化工进展 ›› 2022, Vol. 41 ›› Issue (6): 3314-3323.DOI: 10.16085/j.issn.1000-6613.2021-2363
焦粉高效活化过硫酸盐对苯胺的降解性能
- 1.中国矿业大学资源与地球科学学院,江苏 徐州 221116
2.中国矿业大学国家煤加工与洁净化工程技术研究中心,江苏 徐州 221116
-
收稿日期:2021-11-17修回日期:2021-12-14出版日期:2022-06-10发布日期:2022-06-21 -
通讯作者:徐天缘 -
作者简介:徐天缘(1988—),女,副教授,硕士生导师,研究方向为煤系固废资源化利用。E-mail:xutianyuan@cumt.edu.cn 。 -
基金资助:国家自然科学基金(21806050);江苏省“双创博士”基金(JSSCBS20211232)
Persulfate activation by coke powder for aniline degradation
XU Tianyuan1( ), ZHENG Xi2, WANG Lianjuan2, CHEN Ting2, WEI Xinpeng2
), ZHENG Xi2, WANG Lianjuan2, CHEN Ting2, WEI Xinpeng2
- 1.School of Resource and Geosciences, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
2.National Engineering Research Center of Coal Preparation and Purification, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
-
Received:2021-11-17Revised:2021-12-14Online:2022-06-10Published:2022-06-21 -
Contact:XU Tianyuan
摘要:
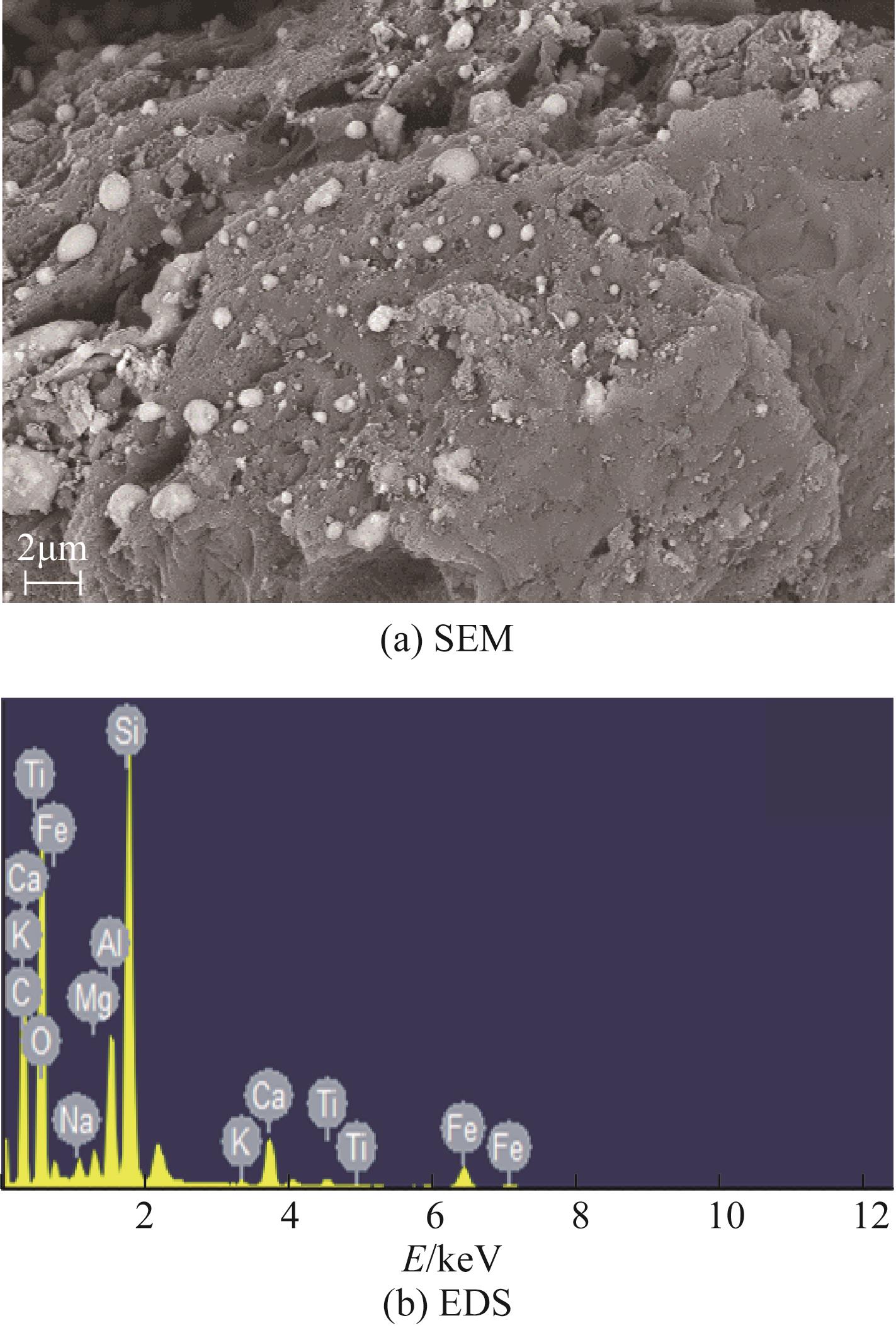
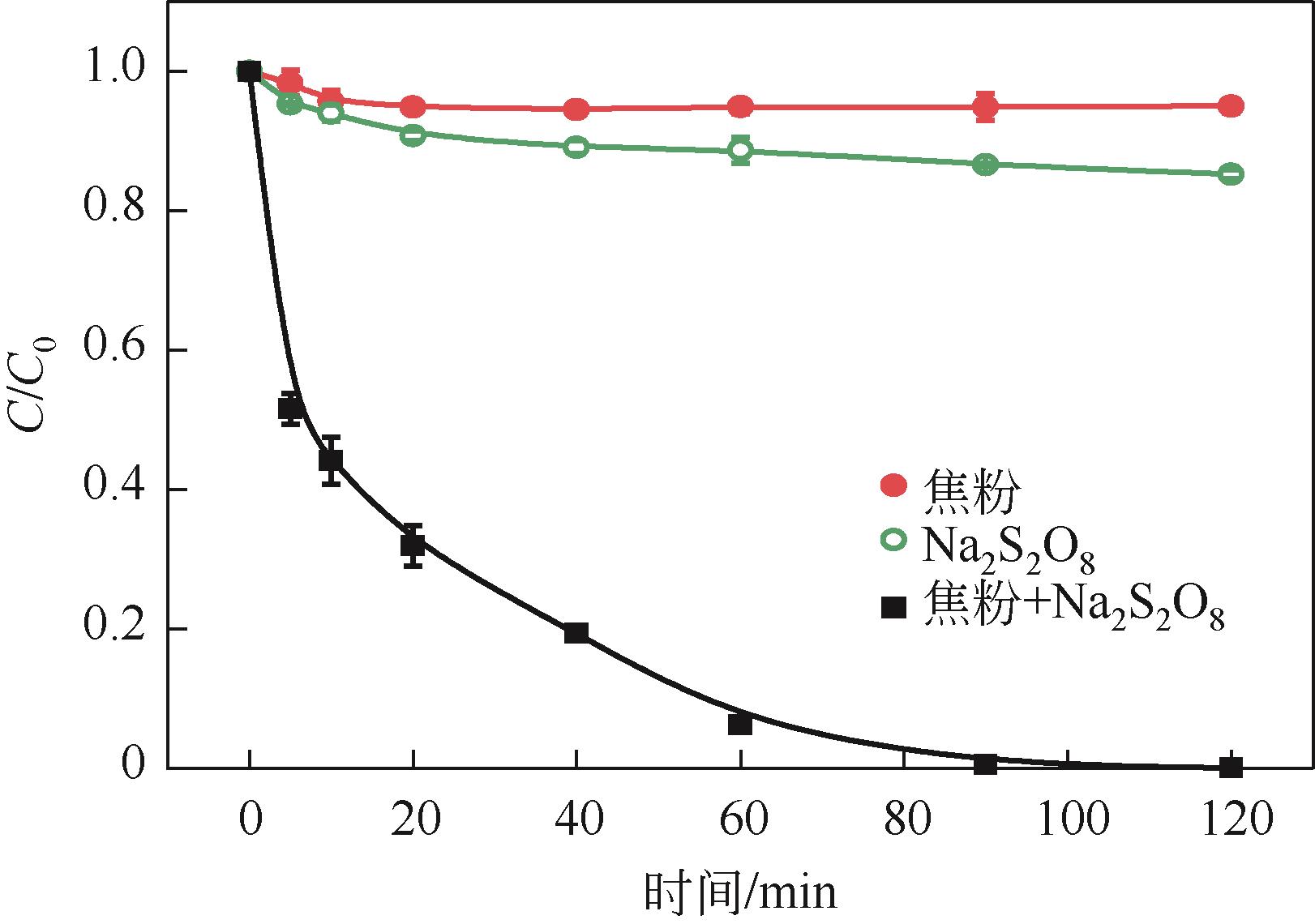
