ReO3 | 叔丁醇 | H2O2 | 70℃,4h | 香草醛 | 90.3 | [ |
| 催化剂类型 | 催化剂 | 溶剂 | 氧化剂 | 反应时间 | 底物(木质素/木质素 模型化合物) | 芳香醛 主要产物 | 转化率 /% | 产率 /% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 金属卟啉催化剂 | CoTBrPP | 氢氧化钠水溶液 | H2O2 | 150℃,30min | 1-(3,4-二甲氧基苯基)-2- (2-甲氧基苯氧基)乙烷-1-醇 | 藜芦醛 | 86 | 20.4 | [ |
| TPPFeCl | — | 叔丁酸 | 过夜 | β-1木质素模型化合物 | 藜芦醛 | — | 50 | [ | |
| TSPCMnCI | 磷酸盐水溶液 | 叔丁酸 | 室温 | 藜芦醇 | 藜芦醛 | — | 16 | [ | |
| 金属-席夫碱配合物 | Co(salen) | 甲醇 | O2 | 室温,40min | 丁香醇 | 丁香醛 | 98 | 36 | [ |
| Co(salen) | 氢氧化钠水溶液 | O2 | 80℃,28h | 藜芦醇 | 藜芦醛 | — | 43 | [ | |
| 多金属氧酸盐 | H5PV2Mo10O40 | [HC4im][HSO4] | O2 | 100℃,5h | 硬木木质素 | 丁香醛 | — | 5 | [ |
| H3PMo12O40 | 水/甲醇 | O2 | 170℃,0.33h | 硫酸盐木质素 | 香草醛 | — | 5.18 | [ | |
| K6[SiV2W10O40] | 水/甲醇 | O2 | 150℃,2MPa | 酶解木质素 | 香草醛 | — | 5.46 | [ | |
| 简单金属盐催化剂 | Cu(OH)2 | — | O2 | 160℃ | 香草醛丙酮 | 丁香醛 | — | 60 | [ |
| FeCl3 | — | O2 | 160℃ | Clason&酸解木质素 | 香草醛 | — | 8.8 | [ | |
| 丁香醛 | — | 0.7 |
表3 均相催化剂在催化木质素/木质素模型化合物中的使用
| 催化剂类型 | 催化剂 | 溶剂 | 氧化剂 | 反应时间 | 底物(木质素/木质素 模型化合物) | 芳香醛 主要产物 | 转化率 /% | 产率 /% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 金属卟啉催化剂 | CoTBrPP | 氢氧化钠水溶液 | H2O2 | 150℃,30min | 1-(3,4-二甲氧基苯基)-2- (2-甲氧基苯氧基)乙烷-1-醇 | 藜芦醛 | 86 | 20.4 | [ |
| TPPFeCl | — | 叔丁酸 | 过夜 | β-1木质素模型化合物 | 藜芦醛 | — | 50 | [ | |
| TSPCMnCI | 磷酸盐水溶液 | 叔丁酸 | 室温 | 藜芦醇 | 藜芦醛 | — | 16 | [ | |
| 金属-席夫碱配合物 | Co(salen) | 甲醇 | O2 | 室温,40min | 丁香醇 | 丁香醛 | 98 | 36 | [ |
| Co(salen) | 氢氧化钠水溶液 | O2 | 80℃,28h | 藜芦醇 | 藜芦醛 | — | 43 | [ | |
| 多金属氧酸盐 | H5PV2Mo10O40 | [HC4im][HSO4] | O2 | 100℃,5h | 硬木木质素 | 丁香醛 | — | 5 | [ |
| H3PMo12O40 | 水/甲醇 | O2 | 170℃,0.33h | 硫酸盐木质素 | 香草醛 | — | 5.18 | [ | |
| K6[SiV2W10O40] | 水/甲醇 | O2 | 150℃,2MPa | 酶解木质素 | 香草醛 | — | 5.46 | [ | |
| 简单金属盐催化剂 | Cu(OH)2 | — | O2 | 160℃ | 香草醛丙酮 | 丁香醛 | — | 60 | [ |
| FeCl3 | — | O2 | 160℃ | Clason&酸解木质素 | 香草醛 | — | 8.8 | [ | |
| 丁香醛 | — | 0.7 |
| 阳极材料 | 溶剂 | 反应条件 | 底物(木质素/木质素模型化合物) | 芳香醛主要产物 | 转化率/% | 产率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| PbO2 | 氢氧化钠水溶液 | 室温 | 硫酸盐木质素 | 香草醛 | 17 | 64 | [ |
| PbO2 | 氢氧化钠水溶液 | 50℃,50mA/cm2 | 白杨木木质素 | 香草醛,丁香醛 | — | 30.4/13.75 | [ |
| Ni | — | 80℃,恒定电流,1.9mA/cm2 | 硫酸盐木质素 | 香草醛 | — | 0.7 | [ |
| Co | — | 80℃,恒定电流,1.9mA/cm2 | 硫酸盐木质素 | 香草醛 | — | 1.4 | [ |
| 钨铬钴合金 | — | 80℃,恒定电流,1.9mA/cm2 | 硫酸盐木质素 | 香草醛 | — | 1.8 | [ |
表4 电化学解聚木质素/木质素模型化合物
| 阳极材料 | 溶剂 | 反应条件 | 底物(木质素/木质素模型化合物) | 芳香醛主要产物 | 转化率/% | 产率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| PbO2 | 氢氧化钠水溶液 | 室温 | 硫酸盐木质素 | 香草醛 | 17 | 64 | [ |
| PbO2 | 氢氧化钠水溶液 | 50℃,50mA/cm2 | 白杨木木质素 | 香草醛,丁香醛 | — | 30.4/13.75 | [ |
| Ni | — | 80℃,恒定电流,1.9mA/cm2 | 硫酸盐木质素 | 香草醛 | — | 0.7 | [ |
| Co | — | 80℃,恒定电流,1.9mA/cm2 | 硫酸盐木质素 | 香草醛 | — | 1.4 | [ |
| 钨铬钴合金 | — | 80℃,恒定电流,1.9mA/cm2 | 硫酸盐木质素 | 香草醛 | — | 1.8 | [ |
| 编号 | 底物 | 催化剂 | 溶剂/反应时间 | 芳香醛单体/% | ||
|---|---|---|---|---|---|---|
| 对羟基苯甲醛 | 香草醛 | 丁香醛 | ||||
| 1 | 桦木木质素 | VO(OiPr)3 | 乙腈/24h | — | 0.34 | 0.3 |
| 2 | 秸秆木质素 | VO(OiPr)2 | 0.21 | 0.23 | 0.1 | |
| 3 | 桦木木质素 | VO(acac)2 | — | 0.22 | 0.18 | |
| 4 | 秸秆木质素 | VO(acac)3 | — | 0.24 | 0.07 | |
| 5 | 桦木木质素 | VO(OiPr)2 | 丙酮∶甲醇=9∶1 | — | 0.35 | 0.57 |
