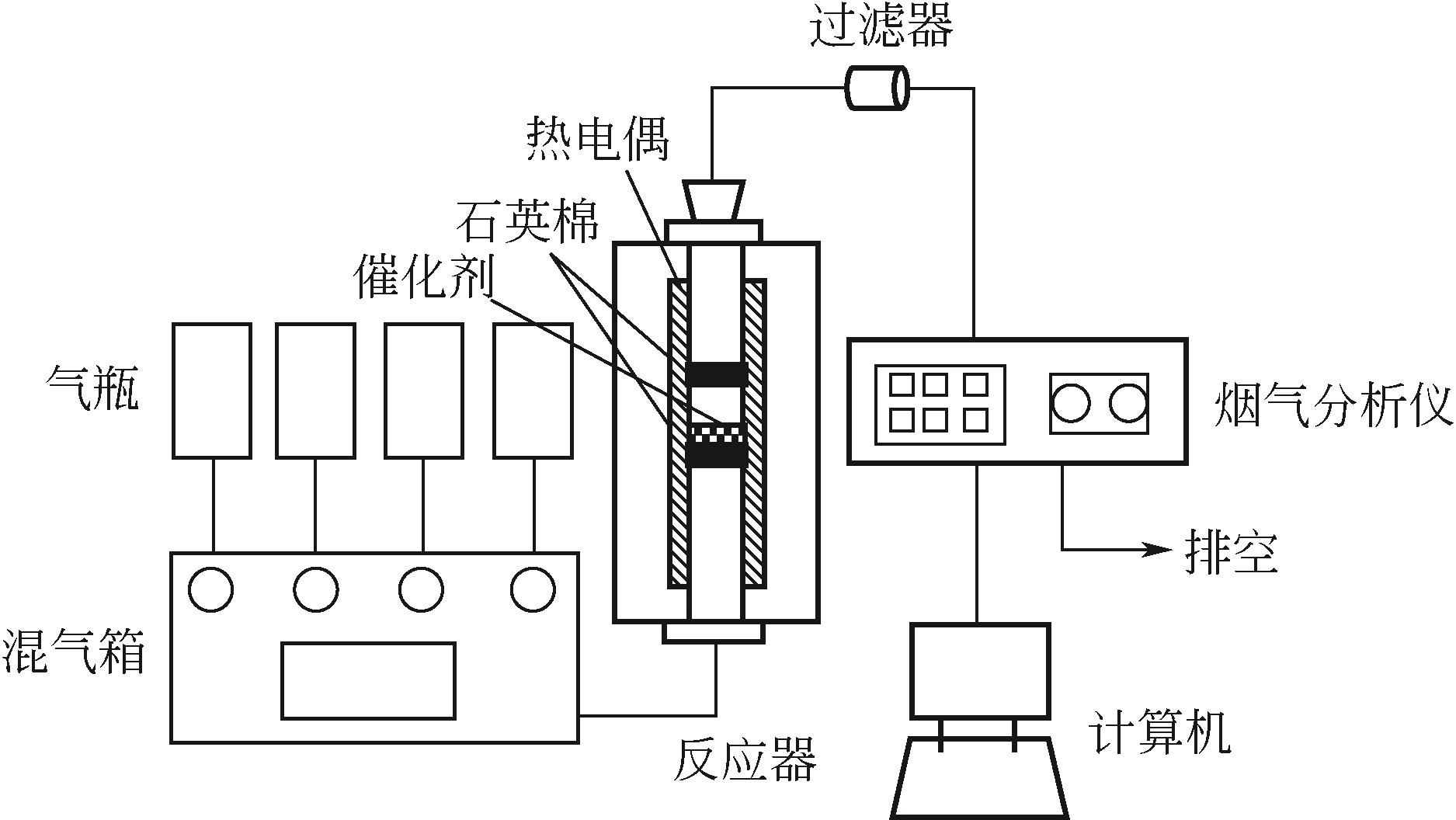
化工进展 ›› 2021, Vol. 40 ›› Issue (10): 5818-5828.DOI: 10.16085/j.issn.1000-6613.2020-2175
机械力-微波活化对稀土尾矿NH3-SCR脱硝性能的影响
侯丽敏1( ), 闫笑2, 乔超越2, 付善聪2, 武文斐1,2(
), 闫笑2, 乔超越2, 付善聪2, 武文斐1,2( )
)
- 1.内蒙古科技大学工业技术研究院,内蒙古自治区白云鄂博矿多金属资源综合利用省部共建国家重点实验室
1.内蒙古 包头 014010,内蒙古科技大学能源与环境学院,内蒙古自治区高效洁净燃烧重点实验室;内蒙古 包头 014010
-
收稿日期:2020-10-30修回日期:2021-04-07出版日期:2021-10-10发布日期:2021-10-25 -
通讯作者:武文斐 -
作者简介:侯丽敏(1988—),女,博士,讲师,研究方向为矿产资源综合利用、矿物催化剂、CLAS。E-mail:neuhlm@163.com 。 -
基金资助:国家重点研发计划(2020YFC1909102);国家自然科学基金(51866013);内蒙古自然科学基金(2020BS05030)
Effect of mechanical force and microwave on the NH3-SCR denitration of rare earth tailings
HOU Limin1( ), YAN Xiao2, QIAO Chaoyue2, FU Shancong2, WU Wenfei1,2(
), YAN Xiao2, QIAO Chaoyue2, FU Shancong2, WU Wenfei1,2( )
)
- 1.Key Laboratory of Integrated Exploitation of Bayan Obo Multi-Metal Resources, Institute of Industrial Technology Research, Inner Mongolia University of Science & Technology, Baotou 014010, Inner Mongolia, China, School of Energy and Environment, Inner Mongolia University of Science & Technology, Baotou 014010, Inner Mongolia, China
2.Key Laboratory of Efficient and Clean Combustion, School of Energy and Environment, Inner Mongolia University of Science & Technology, Baotou 014010, Inner Mongolia, China
-
Received:2020-10-30Revised:2021-04-07Online:2021-10-10Published:2021-10-25 -
Contact:WU Wenfei
摘要:
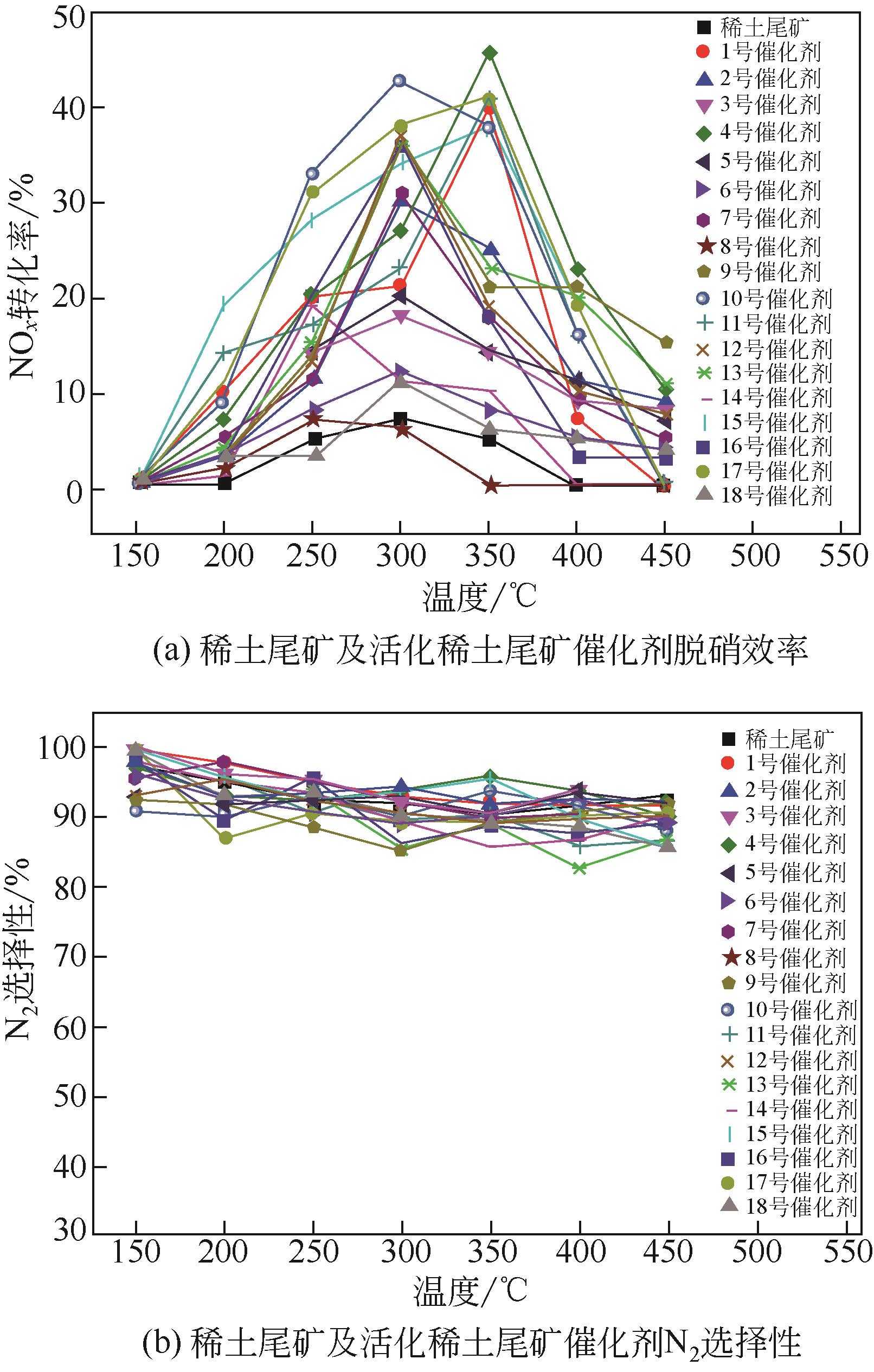
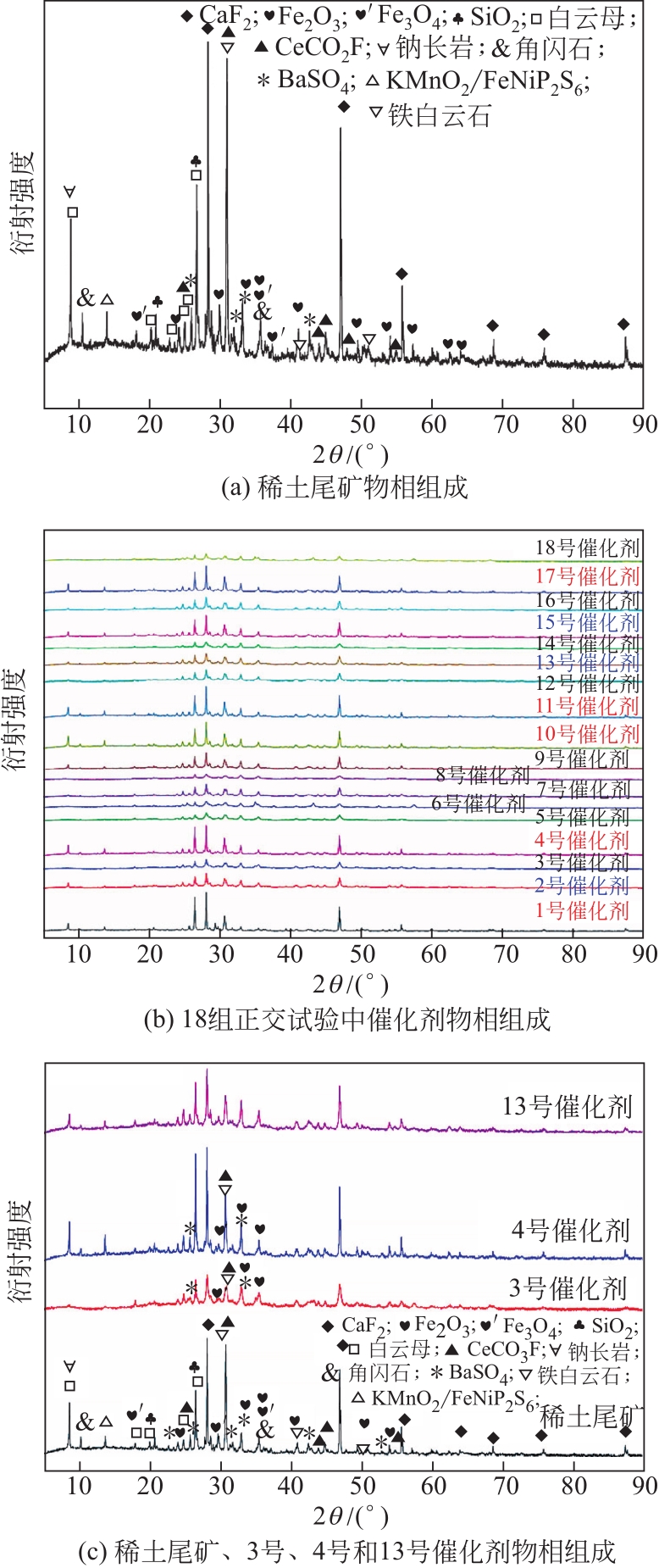
因为稀土尾矿中矿物复杂的连生关系,使其部分矿物在脱硝反应过程中并不能充分暴露发挥作用。本文采用机械力微波活化稀土尾矿,利用正交试验方法研究机械力微波活化参数对稀土尾矿NH3-SCR脱硝性能的影响,借助XRD、SEM-EDS、H2-TPR、NH3-TPD、BET等表征手段分析了机械力微波活化对稀土尾矿性能的影响。实验结果表明,稀土尾矿对活化参数的敏感性为:球料比>转子转速>球磨时间=球直径比>微波焙烧时间=微波焙烧温度=微波焙烧功率,机械力微波活化最优参数为球磨2h、转子转速300r/min、球料比1∶1、球直径比1∶1∶1、微波焙烧温度250℃、微波焙烧时间20min、微波焙烧功率1100W,活化稀土尾矿脱硝效率最高提升了40%。活化后,稀土尾矿催化剂的比表面积、矿物分散度、表面酸性位数量和氧化还原性能均得到了提升,弱酸、中强酸和强酸活性中心均匀分布有利于脱硝反应。赤铁矿暴露程度越高,越有利于稀土尾矿脱硝反应过程。
中图分类号:
引用本文
侯丽敏, 闫笑, 乔超越, 付善聪, 武文斐. 机械力-微波活化对稀土尾矿NH3-SCR脱硝性能的影响[J]. 化工进展, 2021, 40(10): 5818-5828.
HOU Limin, YAN Xiao, QIAO Chaoyue, FU Shancong, WU Wenfei. Effect of mechanical force and microwave on the NH3-SCR denitration of rare earth tailings[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5818-5828.
| 元素 | 质量分数 /% | 元素 | 质量分数 /% | 元素 | 质量分数 /% | 元素 | 质量分数 /% |
|---|---|---|---|---|---|---|---|
| Fe | 17.394 | Ca | 17.798 | Si | 8.804 | Mg | 3.104 |