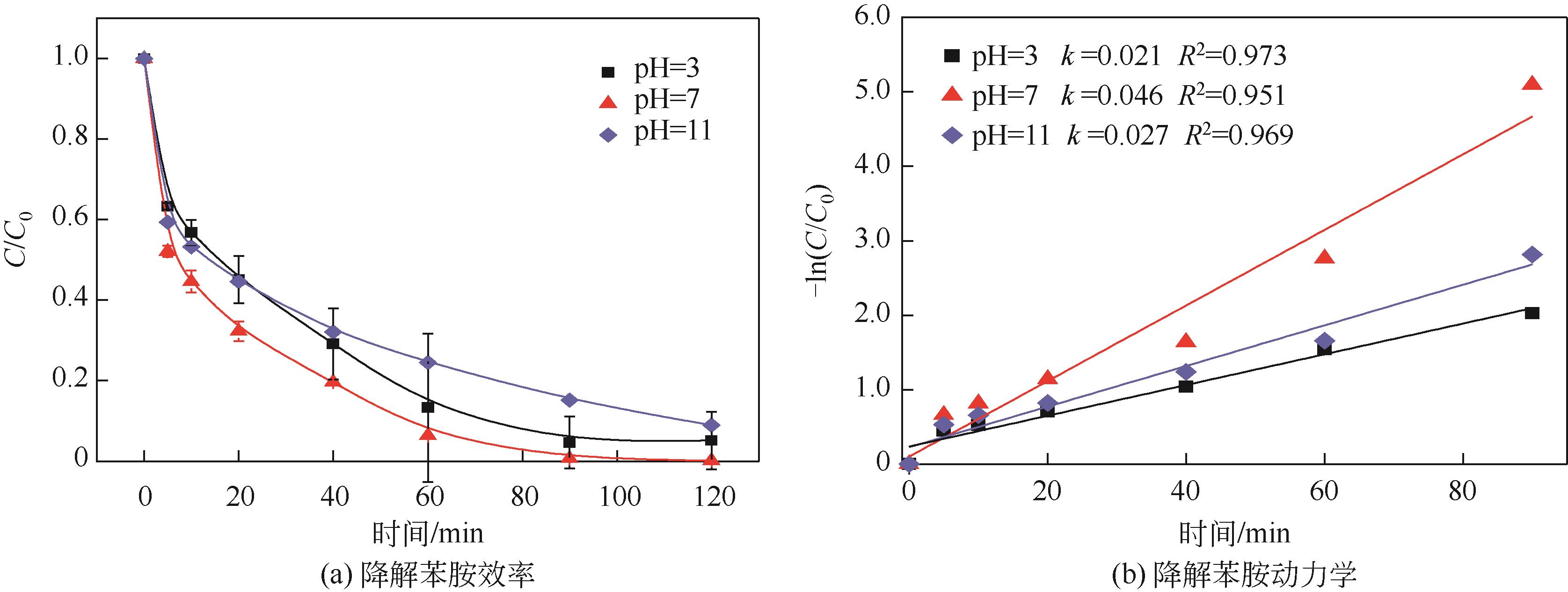
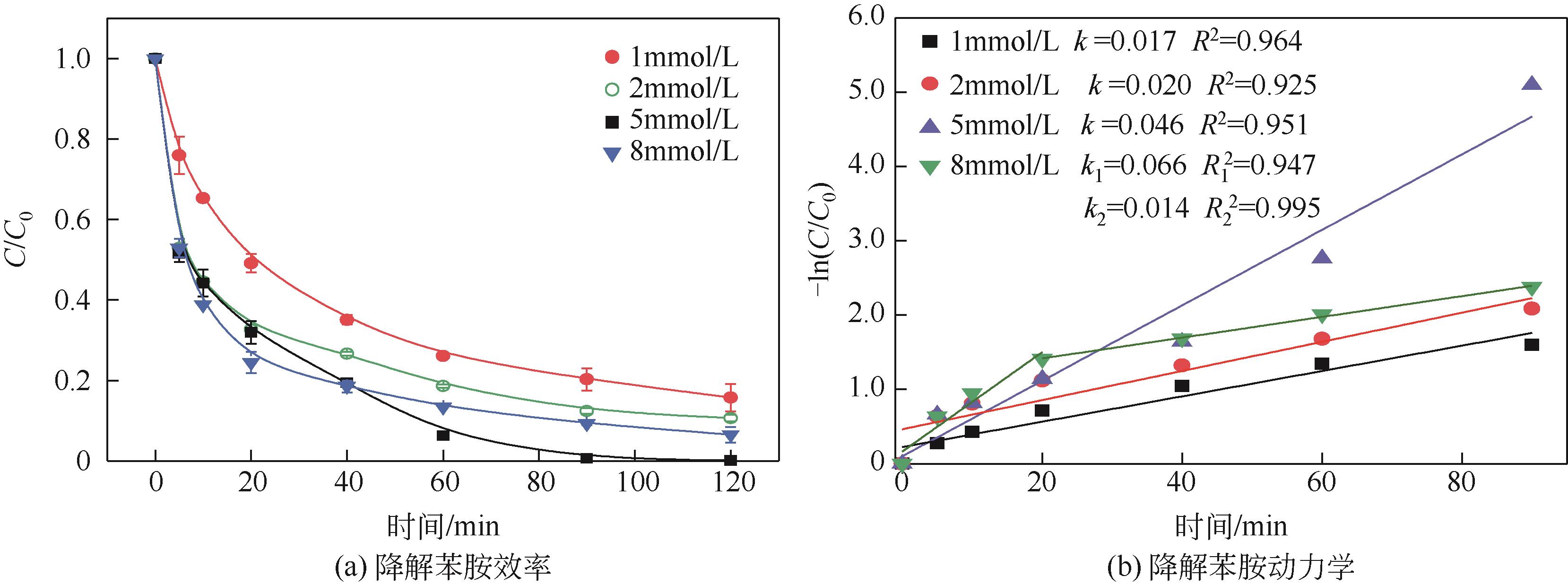
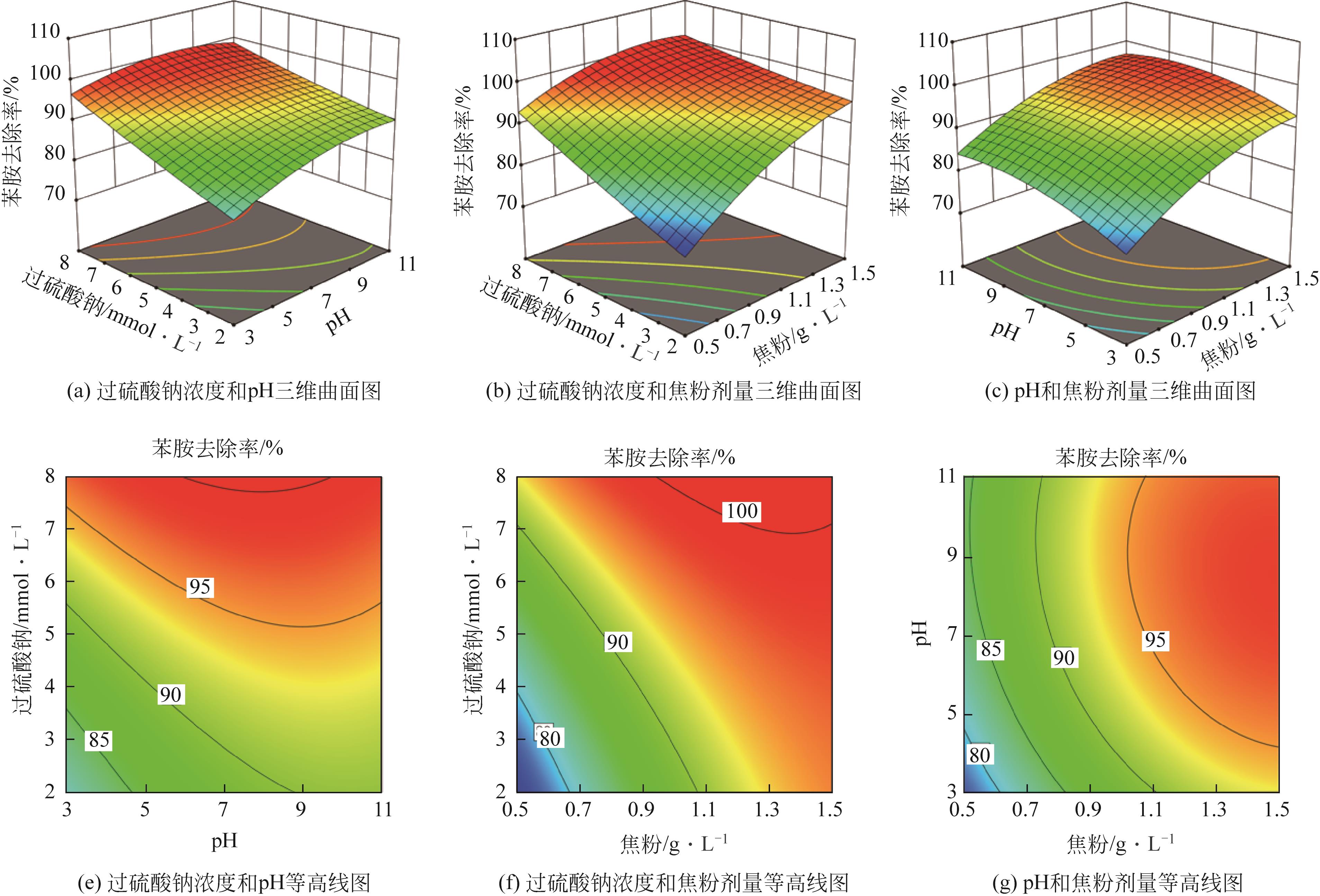
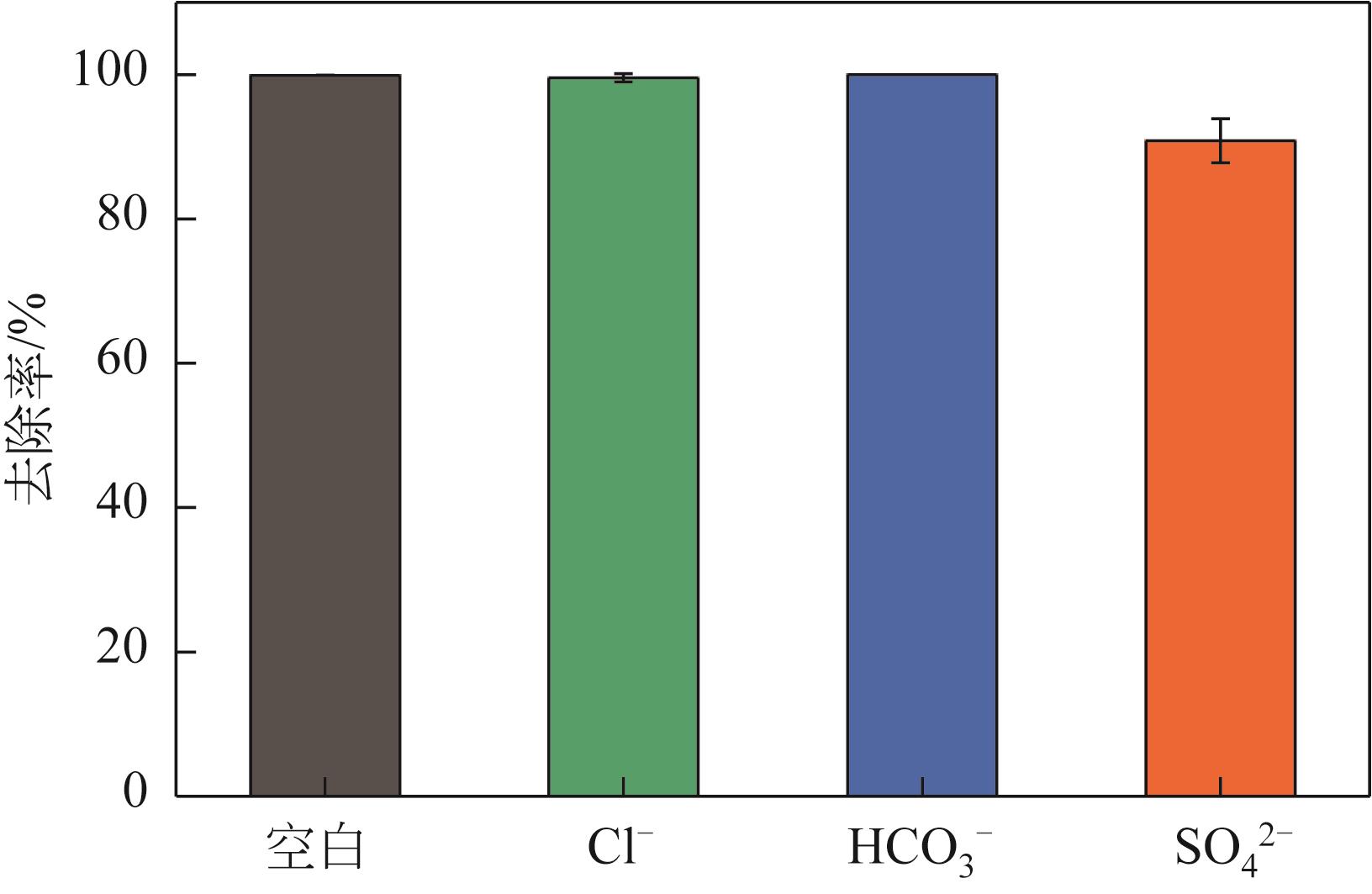
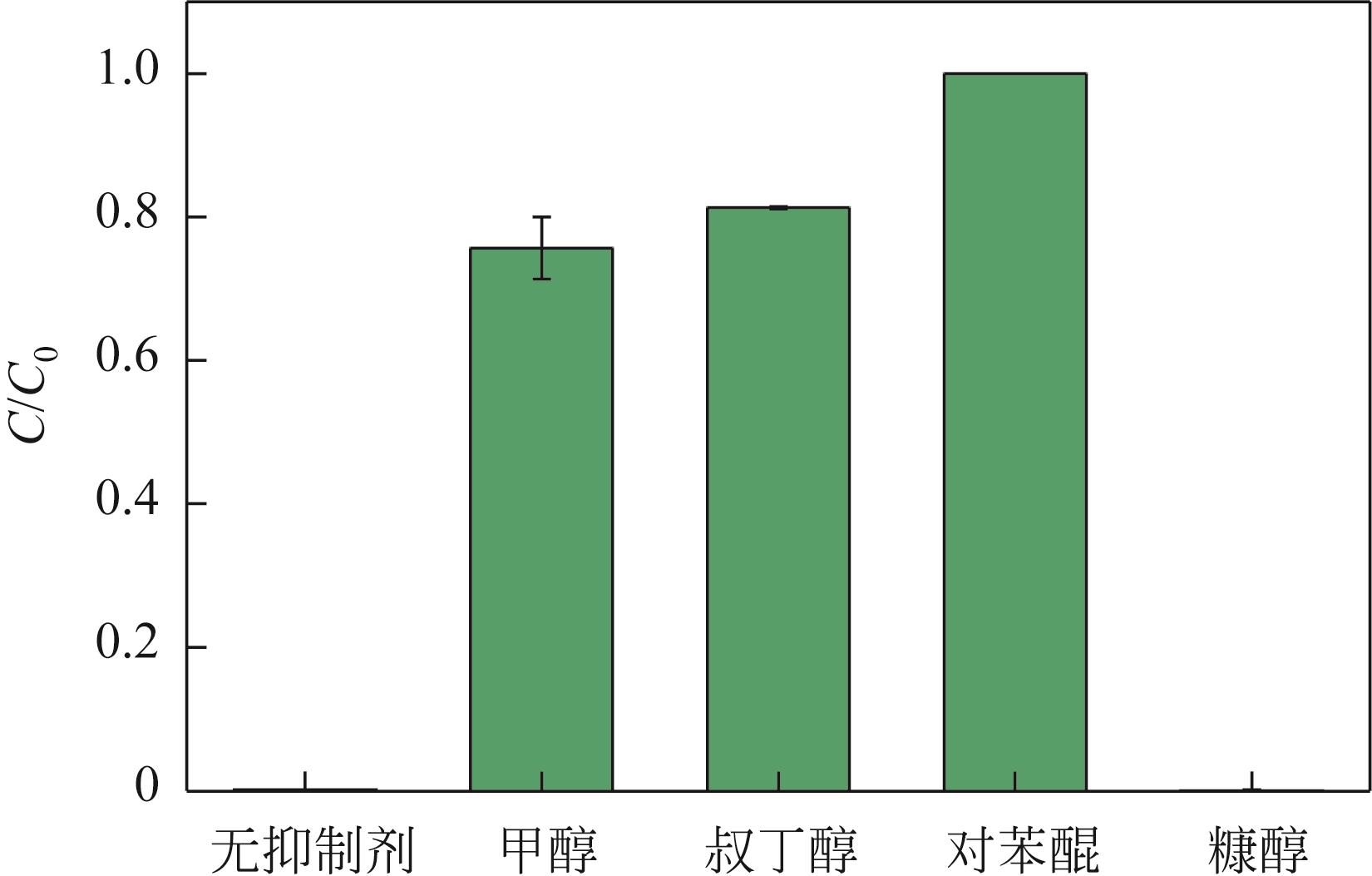
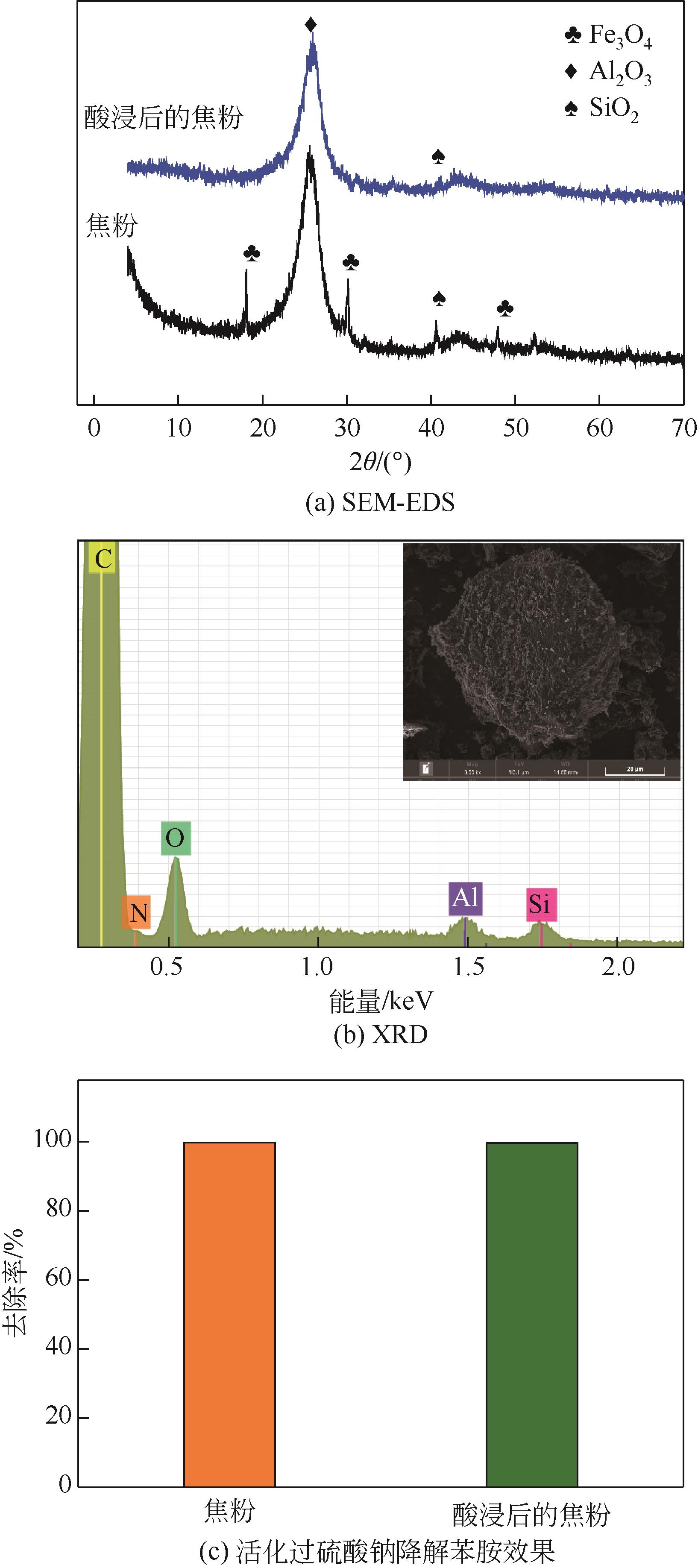
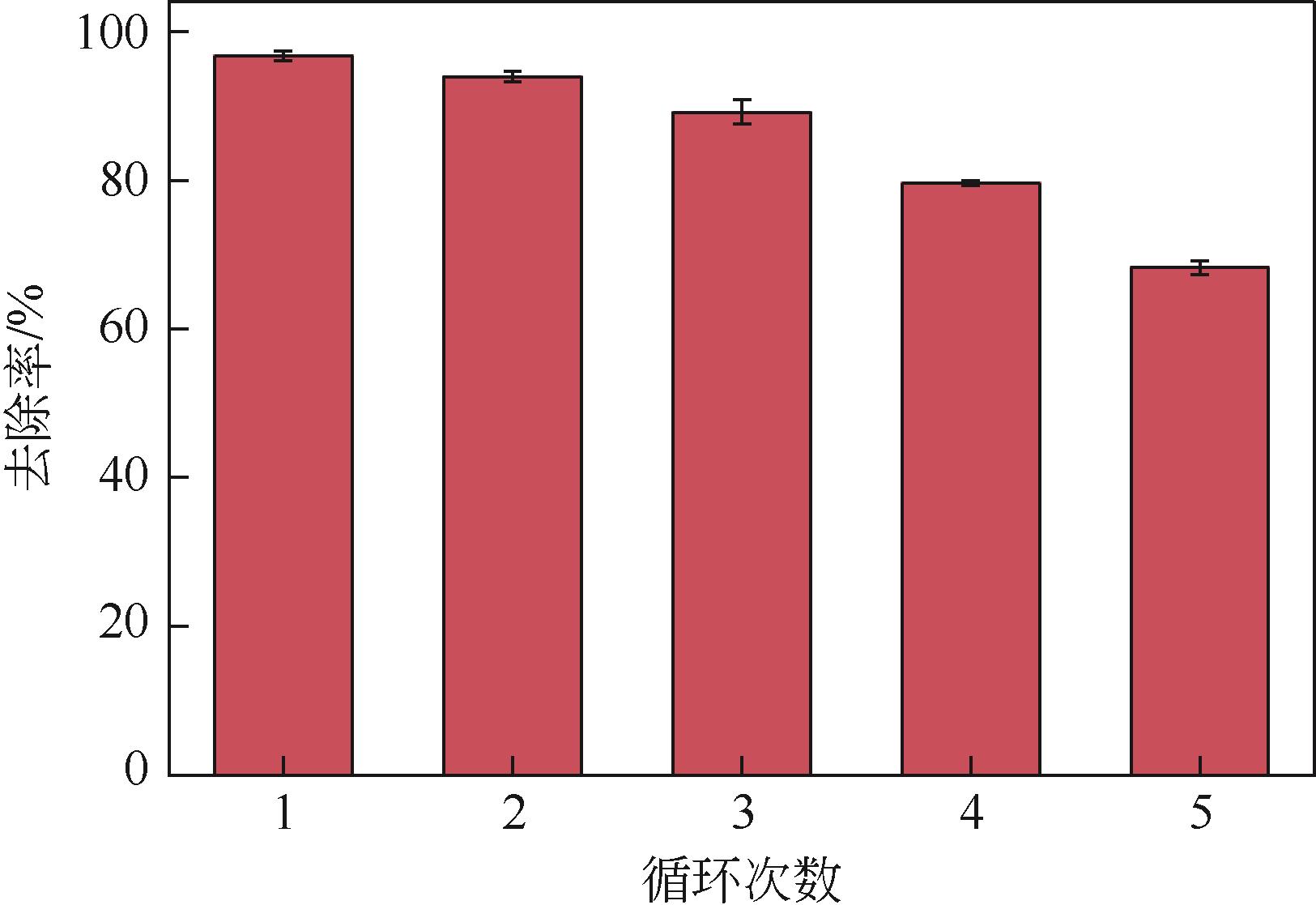
焦粉作为焦化/煤化企业的低附加值副产物,用作污染控制材料是其高价值应用的一条重要途径。本文对焦粉形貌与成分进行表征分析,通过苯胺降解动力学和影响因素之间的交互作用评估焦粉活化过硫酸盐的催化性能,并初步探讨了相关机理。表征结果显示焦粉表面粗糙,有清晰的孔隙。活性测试结果显示,1g/L焦粉可以高效活化5mmol/L过硫酸盐降解20mg/L苯胺,反应120min后苯胺去除率高于99%,其中灰分对苯胺降解无贡献。增加焦粉剂量和过硫酸盐浓度均可提高苯胺的降解效率。焦粉在pH 3~11范围内均可高效活化过硫酸盐降解苯胺,去除率维持在83%以上。此外,焦粉具有应用于修复复杂水体中有机污染物的潜能,在Cl-、HCO
中图分类号:
引用本文
徐天缘, 郑茜, 王连娟, 陈婷, 魏鑫鹏. 焦粉高效活化过硫酸盐对苯胺的降解性能[J]. 化工进展, 2022, 41(6): 3314-3323.
