化工进展 ›› 2021, Vol. 40 ›› Issue (2): 813-823.DOI: 10.16085/j.issn.1000-6613.2020-0687
乙炔氢氯化反应无汞贵金属催化剂的研究进展
代元元1( ), 李杰2, 王志文2, 赵长森2, 解荣永2(
), 李杰2, 王志文2, 赵长森2, 解荣永2( )
)
- 1.上海大学材料基因组工程研究院,上海 200444
2.鄂尔多斯市瀚博科技有限公司,内蒙古 鄂尔多斯 016064
-
收稿日期:2020-04-27修回日期:2020-07-11出版日期:2021-02-05发布日期:2021-02-09 -
通讯作者:解荣永 -
作者简介:代元元(1990—),女,博士,研究方向为绿色催化工程。E-mail:daiyuanyuan@shu.edu.cn 。
Research progress of mercury-free noble metal catalysts for acetylene hydrochlorination
Yuanyuan DAI1( ), Jie LI2, Zhiwen WANG2, Changsen ZHAO2, Rongyong XIE2(
), Jie LI2, Zhiwen WANG2, Changsen ZHAO2, Rongyong XIE2( )
)
- 1.Institute of Materials Genomics Engineering, Shanghai University, Shanghai 200444, China
2.Ordos Hanbo Technology Co. , Ltd. , Ordos 016064, Inner Mongolia, China
-
Received:2020-04-27Revised:2020-07-11Online:2021-02-05Published:2021-02-09 -
Contact:Rongyong XIE
摘要:
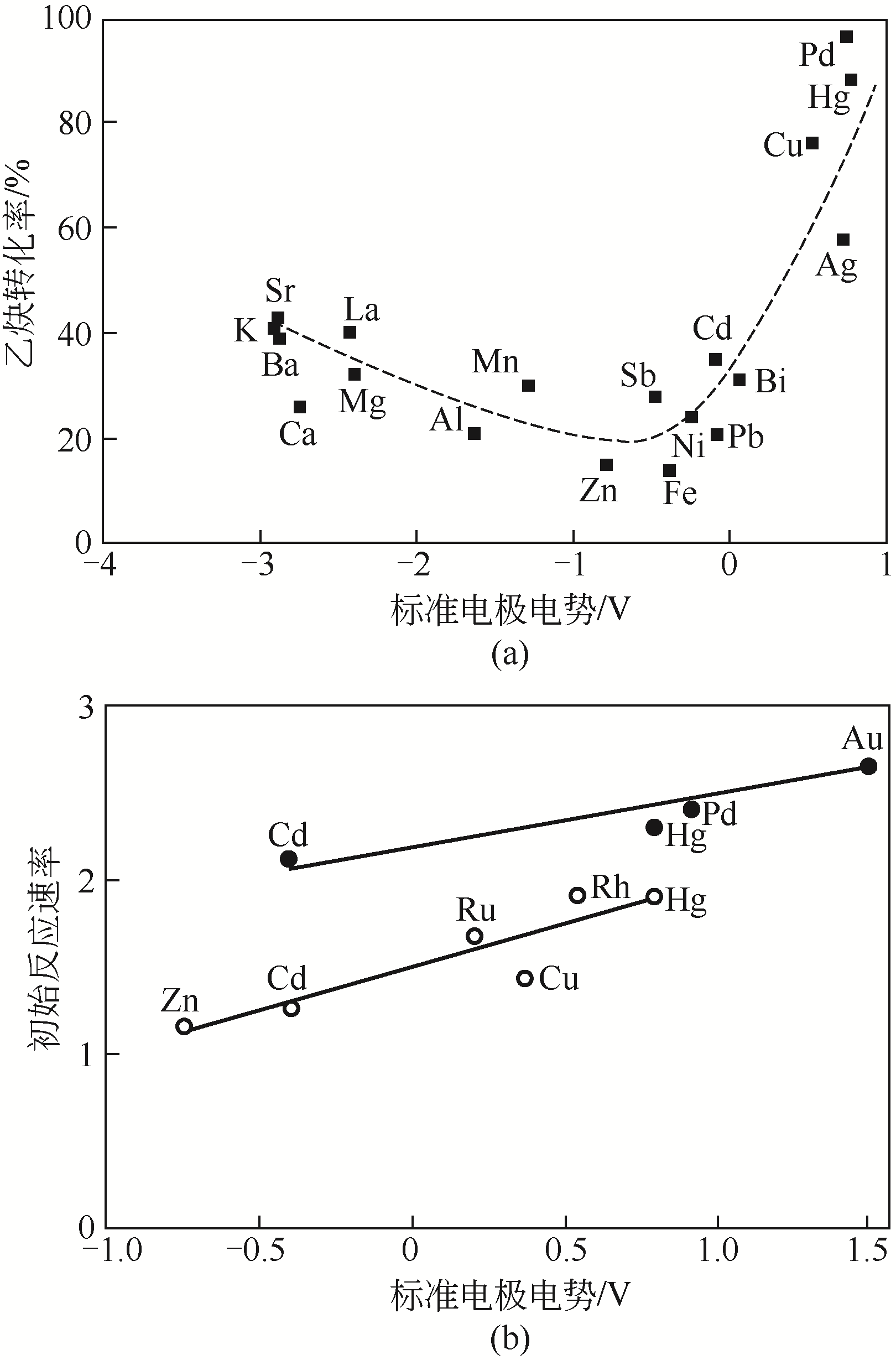
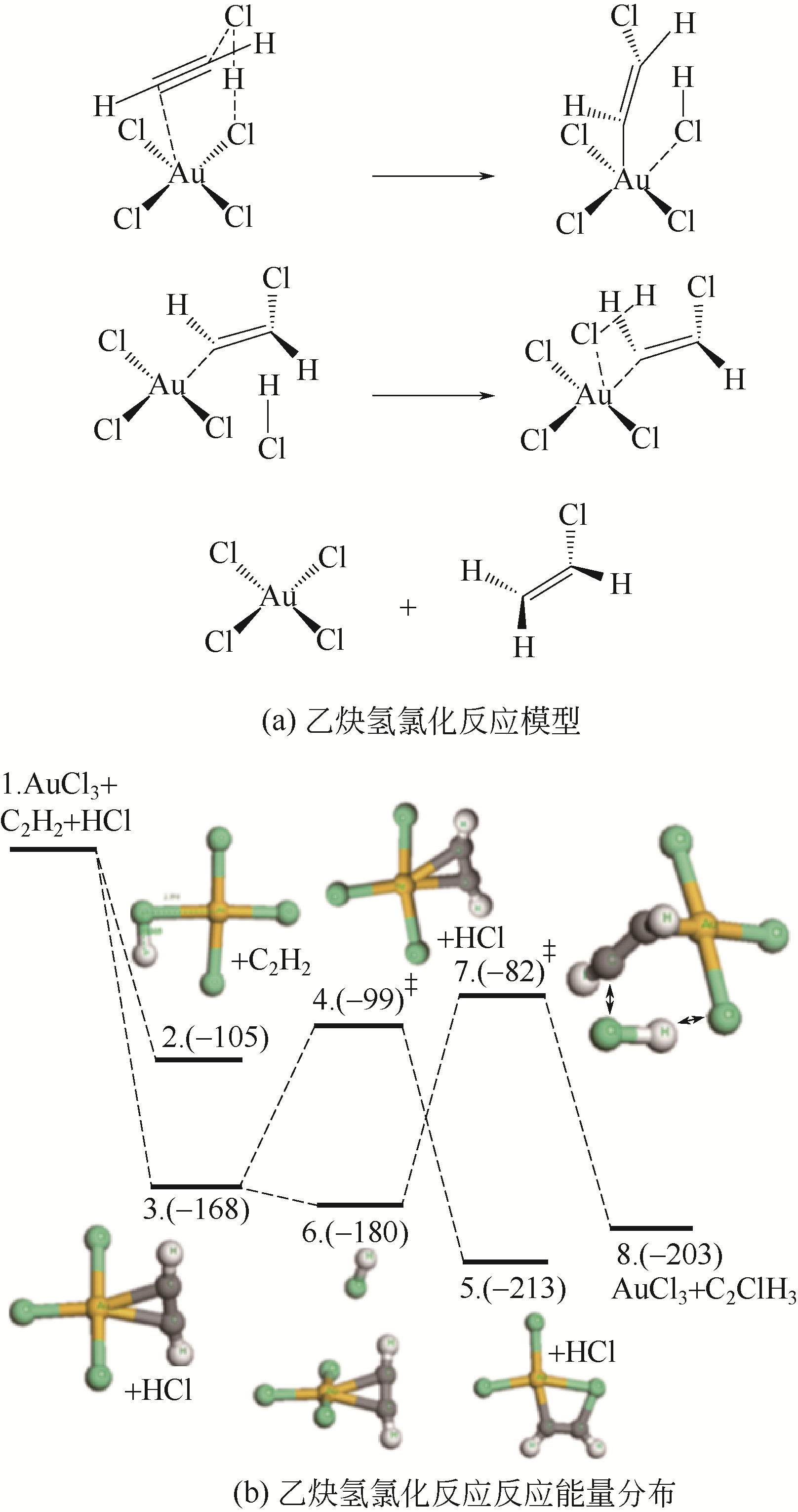