| Ce | 1.783 | Al | 1.485 | Na | 1.336 | P | 1.158 |
| S | 0.718 | Ba | 0.997 | Nd | 0.683 | Mn | 0.926 |
| K | 0.572 | Ti | 0.310 | Nb | 0.112 | Pr | 0.109 |
| Sr | 0.099 | Th | 0.031 | Zn | 0.036 | V | 0.008 |
| Sc | 0.010 | Pb | 0.017 | Cl | 0.077 | Co | 0.005 |
| Pd | 0.003 | I | 0.006 | Zr | 0.004 | Sn | 0.005 |
| Ni | 0.002 | Cr | 0.001 | Rb | 0.002 | Te | 0.0016 |
| As | 0.0004 | W | 0.0005 | Cu | 0.0025 |
表1 稀土尾矿中主要元素的质量分数
| 元素 | 质量分数 /% | 元素 | 质量分数 /% | 元素 | 质量分数 /% | 元素 | 质量分数 /% |
|---|---|---|---|---|---|---|---|
| Fe | 17.394 | Ca | 17.798 | Si | 8.804 | Mg | 3.104 |
| Ce | 1.783 | Al | 1.485 | Na | 1.336 | P | 1.158 |
| S | 0.718 | Ba | 0.997 | Nd | 0.683 | Mn | 0.926 |
| K | 0.572 | Ti | 0.310 | Nb | 0.112 | Pr | 0.109 |
| Sr | 0.099 | Th | 0.031 | Zn | 0.036 | V | 0.008 |
| Sc | 0.010 | Pb | 0.017 | Cl | 0.077 | Co | 0.005 |
| Pd | 0.003 | I | 0.006 | Zr | 0.004 | Sn | 0.005 |
| Ni | 0.002 | Cr | 0.001 | Rb | 0.002 | Te | 0.0016 |
| As | 0.0004 | W | 0.0005 | Cu | 0.0025 |
| 试验序号 | 球磨时间/h | 球磨转速/r·min-1 | 球料比 | 球直径比 | 微波焙烧温度/℃ | 微波焙烧时间/min | 微波焙烧功率/W |
|---|---|---|---|---|---|---|---|
| 1 | 1(2) | 1(300) | 1(1∶1) | 1(5∶3∶1) | 1(150) | 1(5) | 1(300) |
| 2 | 1 | 2(600) | 2(10∶1) | 2(1∶1∶1) | 2(250) | 2(20) | 2(700) |
| 3 | 1 | 3(900) | 3(20∶1) | 3(1∶3∶5) | 3(350) | 3(35) | 3(1100) |
| 4 | 2(8) | 1 | 1 | 2 | 2 | 3 | 3 |
| 5 | 2 | 2 | 2 | 3 | 3 | 1 | 1 |
| 6 | 2 | 3 | 3 | 1 | 1 | 2 | 2 |
| 7 | 3(14) | 1 | 2 | 1 | 3 | 2 | 3 |
| 8 | 3 | 2 | 3 | 2 | 1 | 3 | 1 |
| 9 | 3 | 3 | 1 | 3 | 2 | 1 | 2 |
| 10 | 1 | 1 | 3 | 3 | 2 | 2 | 1 |
| 11 | 1 | 2 | 1 | 1 | 3 | 3 | 2 |
| 12 | 1 | 3 | 2 | 2 | 1 | 1 | 3 |
| 13 | 2 | 1 | 2 | 3 | 1 | 3 | 2 |
| 14 | 2 | 2 | 3 | 1 | 2 | 1 | 3 |
| 15 | 2 | 3 | 1 | 2 | 3 | 2 | 1 |
| 16 | 3 | 1 | 3 | 2 | 3 | 1 | 2 |
| 17 | 3 | 2 | 1 | 3 | 1 | 2 | 3 |
| 18 | 3 | 3 | 2 | 1 | 2 | 3 | 1 |
表2 正交试验方案
| 试验序号 | 球磨时间/h | 球磨转速/r·min-1 | 球料比 | 球直径比 | 微波焙烧温度/℃ | 微波焙烧时间/min | 微波焙烧功率/W |
|---|---|---|---|---|---|---|---|
| 1 | 1(2) | 1(300) | 1(1∶1) | 1(5∶3∶1) | 1(150) | 1(5) | 1(300) |
| 2 | 1 | 2(600) | 2(10∶1) | 2(1∶1∶1) | 2(250) | 2(20) | 2(700) |
| 3 | 1 | 3(900) | 3(20∶1) | 3(1∶3∶5) | 3(350) | 3(35) | 3(1100) |
| 4 | 2(8) | 1 | 1 | 2 | 2 | 3 | 3 |
| 5 | 2 | 2 | 2 | 3 | 3 | 1 | 1 |
| 6 | 2 | 3 | 3 | 1 | 1 | 2 | 2 |
| 7 | 3(14) | 1 | 2 | 1 | 3 | 2 | 3 |
| 8 | 3 | 2 | 3 | 2 | 1 | 3 | 1 |
| 9 | 3 | 3 | 1 | 3 | 2 | 1 | 2 |
