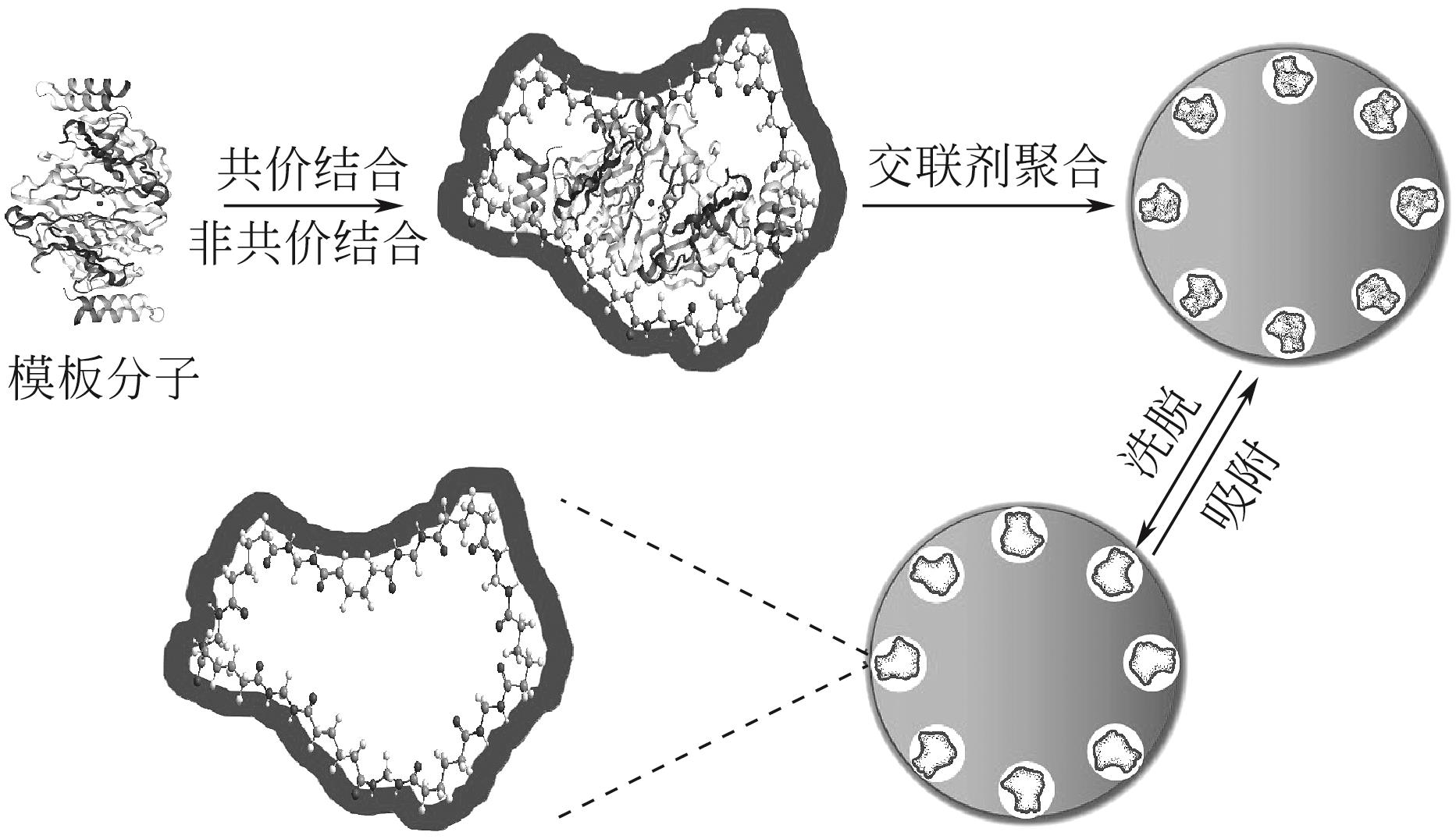
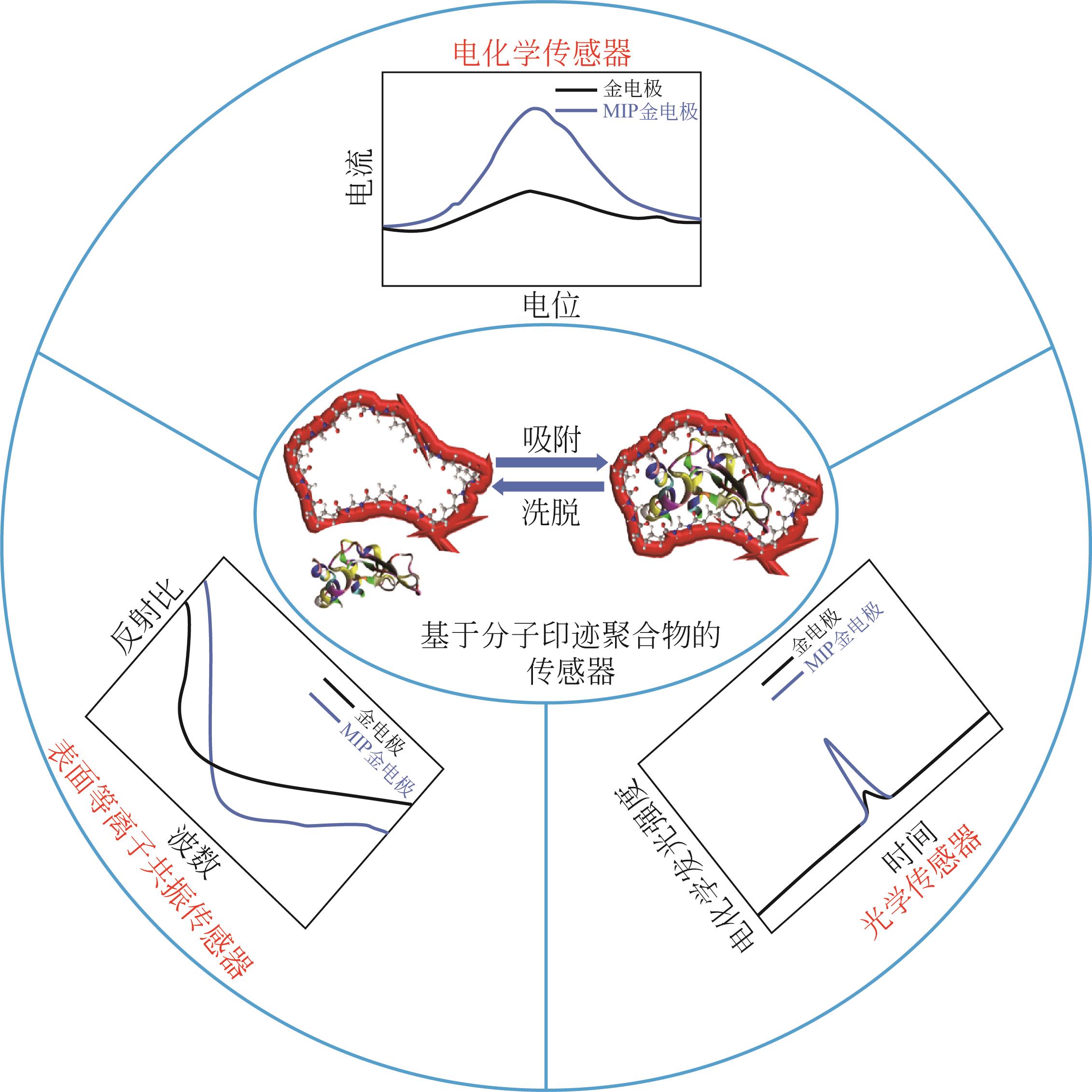
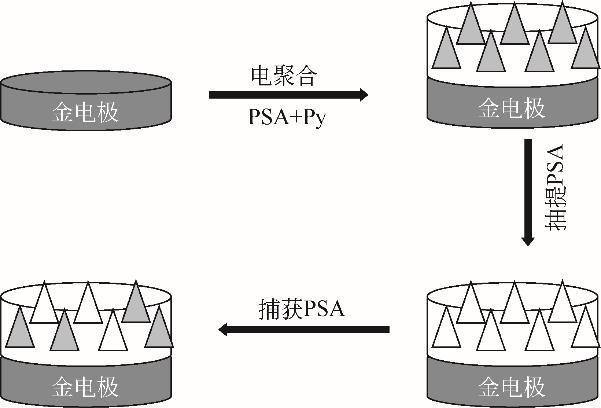
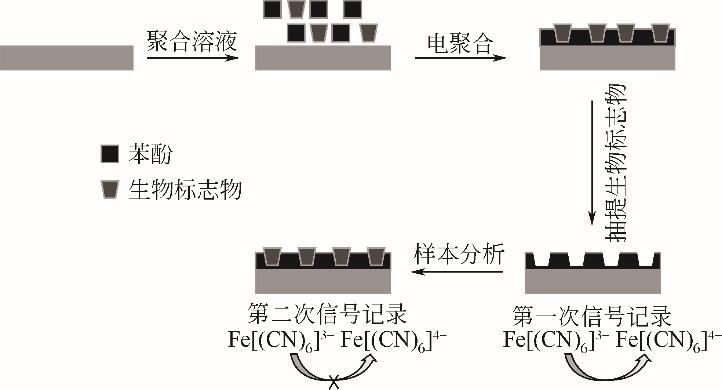
化工进展 ›› 2022, Vol. 41 ›› Issue (S1): 448-460.DOI: 10.16085/j.issn.1000-6613.2022-0617
新型分子印迹生物传感器在癌症生物标志物中的应用
- 北京理工大学生命学院,分子医学与生物诊疗工业和信息化部重点实验室,北京 100081
-
收稿日期:2022-04-11修回日期:2022-06-20出版日期:2022-10-20发布日期:2022-11-10 -
通讯作者:梁阿新 -
作者简介:罗爱芹(1965—),女,博士,教授,研究方向为分子识别与生物传感、生化分析与分离。E-mail:bitluo@bit.edu.cn。 -
基金资助:国家重点研发计划(2019YFA0904104)
Application of novel molecularly imprinted biosensor in cancer marker
LUO Aiqin( ), CAI Yanhui, LIANG Axin(
), CAI Yanhui, LIANG Axin( ), XIE Bingteng
), XIE Bingteng