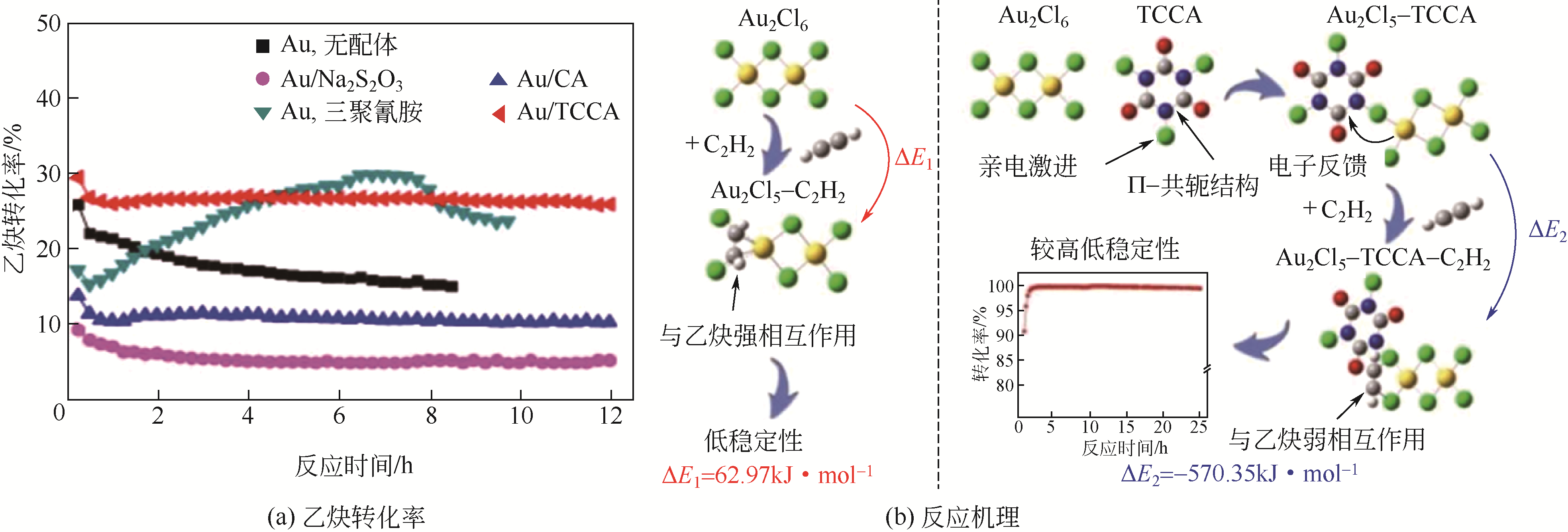
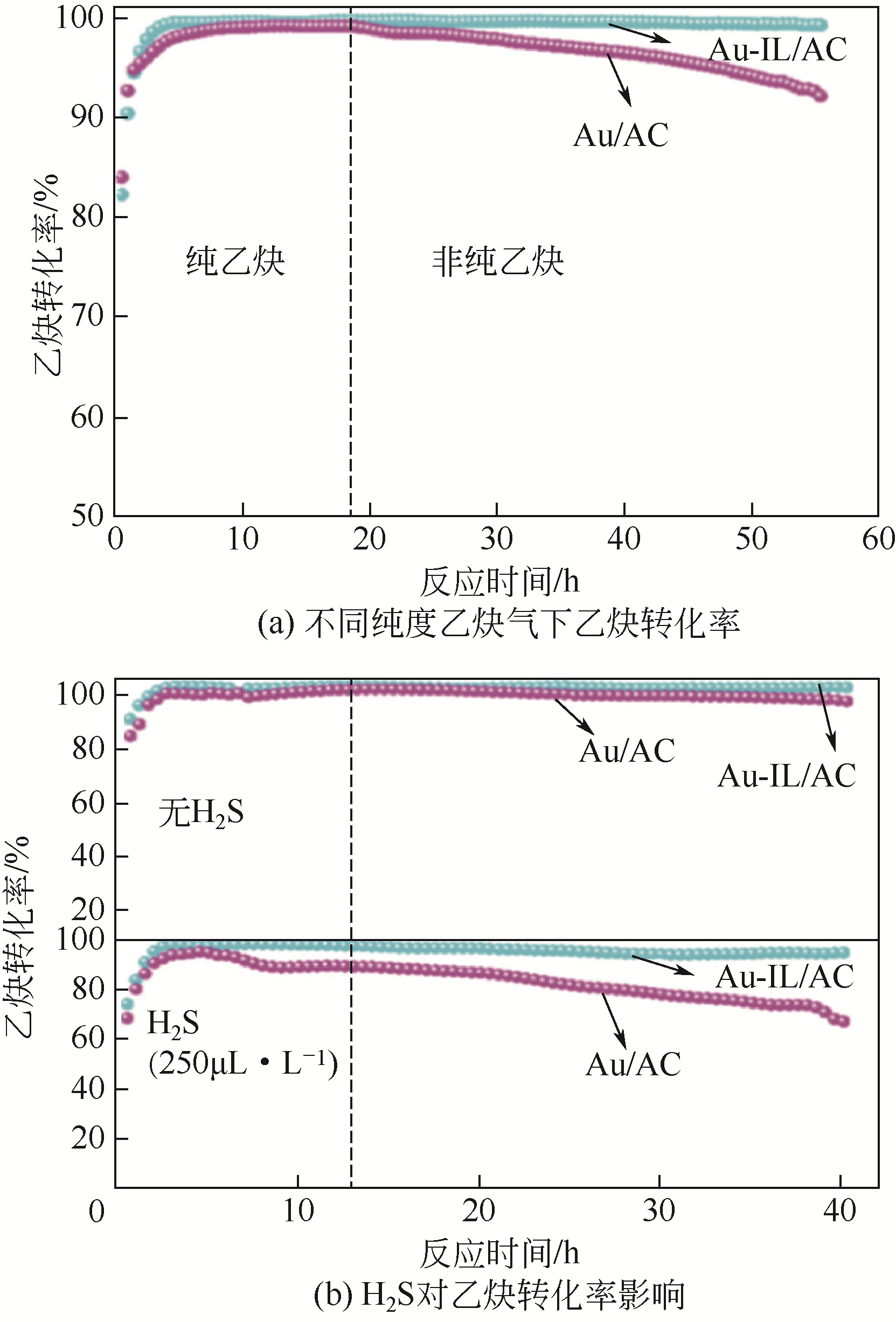
受“水俣公约”和环境保护政策约束,研究和开发新型无汞催化剂是保障我国乙炔法制备聚氯乙烯未来发展的核心环节。无汞催化剂包括贵金属催化剂、非贵金属催化剂和非金属催化剂,其中贵金属催化剂被认为是最具有工业化应用前景的催化剂。本文概述了近年来国内外乙炔氢氯化贵金属Au、Ru、Pd和Pt催化剂的研究进展,重点综述了不同催化剂的活性组分、催化机理及催化剂的改性方法等方面的研究,分析了不同改性方法的作用机制及不同催化剂改性的研究方向。研究表明,添加助剂或多金属复配、配体和离子液体的使用及载体改性均使贵金属催化剂的催化活性和稳定性得到一定程度提高,从而进一步增加了贵金属催化剂工业化应用的可能性。
中图分类号:
引用本文
代元元, 李杰, 王志文, 赵长森, 解荣永. 乙炔氢氯化反应无汞贵金属催化剂的研究进展[J]. 化工进展, 2021, 40(2): 813-823.