| 10 | 1 | 1 | 3 | 3 | 2 | 2 | 1 |
| 11 | 1 | 2 | 1 | 1 | 3 | 3 | 2 |
| 12 | 1 | 3 | 2 | 2 | 1 | 1 | 3 |
| 13 | 2 | 1 | 2 | 3 | 1 | 3 | 2 |
| 14 | 2 | 2 | 3 | 1 | 2 | 1 | 3 |
| 15 | 2 | 3 | 1 | 2 | 3 | 2 | 1 |
| 16 | 3 | 1 | 3 | 2 | 3 | 1 | 2 |
| 17 | 3 | 2 | 1 | 3 | 1 | 2 | 3 |
| 18 | 3 | 3 | 2 | 1 | 2 | 3 | 1 |
| 试验序号 | 球磨时间/h | 球磨转速/r·min-1 | 球料比 | 球直径比 | 微波焙烧温度/℃ | 微波焙烧时间/min | 微波焙烧功率/W | 指标 |
|---|---|---|---|---|---|---|---|---|
| 1 | 1(2) | 1(300) | 1(1∶1) | 1(5∶3∶1) | 1(150) | 1(5) | 1(300) | 0.40 |
| 2 | 1 | 2(600) | 2(10∶1) | 2(1∶1∶1) | 2(250) | 2(20) | 2(700) | 0.30 |
| 3 | 1 | 3(900) | 3(20∶1) | 3(1∶3∶5) | 3(350) | 3(35) | 3(1100) | 0.18 |
| 4 | 2(8) | 1 | 1 | 2 | 2 | 3 | 3 | 0.46 |
| 5 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 0.20 |
| 6 | 2 | 3 | 3 | 1 | 1 | 2 | 2 | 0.12 |
| 7 | 3(14) | 1 | 2 | 1 | 3 | 2 | 3 | 0.32 |
| 8 | 3 | 2 | 3 | 2 | 1 | 3 | 1 | 0.07 |
| 9 | 3 | 3 | 1 | 3 | 2 | 1 | 2 | 0.36 |
| 10 | 1 | 1 | 3 | 3 | 2 | 2 | 1 | 0.43 |
| 11 | 1 | 2 | 1 | 1 | 3 | 3 | 2 | 0.41 |
| 12 | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 0.37 |
| 13 | 2 | 1 | 2 | 3 | 1 | 3 | 2 | 0.36 |
| 14 | 2 | 2 | 3 | 1 | 2 | 1 | 3 | 0.19 |
| 15 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 0.38 |
| 16 | 3 | 1 | 3 | 2 | 3 | 1 | 2 | 0.36 |
| 17 | 3 | 2 | 1 | 3 | 1 | 2 | 3 | 0.41 |
| 18 | 3 | 3 | 2 | 1 | 2 | 3 | 1 | 0.11 |
| T1 | 2.09 | 2.32 | 2.42 | 1.54 | 1.73 | 1.88 | 1.59 | |
| T2 | 1.71 | 1.58 | 1.65 | 2.18 | 1.85 | 1.95 | 1.91 | |
| T3 | 1.62 | 1.52 | 1.35 | 1.94 | 1.84 | 1.59 | 1.92 | |
| 0.348 | 0.387 | 0.403 | 0.257 | 0.288 | 0.313 | 0.265 | ||
| 0.285 | 0.263 | 0.275 | 0.363 | 0.308 | 0.325 | 0.318 | ||
| 0.270 | 0.253 | 0.225 | 0.323 | 0.307 | 0.265 | 0.320 | ||
| R | 0.078 | 0.150 | 0.210 | 0.020 | 0.030 | 0.010 | 0.050 | |
| SSj | 0.0207 | 0.0662 | 0.01015 | 0.03804 | 0.00148 | 0.01214 | 0.01174 | |
| SSt | 0.25678 | |||||||
| SSe | 0.00490 | |||||||
表3 正交试验结果分析
| 试验序号 | 球磨时间/h | 球磨转速/r·min-1 | 球料比 | 球直径比 | 微波焙烧温度/℃ | 微波焙烧时间/min | 微波焙烧功率/W | 指标 |
|---|---|---|---|---|---|---|---|---|
| 1 | 1(2) | 1(300) | 1(1∶1) | 1(5∶3∶1) | 1(150) | 1(5) | 1(300) | 0.40 |
| 2 | 1 | 2(600) | 2(10∶1) | 2(1∶1∶1) | 2(250) | 2(20) | 2(700) | 0.30 |
| 3 | 1 | 3(900) | 3(20∶1) | 3(1∶3∶5) | 3(350) | 3(35) | 3(1100) | 0.18 |
| 4 | 2(8) | 1 | 1 | 2 | 2 | 3 | 3 | 0.46 |
| 5 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 0.20 |
