化工进展 ›› 2022, Vol. 41 ›› Issue (4): 1848-1857.DOI: 10.16085/j.issn.1000-6613.2021-0804
电催化还原二氧化碳制一氧化碳催化剂研究进展
张少阳1( ), 商阳阳1, 赵瑞花1,2, 赵丹丹1, 郭天宇3,4, 杜建平1,4(
), 商阳阳1, 赵瑞花1,2, 赵丹丹1, 郭天宇3,4, 杜建平1,4( ), 李晋平1,4
), 李晋平1,4
- 1.太原理工大学化学化工学院,山西 太原 030024
2.山西昆明烟草有限责任公司,山西 太原 030024
3.太原理工大学环境科学与工程学院,山西 晋中 030600
4.气体能源高效清洁利用山西省重点实验室,山西 太原 030024
-
收稿日期:2021-04-16修回日期:2021-06-27出版日期:2022-04-23发布日期:2022-04-25 -
通讯作者:杜建平 -
作者简介:张少阳(1995—),男,博士研究生,研究方向为纳米催化材料制备及电化学性能。E-mail:651749607@qq.com 。 -
基金资助:国家自然科学基金(51572185);山西省重点研发国际合作项目(201903D421079)
Research progress on catalysts for electrocatalytic reduction of carbon dioxide to carbon monoxide
ZHANG Shaoyang1( ), SHANG Yangyang1, ZHAO Ruihua1,2, ZHAO Dandan1, GUO Tianyu3,4, DU Jianping1,4(
), SHANG Yangyang1, ZHAO Ruihua1,2, ZHAO Dandan1, GUO Tianyu3,4, DU Jianping1,4( ), LI Jinping1,4
), LI Jinping1,4
- 1.College of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.Shanxi Kunming Tobacco Limited Liability Company, Taiyuan 030024, Shanxi, China
3.College of Environmental Science and Engineering, Taiyuan University of Technology, Jinzhong 030600, Shanxi, China
4.Shanxi Key Laboratory of Gas Energy Efficient and Clean Utilization, Taiyuan 030024, Shanxi, China
-
Received:2021-04-16Revised:2021-06-27Online:2022-04-23Published:2022-04-25 -
Contact:DU Jianping
摘要:
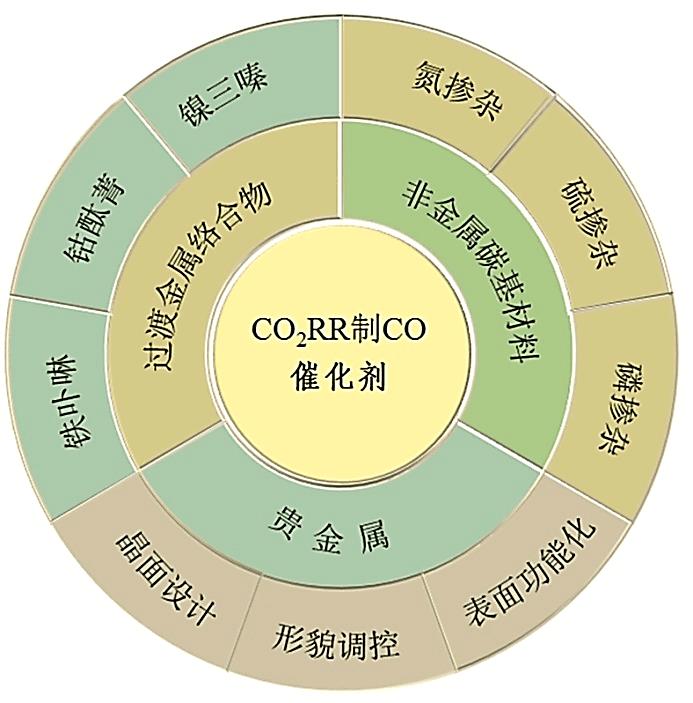
电催化还原CO2作为缓解能源危机和全球变暖的有效途径已成为催化领域的研究热点。然而,不同反应途径的氧化还原电位较为接近,使产物的选择性成为电催化还原CO2所需解决的主要问题。迄今为止,在水性电解质中可实现CO2选择性地转化为一氧化碳(CO)和甲酸(HCOOH)。本文简述了电催化还原CO2制CO的机理,包括CO2吸附过程、二电子转移过程和CO脱附过程。从贵金属的晶面设计、形貌调控和表面功能化对反应活性和产物选择性的影响,铁卟啉、钴酞菁和镍三嗪在还原CO2为CO反应中的电子转移途径,非金属碳基材料中杂原子和碳基质间的耦合效应等方面,重点介绍了近年来贵金属催化剂、过渡金属络合物催化剂和非金属碳基材料催化剂的研究进展,总结了各类催化剂的优缺点。指出在三类电催化还原CO2制CO的催化剂中,非金属碳材料具有较高的CO法拉第效率,尤其是非金属碳材料成本较低、制备简单、结构易调控,在电催化还原中具有潜在的应用优势,是有望实现商业化应用的新型催化剂的候选材料之一。
中图分类号:
引用本文
张少阳, 商阳阳, 赵瑞花, 赵丹丹, 郭天宇, 杜建平, 李晋平. 电催化还原二氧化碳制一氧化碳催化剂研究进展[J]. 化工进展, 2022, 41(4): 1848-1857.