| 6 | 桦木木质素 | VO(acac)2 | — | 0.6 | 0.79 | |
| 7 | 秸秆木质素 | VO(acac)2 | /24h | — | 0.25 | 0.28 |
| 8 | 白杨木质素 | VO(acac)2 | — | 0.2 | 0.26 | |
表5 相同实验条件下不同催化剂与溶剂组合得到的芳香醛及产率对比[77]
| 编号 | 底物 | 催化剂 | 溶剂/反应时间 | 芳香醛单体/% | ||
|---|---|---|---|---|---|---|
| 对羟基苯甲醛 | 香草醛 | 丁香醛 | ||||
| 1 | 桦木木质素 | VO(OiPr)3 | 乙腈/24h | — | 0.34 | 0.3 |
| 2 | 秸秆木质素 | VO(OiPr)2 | 0.21 | 0.23 | 0.1 | |
| 3 | 桦木木质素 | VO(acac)2 | — | 0.22 | 0.18 | |
| 4 | 秸秆木质素 | VO(acac)3 | — | 0.24 | 0.07 | |
| 5 | 桦木木质素 | VO(OiPr)2 | 丙酮∶甲醇=9∶1 | — | 0.35 | 0.57 |
| 6 | 桦木木质素 | VO(acac)2 | — | 0.6 | 0.79 | |
| 7 | 秸秆木质素 | VO(acac)2 | /24h | — | 0.25 | 0.28 |
| 8 | 白杨木质素 | VO(acac)2 | — | 0.2 | 0.26 | |
| 催化剂 | 溶剂 | 反应条件 | 原料(木质素/木质素模型化合物) | 芳香醛主要产物 | 转化率/% | 产率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| TiO2 | 乙醇 | UV,300W | 有机溶剂木质素 | 香草醛,丁香醛 | — | 0.9/14.2 | [ |
| TiO2 | 水 | UV,125W,高压水银灯 | TiO2-木质素混合物 | 香草醛,丁香醛 | — | — | [ |
| Bi1%/Pt1%-TiO2 | — | 太阳能灯,300W,1h | 磺酸盐木质素 | 香草醛 | 62 | 1.5 | [ |
| VO(OiPr)3 | 乙腈 | 6W,LEDs(455nm±5nm),O2,6h | β-1木质素模型化合物 | 丁香醛 | 100 | 61 | [ |
| VO(OiPr)3 | 乙腈 | 6W,LEDs(455nm±5nm),O2,24h | 二𫫇英木质素 | 香草醛 | — | 0.23 | [ |
| 丁香醛 | 0.1 | ||||||
| 对羟基苯甲醛 | 0.21 |
表6 光催化木质素/木质素模型化合物氧化解聚
| 催化剂 | 溶剂 | 反应条件 | 原料(木质素/木质素模型化合物) | 芳香醛主要产物 | 转化率/% | 产率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| TiO2 | 乙醇 | UV,300W | 有机溶剂木质素 | 香草醛,丁香醛 | — | 0.9/14.2 | [ |
| TiO2 | 水 | UV,125W,高压水银灯 | TiO2-木质素混合物 | 香草醛,丁香醛 | — | — | [ |
| Bi1%/Pt1%-TiO2 | — | 太阳能灯,300W,1h | 磺酸盐木质素 | 香草醛 | 62 | 1.5 | [ |
| VO(OiPr)3 | 乙腈 | 6W,LEDs(455nm±5nm),O2,6h | β-1木质素模型化合物 | 丁香醛 | 100 | 61 | [ |
| VO(OiPr)3 | 乙腈 | 6W,LEDs(455nm±5nm),O2,24h | 二𫫇英木质素 | 香草醛 | — | 0.23 | [ |
| 丁香醛 | 0.1 | ||||||
| 对羟基苯甲醛 | 0.21 |
| 1 | WENG Caihong, PENG Xiaowei, HAN Yejun. Depolymerization and conversion of lignin to value-added bioproducts by microbial and enzymatic catalysis[J]. Biotechnology for Biofuels, 2021, 14(1): 84. |
| 2 | BAGHEL Swati, ANANDKUMAR J. Biodepolymerization of Kraft lignin for production and optimization of vanillin using mixed bacterial culture[J]. Bioresource Technology Reports, 2019, 8: 100335. |
| 3 | 沈晓骏, 黄攀丽, 文甲龙, 等. 木质素氧化还原解聚研究现状[J]. 化学进展, 2017, 29(1): 162-178. |
| SHEN Xiaojun, HUANG Panli, WEN Jialong, et al. Research status of lignin oxidative and reductive depolymerization[J]. Progress in Chemistry, 2017, 29(1): 162-178. | |
| 4 | LIU Wujun, JIANG Hong, YU Hanqing. Thermochemical conversion of lignin to functional materials: A review and future directions[J]. Green Chemistry, 2015, 17(11): 4888-4907. |
| 5 | LENG Erwei, GUO Yilin, CHEN Jingwei, et al. A comprehensive review on lignin pyrolysis: Mechanism, modeling and the effects of inherent metals in biomass[J]. Fuel, 2022, 309: 122102. |
| 6 | 张雷, 王海英, 韩洪晶, 等. 木质素催化热解用催化剂的研究进展[J]. 化工进展, 2022, 41(5):2429-2440. |
| ZHANG Lei, WANG Haiying, HAN Hongjing, et al. Development of catalysts for catalytic pyrolysis of lignin[J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2429-2440. | |
| 7 | LIU Zhihua, LE Rosemary K, KOSA Matyas, et al. Identifying and creating pathways to improve biological lignin valorization[J]. Renewable and Sustainable Energy Reviews, 2019, 105: 349-362. |