- Key Laboratory of Molecular Medicine and Biotherapy, the Ministry of Industry and Information Technology, School of Life Science, Beijing Institute of Technology, Beijing 100081, China
-
Received:2022-04-11Revised:2022-06-20Online:2022-10-20Published:2022-11-10 -
Contact:LIANG Axin
摘要:
癌症生物标志物作为一种特定的身体中可测量的指标,可以通过自身产生的变化来监测癌症健康状况,对于癌症的预防、早期诊断和精准治疗具有重要意义。因此,面向典型疾病标志物的快速检测与精准分析已成为科学研究和临床诊断的热点领域。基于分子印迹的生物传感器由于其灵敏度高、响应速度快、特异性强以及成本低等优点,有望成为癌症生物标志物快速检测的新手段,在癌症临床分析领域得到了广泛研究。本综述简要介绍了分子印迹聚合物的合成方法和策略,分子印迹与电化学、表面等离子共振和光学分析集成的传感技术和手段,以及分子印迹生物传感在癌症生物标志物的应用。最后本综述就目前国内外关于分子印迹传感器在分析检测癌症生物标志物领域的应用现状进行了总结和讨论,并展望了其未来发展前景。
中图分类号:
引用本文
罗爱芹, 蔡艳慧, 梁阿新, 解炳腾. 新型分子印迹生物传感器在癌症生物标志物中的应用[J]. 化工进展, 2022, 41(S1): 448-460.