ZHANG Shaoyang, SHANG Yangyang, ZHAO Ruihua, ZHAO Dandan, GUO Tianyu, DU Jianping, LI Jinping. Research progress on catalysts for electrocatalytic reduction of carbon dioxide to carbon monoxide[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1848-1857.
| 1 | XIE Huan, WANG Tanyuan, LIANG Jiashun, et al. Cu-based nanocatalysts for electrochemical reduction of CO2 [J]. Nano Today, 2018, 21: 41-54. |
| 2 | ZHANG Lei, ZHAO Zhijian, GONG Jinlong. Nanostructured materials for heterogeneous electrocatalytic CO2 reduction and their related reaction mechanisms[J]. Angewandte Chemie International Edition, 2017, 56(38): 11326-11353. |
| 3 | WU Jinghua, HUANG Yang, YE Wen, et al. CO2 reduction: from the electrochemical to photochemical approach[J]. Advanced Science, 2017, 4(11): 1700194. |
| 4 | DE Sudipta, DOKANIA Abhay, RAMIREZ Adrian, et al. Advances in the design of heterogeneous catalysts and thermocatalytic processes for CO2 utilization[J]. ACS Catalysis, 2020, 10(23): 14147-14185. |
| 5 | SULTANA Sabiha, SAHOO Prakash Chandra, MARTHA Satyabadi, et al. A review of harvesting clean fuels from enzymatic CO2 reduction[J]. RSC Advances, 2016, 6(50): 44170-44194. |
| 6 | 郑元波, 张前, 石坚, 等. 电催化还原CO2生成多种产物催化剂研究进展[J]. 化工进展, 2022, 41(3): 1209-1223. |
| ZHENG Yuanbo, ZHANG Qian, SHI Jian, et al. Research progress of catalysts for electrocatalytic reduction of CO2 to various products[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1209-1223. | |
| 7 | CHEN Yanping, WEI Jiatong, DUYAR Melis, et al. Carbon-based catalysts for Fischer-Tropsch synthesis[J]. Chemical Society Reviews, 2021, 50(4): 2337-2366. |
| 8 | BAHMANPOUR Ali, SIGNORILE Matteo, Oliver KRÖCHER. Recent progress in syngas production via catalytic CO2 hydrogenation reaction[J]. Applied Catalysis B: Environmental, 2021, 295: 120319. |
| 9 | 华亚妮, 冯少广, 党欣悦, 等. CO2电催化还原产合成气研究进展[J]. 化工进展, 2022, 41(3): 1224-1240. |
| HUA Yani, FENG Shaoguang, DANG Xinyue, et al. Research progress of CO2 electrocatalytic reduction to syngas[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1224-1240. | |
| 10 | PAN Fuping, YANG Yang. Designing CO2 reduction electrode materials by morphology and interface engineering[J]. Energy & Environmental Science, 2020, 13(8): 2275-2309. |
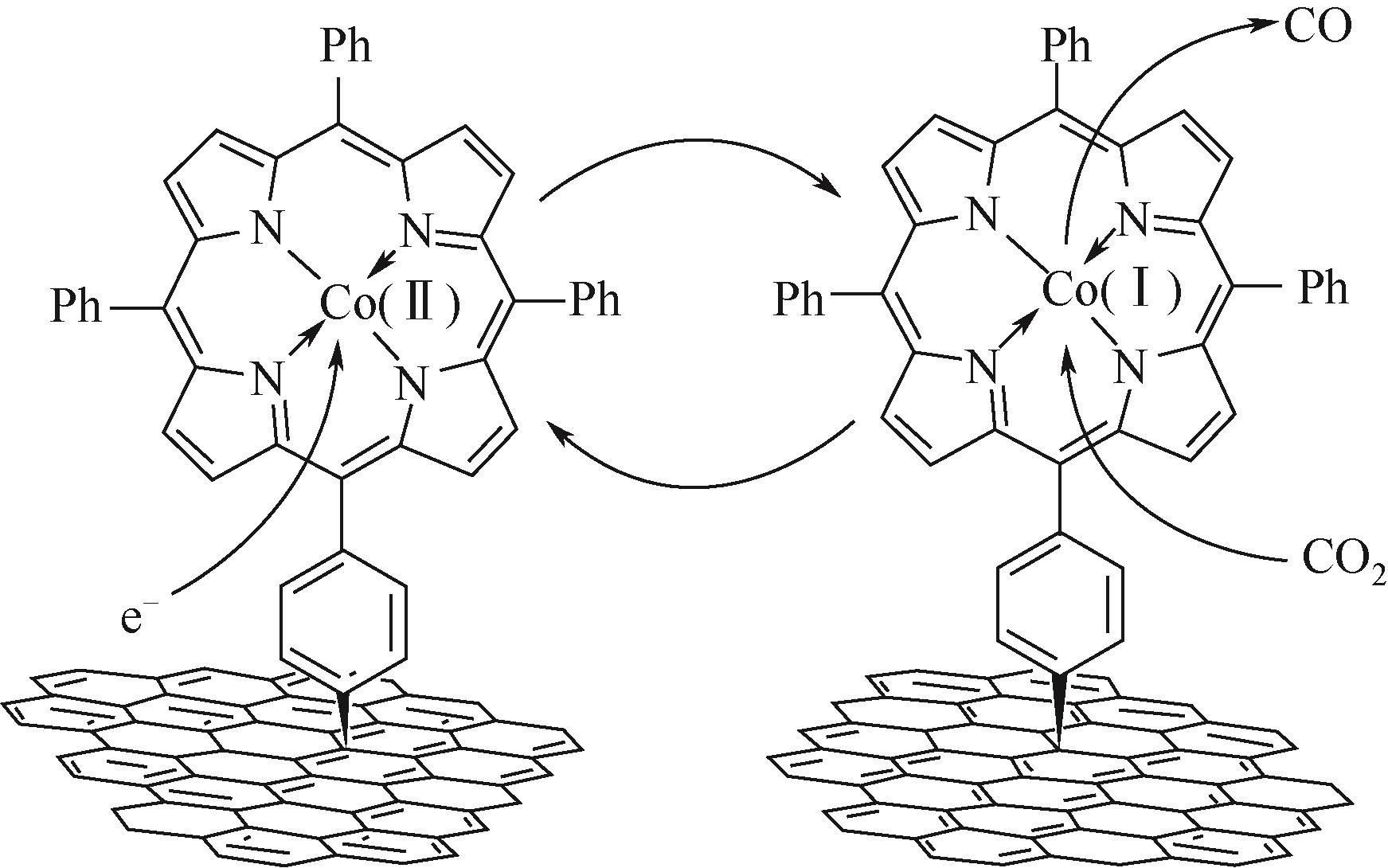
| 11 | XU Shenzhen, CARTER Emily. Theoretical insights into heterogeneous(photo) electrochemical CO2 reduction[J]. Chemical Reviews, 2019, 119(11): 6631-69. |
| 12 | BACK Seoin, YEOM Min Sun, JUNG Yousung. Active sites of Au and Ag nanoparticle catalysts for CO2 electroreduction to CO[J]. ACS Catalysis, 2015, 5(9): 5089-5096. |