| 8 | MA Hongwei, LI Haowei, ZHAO Weijie, et al. Selective depolymerization of lignin catalyzed by nickel supported on zirconium phosphate[J]. Green Chemistry, 2019, 21(3): 658-668. |
| 9 | LIU Chao, WU Shiliang, ZHANG Huiyan, et al. Catalytic oxidation of lignin to valuable biomass-based platform chemicals: A review[J]. Fuel Processing Technology, 2019, 191: 181-201. |
| 10 | CIRIMINNA Rosaria, FIDALGO Alexandra, MENEGUZZO Francesco, et al. Vanillin: The case for greener production driven by sustainability megatrend[J]. ChemistryOpen, 2019, 8(6): 660-667. |
| 11 | BANERJEE Goutam, CHATTOPADHYAY Pritam. Vanillin biotechnology: The perspectives and future[J]. Journal of the Science of Food and Agriculture, 2019, 99(2): 499-506. |
| 12 | TARABANKO V E, CHELBINA Yu V, KUDRYASHEV A V, et al. Separation of vanillin and syringaldehyde produced from lignins[J]. Separation Science and Technology, 2013, 48(1): 127-132. |
| 13 | 彭建军, 张学铭, 陈雪梅, 等. 基于生物质精炼的木质素分离及结构研究进展[C]//中国造纸学会第十九届学术年会论文集. 郑州, 2020: 339-347. |
| WU Miao, PENG Jianjun, ZHANG Xueming, et al. Research progress on lignin separation and structure based on biomass refining[C]//Proceedings of the 19th academic annual meeting of the china paper industry association. Zhengzhou, 2020: 339-347. | |
| 14 | LI Changzhi, ZHAO Xiaochen, WANG Aiqin, et al. Catalytic transformation of lignin for the production of chemicals and fuels[J]. Chemical Reviews, 2015, 115(21): 11559-11624. |
| 15 | ZHANG Chaofeng, WANG Feng. Catalytic lignin depolymerization to aromatic chemicals[J]. Accounts of Chemical Research, 2020, 53(2): 470-484. |
| 16 | SUN Zhuohua, Bálint FRIDRICH, DE SANTI Alessandra, et al. Bright side of lignin depolymerization: Toward new platform chemicals[J]. Chemical Reviews, 2018, 118(2): 614-678. |
| 17 | 邱学青, 楼宏铭, 杨东杰, 等. 工业木质素的改性及其作为精细化工产品的研究进展[J]. 精细化工, 2005, 22(3): 161-167, 197. |
| QIU Xueqing, LOU Hongming, YANG Dongjie, et al. Research progress of industrial lignin modification and its utilization as fine chemicals[J]. Fine Chemicals, 2005, 22(3): 161-167, 197. | |
| 18 | BOARINO Alice, KLOK Harm-Anton. Opportunities and challenges for lignin valorization in food packaging, antimicrobial, and agricultural applications[J]. Biomacromolecules, 2023, 24(3): 1065-1077. |
| 19 | 赵丽莎. 木质素结构及加氢解聚对其抗氧化活性的影响[D]. 广州: 华南理工大学, 2020. |
| ZHAO Lisha. Effect of lignin structure and hydrogenolysis on its antioxidant activity[D]. Guangzhou: South China University of Technology, 2020. | |
| 20 | Alexander STÜCKER, Fokko SCHÜTT, SAAKE Bodo, et al. Lignins from enzymatic hydrolysis and alkaline extraction of steam refined poplar wood: Utilization in lignin-phenol-formaldehyde resins[J]. Industrial Crops and Products, 2016, 85: 300-308. |
| 21 | Mikel OREGUI-BENGOECHEA, AGIRRE Ion, IRIONDO Aitziber, et al. Heterogeneous catalyzed thermochemical conversion of lignin model compounds: An overview[J]. Topics in Current Chemistry, 2019, 377(6): 1-75. |