LUO Aiqin, CAI Yanhui, LIANG Axin, XIE Bingteng. Application of novel molecularly imprinted biosensor in cancer marker[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 448-460.
| 癌症类型 | 生物标志物 | 技术 | 线性范围 | 检出限 | 参考文献 |
|---|---|---|---|---|---|
| 前列腺癌 | PSA | SPR | 0.1~50×10-9g/mL | 91×10-12g/mL | [ |
| 电化学 | 0.01~4×10-9g/mL | 2×10-12g/mL | [ | ||
| SPR | — | — | [ | ||
| SAR | 电化学 | 5.0~1100×10-6mol/L | 0.38×10-6mol/L | [ | |
| 电化学 | 1~100pm | 0.4pm | [ | ||
| 乳腺癌 | HER2-ECD | 电化学 | 10~70×10-9g/mL | 1.6×10-9g/mL | [ |
| 电化学 | 5~50U/mL | 1.5U/mL | [ | ||
| CA 15-3 | 电化学 | 1.44~13.2U/mL | 1.07U/mL | [ | |
| 光学 | 0.0005~40U/mL | 50μU/mL | [ | ||
| 电化学 | 0.1~100×10-6g/mL | 0.08×10-9g/mL | [ | ||
| CEA | 电化学 | — | 0.1×10-12g/mL | [ | |
| — | 1~500×10-9g/mL | 0.32×10-9g/mL | [ | ||
| 肝癌 | AFP | 光学 | 0.025~100×10-9g/mL | 2.5×10-12g/mL | [ |
| 0.01~100×10-9g/mL | 1.2×10-12g/mL | ||||
| 0.001~25×10-9g/mL | 0.05×10-12g/mL | ||||
| 0.005~25×10-9g/mL | 0.015×10-12g/mL | ||||
| 光学 | 20~125μg/L | 9.65μg/L | [ | ||
| 光学 | 10~100×10-9g/mL | 0.474×10-9g/mL | [ | ||
| 其他 | Anti SARS-CoV-2 | 电化学 | 111×10-15mol/L | 15×10-15mol/L | [ |
| IL-2 | 光学 | 35×10-15g/mL~39×10-12g/mL | 5.91×10-15g/mL | [ |
表1 基于MIP传感器对癌症生物标志物检测对比
| 癌症类型 | 生物标志物 | 技术 | 线性范围 | 检出限 | 参考文献 |
|---|---|---|---|---|---|
| 前列腺癌 | PSA | SPR | 0.1~50×10-9g/mL | 91×10-12g/mL | [ |
| 电化学 | 0.01~4×10-9g/mL | 2×10-12g/mL | [ | ||
| SPR | — | — | [ | ||
| SAR | 电化学 | 5.0~1100×10-6mol/L | 0.38×10-6mol/L | [ | |
| 电化学 | 1~100pm | 0.4pm | [ | ||
| 乳腺癌 | HER2-ECD | 电化学 | 10~70×10-9g/mL | 1.6×10-9g/mL | [ |
| 电化学 | 5~50U/mL | 1.5U/mL | [ | ||
| CA 15-3 | 电化学 | 1.44~13.2U/mL | 1.07U/mL | [ | |
| 光学 | 0.0005~40U/mL | 50μU/mL | [ | ||
| 电化学 | 0.1~100×10-6g/mL | 0.08×10-9g/mL | [ | ||
| CEA | 电化学 | — | 0.1×10-12g/mL | [ | |
| — | 1~500×10-9g/mL | 0.32×10-9g/mL | [ | ||
| 肝癌 | AFP | 光学 | 0.025~100×10-9g/mL | 2.5×10-12g/mL | [ |
| 0.01~100×10-9g/mL | 1.2×10-12g/mL | ||||
| 0.001~25×10-9g/mL | 0.05×10-12g/mL | ||||
| 0.005~25×10-9g/mL | 0.015×10-12g/mL | ||||
| 光学 | 20~125μg/L | 9.65μg/L | [ | ||
| 光学 | 10~100×10-9g/mL | 0.474×10-9g/mL | [ | ||
| 其他 | Anti SARS-CoV-2 | 电化学 | 111×10-15mol/L | 15×10-15mol/L | [ |
| IL-2 | 光学 | 35×10-15g/mL~39×10-12g/mL | 5.91×10-15g/mL | [ |
| 1 | OH C M, LEE D, KONG H J, et al. Causes of death among cancer patients in the era of cancer survivorship in Korea: Attention to the suicide and cardiovascular mortality[J]. Cancer Medicine, 2020, 9(5): 1741-1752. |
| 2 | RAHIB L, WEHNER M R, MATRISIAN L M, et al. Estimated projection of US cancer incidence and death to 2040[J]. JAMA Network Open, 2021, 4(4): e214708. |
| 3 | HUANG Jie, CHEN Xinxin, FU Xuekun, et al. Advances in aptamer-based biomarker discovery[J]. Frontiers in Cell and Developmental Biology, 2021, 9: 659760-659771. |
| 4 | MATZKE L A, WATSON P H. Biobanking for cancer biomarker research: issues and solutions[J]. Biomarker Insights, 2020, 15: 1177271920965522. |
| 5 | CONWAY S R, WONG H R. Biomarker panels in critical care[J]. Critical Care Clinics, 2020, 36(1): 89-104. |
| 6 | FENG Ziding, PEPE M S. Adding rigor to biomarker evaluations-EDRN experience[J]. Cancer Epidemiology Biomarkers & Prevention, 2020, 29(12): 2575-2582. |
| 7 | CASTELLANOS E H, ORLANDO A, MA X, et al. Evaluating the impact of oncology care model reporting requirements on biomarker testing and treatment[J]. Jco Oncology Practice, 2020, 16(10): E1216-E1221. |
| 8 | MEARELLI F, BARBATI G, MORAS C, et al. Soluble Fc gamma RIA expressed on monocytes (sCD64): a new serum biomarker of acute kidney injury in patients with suspected infection at emergency department admission[J]. Cytokine, 2021, 148: 155661-155665. |
| 9 | GUSEV A, BUKO A, OGA T, et al. PD52-09 development of a urinary metabolomic signature for prostate cancer using capillary electrophoresis mass spectrometry[J]. Journal of Urology, 2020, 203: E1093. |
| 10 | KONDO T. Cancer biomarker development and two-dimensional difference gel electrophoresis (2D-DIGE)[J]. Biochimica Et Biophysica Acta-Proteins and Proteomics, 2019, 1867(1): 2-8. |
| 11 | FUNAI K, HONZAWA K, SUZUKI M, et al. Urinary fluorescent metabolite O-aminohippuric acid is a useful biomarker for lung cancer detection[J]. Metabolomics, 2020, 16(10): 101. |