XU Tianyuan, ZHENG Xi, WANG Lianjuan, CHEN Ting, WEI Xinpeng. Persulfate activation by coke powder for aniline degradation[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3314-3323.
| 序号 | 代码 | 因素 | 水平 | ||
|---|---|---|---|---|---|
| -1 | 0 | 1 | |||
| 1 | X1 | 焦粉/g·L-1 | 0.5 | 1 | 1.5 |
| 2 | X2 | pH | 3 | 7 | 11 |
| 3 | X3 | 过硫酸钠/mmol·L-1 | 2 | 5 | 8 |
表1 BBD设计因素与代码
| 序号 | 代码 | 因素 | 水平 | ||
|---|---|---|---|---|---|
| -1 | 0 | 1 | |||
| 1 | X1 | 焦粉/g·L-1 | 0.5 | 1 | 1.5 |
| 2 | X2 | pH | 3 | 7 | 11 |
| 3 | X3 | 过硫酸钠/mmol·L-1 | 2 | 5 | 8 |
| 元素 | 质量分数/% | 摩尔分数/% |
|---|---|---|
| C | 35.66 | 48.06 |
| O | 38.37 | 38.82 |
| Si | 12.20 | 7.03 |
| Al | 3.72 | 2.23 |
| Fe | 4.82 | 1.40 |
| Ca | 2.97 | 1.20 |
| Mg | 0.78 | 0.52 |
| Na | 0.63 | 0.44 |
| Ti | 0.64 | 0.22 |
| K | 0.21 | 0.09 |
表2 焦粉的元素组成
| 元素 | 质量分数/% | 摩尔分数/% |
|---|---|---|
| C | 35.66 | 48.06 |
| O | 38.37 | 38.82 |
| Si | 12.20 | 7.03 |
| Al | 3.72 | 2.23 |
| Fe | 4.82 | 1.40 |
| Ca | 2.97 | 1.20 |
| Mg | 0.78 | 0.52 |
| Na | 0.63 | 0.44 |
| Ti | 0.64 | 0.22 |
| K | 0.21 | 0.09 |
| 方差来源 | 平方和 | 自由度 | 均方 | F | P>F |
|---|---|---|---|---|---|
| Model | 923.03 | 9 | 102.56 | 24.63 | 0.0002 |
| A | 425.34 | 1 | 425.34 | 102.16 | < 0.0001 |
| B | 65.25 | 1 | 65.25 | 15.67 | 0.0055 |
| C | 287.98 | 1 | 287.98 | 69.17 | < 0.0001 |
| AB | 3.12 | 1 | 3.12 | 0.7503 | 0.4151 |
| AC | 42.54 | 1 | 42.54 | 10.22 | 0.0151 |
| BC | 11.29 | 1 | 11.29 | 2.71 | 0.1436 |
| A2 | 50.43 | 1 | 50.43 | 12.11 | 0.0103 |
| B2 | 32.47 | 1 | 32.47 | 7.80 | 0.0268 |
| C2 | 1.06 | 1 | 1.06 | 0.2556 | 0.6287 |
| 残差 | 29.14 | 7 | 4.16 | ||
| 拟合不足 | 29.14 | 3 | 9.71 | ||
| 纯误差 | 0 | 4 | 0 | ||
| 总误差 | 952.18 | 16 | |||
| R2= 0.969 |
表3 二次回归模型的方差分析(以苯胺去除率为响应对象)
| 方差来源 | 平方和 | 自由度 | 均方 | F | P>F |
|---|---|---|---|---|---|
| Model | 923.03 | 9 | 102.56 | 24.63 | 0.0002 |
| A | 425.34 | 1 | 425.34 | 102.16 | < 0.0001 |
| B | 65.25 | 1 | 65.25 | 15.67 | 0.0055 |
| C | 287.98 | 1 | 287.98 | 69.17 | < 0.0001 |
| AB | 3.12 | 1 | 3.12 | 0.7503 | 0.4151 |
| AC | 42.54 | 1 | 42.54 | 10.22 | 0.0151 |
| BC | 11.29 | 1 | 11.29 | 2.71 | 0.1436 |
| A2 | 50.43 | 1 | 50.43 | 12.11 | 0.0103 |
| B2 | 32.47 | 1 | 32.47 | 7.80 | 0.0268 |
| C2 | 1.06 | 1 | 1.06 | 0.2556 | 0.6287 |
| 残差 | 29.14 | 7 | 4.16 | ||
| 拟合不足 | 29.14 | 3 | 9.71 | ||
| 纯误差 | 0 | 4 | 0 | ||
| 总误差 | 952.18 | 16 | |||
| R2= 0.969 |
| 1 | 武恒平, 韦朝海, 任源, 等. 焦化废水预处理及其特征污染物的变化分析[J]. 化工进展, 2017, 36(10): 3911-3920. |
| WU Hengping, WEI Chaohai, REN Yuan, et al. Analysis of typical pollutants and its removal characteristics in the pretreatment of coking wastewater[J]. Chemical Industry and Engineering Progress, 2017, 36(10): 3911-3920. | |
| 2 | 胡记杰, 肖俊霞, 任源, 等. 焦化废水原水中有机污染物的活性炭吸附过程解析[J]. 环境科学, 2008, 29(6): 1567-1571. |
| HU Jijie, XIAO Junxia, REN Yuan, et al. Adsorption process of organic contaminant in untreated coking wastewater by powdered activated carbon[J]. Environmental Science, 2008, 29(6): 1567-1571. | |
| 3 | MOHAMMED M, MEKALA L P, CHINTALAPATI S, et al. New insights into aniline toxicity: aniline exposure triggers envelope stress and extracellular polymeric substance formation in Rubrivivax benzoatilyticus JA2[J]. Journal of Hazardous Materials, 2020, 385: 121571. |
| 4 | O’SHEA K E, DIONYSIOU D D. Advanced oxidation processes for water treatment[J]. The Journal of Physical Chemistry Letters, 2012, 3(15): 2112-2113. |
| 5 |
张轩, 宋小三, 赵珀, 等. 高级氧化技术处理1,4-二 烷污染研究进展[J]. 化工进展, 2021, 40(S2): 380-388. 烷污染研究进展[J]. 化工进展, 2021, 40(S2): 380-388.