| 22 | PANDEY M P, KIM C S. Lignin depolymerization and conversion: A review of thermochemical methods[J]. Chemical Engineering & Technology, 2011, 34(1): 29-41. |
| 23 | DEEPAK Raikwar, SAPTARSHI Majumdar, DEBAPRASAD Shee. Effects of solvents in the depolymerization of lignin into value-added products: A review[J]. Biomass Conversion and Biorefinery, 2023, 13(13): 11383-11416. |
| 24 | CHIO Chonlong, SAIN Mohini, QIN Wensheng. Lignin utilization: A review of lignin depolymerization from various aspects[J]. Renewable and Sustainable Energy Reviews, 2019, 107: 232-249. |
| 25 | KUMAR Avnish, BISWAS Bijoy, KAUR Ramandeep, et al. Hydrothermal oxidative valorisation of lignin into functional chemicals: A review[J]. Bioresource Technology, 2021, 342: 126016. |
| 26 | YAMAMURA Masaomi, HATTORI Takefumi, SUZUKI Shiro, et al. Microscale alkaline nitrobenzene oxidation method for high-throughput determination of lignin aromatic components[J]. Plant Biotechnology, 2010, 27(4): 305-310. |
| 27 | 周姚红, 张晓华, 熊万明. 木质素催化氧化制备芳香醛研究进展[J]. 精细化工, 2022, 39(3): 442-453. |
| ZHOU Yaohong, ZHANG Xiaohua, XIONG Wanming. Research progress of preparation of aromatic aldehydes by catalytic oxidation of lignin[J]. Fine Chemicals, 2022, 39(3): 442-453. | |
| 28 | DENG Weiping, ZHANG Hongxi, WU Xuejiao, et al. Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles[J]. Green Chemistry, 2015, 17(11): 5009-5018. |
| 29 | IRMAK Sibel, KANG Juhyon, WILKINS Mark. Depolymerization of lignin by wet air oxidation[J]. Bioresource Technology Reports, 2020, 9: 100377. |
| 30 | ERDOCIA Xabier, PRADO Raquel, Javier FERNÁNDEZ-RODRÍGUEZ, et al. Depolymerization of different organosolv lignins in supercritical methanol, ethanol, and acetone to produce phenolic monomers[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 1373-1380. |
| 31 | 马春慧, 孙晋德, 李伟, 等. 离子液体在木质素解聚领域的应用进展[J]. 林业工程学报, 2021, 6(5): 14-26. |
| MA Chunhui, SUN Jinde, LI Wei, et al. Application progress of ionic liquids in the field of lignin depolymerization[J]. Journal of Forestry Engineering, 2021, 6(5): 14-26. | |
| 32 | PENG Mingming, NAKABAYASHI Manaka, KIM Kihoon, et al. Lignin depolymerization with alkaline ionic liquids and ethylene glycol in a continuous flow reactor [J]. Fuel, 2023, 335: 126960. |
| 33 | 候其东, 鞠美庭, 李维尊, 等. 基于离子液体的生物质组分分离研究进展[J]. 化工进展,2016,35(10):3022-3031. |
| HOU Qidong, JU Meiting, LI Weizun,et al. Research progress on biomass fractionation using ionic liquids[J]. Chemical Industry and Engineering Progress, 2016, 35(10):3022-3031. | |
| 34 | FRANCO Ana, DE Sudipta, BALU Alina M, et al. Selective oxidation of isoeugenol to vanillin over mechanochemically synthesized aluminosilicate supported transition metal catalysts[J]. ChemistrySelect, 2017, 2(29): 9546-9551. |
| 35 | ZAKZESKI Joseph, BRUIJNINCX Pieter C A, JONGERIUS Anna L, et al. The catalytic valorization of lignin for the production of renewable chemicals[J]. Chemical Reviews, 2010, 110(6): 3552-3599. |
| 36 | 刘思洁. 过渡金属(Co, Ni, Mo)催化木质素解聚研究[D]. 广州: 华南理工大学, 2019. |
| LIU Sijie. Catalytic depolymerization of lignin using the transition metal(Co, Ni, Mo) catalysts[D]. Guangzhou: South China University of Technology, 2019. | |