| 12 | HAN Jialing, CHENG Longhao, ZHU Ya, et al. Covalent-assembly based fluorescent probes for detection of hNQO1 and imaging in living cells[J]. Frontiers in Chemistry, 2020, 8:756-765. |
| 13 | LI Xueqi, PAN Yutong, CHEN Huan, et al. Specific near-infrared probe for ultrafast imaging of lysosomal beta-galactosidase in ovarian cancer cells[J]. Analytical Chemistry, 2020, 92(8): 5772-5779. |
| 14 | YOU Li, ZHEN Qin, ZHANG Fengli, et al. Two-color fluorescent proteins reporting survivin regulation in breast cancer cells for high throughput drug screening[J]. Biotechnology and Bioengineering, 2022, 119(3): 1004-1017. |
| 15 | LEE Mingjun, LEE Enxue, KIM Taihuan, et al. Detection of thioredoxin-1 using ultra-sensitive ELISA with enzyme-encapsulated human serum albumin nanoparticle[J]. Nano Convergence, 2019, 6(1): 37-44. |
| 16 | SONG Minkyo, HILDESHEIM A, SHIELS M S. Premature years of life lost due to cancer in the United States in 2017[J]. Cancer Epidemiology Biomarkers & Prevention, 2020, 29(12): 2591-2598. |
| 17 | YANG Pengcheng, ZHENG Yongqiang, CHEN Jiayuan, et al. Immediate risk of non-cancer deaths after a cancer diagnosis[J]. BMC Cancer, 2021, 21(1): 963-976. |
| 18 | SOBSEY C A, IBRAHIM S, RICHARD V R, et al. Targeted and untargeted proteomics approaches in biomarker development[J]. Proteomics, 2020, 20(9): 1900029. |
| 19 | WANG Laixi, TONG Xin, LI Chao, et al. Glycoengineering of antibodies for modulating functions[J]. Annual Review Biochemistry, 2019, 88: 433–459. |
| 20 | MENG Q Y, XIE B H, MA X Y, et al. Rational design of ER alpha targeting hypoxia turn-on fluorescent probes with antiproliferative activity for breast cancer[J]. Chemical Communications, 2020, 56(72): 10493-10496. |
| 21 | MOHAN S, LAWTON R, PALMER C, et al. Competitive ELISA method for novel estrogen-negative breast cancer biomarker quantitation[J]. Journal of Immunological Methods, 2019, 474: 112671-112677. |
| 22 | AHMAD L, SALMON L, KORRI-YOUSSOUFI H. Electrochemical detection of the human cancer biomarker ‘autocrine motility factor-phosphoglucose isomerase’ based on a biosensor formed with a monosaccharidic inhibitor[J]. Sensors and Actuators B: Chemical, 2019, 299: 126933-126942. |
| 23 | ASADI H, RAMASAMY R P. Graphene-based electrochemical biosensor for impedimetric detection of miRNAs as potential cancer biomarkers[J]. Journal of the Electrochemical Society, 2020, 167(16): 167523-167532. |
| 24 | BRANELLA G M, SPENCER H T. Natural receptor- and ligand-based chimeric antigen receptors: strategies using natural ligands and receptors for targeted cell killing[J]. Cells, 2021, 11(1): 21-25. |
| 25 | DING Xiaochu, HEIDEN P A. Recent developments in molecularly imprinted nanoparticles by surface imprinting techniques[J]. Macromolecular Materials and Engineering, 2014, 299(3): 268-282. |
| 26 | HISAMATSU Y, OTANI K, TAKASE H, et al. Fluorescence response and self-assembly of a tweezer-type synthetic receptor triggered by complexation with heme and its catabolites[J]. Chemistry: a European Journal, 2021, 27(21): 6489-6499. |
| 27 | MANHAS J, EDELSTEIN H I, LEONARD J N, et al. The evolution of synthetic receptor systems[J]. Nature Chemical Biology, 2022, 18(3): 244-255. |
| 28 | CHEN R N, KANG S H, LI J, et al. Comparison and recent progress of molecular imprinting technology and dummy template molecular imprinting technology[J]. Analytical Methods, 2021, 13(39): 4538-4556. |
| 29 | NDUNDA E N. Molecularly imprinted polymers—A closer look at the control polymer used in determining the imprinting effect: a mini review[J]. Journal of Molecular Recognition, 2020, 33(11): e2855. |
| 30 | WAN Qingqing, LIU Hui, DENG Zhiwei, et al. A critical review of molecularly imprinted solid phase extraction technology[J]. Journal of Polymer Research, 2021, 28(10): 401-417. |
| 31 | LIU Chang, CAO Yichuan, ZHAO Tian, et al. A novel multi-purpose MIP for SPE-HPLC and QCM detection of carbaryl residues in foods[J]. Food Analytical Methods, 2021, 14(2): 331-343. |
| 32 | MATHEW D, THOMAS B, DEVAKY K S. Design, synthesis and characterization of enzyme-analogue-built polymer catalysts as artificial hydrolases[J]. Artificial Cells Nanomedicine and Biotechnology, 2019, 47(1): 1149-1172. |