| 13 | ZHU Dongdong, LIU Jinlong, QIAO Shizhang. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide[J]. Advanced Materials, 2016, 28(18): 3423-3452. |
| 14 | GAO Feiyue, BAO Ruicheng, GAO Minrui, et al. Electrochemical CO2-to-CO conversion: electrocatalysts, electrolytes, and electrolyzers[J]. Journal of Materials Chemistry A, 2020, 8(31): 15458-15478. |
| 15 | FRANCO Federico, RETTENMAIER Clara, JEON Hyo Sang, et al. Transition metal-based catalysts for the electrochemical CO2 reduction: from atoms and molecules to nanostructured materials[J]. Chemical Society Reviews, 2020, 49(19): 6884-6946. |
| 16 | WU Jingjie, SHARIFI Tiva, GAO Ying, et al. Emerging carbon-based heterogeneous catalysts for electrochemical reduction of carbon dioxide into value-added chemicals[J]. Advanced Materials, 2019, 31(13): 1804257. |
| 17 | GOYAL Akansha, MARCANDALLI Giulia, MINTS Vladislav, et al. Competition between CO2 reduction and hydrogen evolution on a gold electrode under well-defined mass transport conditions[J]. Journal of the American Chemical Society, 2020, 142(9): 4154-4161. |
| 18 | DONG Cunku, FU Jianyu, LIU Hui, et al. Tuning the selectivity and activity of Au catalysts for carbon dioxide electroreduction via grain boundary engineering: a DFT study[J]. Journal of Materials Chemistry A, 2017, 5(15): 7184-7190. |
| 19 | HOSSAIN Nur, LIU Zhonggang, WEN Jiali, et al. Enhanced catalytic activity of nanoporous Au for the efficient electrochemical reduction of carbon dioxide[J]. Applied Catalysis B: Environmental, 2018, 236: 483-489. |
| 20 | YANG Dongrui, LIU Ling, ZHANG Qian, et al. Importance of Au nanostructures in CO2 electrochemical reduction reaction[J]. Science Bulletin, 2020, 65(10): 796-802. |
| 21 | MA Zhongqiao, LIAN Cheng, NIU Dongfang, et al. Enhancing CO2 electroreduction with Au/pyridine/carbon nanotubes hybrid structures[J]. ChemSusChem, 2019, 12(8): 1724-1731. |
| 22 | CLARK Ezra, RINGE Stefan, TANG Michael, et al. Influence of atomic surface structure on the activity of Ag for the electrochemical reduction of CO2 to CO[J]. ACS Catalysis, 2019, 9(5): 4006-4014. |
| 23 | PENG Xiong, KARAKALOS Stavros, MUSTAIN William. Preferentially oriented Ag nanocrystals with extremely high activity and faradaic efficiency for CO2 electrochemical reduction to CO[J]. ACS Applied Materials & Interfaces, 2018, 10(2): 1734-1742. |