Yuanyuan DAI, Jie LI, Zhiwen WANG, Changsen ZHAO, Rongyong XIE. Research progress of mercury-free noble metal catalysts for acetylene hydrochlorination[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 813-823.
| 催化剂 | 温度 /℃ | 空速 /h-1 | 运行时间 /h | 转化率 /% | 选择性 /% | 参考文献 |
|---|---|---|---|---|---|---|
| Au(0.5%)-Cu/AC | 160 | 50 | 200 | 99.5 | 99.5 | [ |
| Au(1%)-Sn/AC | 170 | 720 | 48 | 95 | 99 | [ |
| Au(0.3%)-Bi/AC | 180 | 600 | 5 | 85 | — | [ |
| Au(1%)-Cs/AC | 180 | 50 | 500 | 99.5 | 99.9 | [ |
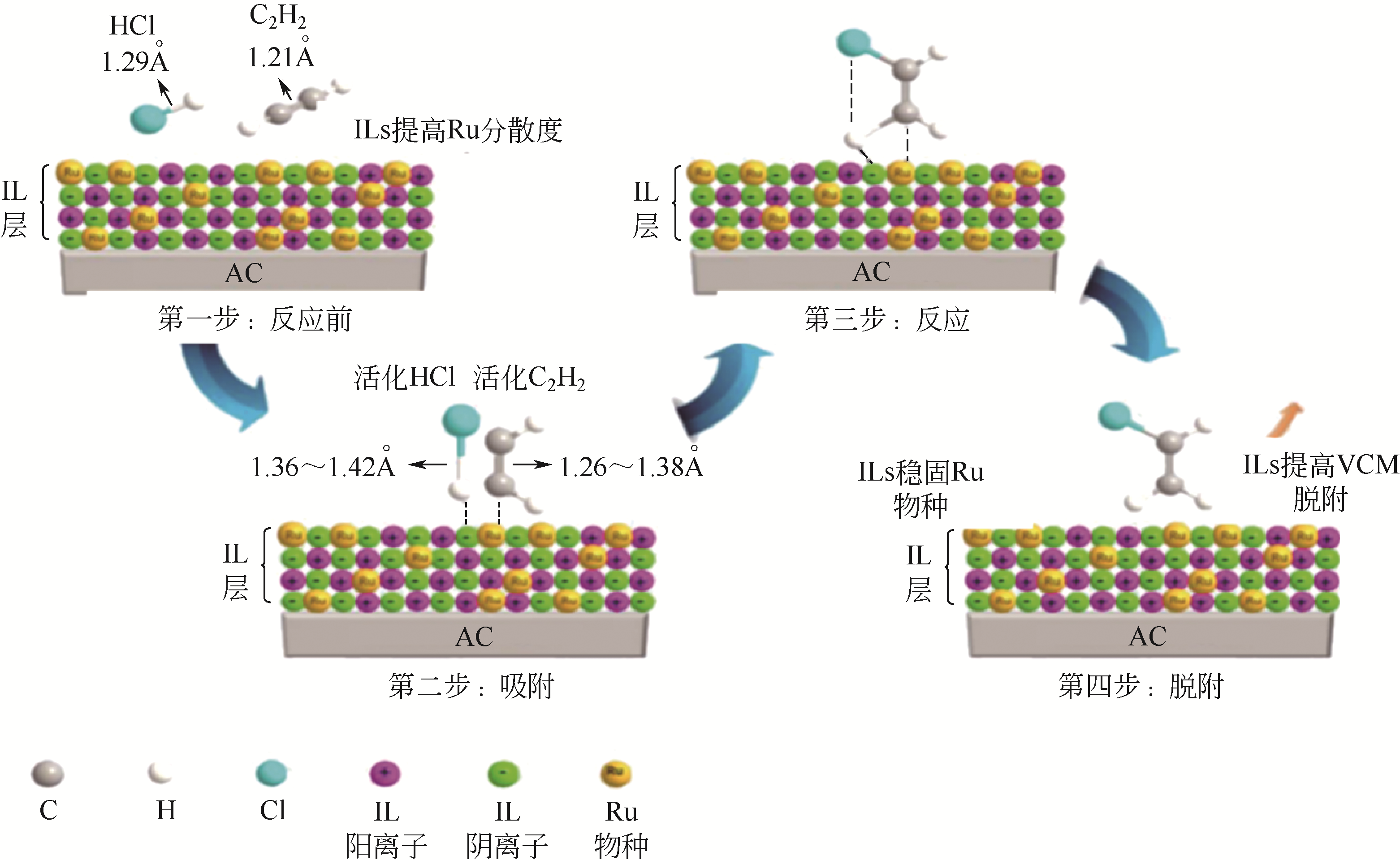
| Au(0.2%)-Cu-K/AC | 165 | 40 | 1600 | 89 | 99.7 | [ |
| Au(0.25%)-Cu-Cs/AC | 180 | 50 | 600 | 98.5 | 99.9 | [ |
| Au(1%)-In-Cs/AC | 180 | 1480 | 50 | 89.1 | 99.9 | [ |
| Au(1%)-Li/AC | 180 | 600 | 48 | 91.3 | 99.9 | [ |
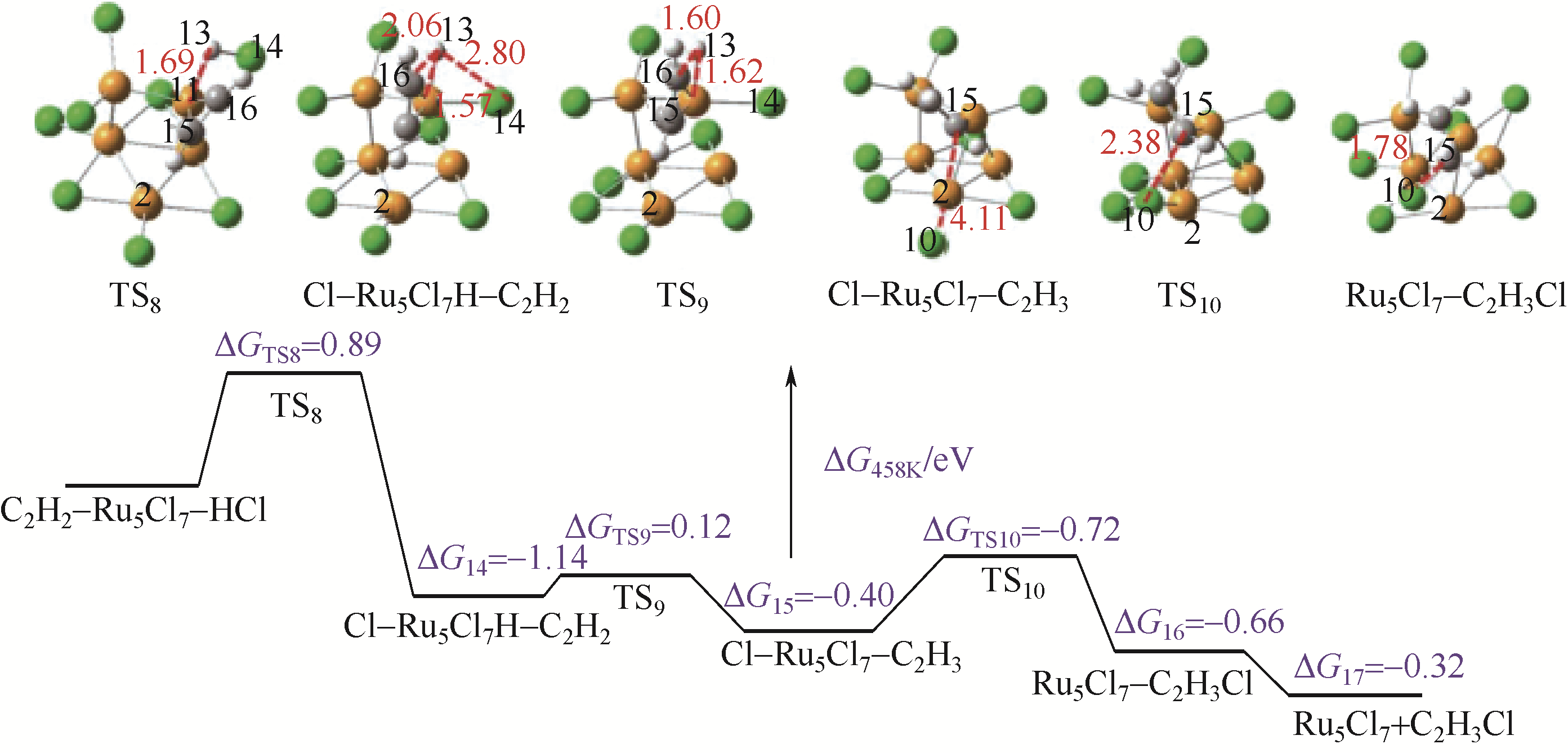
| Au(1%)-Ba/AC | 200 | 360 | 86 | 92.3 | 99.9 | [ |
| 1Au(1.5%)-Ni/CSs | 170 | 900 | 46 | 97 | 99.9 | [ |
表1 不同助金属对Au基催化剂的催化性能影响
| 催化剂 | 温度 /℃ | 空速 /h-1 | 运行时间 /h | 转化率 /% | 选择性 /% | 参考文献 |
|---|---|---|---|---|---|---|
| Au(0.5%)-Cu/AC | 160 | 50 | 200 | 99.5 | 99.5 | [ |
| Au(1%)-Sn/AC | 170 | 720 | 48 | 95 | 99 | [ |
| Au(0.3%)-Bi/AC | 180 | 600 | 5 | 85 | — | [ |
| Au(1%)-Cs/AC | 180 | 50 | 500 | 99.5 | 99.9 | [ |
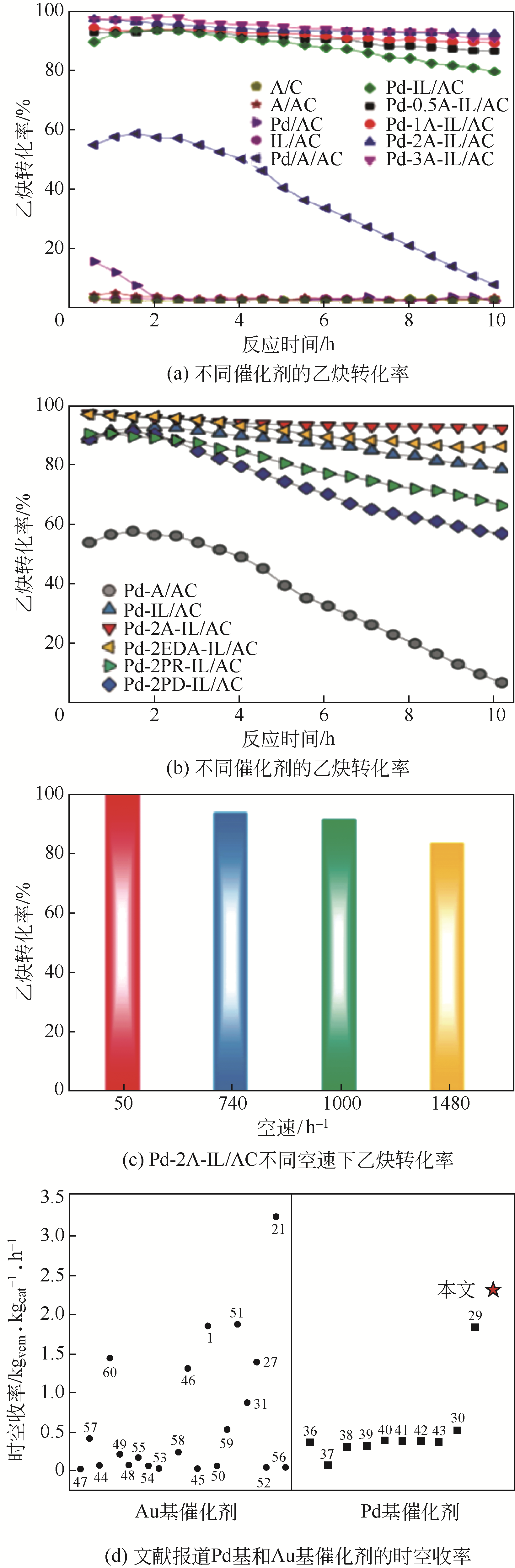
| Au(0.2%)-Cu-K/AC | 165 | 40 | 1600 | 89 | 99.7 | [ |
| Au(0.25%)-Cu-Cs/AC | 180 | 50 | 600 | 98.5 | 99.9 | [ |
| Au(1%)-In-Cs/AC | 180 | 1480 | 50 | 89.1 | 99.9 | [ |