| 33 | ANSARI S, MASOUM S. Molecularly imprinted polymers for capturing and sensing proteins: Current progress and future implications[J]. Trac-Trends in Analytical Chemistry, 2019, 114: 29-47. |
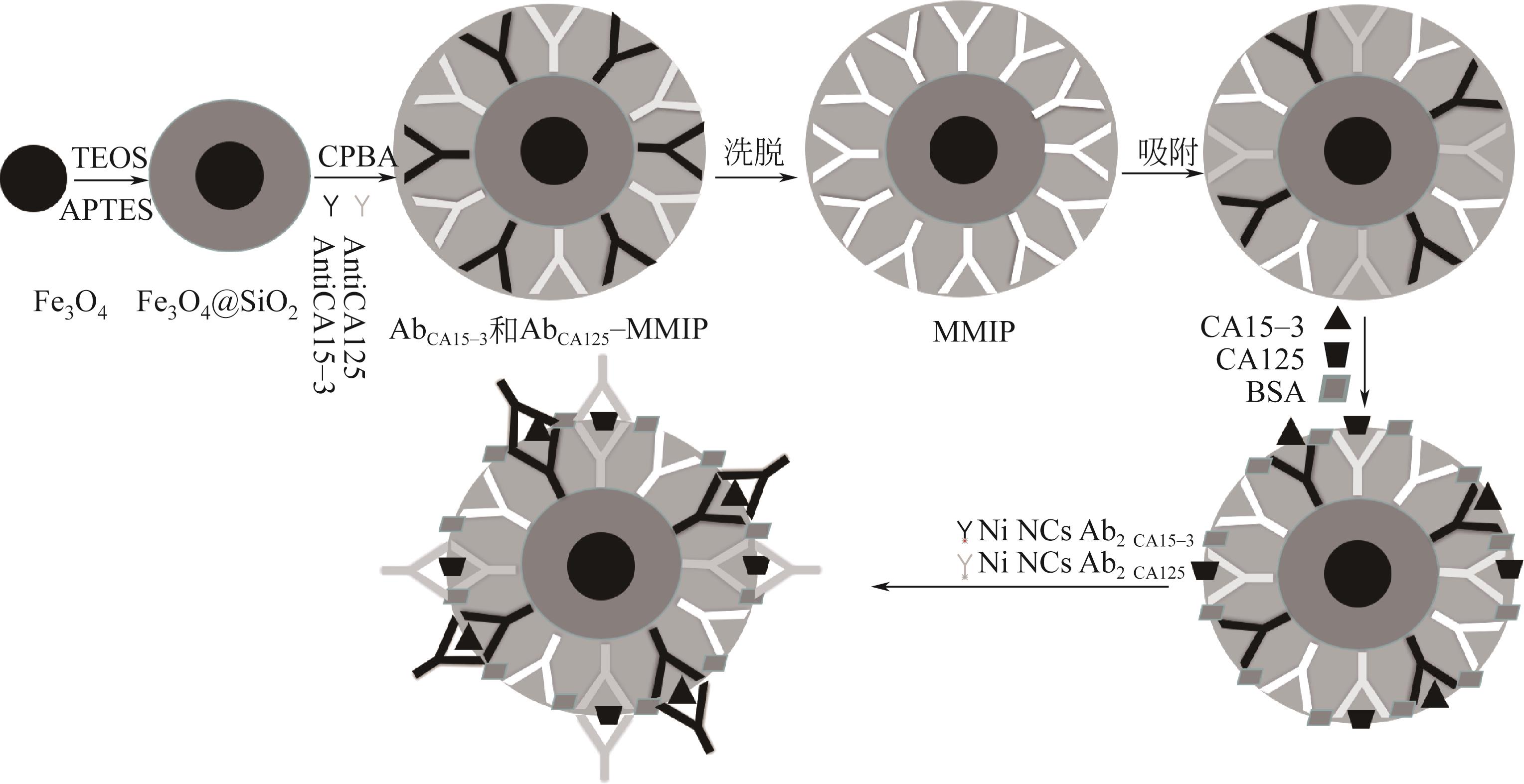
| 34 | RICO Y A, CARRASCO S. Molecularly imprinted polymer-based hybrid materials for the development of optical sensors[J]. Polymers, 2019, 11(7): 1173-1217. |
| 35 | NAWAZ N, ABU B N, MAHMUD H, et al. Molecularly imprinted polymers-based DNA biosensors[J]. Analytical Biochemistry, 2021, 630:114328-114343. |
| 36 | UNGER C, LIEBERZEIT P A. Molecularly imprinted thin film surfaces in sensing: Chances and challenges[J]. Reactive & Functional Polymers, 2021, 161: 104855-104865. |
| 37 | BELBRUNO J J. Molecularly imprinted polymers[J]. Chemical reviews, 2019, 119(1): 94-119. |
| 38 | DONATO L, NASSER I I, MAJDOUB M, et al. Green chemistry and molecularly imprinted membranes[J]. Membranes, 2022, 12(5): 472-505. |
| 39 | BEATRIZ F C, ALEX D B, SOLEDAD C. Molecularly imprinted polymer micro- and nano-particles: A review[J]. Molecules, 2020, 25(20): 4740-4762. |
| 40 | ROUHANI S, NAHAVANDIFARD F. Molecular imprinting-based fluorescent optosensor using a polymerizable 1,8-naphthalimide dye as a florescence functional monomer[J]. Sensors and Actuators B-Chemical, 2014, 197: 185-192. |
| 41 | WANG Jinfang, ZHOU Liangmo, LIU Xueliang, et al. Effect of functional monomer on chiral separation ability of molecular imprinted chiral stationary phase[J]. Acta Chimica Sinica, 2000, 58(3): 351-355. |
| 42 | HASANAH A N, SAFITRI N, ZULFA A, et al. Factors affecting preparation of molecularly imprinted polymer and methods on finding template-monomer interaction as the key of selective properties of the materials[J]. Molecules, 2021, 26(18): 5612-5634. |
| 43 | LI Runfa, FENG Yonghai, PAN Guoqing, et al. Advances in molecularly imprinting technology for bioanalytical applications[J]. Sensors, 2019, 19(1): 177-211. |
| 44 | MONDAL P, SAMANTA N S, MEGHNANI V, et al. Selective glucose permeability in presence of various salts through tunable pore size of pH responsive PVDF-co-HFP membrane[J]. Separation and Purification Technology, 2019, 221: 249-260. |
| 45 | JANTARAT C, ATTAKITMONGKOL K, NICHSAPA S, et al. Molecularly imprinted bacterial cellulose for sustained-release delivery of quercetin[J]. Journal of Biomaterials Science-Polymer Edition, 2020, 31(15): 1961-1976. |
| 46 | LAH N F C, AHMAD A L, AMRI M H M, et al. Configuration of molecularly imprinted polymers for specific uptake of pharmaceutical in aqueous media through radical polymerization method[J]. Journal of Polymer Research, 2022, 29(2): 41-55. |
| 47 | GONG Jinlong, LIPOMI D J, DENG Jiangdong, et al. Micro-and nanopatterning of inorganic and polymeric substrates by indentation lithography[J]. Nano letters, 2010, 10(7): 2702-2708. |
| 48 | QIN Dong, XIA Younan, WHITESIDES G M. Soft lithography for micro-and nanoscale patterning[J]. Nature Protocols, 2010, 5(3): 491. |
| 49 | GAVRILA A M, IORDACHE T V, SANDU T, et al. Molecularly imprinted polymer pearls obtained by phase inversion for the selective recognition of hypericin[J]. Materiale Plastice, 2019, 56(2): 315-320. |
| 50 | DINC M, ESEN C, MIZAIKOFF B. Recent advances on core-shell magnetic molecularly imprinted polymers for biomacromolecules[J]. Trac-Trends in Analytical Chemistry, 2019, 114: 202-217. |
| 51 | LATIF U, MUJAHID A, ZAHID M, et al. Nanostructured molecularly imprinted photonic polymers for sensing applications[J]. Current Nanoscience, 2020, 16(4): 495-503. |
| 52 | LIU W F, HOLDSWORTH C, YE L. Synthesis of molecularly imprinted polymers using a functionalized initiator for chiral-selective recognition of propranolol[J]. Chirality, 2020, 32(3): 370-377. |
| 53 | DOAA R, MOHAMED G A, FARGHALI A A, et al. Strategies for molecular imprinting and the evolution of MIP nanoparticles as plastic antibodies—Synthesis and applications[J]. International Journal of Molecular Sciences, 2019, 20(24): 6304-6325. |
| 54 | BODOKI A E, IACOB B C, GLIGA L E, et al. Improved enantioselectivity for atenolol employing pivot based molecular imprinting[J]. Molecules, 2018, 23(8): 1875-1895. |