| 24 | LIU Shaoqing, WU Shuwen, GAO Minrui, et al. Hollow porous Ag spherical catalysts for highly efficient and selective electrocatalytic reduction of CO2 to CO[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(17): 14443-14450. |
| 25 | 何志桥, 魏榕飞, 严婷婷, 等. 磷酸钠水溶液中氧化还原循环制备三维银电极还原CO2生成CO[J]. 化工学报, 2017, 68(12): 4809-4815. |
| HE Zhiqiao, WEI Rongfei, YAN Tingting, et al. Preparation of three-dimensional Ag electrode by oxidation-reduction cycle method in sodium phosphate aqueous solution for reduction CO2 to CO[J]. CIESC Journal, 2017, 68(12): 4809-4815. | |
| 26 | ABEYWEERA Sasitha, YU Jie, PERDEW John, et al. Hierarchically 3D porous Ag nanostructures derived from silver benzenethiolate nanoboxes: enabling CO2 reduction with a near-unity selectivity and mass-specific current density over 500A/g[J]. Nano Letters, 2020, 20(4): 2806-2811. |
| 27 | LEE Sung Min, LEE Hyunju, KIM Junhyeong, et al. All-water-based solution processed Ag nanofilms for highly efficient electrocatalytic reduction of CO2 to CO[J]. Applied Catalysis B: Environmental, 2019, 259: 118045. |
| 28 | QIU Weibin, LIANG Ruping, LUO Yonglan, et al. A Br- anion adsorbed porous Ag nanowire film: in situ electrochemical preparation and application toward efficient CO2 electroreduction to CO with high selectivity[J]. Inorganic Chemistry Frontiers, 2018, 5(9): 2238-2241. |
| 29 | WEI Li, LI Hao, CHEN Junsheng, et al. Thiocyanate-modified silver nanofoam for efficient CO2 reduction to CO[J]. ACS Catalysis, 2020, 10(2): 1444-1453. |
| 30 | ZHU Wenlei, KATTEL Shyam, JIAO Feng, et al. Shape-controlled CO2 electrochemical reduction on nanosized Pd hydride cubes and octahedra[J]. Advanced Energy Materials, 2019, 9(9): 1802840. |
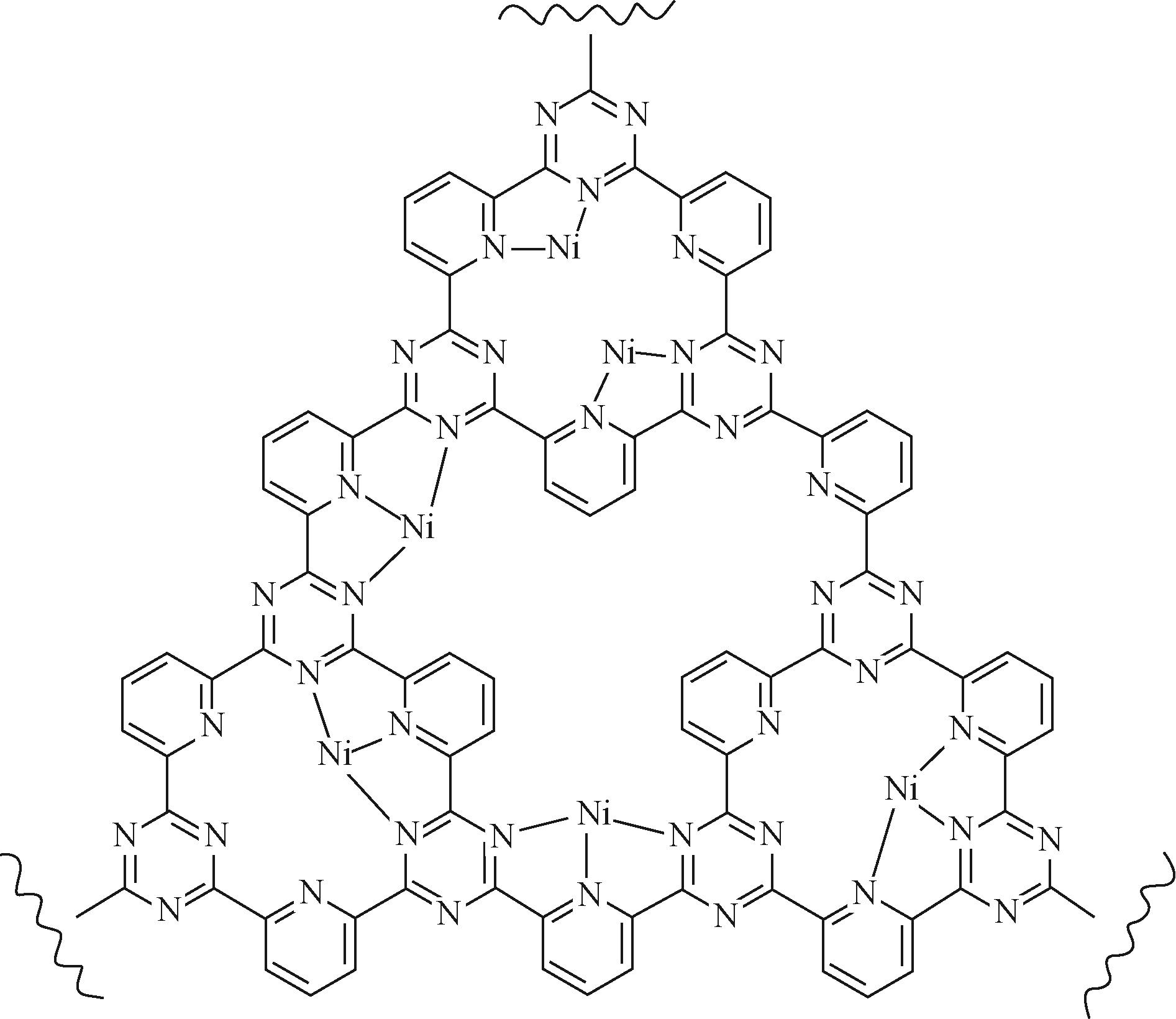
| 31 | HUANG Hongwen, JIA Huanhuan, LIU Zhao, et al. Understanding of strain effects in the electrochemical reduction of CO2: using Pd nanostructures as an ideal platform[J]. Angewandte Chemie International Edition, 2017, 56(13): 3594-3598. |
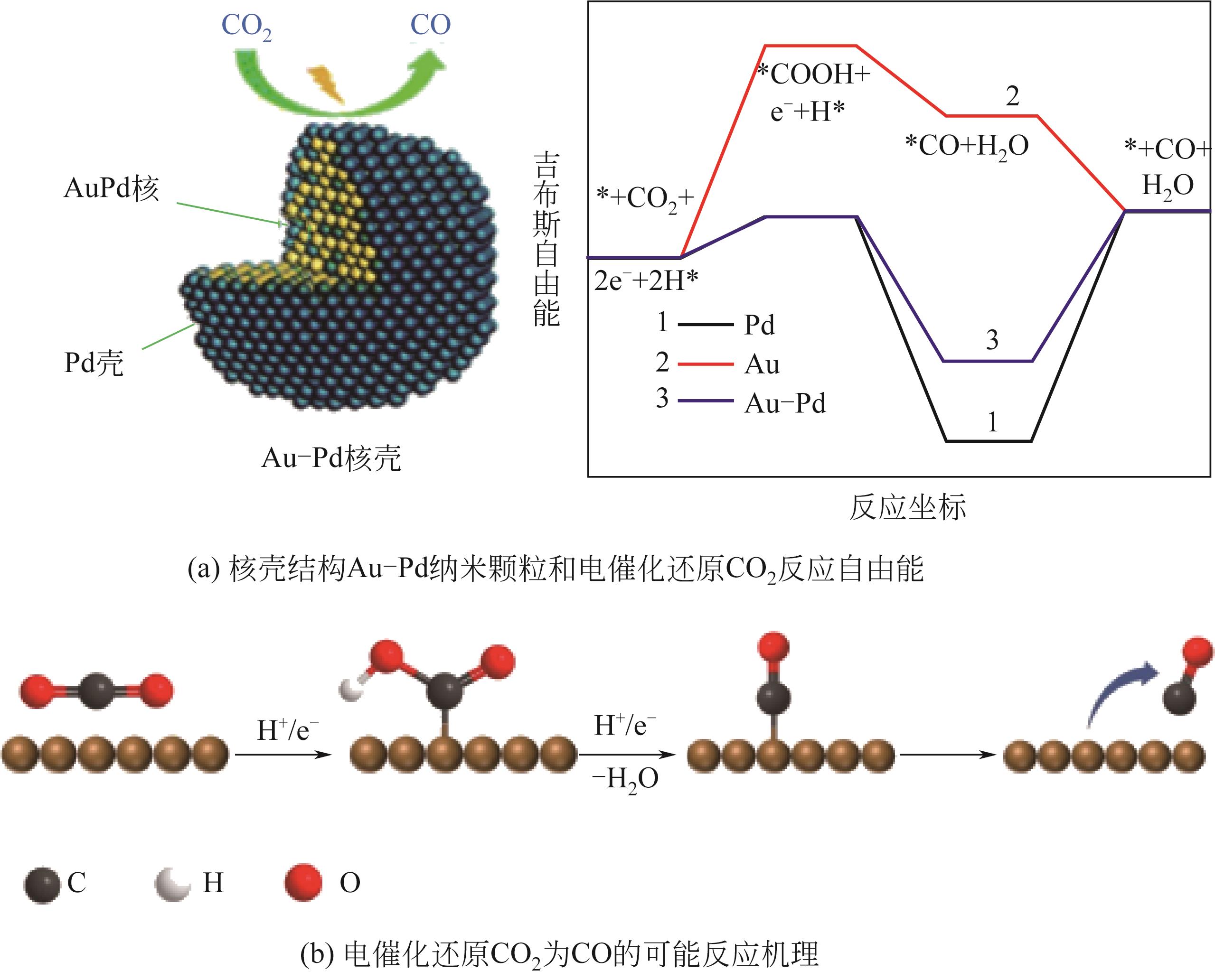
| 32 | ZHU Shangqian, QIN Xueping, WANG Qi, et al. Composition-dependent CO2 electrochemical reduction activity and selectivity on Au-Pd core-shell nanoparticles[J]. Journal of Materials Chemistry A, 2019, 7(28): 16954-16961. |
| 33 | ZHU Shangqian, WANG Qi, QIN Xueping, et al. Tuning structural and compositional effects in Pd-Au nanowires for highly selective and active CO2 electrochemical reduction reaction[J]. Advanced Energy Materials, 2018, 8(32): 1802238. |
| 34 | Yeongdong MUN, LEE Seunghyun, CHO Ara, et al. Cu-Pd alloy nanoparticles as highly selective catalysts for efficient electrochemical reduction of CO2 to CO[J]. Applied Catalysis B: Environmental, 2019, 246: 82-88. |