| 6 | 2 | 3 | 3 | 1 | 1 | 2 | 2 | 0.12 |
| 7 | 3(14) | 1 | 2 | 1 | 3 | 2 | 3 | 0.32 |
| 8 | 3 | 2 | 3 | 2 | 1 | 3 | 1 | 0.07 |
| 9 | 3 | 3 | 1 | 3 | 2 | 1 | 2 | 0.36 |
| 10 | 1 | 1 | 3 | 3 | 2 | 2 | 1 | 0.43 |
| 11 | 1 | 2 | 1 | 1 | 3 | 3 | 2 | 0.41 |
| 12 | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 0.37 |
| 13 | 2 | 1 | 2 | 3 | 1 | 3 | 2 | 0.36 |
| 14 | 2 | 2 | 3 | 1 | 2 | 1 | 3 | 0.19 |
| 15 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 0.38 |
| 16 | 3 | 1 | 3 | 2 | 3 | 1 | 2 | 0.36 |
| 17 | 3 | 2 | 1 | 3 | 1 | 2 | 3 | 0.41 |
| 18 | 3 | 3 | 2 | 1 | 2 | 3 | 1 | 0.11 |
| T1 | 2.09 | 2.32 | 2.42 | 1.54 | 1.73 | 1.88 | 1.59 | |
| T2 | 1.71 | 1.58 | 1.65 | 2.18 | 1.85 | 1.95 | 1.91 | |
| T3 | 1.62 | 1.52 | 1.35 | 1.94 | 1.84 | 1.59 | 1.92 | |
| 0.348 | 0.387 | 0.403 | 0.257 | 0.288 | 0.313 | 0.265 | ||
| 0.285 | 0.263 | 0.275 | 0.363 | 0.308 | 0.325 | 0.318 | ||
| 0.270 | 0.253 | 0.225 | 0.323 | 0.307 | 0.265 | 0.320 | ||
| R | 0.078 | 0.150 | 0.210 | 0.020 | 0.030 | 0.010 | 0.050 | |
| SSj | 0.0207 | 0.0662 | 0.01015 | 0.03804 | 0.00148 | 0.01214 | 0.01174 | |
| SSt | 0.25678 | |||||||
| SSe | 0.00490 | |||||||
| 影响因素 | 离差平方和 | 自由度 | 方差 | 统计量 | 重要程度 |
|---|---|---|---|---|---|
| 球磨时间 | 0.02074 | 2 | 0.01037 | 4.23356 | [*] |
| 转子转速 | 0.66200 | 2 | 0.03309 | 13.20567 | (*) |
| 球料比 | 0.10150 | 2 | 0.05077 | 20.72335 | * |
| 球直径比 | 0.03800 | 2 | 0.01902 | 7.764172 | [*] |
| 微波焙烧温度 | 0.00150 | 2 | 0.00074 | 0.30158 | |
| 微波焙烧时间 | 0.01210 | 2 | 0.00607 | 2.47845 | |
| 微波焙烧功率 | 0.01170 | 2 | 0.00587 | 2.39682 | |
| 误差 | 0.00490 | 2 | 0.00245 | — |
表4 方差分析
| 影响因素 | 离差平方和 | 自由度 | 方差 | 统计量 | 重要程度 |
|---|---|---|---|---|---|
| 球磨时间 | 0.02074 | 2 | 0.01037 | 4.23356 | [*] |
| 转子转速 | 0.66200 | 2 | 0.03309 | 13.20567 | (*) |
| 球料比 | 0.10150 | 2 | 0.05077 | 20.72335 | * |
| 球直径比 | 0.03800 | 2 | 0.01902 | 7.764172 | [*] |
| 微波焙烧温度 | 0.00150 | 2 | 0.00074 | 0.30158 | |
| 微波焙烧时间 | 0.01210 | 2 | 0.00607 | 2.47845 | |
| 微波焙烧功率 | 0.01170 | 2 | 0.00587 | 2.39682 | |
| 误差 | 0.00490 | 2 | 0.00245 | — |
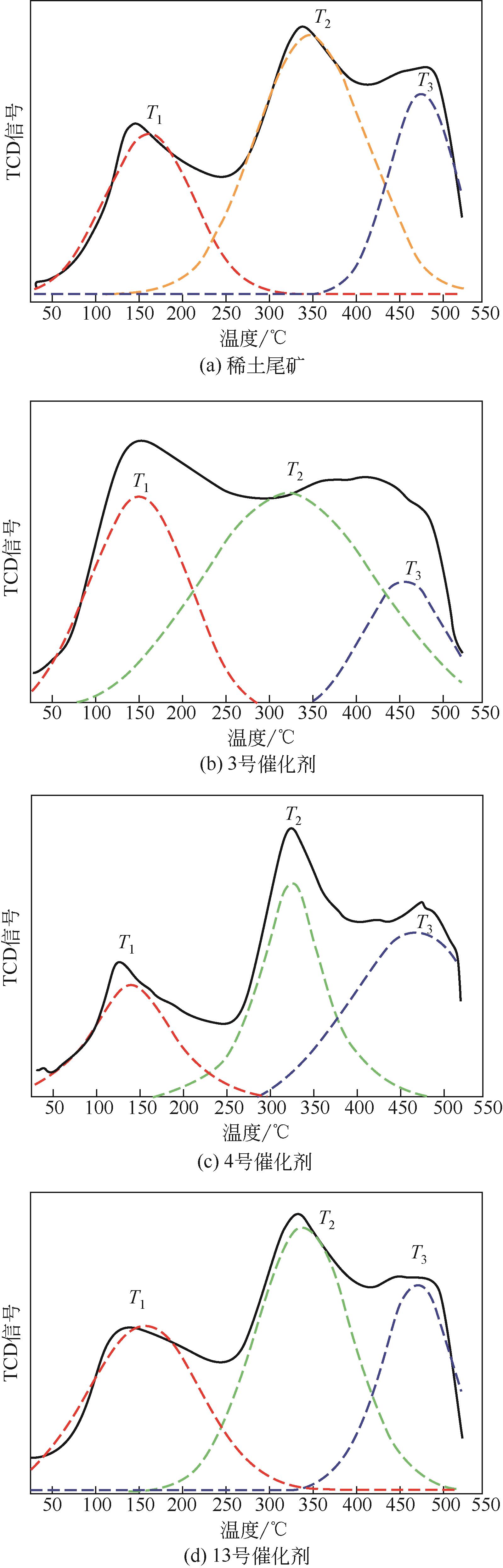
| 催化剂 | 峰值温度/℃ | 峰面积 | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | S1 | S2 | S3 | |
| 稀土尾矿 | 159 | 253 | 477 | 216 | 432 | 209 |
| 3号催化剂 | 147 | 316 | 464 | 578 | 1021 | 303 |
| 4号催化剂 | 137 | 327 | 473 | 226 | 291 | 350 |
| 13号催化剂 | 159 | 338 | 474 | 373 | 515 | 103 |
表5 稀土尾矿及活化稀土尾矿催化剂表面酸性位种类定量分析