| Au(1%)-Li/AC | 180 | 600 | 48 | 91.3 | 99.9 | [ |
| Au(1%)-Ba/AC | 200 | 360 | 86 | 92.3 | 99.9 | [ |
| 1Au(1.5%)-Ni/CSs | 170 | 900 | 46 | 97 | 99.9 | [ |
| Au前体配合物 | Au负载量/% | 转化率/% |
|---|---|---|
| Au[CS(NH2)2]2 | 0.1 | 95 |
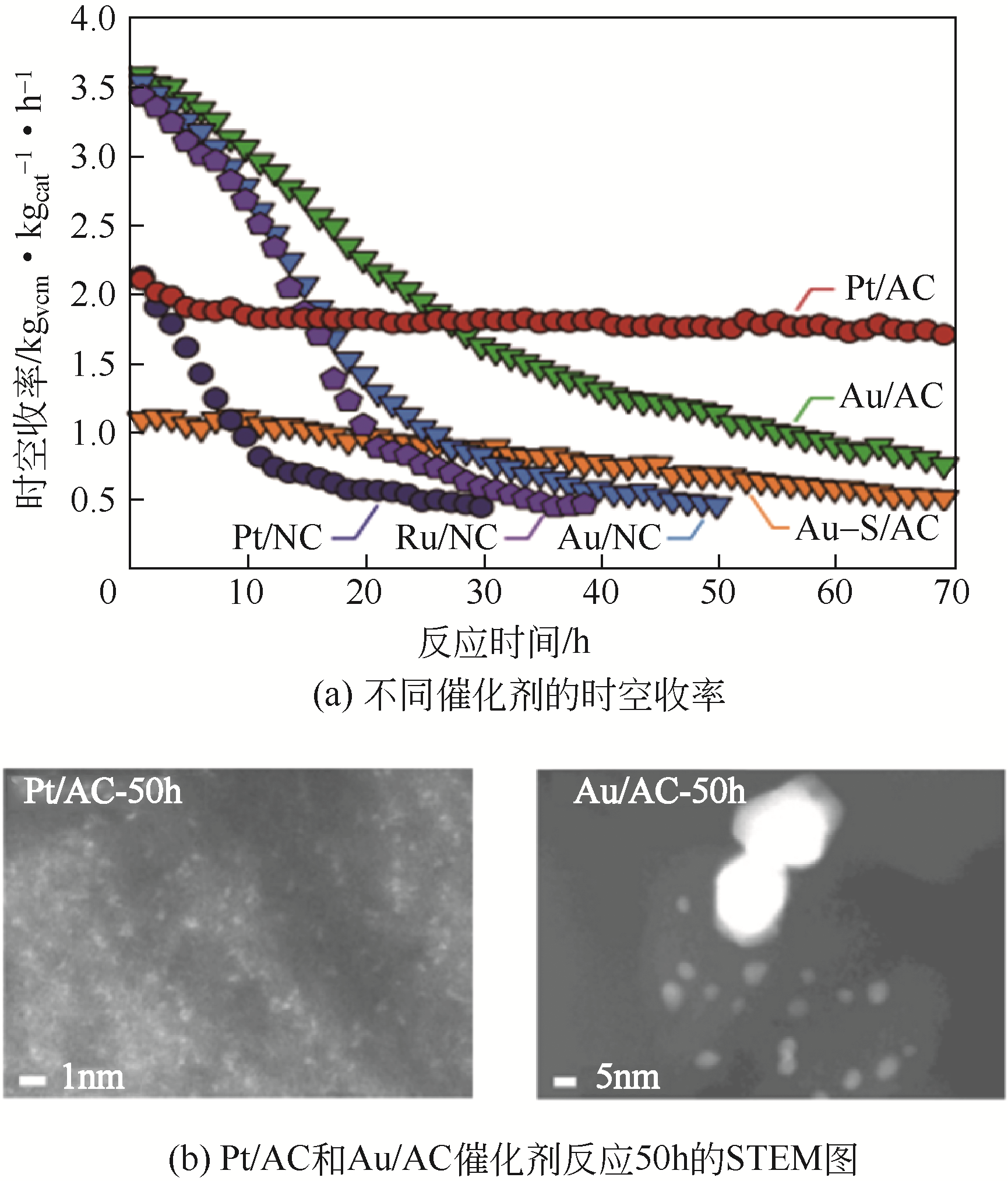
| Na3Au(S2O3)2 | 0.1 | 86 |
| KAu(CN)2 | 0.1 | 85 |
| (NH4)3Au(S2O3)2 | 0.1 | 75 |
| KAu(SCN)4 | 0.1 | 74 |
| Ca3[Au(S2O3)2]2 | 0.1 | 74 |
| KAu(CN)4 | 0.1 | 69 |
| Au(NCNH2)2 | 0.1 | 55 |
| HAuCl4+Aqua regia | 1 | 52 |
| HAu(C3Cl3N3O3)3Cl | 1 | 52 |
| [Au(P(NCH2CH2OCH2CH2)3)2]NO3 | 1 | 33 |
| [(AuCl)2dppe] | 1 | 14 |
| [Au(en)2]Cl3 | 1 | 14 |
| HAuCl4+H2O | 1 | 11 |
| 炭黑挤出物(无Au) | 0 | 7 |
表2 不同配体对Au基催化剂的催化性能影响[30]
| Au前体配合物 | Au负载量/% | 转化率/% |
|---|---|---|
| Au[CS(NH2)2]2 | 0.1 | 95 |
| Na3Au(S2O3)2 | 0.1 | 86 |
| KAu(CN)2 | 0.1 | 85 |
| (NH4)3Au(S2O3)2 | 0.1 | 75 |
| KAu(SCN)4 | 0.1 | 74 |
| Ca3[Au(S2O3)2]2 | 0.1 | 74 |
| KAu(CN)4 | 0.1 | 69 |
| Au(NCNH2)2 | 0.1 | 55 |
| HAuCl4+Aqua regia | 1 | 52 |
| HAu(C3Cl3N3O3)3Cl | 1 | 52 |
| [Au(P(NCH2CH2OCH2CH2)3)2]NO3 | 1 | 33 |
| [(AuCl)2dppe] | 1 | 14 |
| [Au(en)2]Cl3 | 1 | 14 |
| HAuCl4+H2O | 1 | 11 |
| 炭黑挤出物(无Au) | 0 | 7 |
| 1 | ZHONG J W, XU Y P, LIU Z M. Heterogeneous non-mercury catalysts for acetylene hydrochlorination: progress, challenges, and opportunities[J]. Green Chemistry, 2018, 20: 2412-2427. |
| 2 | YANG L F, YANG Q W, HU J Y, et al. Metal nanoparticles in ionic liquid-cosolvent biphasic systems as active catalysts for acetylene hydrochlorination[J]. AIChE Journal, 2018, 64(7): 2536-2544. |