| 35 | ZHU Wenjin, ZHANG Lei, YANG Piaoping, et al. Morphological and compositional design of Pd-Cu bimetallic nanocatalysts with controllable product selectivity toward CO2 electroreduction[J]. Small, 2018, 14(7): 1703314. |
| 36 | CUI Meiyang, JOHNSON Grayson, ZHANG Zhiyong, et al. AgPd nanoparticles for electrocatalytic CO2 reduction: bimetallic composition-dependent ligand and ensemble effects[J]. Nanoscale, 2020, 12(26): 14068-14075. |
| 37 | CAO Zhi, DERRICK Jeffrey, XU Jun, et al. Chelating N-heterocyclic carbene ligands enable tuning of electrocatalytic CO2 reduction to formate and carbon monoxide: surface organometallic chemistry[J]. Angewandte Chemie International Edition, 2018, 57(18): 4981-4985. |
| 38 | XIA Rong, ZHANG Sheng, MA Xinbin, et al. Surface-functionalized palladium catalysts for electrochemical CO2 reduction[J]. Journal of Materials Chemistry A, 2020, 8(31): 15884-15890. |
| 39 | GU Jun, HSU Chia-shuo, BAI Lichen, et al. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO[J]. Science, 2019, 364(6445): 1091. |
| 40 | WANG Xiaoqian, CHEN Zhao, ZHAO Xuyan, et al. Regulation of coordination number over single Co sites: triggering the efficient electroreduction of CO2 [J]. Angewandte Chemie International Edition, 2018, 57(7): 1944-1948. |
| 41 | ZHAO Changming, DAI Xinyao, YAO Tao, et al. Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2 [J]. Journal of the American Chemical Society, 2017, 139(24): 8078-8081. |
| 42 | HE Qun, LEE Ji Hoon, LIU Daobin, et al. Accelerating CO2 electroreduction to CO over Pd single-atom catalyst[J]. Advanced Functional Materials, 2020, 30(17): 2000407. |
| 43 | HUAI Mingming, YIN Zhenglei, WEI Fengyuan, et al. Electrochemical CO2 reduction on heterogeneous cobalt phthalocyanine catalysts with different carbon supports[J]. Chemical Physics Letters, 2020, 754: 137655. |
| 44 | HU Bihua, XIE Weiwei, LI Ruchun, et al. How does the ligands structure surrounding metal-N4 of Co-based macrocyclic compounds affect electrochemical reduction of CO2 performance?[J]. Electrochimica Acta, 2020, 331: 135283. |
| 45 | MARIANOV Aleksei, JIANG Yijiao. Covalent ligation of Co molecular catalyst to carbon cloth for efficient electroreduction of CO2 in water[J]. Applied Catalysis B: Environmental, 2019, 244: 881-888. |
| 46 | HAN Na, WANG Yu, MA Lu, et al. Supported cobalt polyphthalocyanine for high-performance electrocatalytic CO2 reduction[J]. Chem, 2017, 3(4): 652-664. |