| 催化剂 | 峰值温度/℃ | 峰面积 | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | S1 | S2 | S3 | |
| 稀土尾矿 | 159 | 253 | 477 | 216 | 432 | 209 |
| 3号催化剂 | 147 | 316 | 464 | 578 | 1021 | 303 |
| 4号催化剂 | 137 | 327 | 473 | 226 | 291 | 350 |
| 13号催化剂 | 159 | 338 | 474 | 373 | 515 | 103 |
| 1 | BONINGARI T, SMIRNIOTIS P G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement[J]. Current Opinion in Chemical Engineering, 2016, 13: 133-141. |
| 2 | 陆强, 裴鑫琦, 徐明新, 等. SCR脱硝催化剂抗砷中毒改性优化与再生研究进展[J]. 化工进展, 2021, 40(5): 2365-2374. |
| LU Qiang, PEI Xinqi, XU Mingxin, et al. Progress in the development and regeneration of SCR catalysts for anti-arsenic poisoning[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2365-2374. | |
| 3 | 环境保护部. 钢铁烧结、球团工业大气污染物排放标准: [S]. 北京: 中国环境科学出版社, 2012. |
| Minsitey of Environment Protection of the People’s Republic of China. Emission standard of air pollutants for sintering and pelletizing of iron and steel industry: [S]. Beijing: China Environment Science Press, 2012. | |
| 4 | DELMAS R, SER A D, JAMBERT C. Global inventory of NOx sources[J]. Nutrient Cycling in Agroecosystems, 1997, 48(1): 51-60. |
| 5 | 刘勇军, 王雪娇, 巩梦丹, 等. 氮氧化物控制技术现状与进展[J]. 四川环境, 2014, 33(6): 115-117. |
| LIU Yongjun, WANG Xuejiao, GONG Mengdan, et al. Current situation and progress of nitrogen oxide pollution control technology[J]. Sichuan Environment, 2014, 33(6): 115-117. | |
| 6 | FU M F, LI C T, LU P, et al. A review on selective catalytic reduction of NOx by supported catalysts at 100-300℃—Catalysts, mechanism, kinetics[J]. Catalysis Science & Technology, 2014, 4(1): 14-25. |
| 7 | LI J, CHANG H, MA L, et al. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review[J]. Catalysis Today, 2011, 175(1): 147-156. |
| 8 | HUANG B, HUANG R, JIN D, et al. Low temperature SCR of NO with NH3 over carbon nanotubes supported vanadium oxides[J]. Catalysis Today, 2007, 126(3-4): 279-283. |
| 9 | WNAG X B, GUI K T. Fe2O3 particles as superior catalysts for low temperature selective catalytic reduction of NO with NH3[J]. Journal of Environmental Sciences, 2013, 25(12): 2469-2475. |
| 10 | KAPTEIJN F, SINGOREDJO L, ANDREINI A, et al. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia[J]. Applied Catalysis B: Environmental, 1994, 3(2/3): 173-189. |
| 11 | WANG X B, WU S G, ZOU W X, et al. Fe-Mn/Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3[J]. Chinese Journal of Catalysis, 2016, 37(8): 1314-1323. |