| 3 | 乔贤亮, 关庆鑫, 李伟. 乙炔氢氯化无汞催化剂研究进展[J]. 中国科学:化学, 2019, 49(11): 1385-1400. |
| QIAO Xianliang, GUAN Qingxin, LI Wei. Research progress on mercury-free catalysts for acetylene hydrochlorination[J]. Scientia Sinica Chimica, 2019, 49(11): 1385-1400. | |
| 4 | 申玉海, 朱峰云, 李保瑞, 等. 乙炔氢氯化合成氯乙烯催化剂的研究进展[J]. 工程塑料应用, 2019, 47(5): 153-158. |
| SHEN Yuhai, ZHU Fengyun, LI Baorui, et al. Development of researches on catalysts for acetylene hydrochloride to synthesis chloroethylene[J]. Engineering Plastics Application, 2019, 47(5):153-158. | |
| 5 | SMITH D M, WALSH P M, SLAGER T L. Studies of silica-supported metal chloride catalysts for the vapor-phase hydrochlorination of acetylene[J]. Journal of Catalysis, 1968, 11: 113-130. |
| 6 | SHINODA K. The vapor-phase hydrochlorination of acetylene over metal chlorides supported on activate carbon[J]. Chemistry Letters, 1975, 219-220. |
| 7 | NKOSI B, HUTCHINGS G J. Vapour phase hydrochlorination of acetylene with group Ⅷ and ⅠB metal chloride catalysts[J]. Applied Catalysis, 1988, 43: 33-39. |
| 8 | HUTCHINGS G J. Vapor phase hydrochlorination of acetylene correlation of catalytic activity of supported metal chloride catalysts[J]. Journal of Catalysis, 1985, 96: 292-295. |
| 9 | CONTE M, CARLEY A F, HEIRENE C, et al. Hydrochlorination of acetylene using a supported gold catalyst: a study of the reaction mechanism[J]. Journal of Catalysis, 2007, 250(2): 231-239. |