| 47 | ABDINEJAD Maryam, Caitlin DAO, ZHANG Xiao-an, et al. Enhanced electrocatalytic activity of iron amino porphyrins using a flow cell for reduction of CO2 to CO[J]. Journal of Energy Chemistry, 2021, 58: 162-169. |
| 48 | CHOI Jaecheol, KIM Jeonghun, WAGNER Pawel, et al. Highly ordered mesoporous carbon/iron porphyrin nanoreactor for the electrochemical reduction of CO2 [J]. Journal of Materials Chemistry A, 2020, 8(30): 14966-14974. |
| 49 | CHOI Jaecheol, WAGNER Pawel, JALILI Rouhollah, et al. A porphyrin/graphene framework: a highly efficient and robust electrocatalyst for carbon dioxide reduction[J]. Advanced Energy Materials, 2018, 8(26): 1801280. |
| 50 | CHOI Jaecheol, KIM Jeonghun, WAGNER Pawel, et al. Energy efficient electrochemical reduction of CO2 to CO using a three-dimensional porphyrin/graphene hydrogel[J]. Energy & Environmental Science, 2019, 12(2): 747-755. |
| 51 | MAURIN Antoine, ROBERT Marc. Catalytic CO2-to-CO conversion in water by covalently functionalized carbon nanotubes with a molecular iron catalyst[J]. Chemical Communications, 2016, 52(81): 12084-12087. |
| 52 | MOHAMED Eman, ZAHRAN Zaki, NARUTA Yoshinori. Efficient heterogeneous CO2 to CO conversion with a phosphonic acid fabricated cofacial iron porphyrin dimer[J]. Chemistry of Materials, 2017, 29(17): 7140-7150. |
| 53 | XIA Yujian, KASHTANOV Stepan, YU Pengfei, et al. Identification of dual-active sites in cobalt phthalocyanine for electrochemical carbon dioxide reduction[J]. Nano Energy, 2020, 67: 104163. |
| 54 | ZHANG Zheng, XIAO Jianping, CHEN Xuejiao, et al. Reaction mechanisms of well-defined metal-N4 sites in electrocatalytic CO2 reduction[J]. Angewandte Chemie International Edition, 2018, 57(50): 16339-16342. |
| 55 | CHEN Jiacheng, ZHU Minghui, LI Jiayu, et al. Structure-activity relationship of the polymerized cobalt phthalocyanines for electrocatalytic carbon dioxide reduction[J]. The Journal of Physical Chemistry C, 2020, 124(30): 16501-16507. |
| 56 | Natalia MORLANÉS, TAKANABE Kazuhiro, RODIONOV Valentin. Simultaneous reduction of CO2 and splitting of H2O by a single immobilized cobalt phthalocyanine electrocatalyst[J]. ACS Catalysis, 2016, 6(5): 3092-3095. |
| 57 | WANG Min, TORBENSEN Kristian, SALVATORE Danielle, et al. CO2 electrochemical catalytic reduction with a highly active cobalt phthalocyanine[J]. Nature Communications, 2019, 10(1): 3602. |
| 58 | ZHANG Xing, WU Zishan, ZHANG Xiao, et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures[J]. Nature Communications, 2017, 8(1): 14675. |