| 55 | LAH N F C, AHMAD A L, LOW S C, et al. The role of porogen-polymer complexation in atrazine imprinted polymer to work as an electrochemical sensor in water[J]. Journal of Environmental Chemical Engineering, 2019, 7(6): 103500-103508. |
| 56 | LIU Benchi, MA Yurong, ZHOU Fei, et al. Voltammetric determination of sulfadiazine based on molecular imprinted electrochemical sensor[J]. International Journal of Electrochemical Science, 2020, 15(10): 9590-9596. |
| 57 | CIMEN D. Testosterone Imprinted poly(HEMA-MAA) Nanoparticles based surface plasmon resonance sensor for detection of testosterone[J]. Chemistryselect, 2022, 7(5): e202103949. |
| 58 | OZKAN A, ATAR N, Yola M L. Enhanced surface plasmon resonance (SPR) signals based on immobilization of core-shell nanoparticles incorporated boron nitride nanosheets: Development of molecularly imprinted SPR nanosensor for anticancer drug, etoposide[J]. Biosensors & Bioelectronics, 2019, 130: 293-298. |
| 59 | TOPCU A A, OZGUR E, YILMAZ F, et al. Real time monitoring and label free creatinine detection with artificial receptors[J]. Materials Science and Engineering B: Advanced Functional Solid-State Materials, 2019, 244: 6-11. |
| 60 | AHMAD O S, BEDWELL T S, ESEN C, et al. Molecularly imprinted polymers in electrochemical and optical sensors[J]. Trends in Biotechnology, 2019, 37(3): 294-309. |
| 61 | FANG Ling, JIA Mingxuan, ZHAO Haiping, et al. Molecularly imprinted polymer-based optical sensors for pesticides in foods: Recent advances and future trends[J]. Trends in Food Science & Technology, 2021, 116: 387-404. |
| 62 | ZHANG Xin, YANG Shu, JIANG Rui, et al. Fluorescent molecularly imprinted membranes as biosensor for the detection of target protein[J]. Sensors and Actuators B: Chemical, 2018, 254: 1078-1086. |
| 63 | LIANG Axin, TANG Bo, HOU Huipeng, et al. A novel CuFe2O4 nanospheres molecularly imprinted polymers modified electrochemical sensor for lysozyme determination[J]. Journal of Electroanalytical Chemistry, 2019, 853: 113465. |
| 64 | LIANG Axin, HOU Huipeng, TANG Shanshan, et al. An advanced molecularly imprinted electrochemical sensor for the highly sensitive and selective detection and determination of human IgG[J]. Bioelectrochemistry, 2021, 137: 107671. |
| 65 | KHALIL A, AHMAD Z, TOUATI F, et al. N719-Dye based electrochemical light and temperature sensor[J]. International Journal of Electrochemical Science, 2020, 15(1): 311-318. |
| 66 | SUI Y, ZORMAN C A. Review-inkjet printing of metal structures for electrochemical sensor applications[J]. Journal of the Electrochemical Society, 2020, 167(3): doi:10.1149/1945-7111/ab72H. |
| 67 | JALALVAND A R, ZANGENEH M M, JALILI F, et al. An elegant technology for ultrasensitive impedimetric and voltammetric determination of cholestanol based on a novel molecularly imprinted electrochemical sensor[J]. Chemistry and Physics of Lipids, 2020, 229: 104895-104903. |
| 68 | LIU Yanrui, WEI Meiting, HU Yue, et al. An electrochemical sensor based on a molecularly imprinted polymer for determination of anticancer drug Mitoxantrone[J]. Sensors and Actuators B: Chemical, 2018, 255: 544-551. |
| 69 | ZHOU Tingting, FENG Yaqian, ZHOU Linxi, et al. Selective and sensitive detection of tetrabromobisphenol-A in water samples by molecularly imprinted electrochemical sensor[J]. Sensors and Actuators B—Chemical, 2016, 236: 153-162. |
| 70 | EDDIN F B K, FEN Y W. The principle of nanomaterials based surface plasmon resonance biosensors and its potential for dopamine detection[J]. Molecules, 2020, 25(12): 2769-2789. |
| 71 | CAMARCA A, VARRIALE A, CAPO A, et al. Emergent biosensing technologies based on fluorescence spectroscopy and surface plasmon resonance[J]. Sensors, 2021, 21(3): 906-940. |
| 72 | CHOI Jinha, LEE Jinho, Joohyung SON, et al. Noble metal-assisted surface plasmon resonance immunosensors[J]. Sensors, 2020, 20(4): 1003-1022. |
| 73 | SHAH K, SHARMA N K. Theoretical study on fiber optic SPR sensor using indium nitride[J]. Indian Journal of Physics, 2022, 96(1): 275-279. |
| 74 | ARCADIO F, ZENI L G, PERRI C, et al. Bovine serum albumin protein detection by a removable SPR chip combined with a specific MIP receptor[J]. Chemosensors, 2021, 9(8): 218-227. |