| 10 | MALTA G, KONDRAT S A, FREAKLEY S J, et al. Identification of single-site gold catalysis in acetylene hydrochlorination[J]. Science, 2017, 355: 1399-1403. |
| 11 | KAISER S K, LIN R, MITCHELL S, et al. Controlling the speciation and reactivity of carbon-supported gold nanostructures for catalysed acetylene hydrochlorination[J]. Chemical Science, 2019, 10(2): 359-369. |
| 12 | ZHU M Y, WANG Q Q, CHEN K, et al. Development of a heterogeneous non-mercury catalyst for acetylene hydrochlorination[J]. ACS Catalysis, 2015, 5(9): 5306-5316. |
| 13 | ZHAO J, ZHANG T T, DI X X, et al. Activated carbon supported ternary gold-cesium(Ⅰ)-indium(Ⅲ) catalyst for the hydrochlorination of acetylene[J]. Catalysis Science & Technology, 2015, 5(11): 4973-4984. |
| 14 | LI G B, LI W, ZHANG J L. Non-mercury catalytic acetylene hydrochlorination over activated carbon-supported Au catalysts promoted by CeO2[J]. Catalysis Science & Technology, 2016, 6(6): 1821-1828. |
| 15 | HUANG C F, ZHU M Y, KANG L H, et al. Active carbon supported TiO2-AuCl3/AC catalyst with excellent stability for acetylene hydrochlorination reaction[J]. Chemical Engineering Journal, 2014, 242: 69-75. |
| 16 | XU H Y, LUO J J, XU S Y, et al. Promoter effect of La2O3 on gold catalyst with different textural structures[J]. Journal of Energy Chemistry, 2016, 25(5): 854-860. |