| 59 | CHOI Jaecheol, WAGNER Pawel, GAMBHIR Sanjeev, et al. Steric modification of a cobalt phthalocyanine/graphene catalyst to give enhanced and stable electrochemical CO2 reduction to CO[J]. ACS Energy Letters, 2019, 4(3): 666-672. |
| 60 | ZHU Minghui, CHEN Jiacheng, HUANG Libei, et al. Covalently grafting cobalt porphyrin onto carbon nanotubes for efficient CO2 electroreduction[J]. Angewandte Chemie International Edition, 2019, 58(20): 6595-6599. |
| 61 | ZHU Minghui, CHEN Jiacheng, GUO Rong, et al. Cobalt phthalocyanine coordinated to pyridine-functionalized carbon nanotubes with enhanced CO2 electroreduction[J]. Applied Catalysis B: Environmental, 2019, 251: 112-118. |
| 62 | PUGLIESE Silvia, HUAN Ngoc Tran, FORTE Jérémy, et al. Functionalization of carbon nanotubes with nickel cyclam for the electrochemical reduction of CO2 [J]. ChemSusChem, 2020, 13(23): 6449-6456. |
| 63 | LIU Jinhang, YANG Liming, GANZ Eric. Electrocatalytic reduction of CO2 by two-dimensional transition metal porphyrin sheets[J]. Journal of Materials Chemistry A, 2019, 7(19): 11944-11952. |
| 64 | SU Panpan, IWASE Kazuyuki, HARADA Takashi, et al. Covalent triazine framework modified with coordinatively-unsaturated Co or Ni atoms for CO2 electrochemical reduction[J]. Chemical Science, 2018, 9(16): 3941-3947. |
| 65 | FENG Shaohua, ZHENG Wanzhen, ZHU Jingke, et al. Porous metal-porphyrin triazine-based frameworks for efficient CO2 electroreduction[J]. Applied Catalysis B: Environmental, 2020, 270: 118908. |
| 66 | ZHANG Xiao, WANG Yang, GU Meng, et al. Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2 reduction[J]. Nature Energy, 2020, 5(9): 684-692. |
| 67 | DUAN Xiaochuan, XU Jiantie, WEI Zengxi, et al. Metal-free carbon materials for CO2 electrochemical reduction[J]. Advanced Materials, 2017, 29(41): 1701784. |
| 68 | VASILEFF Anthony, ZHENG Yao, QIAO Shizhang. Carbon solving carbon’s problems: recent progress of nanostructured carbon-based catalysts for the electrochemical reduction of CO2 [J]. Advanced Energy Materials, 2017, 7(21): 1700759. |
| 69 | SHARMA Pranav, WU Jingjie, YADAV Ram Manohar, et al. Nitrogen-doped carbon nanotube arrays for high-efficiency electrochemical reduction of CO2: on the understanding of defects, defect density, and selectivity[J]. Angewandte Chemie International Edition, 2015, 54(46): 13701-13705. |
| 70 | SIAHROSTAMI Samira, JIANG Kun, KARAMAD Mohammadreza, et al. Theoretical investigations into defected graphene for electrochemical reduction of CO2 [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 11080-11085. |