| 37 | SALES Fernando G, MARANHÃO Laísse C A, FILHO Nelson M Lima, et al. Experimental evaluation and continuous catalytic process for fine aldehyde production from lignin[J]. Chemical Engineering Science, 2007, 62(18/19/20): 5386-5391. |
| 38 | SALES Fernando G, MARANHÃO Laísse C A, LIMA FILHO Nelson M, et al. Kinetic evaluation and modeling of lignin catalytic wet oxidation to selective production of aromatic aldehydes[J]. Industrial & Engineering Chemistry Research, 2006, 45(20): 6627-6631. |
| 39 | 安宏宇. 钙钛矿催化剂的掺杂改性及其催化热解木质素制备含氧化合物[D]. 大庆: 东北石油大学, 2018. |
| AN Hongyu. The doping of perovskite catalyst and its catalytic pyrolysis lignin to prepare the oxygenation[D]. Daqing: Northeast Petroleum University, 2018. | |
| 40 | DENG Haibo, LIN Lu, SUN Yong, et al. Activity and stability of perovskite-type oxide LaCoO3 catalyst in lignin catalytic wet oxidation to aromatic aldehydes process[J]. Energy & Fuels, 2009, 23(1): 19-24. |
| 41 | ZHANG Junhua, DENG Haibo, LIN Lu. Wet aerobic oxidation of lignin into aromatic aldehydes catalysed by a perovskite-type oxide: LaFe(1-x)Cu(x)O3 (x=0, 0.1, 0.2)[J]. Molecules, 2009, 14(8): 2747-2757. |
| 42 | Ajay JHA, PATIL Kashinath R, RODE Chandrashekhar V. Mixed Co-Mn oxide-catalysed selective aerobic oxidation of vanillyl alcohol to vanillin in base-free conditions[J]. ChemPlusChem, 2013, 78(11): 1384-1392. |
| 43 | ZAKZESKI Joseph, Agnieszka DĘBCZAK, BRUIJNINCX Pieter C A, et al. Catalytic oxidation of aromatic oxygenates by the heterogeneous catalyst Co-ZIF-9[J]. Applied Catalysis A: General, 2011, 394(1/2): 79-85. |
| 44 | HERRMANN Wolfgang A, WESKAMP Thomas, ZOLLER Jochen P, et al. Methyltrioxorhenium: Oxidative cleavage of CC-double bonds and its application in a highly efficient synthesis of vanillin from biological waste[J]. Journal of Molecular Catalysis A: Chemical, 2000, 153(1/2): 49-52. |
| 45 | GU Xiaoli, CHENG Kanghua, MING He, et al. La-modified SBA-15/H2O2 systems for the microwave assisted oxidation of organosolv beech wood lignin[J]. Maderas Ciencia y Tecnología, 2012, 14(1): 31-41. |
| 46 | MATE V R, JHA A, JOSHI U D, et al. Effect of preparation parameters on characterization and activity of Co3O4 catalyst in liquid phase oxidation of lignin model substrates[J]. Applied Catalysis A: General, 2014, 487: 130-138. |
| 47 | 何金义, 朱凯. 甲基三氧化铼催化过氧化氢氧化异丁香酚合成香兰素的研究[J]. 应用化工, 2019, 48(3): 550-553. |
| HE Jinyi, ZHU Kai. Synthesis of vanillin by catalytic hydrogen peroxide oxidation of iso-eugenol catalyzed by methyl-trioxide[J]. Applied Chemical Industry, 2019, 48(3): 550-553. | |
| 48 | XU Wenbiao, LI Xiangyu, SHI Junyou. Oxidative depolymerization of cellulolytic enzyme lignin over silicotungvanadium polyoxometalates[J]. Polymers, 2019, 11(3): 564. |
| 49 | XIE Jinfeng, MA Guanfeng, OUYANG Xinping, et al. Metalloporphyrin as a biomimetic catalyst for the catalytic oxidative degradation of lignin to produce aromatic monomers[J]. Waste and Biomass Valorization, 2020, 11(8): 4481-4489. |