| 75 | BAKHSHPOUR M, GÖKTÜRK I, BERELI N, et al. Selective detection of penicillin g antibiotic in milk by molecularly imprinted polymer-based plasmonic SPR sensor[J]. Biomimetics, 2021, 6(4): 72-87. |
| 76 | CHIU Nanfu, TAI Mingjung, DEVI T N, et al. Immunoassay-amplified responses using a functionalized mos2-based spr biosensor to detect PAPP-A2 in maternal serum samples to screen for fetal down’s syndrome[J]. Int. J. Nanomedicine, 2021, 16: 2715-2733. |
| 77 | RAO Xing, ZHAO Lin, XU Lukui, et al. Review of optical humidity sensors[J]. Sensors, 2021, 21(23): 8049-8111. |
| 78 | MORADI A, SRINIVASAN S, CLEMENTS J, et al. Beyond the biomarker role: Prostate-specific antigen (PSA) in the prostate cancer microenvironment[J]. Cancer and Metastasis Reviews, 2019, 38(3): 333-346. |
| 79 | YAZDANI Z, YADEGARI H, HELI H. A molecularly imprinted electrochemical nanobiosensor for prostate specific antigen determination[J]. Analytical Biochemistry, 2019, 566: 116-125. |
| 80 | PUNDIR C S, DESWAl R, KUMAR P. Quantitative analysis of sarcosine with special emphasis on biosensors: A review[J]. Biomarkers, 2019, 24(5): 415-422. |
| 81 | SHEYDAEI O, KHAJEHSHARIFI H, RAJABI H R. Rapid and selective diagnose of Sarcosine in urine samples as prostate cancer biomarker by mesoporous imprinted polymeric nanobeads modified electrode[J]. Sensors and Actuators B-Chemical, 2020, 309: 127559. |
| 82 | TANG Pingping, WANG Yaobin, HE Feiyu. Electrochemical sensor based on super-magnetic metal–organic framework@ molecularly imprinted polymer for Sarcosine detection in urine[J]. Journal of Saudi Chemical Society, 2020, 24(8): 620-630. |
| 83 | PERNAS S, TOLANEY S M. Targeting HER2 heterogeneity in early-stage breast cancer[J]. Current Opinion in Oncology, 2020, 32(6): 545-554. |
| 84 | PACHECO J G, REBELO P, FREITAS M, et al. Breast cancer biomarker (HER2-ECD) detection using a molecularly imprinted electrochemical sensor[J]. Sensors and Actuators B-Chemical, 2018, 273: 1008-1014. |
| 85 | HING J X, MOK C W, TAN P T, et al. Clinical utility of tumour marker velocity of cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) in breast cancer surveillance[J]. Breast, 2020, 52: 95-101. |
| 86 | PACHECO J G, SILVA M S, FREITAS M, et al. Molecularly imprinted electrochemical sensor for the point-of-care detection of a breast cancer biomarker (CA 15-3) [J]. Sensors and Actuators B-Chemical, 2018, 256: 905-912. |
| 87 | SANTOS A R T, MOREIRA F T C, HELGUERO Luísa A, et al. Antibody biomimetic material made of pyrrole for CA 15-3 and its application as sensing material in ion-selective electrodes for potentiometric detection[J]. Biosensors, 2018, 8(1): 8. |
| 88 | CLEVERS M R, KASTELIJN E A, PETERS B J, et al. Molecular biopsy of human tumors[J]. Anticancer Research, 2021, 41(2): 869-876. |
| 89 | JIANG Jingjing, JI Lixia, YANG Zang, et al. Electrochemistry/photoelectrochemistry-based immunosensing and aptasensing of carcinoembryonic antigen[J]. Sensors, 2021, 21(22): 7742-7765. |
| 90 | CARNEIRO L P T, FERREIRA N S, TAVARES A P, et al. A passive direct methanol fuel cell as transducer of an electrochemical sensor, applied to the detection of carcinoembryonic antigen[J]. Biosensors and Bioelectronics, 2021, 175: 112877. |
| 91 | MOREIRA F T, SALES M G F. Autonomous biosensing device merged with photovoltaic technology for cancer biomarker detection[J]. Journal of Electroanalytical Chemistry, 2019, 855: 113611. |
| 92 | QI Ji, LI Bowei, ZHOU Na, et al. The strategy of antibody-free biomarker analysis by in-situ synthesized molecularly imprinted polymers on movable valve paper-based device[J]. Biosensors and Bioelectronics, 2019, 142: 111533. |
| 93 | JANIK E, BARTOS M, NIEMCEWICZ M, et al. SARS-CoV-2: Outline, prevention, and decontamination[J]. Pathogens, 2021, 10(2): 114-129. |
| 94 | KANAKAN A, MISHRA N, VASUDEVAN J S, et al. Threading the pieces together: integrative perspective on SARS-CoV-2[J]. Pathogens, 2020, 9(11): 912-944. |
| 95 | SAGNELLI C, CELIA B, MONARI C, et al. Management of SARS-CoV-2 pneumonia[J]. Journal of Medical Virology, 2021, 93(3): 1276-1287. |
| 96 | SINGH J, PANDIT P, MCARTHUR A G, et al. Evolutionary trajectory of SARS-CoV-2 and emerging variants[J]. Virology Journal, 2021, 18(1):166-187. |
| 97 | RAZIQ A, KIDAKOVA A, BOROZNJAK R, et al. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen[J]. Biosensors and Bioelectronics, 2021, 178: 113029. |