| 12 | 章贤臻, 王世磊, 李运姣, 等. 天然锰矿低温NH3-SCR烟气脱硝实验研究[J]. 矿产保护与利用, 2019, 39(3): 167-172. |
| ZHANG Xianzhen, WANG Shilei, LI Yunjiao, et al. Technological study of selective catalytic reduction of NO with NH3 over natural manganese ore catalysts at low temperature[J]. Conservation and Utilization of Mineral Resources, 2019, 39(3): 167-172. | |
| 13 | 徐永鹏, 刘海波, 陈冬, 等. 酸浸天然锰矿石低温氧化脱硝性能研究[J]. 合肥工业大学学报(自然科学版), 2017, 40(8): 1133-1138, 1143. |
| XU Yongpeng, LIU Haibo, CHEN Dong, et al. Performance of acid dipping of natural manganese ore for denitration with low temperature oxidation[J]. Journal of Hefei University of Technology (Natural Science), 2017, 40(8): 1133-1138, 1143. | |
| 14 | 卢慧霞, 归柯庭. 铁矿石SCR低温脱硝催化剂的改性研究[J]. 动力工程学报, 2017, 37(9): 726-731. |
| LU Huixia, GUI Keting. Study on modification of iron ore catalysts for low-temperature SCR denitrification[J]. Journal of Chinese Society of Power Engineering, 2017, 37(9): 726-731. | |
| 15 | ZHANG Y, LIN H, DONG Y B, et al. Coupling relationship between multicomponent recovery of rare earth tailings[J]. Rare Metals, 2017, 36(3): 220-228. |
| 16 | WANG L, LIANG T. Accumulation and fractionation of rare earth elements in atmospheric particulates around a mine tailing in Baotou, China[J]. Atmospheric Environment, 2014, 88: 23-29. |
| 17 | 郑强. 综合回收白云鄂博弱磁尾矿中铁、稀土、氟和磷的研究[D]. 沈阳: 东北大学, 2017. |
| ZHENG Qiang. Studies on comprehensive recovery of iron, rare earth, fluorine, and phosphorus from Bayan Obo weakly magnetic tailings[D]. Shenyang: Northeastern University, 2017. | |
| 18 | 付强, 金建文, 李磊. 白云鄂博尾矿库中铁的赋存状态研究[J]. 矿冶, 2017, 26(3): 94-98. |
| FU Qiang, JIN Jianwen, LI Lei. The study of iron occurrence state in Baiyun Obo tailings[J]. Mining & Metallurgy, 2017, 26(3): 94-98. | |
| 19 | 张悦, 林海, 董颖博, 等. 白云鄂博地区尾矿中铁、铌、稀土、萤石综合回收研究[J]. 稀有金属, 2017, 41(7): 799-809. |
| ZHANG Yue, LIN Hai, DONG Yingbo, et al. Comprehensive recovery of iron, niobium, rare earth and fluorite in Bayan Obo tailings[J]. Chinese Journal of Rare Metals, 2017, 41(7): 799-809. | |
| 20 | 付强, 金建文, 李磊, 等. 白云鄂博尾矿中稀土的赋存状态研究[J]. 稀土, 2017, 38(5): 103-110. |
| FU Qiang, JIN Jianwen, LI Lei, et al. The study of REE occurrence state in Bayan Obo tailings[J]. Chinese Rare Earths, 2017, 38(5): 103-110. | |
| 21 | 宋增凯, 陈菓, 彭金辉, 等. 微波加热技术在典型冶金工艺中的应用研究进展[J]. 矿冶, 2014, 23(3): 57-63. |