| 50 | FANG Zhen, MEIER Mark S. Toward the oxidative deconstruction of lignin: Oxidation of β-1 and β-5 linkages[J]. Organic & Biomolecular Chemistry, 2018, 16(13): 2330-2341. |
| 51 | 林泽英. 杂多酸离子液体催化木质素选择性氧化研究[D]. 广州: 华南理工大学, 2020. |
| LIN Zeying. Selective oxidation of lignin catalyzed by polyoxometalate ionic liquids[D]. Guangzhou: South China University of Technology, 2020. | |
| 52 | CANEVALI Carmen, ORLANDI Marco, PARDI Luca, et al. Oxidative degradation of monomeric and dimeric phenylpropanoids: Reactivity and mechanistic investigation[J]. Journal of the Chemical Society, Dalton Transactions, 2002(15): 3007-3014. |
| 53 | ZULETA Ernesto C, GOENAGA Gabriel A, ZAWODZINSKI Thomas A, et al. Deactivation of Co-Schiff base catalysts in the oxidation of para-substituted lignin models for the production of benzoquinones[J]. Catalysis Science & Technology, 2020, 10(2): 403-413. |
| 54 | KERVINEN Kaisa, KORPI Heikki, GERBRAND MESU J, et al. Mechanistic insights into the oxidation of veratryl alcohol with Co(salen) and oxygen in aqueous media: An in situ spectroscopic study[J]. European Journal of Inorganic Chemistry, 2005, 2005(13): 2591-2599. |
| 55 | 李一鸣. 多金属氧酸盐的设计合成及在木质纤维素转化中的性能研究[D]. 长春: 东北师范大学, 2020. |
| LI Yiming. Design and synthesis of polyoxometalates and their activities in lignocellulose conversion[D]. Changchun: Northeast Normal University, 2020. | |
| 56 | 张俊旺. 多金属氧酸盐-离子液体氧化解聚木质素的研究[D]. 大连: 大连工业大学, 2020. |
| ZHANG Junwang. Study on oxidative depolymerization of lignin by polyoxometalates-ionic liquids[D]. Dalian: Dalian Polytechnic University, 2020. | |
| 57 | KIM Yong Sik, CHANG Houmin, KADLA John F. Polyoxometalate (POM) oxidation of lignin model compounds[J]. Holzforschung, 2008, 62(1): 38-49. |
| 58 | VOITL Tobias, VON ROHR Philipp Rudolf. Oxidation of lignin using aqueous polyoxometalates in the presence of alcohols[J]. ChemSusChem, 2008, 1(8/9): 763-769. |
| 59 | DE GREGORIO Gilbert F, PRADO Raquel, VRIAMONT Charles, et al. Oxidative depolymerization of lignin using a novel polyoxometalate-protic ionic liquid system[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(11): 6031-6036. |
| 60 | CUI Futong, DOLPHIN David. Metallophthalocyanines as possible lignin peroxidase models[J]. Bioorganic & Medicinal Chemistry, 1995, 3(5): 471-477. |
| 61 | TARABANKO V E, PETUKHOV D V, SELYUTIN G E. New mechanism for the catalytic oxidation of lignin to vanillin[J]. Kinetics and Catalysis, 2004, 45(4): 569-577. |
| 62 | XIANG Q, LEE Y Y. Production of oxychemicals from precipitated hardwood lignin[J]. Applied Biochemistry and Biotechnology, 2001, 91/92/93: 71-80. |
| 63 | PARTENHEIMER W. Methodology and scope of metal/bromide autoxidation of hydrocarbons[J]. Catalysis Today, 1995, 23(2): 69-158. |
| 64 | 马春慧, 张继芳, 李伟, 等. 电化学催化木质素解聚的研究进展[J]. 林产化学与工业, 2022, 42(1): 110-122. |
| MA Chunhui, ZHANG Jifang, LI Wei, et al. Research progress in electrochemically catalyzed depolymerization of lignin[J]. Chemistry and Industry of Forest Products, 2022, 42(1): 110-122. | |