|
| ZHANG Xuan, SONG Xiaosan, ZHAO Po, et al. A critical review of advanced oxidation technology to treat 1,4-dioxane pollution[J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 380-388. | |
| 6 | YANG Shiying, LI Lei, XIAO Tuo, et al. Promoting effect of ammonia modification on activated carbon catalyzed peroxymonosulfate oxidation[J]. Separation and Purification Technology, 2016, 160: 81-88. |
| 7 | 田婷婷, 李朝阳, 王召东, 等. 过渡金属活化过硫酸盐降解有机废水技术研究进展[J]. 化工进展, 2021, 40(6): 3480-3488. |
| TIAN Tingting, LI Chaoyang, WANG Shaodong, et al. Research progress of transition metal activated persulfate to degrade organic wastewater[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3480-3488. | |
| 8 | 孙金龙, 张宇, 刘福跃, 等. 基于碳基催化剂活化过二硫酸盐降解有机污染物的研究进展[J]. 化工进展, 2021, 40(3): 1653-1666. |
| SUN Jinlong, ZHANG Yu, LIU Fuyue, et al. Research progress in degradation of organic pollutants by activation of persulfates with carbon-based catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1653-1666. | |
| 9 | OLMEZ-HANCI T, ARSLAN-ALATON I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol[J]. Chemical Engineering Journal, 2013, 224: 10-16. |
| 10 | MATTA R, TLILI S, CHIRON S, et al. Removal of carbamazepine from urban wastewater by sulfate radical oxidation[J]. Environmental Chemistry Letters, 2011, 9(3): 347-353. |
| 11 | WANG Jianlong, WANG Shizong. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. |
| 12 | LIU H Z, BRUTON T A, LI W, et al. Oxidation of benzene by persulfate in the presence of Fe(Ⅲ)- and Mn(Ⅳ)-containing oxides: stoichiometric efficiency and transformation products[J]. Environmental Science & Technology, 2016, 50(2): 890-898. |
| 13 | CHEN Hao, CARROLL K C. Metal-free catalysis of persulfate activation and organic-pollutant degradation by nitrogen-doped graphene and aminated graphene[J]. Environmental Pollution, 2016, 215: 96-102. |
| 14 | CHEN Xiao, OH W D, LIM T T. Graphene- and CNTs-based carbocatalysts in persulfates activation: material design and catalytic mechanisms[J]. Chemical Engineering Journal, 2018, 354: 941-976. |
| 15 | JIANG Lili, ZHANG Ying, ZHOU Minghua, et al. Oxidation of Rhodamine B by persulfate activated with porous carbon aerogel through a non-radical mechanism[J]. Journal of Hazardous Materials, 2018, 358: 53-61. |
| 16 | XIAO Pengfei, AN Lu, WU Dedong. The use of carbon materials in persulfate-based advanced oxidation processes: a review[J]. New Carbon Materials, 2020, 35(6): 667-683. |
| 17 | 姜记威, 张诗轩, 曾文炉, 等. 生物炭基材料在抗生素废水处理中的研究进展[J]. 化工进展, 2021, 40(S2): 389-401. |
| JIANG Jiwei, ZHANG Shixuan, ZENG Wenlu, et al. Research progress on biochar-based materials for the treatment of antibiotic wastewater[J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 389-401. | |
| 18 | JIA Hanzhong, ZHAO Song, ZHU Kecheng, et al. Activate persulfate for catalytic degradation of adsorbed anthracene on coking residues: role of persistent free radicals[J]. Chemical Engineering Journal, 2018, 351: 631-640. |
| 19 | 冯建祥, 陈攀攀, 石朝益, 等. Ni/焦粉催化CO2重整焦炉煤气的研究[J]. 天然气化工(C1化学与化工), 2017, 42(1): 52-57. |
| FENG Jianxiang, CHEN Panpan, SHI Zhaoyi, et al. CO2-COG reforming over Ni/coke powder catalyst[J]. Natural Gas Chemical Industry, 2017, 42(1): 52-57. | |
| 20 | GAO Qieyuan, WANG Lei, LI Zhipeng, et al. Adsorptive removal of pyridine in simulation wastewater using coke powder[J]. Processes, 2019, 7(7): 459. |
| 21 | 唐正, 赵松, 钱雅洁, 等. 生物炭持久性自由基形成机制及环境应用研究进展[J]. 化工进展, 2020, 39(4): 1521-1527. |
| TANG Zheng, ZHAO Song, QIAN Yajie, et al. Formation mechanisms and environmental applications of persistent free radicals in biochar: a review[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1521-1527. | |
| 22 | GHAUCH A, TUQAN A M, KIBBI N. Ibuprofen removal by heated persulfate in aqueous solution: a kinetics study[J]. Chemical Engineering Journal, 2012, 197: 483-492. |
| 23 | GUO Shengpeng, WANG Qing, LUO Chengjie, et al. Hydroxyl radical-based and sulfate radical-based photocatalytic advanced oxidation processes for treatment of refractory organic matter in semi-aerobic aged refuse biofilter effluent arising from treating landfill leachate[J]. Chemosphere, 2020, 243: 125390. |
| 24 | XIA Jinsong, ZHANG Na, CHONG Shaokun, et al. Three-dimensional porous graphene-like sheets synthesized from biocarbon via low-temperature graphitization for a supercapacitor[J]. Green Chemistry, 2018, 20(3): 694-700. |