| SONG Zengkai, CHEN Guo, PENG Jinhui, et al. Research progress of application of microwave heating in typical metallurgical technology[J]. Mining & Metallurgy, 2014, 23(3): 57-63. | |
| 22 | GONG Z J, WU W F, ZHAO Z W, et al. Synergy between ferric oxide and rare earth oxides in rare earth tailings for the denitration of semi-coke[J]. Catalysis Today, 2018, 318: 175-179. |
| 23 | GONG Z J, WU W F, ZHAO Z W, et al. Combination of catalytic combustion and catalytic denitration on semi-coke with Fe2O3 and CeO2[J]. Catalysis Today, 2018, 318: 59-65. |
| 24 | 龚志军, 武文斐, 张凯, 等. 白云鄂博稀土矿物催化半焦燃烧与脱硝特性的研究[J]. 中国稀土学报, 2018, 36(5): 564-570. |
| GONG Zhijun, WU Wenfei, ZHANG Kai, et al. Characteristics of char combustion and NOx abatement catalyzed with rare earth ore[J]. Journal of the Chinese Society of Rare Earths, 2018, 36(5): 564-570. | |
| 25 | QI G, YANG R T, CHANG R. MnOx-CeOx mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures[J]. Applied Catalysis B: Environmental, 2004, 51(2): 93-106. |
| 26 | 程蕾. 高能球磨法制备Mg2TiO4、MgO纳米粉体及其陶瓷的微波介电性能研究[D]. 西安: 陕西师范大学, 2013. |
| CHENG Lei. Microwave dielectric properties of Mg2TiO4 and MgO nano powders and the ceramics prepared by high energy ball milling[D]. Xi’an: Shaanxi Normal University, 2013. | |
| 27 | 吴清军. 高能球磨法制备SiC/Al复合材料[D]. 昆明: 昆明理工大学, 2012. |
| WU Qingjun. SiC/Al composites prepared by high energy ball milling[D]. Kunming: Kunming University of Science and Technology, 2012. | |
| 28 | WANG X Y, ZHENG J S, FU R, et al. Effect of microwave power and irradiation time on the performance of Pt/C catalysts synthesized by pulse-microwave assisted chemical reduction[J]. Chinese Journal of Catalysis, 2011, 32(4): 599-605. |
| 29 | 黄宇坤. 微波强化分解包头稀土矿清洁工艺的基础研究[D]. 沈阳: 东北大学, 2017. |
| HUANG Yukun. A fundamental study on a cleaning process of mixed rare earth ore from Baotou decomposed with microwave heating[D]. Shenyang: Northeastern University, 2017. | |
| 30 | 雷鹰. 微波强化还原低品位钛精矿新工艺及理论研究[D]. 昆明: 昆明理工大学, 2011. |
| LEI Ying. New technology and theoretical study on microwave enhanced reduction of low grade titanium concentrate[D]. Kunming: Kunming University of Science and Technology, 2011. | |
| 31 | 滕兆龙. 锰基复合柱撑蒙脱土催化剂制备与脱硝活性研究[D]. 沈阳: 东北大学, 2018. |
| TENG Zhaolong. Preparation and denitration activity of manganese base composite pillared clay catalyst[D]. Shenyang: Northeastern University, 2018. | |