| 65 | YANG C, MALDONADO S, STEPHENSON C R J. Electrocatalytic lignin oxidation[J]. ACS Catalysis, 2021, 11(16): 10104-10114. |
| 66 | FANG Zhiyong, LI Fuhua, WANG Mei, et al. Selective electrocatalytic upgrading of lignin to aryl aldehydes and carboxylic acids over dodecyl sulfate-intercalated CoS nanocones[J]. Applied Catalysis B: Environmental, 2023, 323: 122149. |
| 67 | DU Xu, ZHANG Haichuan, SULLIVAN Kevin P, et al. Electrochemical lignin conversion[J]. ChemSusChem, 2020, 13(17): 4318-4343. |
| 68 | SCHMITT Dominik, REGENBRECHT Carolin, HARTMER Marius, et al. Highly selective generation of vanillin by anodic degradation of lignin: A combined approach of electrochemistry and product isolation by adsorption[J]. Beilstein Journal of Organic Chemistry, 2015, 11: 473-480. |
| 69 | PARPOT P, BETTENCOURT A P, CARVALHO A M, et al. Biomass conversion: Attempted electrooxidation of lignin for vanillin production[J]. Journal of Applied Electrochemistry, 2000, 30(6): 727-731. |
| 70 | WANG Yongsheng, YANG Fang, LIU Zhihua, et al. Electrocatalytic degradation of aspen lignin over Pb/PbO2 electrode in alkali solution[J]. Catalysis Communications, 2015, 67: 49-53. |
| 71 | TOLBA Rasha, TIAN Min, WEN Jiali, et al. Electrochemical oxidation of lignin at IrO2-based oxide electrodes[J]. Journal of Electroanalytical Chemistry, 2010, 649(1/2): 9-15. |
| 72 | 许仃仃. 针叶木硫酸盐木素的电化学降解及改性研究[D]. 济南: 齐鲁工业大学, 2019. |
| XU Dingding. Electrochemical degradation and modification of softwood kraft lignin[D]. Jinan: Qilu University of Technology, 2019. | |
| 73 | UĞURLU M, KARAOĞLU M H. TiO2 supported on sepiolite: Preparation, structural and thermal characterization and catalytic behaviour in photocatalytic treatment of phenol and lignin from olive mill wastewater[J]. Chemical Engineering Journal, 2011, 166(3): 859-867. |
| 74 | CAO Yang, CHEN Season S, ZHANG Shicheng, et al. Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery[J]. Bioresource Technology, 2019, 291: 121878. |
| 75 | PRADO Raquel, ERDOCIA Xabier, LABIDI Jalel. Effect of the photocatalytic activity of TiO2 on lignin depolymerization[J]. Chemosphere, 2013, 91(9): 1355-1361. |
| 76 | GONG Jianyu, IMBAULT Alexander, FARNOOD Ramin. The promoting role of bismuth for the enhanced photocatalytic oxidation of lignin on Pt-TiO2 under solar light illumination[J]. Applied Catalysis B: Environmental, 2017, 204: 296-303. |
| 77 | LIU Huifang, LI Hongji, LUO Nengchao, et al. Visible-light-induced oxidative lignin C-C bond cleavage to aldehydes using vanadium catalysts[J]. ACS Catalysis, 2020, 10(1): 632-643. |
| 78 | NAIR Vaishakh, DHAR Piyali, VINU R. Production of phenolics via photocatalysis of ball milled lignin-TiO2 mixtures in aqueous suspension[J]. RSC Advances, 2016, 6(22): 18204-18216. |
| 79 | 王晶, 倪金荧, 王利群, 等. 一株木质素降解细菌的筛选及其降解途径[J]. 化工进展,2021,40(7): 4021-4026. |
| WANG Jing, NI Jinying, WANG Liqun, et al. Screening of a lignin degrading bacterium and its degradation pathway[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 4021-4026. | |