| 98 | ERTÜRK G, ÖZEN H, TÜMER M A, et al. Microcontact imprinting based surface plasmon resonance (SPR) biosensor for real-time and ultrasensitive detection of prostate specific antigen (PSA) from clinical samples[J]. Sensors and Actuators B-Chemical, 2016, 224: 823-832. |
| 99 | MATSUMOTO H, SUNAYAMA H, KITAYAMA Y, et al. Site-specific post-imprinting modification of molecularly imprinted polymer nanocavities with a modifiable functional monomer for prostate cancer biomarker recognition[J]. Science and Technology of Advanced Materials, 2019, 20(1): 305-312. |
| 100 | BAHARI D, BABAMIRI B, SALIMI A. Ultrasensitive molecularly imprinted fluorescence sensor for simultaneous determination of CA125 and CA15-3 in human serum and OVCAR-3 and MCF-7 cells lines using Cd and Ni nanoclusters as new emitters[J]. Analytical and Bioanalytical Chemistry, 2021, 413(15): 4049-4061. |
| 101 | LUO Ping, WU Sanyun, YU Yalan, et al. Current status and perspective biomarkers in AFP negative HCC: towards screening for and diagnosing hepatocellular carcinoma at an earlier stage[J]. Pathology & Oncology Research, 2020, 26(2): 599-603. |
| 102 | WANG Ting, ZHANG Kunhe. New blood biomarkers for the diagnosis of AFP-negative hepatocellular carcinoma[J]. Frontiers in Oncology, 2020, 10: 1316. |
| 103 | KAWAHARA I, FUKUZAWA H, URUSHIHARA N. AFP-L3 as a prognostic predictor of recurrence in hepatoblastoma: A pilot study[J]. Pediatric Hematology Oncology, 2021, 43: e76-e79. |
| 104 | BO Lin, XU Dong, WANG Qiujiao, et al. AFP-inhibiting fragments for drug delivery: the promise and challenges of targeting therapeutics to cancers[J]. Frontiers in Cell and Developmental Biology, 2021, 9: doi:10.3389/fcell.2021.635476. |
| 105 | LAI Yuxuan, ZHANG Chuanxiang, DENG Yan, et al. A novel α-fetoprotein-MIP immunosensor based on AuNPs/PTh modified glass carbon electrode[J]. Chinese Chemical Letters, 2019, 30(1): 160-162. |
| 106 | TAWFIK S M, ELMASRY M R, SHARIPOV M, et al. Dual emission nonionic molecular imprinting conjugated polythiophenes-based paper devices and their nanofibers for point-of-care biomarkers detection[J]. Biosensors and Bioelectronics, 2020, 160: 112211. |
| 107 | MA Runtian, ZHAO Xiaobo, SUN Xiaoyu, et al. A fluorescent molecularly imprinted device for the on-line analysis of AFP in human serum[J]. Journal of Materials Chemistry B, 2019, 7(40): 6187-6194. |
| 108 | CHEN Lina, SUN Chenghong, PAN Linli, et al. A biomimetic fluorescent nanosensor based on imprinted polymers modified with carbon dots for sensitive detection of alpha-fetoprotein in clinical samples[J]. Analyst, 2019, 144: 6760-6772. |
| 109 | JING H, HETTICH M, GAEDICKE S, et al. Combination treatment with hypofractionated radiotherapy plus IL-2/anti-IL-2 complexes and its theranostic evaluation[J]. Journal for Immunotherapy of Cancer, 2019, 7: 55-71. |
| 110 | PILOTO A M L, RIBEIRO D S, RODRIGUES S S M, et al. Label-free quantum dot conjugates for human protein IL-2 based on molecularly imprinted polymers[J]. Sensors and Actuators B-Chemical, 2020, 304: 127343. |
| 111 | REBECCA L S, KIMBERLY D M, HANNAH E F, et al. Cancer statistics[J]. A Cancer Journal for Clinicians, 2022, 72(1): 7-33. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [4] | 雷伟, 姜维佳, 王玉高, 和明豪, 申峻. N、S共掺杂煤基碳量子点的电化学氧化法制备及用于Fe3+检测[J]. 化工进展, 2023, 42(9): 4799-4807. |
| [5] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [6] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [7] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [8] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [9] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [10] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [11] | 秦凯, 杨仕林, 李俊, 储震宇, 薄翠梅. 基于卡尔曼滤波算法的葡萄糖酶生物传感器高精度检测方法[J]. 化工进展, 2023, 42(6): 3177-3186. |
| [12] | 张鹏, 潘原. 单原子催化剂在电催化氧还原直接合成过氧化氢中的研究进展[J]. 化工进展, 2023, 42(6): 2944-2953. |
| [13] | 陈少华, 王义华, 胡强飞, 胡坤, 陈立爱, 李洁. 电化学修饰电极在检测Cr(Ⅵ)中的研究进展[J]. 化工进展, 2023, 42(5): 2429-2438. |
| [14] | 李华华, 李逸航, 金北辰, 李隆昕, 成少安. 厌氧氨氧化-生物电化学耦合废水处理系统的研究进展[J]. 化工进展, 2023, 42(5): 2678-2690. |
| [15] | 郭朋举, 何小波, 银凤翔. 电催化氮还原合成氨MOF基催化剂研究进展[J]. 化工进展, 2023, 42(4): 1797-1810. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||