| 32 | 刘文卿. 实验设计[M]. 北京: 清华大学出版社, 2007. |
| LIU Wenqing. Experimental design[M]. Beijing: Tsinghua University Press, 2007. | |
| 33 | 左亚军. 基于正交试验法的注塑机合模机构的优化设计研究[D]. 广州: 广东工业大学, 2012. |
| ZUO Yajun. Research on optimal design of clamping mechanism for an injection machine using orthogonal design method[D]. Guangzhou: Guangdong University of Technology, 2012. | |
| 34 | 成岳, 夏光华. 科学研究与工程试验设计方法[M]. 武汉: 武汉理工大学出版社, 2005. |
| CHENG Yue, XIA Guanghua. Design method of scientific research and engineering experiment[M]. Wuhan: Wuhan University of Technology Press, 2005. | |
| 35 | WEI M Q, YU Q B, MU T T, et al. Preparation and characterization of waste ion-exchange resin-based activated carbon for CO2 capture[J]. Adsorption, 2016, 22(3): 385-396. |
| 36 | WEI M Q, YU Q B, DUAN W J, et al. CO2 adsorption and desorption performance of waste ion-exchange resin-based activated carbon[J]. Environmental Progress & Sustainable Energy, 2018, 37(2): 703-711. |
| 37 | 杨洋, 胡准, 米容立, 等. Mn负载量对nMnOx/TiO2催化剂NH3-SCR催化性能的影响[J]. 分子催化, 2020, 34(4): 313-322. |
| YANG Yang, HU Zhun, MI Rongli, et al. Effect of Mn loading on catalytic performance of nMnOx/TiO2 in NH3-SCR reaction[J]. Journal of Molecular Catalysis, 2020, 34(4): 313-322. | |
| 38 | 周超, 赵阳, 徐佳, 等. pH值对浸渍法制备的铈钨钛脱硝催化剂的影响[J]. 稀土, 2020, 41(5): 59-69. |
| ZHOU Chao, ZHAO Yang, XU Jia, et al. Effect of pH on denitration performance of CeWTi catalyst[J]. Chinese Rare Earths, 2020, 41(5): 59-69. | |
| 39 | ZHU X B, WANG Y L, HUANG Y, et al. Selective catalytic reduction of NO with NH3 over Ce-W-Ti oxide catalysts prepared by solvent combustion method[J]. Applied Sciences, 2018, 8(12): 2430-2439. |
| 40 | 余雅昕. 铁基NH3-SCR催化剂的低温脱硝性能及抗SO2中毒机制研究[D]. 南京: 南京大学, 2020. |
| YU Yaxin. Study on the mechanism of SO2 resistance and the catalytic performance in low-temperature selective catalytic reduction of NO by NH3 over Fe-based catalysts[D]. Nanjing: Nanjing University, 2020. | |
| 41 | 黄秀兵, 王鹏, 陶进长, 等. CeO2修饰Mn-Fe-O复合材料及其NH3-SCR脱硝催化性能[J]. 无机材料学报, 2020, 35(5): 573-580. |
| HUANG Xiubing, WANG Peng, TAO Jinzhang, et al. CeO2 modified Mn-Fe-O composites and their catalytic performance for NH3-SCR of NO[J]. Journal of Inorganic Materials, 2020, 35(5): 573-580. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [14] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [15] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||