| 80 | SAINSBURY Paul D, MINEYEVA Yelena, MYCROFT Zoe, et al. Chemical intervention in bacterial lignin degradation pathways: Development of selective inhibitors for intradiol and extradiol catechol dioxygenases[J]. Bioorganic Chemistry, 2015, 60: 102-109. |
| 81 | 梁丛颖, 林璐. 环境微生物介导的木质素代谢及其资源化利用研究进展[J]. 微生物学通报, 2020, 47(10): 3380-3392. |
| LIANG Congying, LIN Lu. Environmental microorganisms driven lignin biodegradation and their roles in lignin utilization[J]. Microbiology China, 2020, 47(10): 3380-3392. | |
| 82 | 唐亮, 廖强, 夏奡, 等. 仿生酶菌协同体系预处理木质素机理及特性[J]. 化工进展,2021,40(10): 5378-5387. |
| TANG Liang, LIAO Qiang, XIA Ao, et al. Mechanism and characteristics of nature inspired enzyme-fungi synergistic system for lignin pretreatment[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5378-5387. | |
| 83 | 徐杰. 光催化材料结构调控和解聚木质素研究[D]. 南京: 南京林业大学, 2021. |
| XU Jie. Study on the structure regulation of photocatalytic materials and the depolymerization of lignin[D]. Nanjing: Nanjing Forestry University, 2021. | |
| 84 | Lucía PENÍN, GIGLI Matteo, SABUZI Federica, et al. Biomimetic vanadate and molybdate systems for oxidative upgrading of iono- and organosolv hard- and softwood lignins[J]. Processes, 2020, 8(9): 1161. |
| 85 | CRESTINI Claudia, JURASEK Lubo, ARGYROPOULOS Dimitris S. On the mechanism of the laccase-mediator system in the oxidation of lignin[J]. Chemistry—A European Journal, 2003, 9(21): 5371-5378. |
| 86 | BOHLIN Christina, PERSSON Per, GORTON Lo, et al. Product profiles in enzymic and non-enzymic oxidations of the lignin model compound erythro-1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol[J]. Journal of Molecular Catalysis B: Enzymatic, 2005, 35(4/5/6): 100-107. |
| [1] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [2] | 关红玲, 杨辉, 井红权, 刘玉琼, 谷守玉, 王好斌, 侯翠红. 木质素基控释材料及其在药物输送和肥料控释中的应用[J]. 化工进展, 2023, 42(7): 3695-3707. |
| [3] | 于丁一, 李圆圆, 王晨钰, 纪永升. pH响应性木质素水凝胶的制备及药物控释[J]. 化工进展, 2023, 42(6): 3138-3146. |
| [4] | 任建鹏, 吴彩文, 刘慧君, 吴文娟. 木质素-聚苯胺复合材料的制备及对刚果红的吸附[J]. 化工进展, 2023, 42(6): 3087-3096. |
| [5] | 杨程瑞雪, 黄琪媛, 冉建速, 崔耘通, 王健健. 磷酸修饰二氧化硅负载钯催化剂用于木质素衍生物高效水相低温加氢脱氧[J]. 化工进展, 2023, 42(10): 5179-5190. |
| [6] | 张鹏, 王绍庆, 李志合, 张安东, 高亮, 万震, 宋宁. 赤泥/木质素共热解制备复合吸附材料及其性能[J]. 化工进展, 2022, 41(S1): 407-414. |
| [7] | 蒲福龙, 伍尚炜, 郑映玲, 郑玉意, 侯雪丹. 基于乳酸的深度共熔溶剂提取秸秆木质素对纤维素酶水解效率的影响[J]. 化工进展, 2022, 41(9): 4937-4945. |
| [8] | 龙垠荧, 杨健, 管敏, 杨怡洛, 程正柏, 曹海兵, 刘洪斌, 安兴业. 木质素基材料在混合型超级电容器电极材料中的研究进展[J]. 化工进展, 2022, 41(9): 4855-4865. |
| [9] | 汪兴, 赵子龙, 张小山, 王宏杰, 董文艺, 陈慧慧. 制备条件对生物炭载铁催化剂催化破络Ni-EDTA性能及活性组分浸出的影响[J]. 化工进展, 2022, 41(9): 4831-4839. |
| [10] | 张丽珠, 王欢, 李琼, 杨东杰. 木质素衍生吸附材料及其在废水处理中的应用研究进展[J]. 化工进展, 2022, 41(7): 3731-3744. |
| [11] | 张伟, 安兴业, 刘利琴, 龙垠荧, 张昊, 程正柏, 曹海兵, 刘洪斌. 木质素纳米颗粒/天然纤维基活性碳纤维材料的制备及其电化学性能[J]. 化工进展, 2022, 41(7): 3770-3783. |
| [12] | 娄瑞, 刘钰, 田杰, 张亚男. 纳米木质素基多孔炭的制备及其电化学性能[J]. 化工进展, 2022, 41(6): 3170-3177. |
| [13] | 申琪, 薛雨源, 杨涛伟, 张妍, 李胜任. 木质素荧光研究进展[J]. 化工进展, 2022, 41(5): 2672-2685. |
| [14] | 王鲁元, 金春江, 陈惠敏, 程星星, 安东海, 张兴宇, 孙荣峰, 耿文广. 一步热解活化法制备纳米木质素基多孔炭材料[J]. 化工进展, 2022, 41(5): 2582-2592. |
| [15] | 张雷, 王海英, 韩洪晶, 陈彦广, 王程昊. 木质素催化热解用催化剂的研究进展[J]. 化工进展, 2022, 41(5): 2429-2440. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||