| 71 | XUE Xiaoyi, YANG Hui, YANG Tao, et al. N,P-coordinated fullerene-like carbon nanostructures with dual active centers toward highly-efficient multi-functional electrocatalysis for CO2RR, ORR and Zn-air battery[J]. Journal of Materials Chemistry A, 2019, 7(25): 15271-15277. |
| 72 | YUE Yawei, SUN Yangye, TANG Can, et al. Ranking the relative CO2 electrochemical reduction activity in carbon materials[J]. Carbon, 2019, 154: 108-114. |
| 73 | JHONG Hueiru-ru Molly, TORNOW Claire, SMID Bretislav, et al. A nitrogen-doped carbon catalyst for electrochemical CO2 conversion to CO with high selectivity and current density[J]. ChemSusChem, 2017, 10(6): 1094-1099. |
| 74 | LIU Tianfu, Sajjad ALI, LIAN Zan, et al. Phosphorus-doped onion-like carbon for CO2 electrochemical reduction: the decisive role of the bonding configuration of phosphorus[J]. Journal of Materials Chemistry A, 2018, 6(41): 19998-20004. |
| 75 | KUMAR Bijandra, ASADI Mohammad, PISASALE Davide, et al. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction[J]. Nature Communications, 2013, 4(1): 2819. |
| 76 | LIU Song, YANG Hongbin, HUANG Xiang, et al. Identifying active sites of nitrogen-doped carbon materials for the CO2 reduction reaction[J]. Advanced Functional Materials, 2018, 28(21): 1800499. |
| 77 | LIU Weiqi, QI Jiawei, BAI Peiyao, et al. Utilizing spatial confinement effect of N atoms in micropores of coal-based metal-free material for efficiently electrochemical reduction of carbon dioxide[J]. Applied Catalysis B: Environmental, 2020, 272: 118974. |
| 78 | CHEN Shuo, LIU Tianfu, OLANRELE Samson, et al. Boosting electrocatalytic activity for CO2 reduction on nitrogen-doped carbon catalysts by co-doping with phosphorus[J]. Journal of Energy Chemistry, 2021, 54: 143-150. |
| 79 | PAN Fuping, LI Boyang, DENG Wei, et al. Promoting electrocatalytic CO2 reduction on nitrogen-doped carbon with sulfur addition[J]. Applied Catalysis B: Environmental, 2019, 252: 240-249. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [7] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [8] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [9] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [10] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [11] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [12] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [13] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [14] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [15] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||