| 25 | EUGENE A J, GUZMAN M I. Production of singlet oxygen (1O2) during the photochemistry of aqueous pyruvic acid: the effects of pH and photon flux under steady-state O2 (aq) concentration[J]. Environmental Science & Technology, 2019, 53(21): 12425-12432. |
| 26 | OEBBEKE M, SIEFKER C, WAGNER B, et al. Fragment binding to kinase hinge:if charge distribution and local pKa shifts mislead popular bioisosterism concepts[J]. Angewandte Chemie International Edition, 2021, 60(1): 252-258. |
| 27 | SANTOS A, FERNANDEZ J, RODRIGUEZ S, et al. Abatement of chlorinated compounds in groundwater contaminated by HCH wastes using ISCO with alkali activated persulfate[J]. Science of the Total Environment, 2018, 615: 1070-1077. |
| 28 | XU Tianyuan, LIU Yun, GE Fei, et al. Application of response surface methodology for optimization of azocarmine B removal by heterogeneous photo-Fenton process using hydroxy-iron-aluminum pillared bentonite[J]. Applied Surface Science, 2013, 280: 926-932. |
| 29 | YAN Zhiming, GU Yong, WANG Xing, et al. Degradation of aniline by ferrous ions activated persulfate: impacts, mechanisms, and by-products[J]. Chemosphere, 2021, 268: 129237. |
| 30 | ZHAO Yan, ZHAO Yongsheng, LI Qin, et al. Effect of common inorganic ions on aniline degradation in groundwater by activated persulfate with ferrous iron[J]. Water Supply, 2016, 16(3): 667-674. |
| 31 | WANG Jun, LI Bin, LI Yang, et al. Easily regenerated CuO/γ-Al2O3 for persulfate-based catalytic oxidation: insights into the deactivation and regeneration mechanism[J]. ACS Applied Materials & Interfaces, 2021, 13(2): 2630-2641. |
| 32 | DING Su, WAN Jinquan, WANG Yan, et al. Activation of persulfate by molecularly imprinted Fe-MOF-74@SiO2 for the targeted degradation of dimethyl phthalate: effects of operating parameters and chlorine[J]. Chemical Engineering Journal, 2021, 422: 130406. |
| 33 | ZHAO Y S, SUN C, SUN J Q, et al. Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process[J]. Separation and Purification Technology, 2015, 142: 182-188. |
| 34 | CHEN Xiaojing, GUO Yanxia, CUI Jinglei, et al. Activated carbon preparation with the addition of coke-making by-product—coke powder: texture evolution and mechanism[J]. Journal of Cleaner Production, 2019, 237: 117812. |
| 35 | 肖鹏飞, 安璐, 韩爽. 炭质材料在活化过硫酸盐高级氧化技术中的应用进展[J]. 化工进展, 2020, 39(8): 3293-3306. |
| XIAO Pengfei, AN Lu, HAN Shuang. Research advances on applying carbon materials to activate persulfate in advanced oxidation technology[J]. Chemical Industry and Engineering Progress, 2020, 39(8): 3293-3306. | |
| 36 | REN Wei, XIONG Liangliang, YUAN Xuehong, et al. Activation of peroxydisulfate on carbon nanotubes: electron-transfer mechanism[J]. Environmental Science & Technology, 2019, 53(24): 14595-14603. |
| 37 | TANG Lin, LIU Yani, WANG Jiajia, et al. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: electron transfer mechanism[J]. Applied Catalysis B: Environmental, 2018, 231: 1-10. |
| [1] | 王琦, 寇丽红, 王冠宇, 王吉坤, 刘敏, 李兰廷, 王昊. 焦化废水生物出水中可溶解性有机物的分子识别[J]. 化工进展, 2023, 42(9): 4984-4993. |
| [2] | 李坡, 张珊珊, 施锦秋, 高航, 王明新. 活化过硫酸盐修复苯胺污染地下水及其环境风险[J]. 化工进展, 2022, 41(5): 2753-2760. |
| [3] | 齐亚兵. 活化过硫酸盐高级氧化法降解抗生素的研究进展[J]. 化工进展, 2022, 41(12): 6627-6643. |
| [4] | 张轩, 宋小三, 赵珀, 董元华, 刘云. 高级氧化技术处理1,4-二 烷污染研究进展[J]. 化工进展, 2021, 40(S2): 380-388. 烷污染研究进展[J]. 化工进展, 2021, 40(S2): 380-388. |
| [5] | 孙培杰, 王林平, 徐乐瑾. 焦化废水中氰化物的处理技术研究进展[J]. 化工进展, 2021, 40(S1): 386-396. |
| [6] | 袁雨婷, 冯勇超, 易红宏, 唐晓龙, 于庆君, 张媛媛, 隗晶慧, 孟宪政. 体相超疏水材料及其在大气污染控制领域的应用研究进展[J]. 化工进展, 2021, 40(8): 4327-4345. |
| [7] | 杨丙衡, 安路阳, 张立涛, 宋迪慧, 刘合鑫. 中间层为聚吡咯的复合电极深度处理焦化废水[J]. 化工进展, 2020, 39(10): 4256-4267. |
| [8] | 陈聪, 刘建忠, 徐发锐. 焦化废水制备水煤浆的成浆性能[J]. 化工进展, 2019, 38(06): 2986-2991. |
| [9] | 黄智辉, 纪志永, 陈希, 郭小甫, 王士钊, 袁俊生. 过硫酸盐高级氧化降解水体中有机污染物研究进展[J]. 化工进展, 2019, 38(05): 2461-2470. |
| [10] | 王丰, 孔巧平, 周红桃, 付炳炳, 吴海珍, 任源, 韦朝海. 炭质吸附剂孔径分布与焦化废水有机组分分离的相关性[J]. 化工进展, 2018, 37(08): 3252-3259. |
| [11] | 张欢, 钟鹭斌, 陈进生, 郑煜铭. 船舶尾气脱硫脱硝技术研究进展[J]. 化工进展, 2016, 35(11): 3650-3657. |
| [12] | 黄欣怡, 张珺婷, 王凡, 贺文智, 李光明. 餐厨垃圾资源化利用及其过程污染控制研究进展[J]. 化工进展, 2016, 35(09): 2945-2951. |
| [13] | 杨 丽1,廖传华2,朱跃钊2,陈海军2,金勤芳1. 微纳米气泡特性及在环境污染控制中的应用[J]. 化工进展, 2012, 31(06): 1333-1337. |
| [14] | 黄会静1,韦朝海1,吴超飞1,冯春华1,吴海珍2,卢 彬3. 焦化废水生物处理A/O/H/O工艺中氰化物的去除特性 [J]. 化工进展, 2011, 30(5): 1141-. |
| [15] | 夏 芳,韦朝海,吴超飞,胡 芸,罗汉金. H2O2/UV流化床氧化焦化废水尾水中惰性成分的可行性考察 [J]. 化工进展, 2011, 30(5): 1135-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||