| 17 | YE L, DUAN X, WU S, et al. Self-regeneration of Au/CeO2 based catalysts with enhanced activity and ultra-stability for acetylene hydrochlorination[J]. Nature Communications, 2019, 10(1): 914. |
| 18 | CONTE M, CARLEY A, ATTARD G, et al. Hydrochlorination of acetylene using supported bimetallic Au-based catalysts[J]. Journal of Catalysis, 2008, 257(1): 190-198. |
| 19 | ZHANG H Y, DAI BM WANG X G, et al. Non-mercury catalytic acetylene hydrochlorination over bimetallic Au-Co(iii)/SAC catalysts for vinyl chloride monomer production[J]. Green Chemistry, 2013, 15(3): 829. |
| 20 | ZHANG H Y, DAI B, LI W, et al. Non-mercury catalytic acetylene hydrochlorination over spherical activated-carbon-supported Au- Co(Ⅲ)-Cu(Ⅱ) catalysts[J]. Journal of Catalysis, 2014, 316: 141-148. |
| 21 | WANG S J, SHEN B X, SONG Q L. Kinetics of acetylene hydrochlorination over bimetallic Au-Cu/C catalyst[J]. Catalysis Letters, 2009, 134: 102-109. |
| 22 | DONG Y Z, ZHANG H Y, LI W, et al. Bimetallic Au-Sn/AC catalysts for acetylene hydrochlorination[J]. Journal of Industrial and Engineering Chemistry, 2016, 35: 177-184. |
| 23 | ZHOU K, WANG W, ZHAO Z, et al. Synergistic gold-bismuth catalysis for non-mercury hydrochlorination of acetylene to vinyl chloride monomer[J]. ACS Catalysis, 2014, 4(9): 3112-3116. |
| 24 | ZHAO J, XU J T, XU J H, et al. Activated-carbon-supported gold-cesium as highly effective catalysts for hydrochlorination of acetylene to vinyl chloride[J]. ChemPlusChem, 2015, 80(1): 196-201. |
| 25 | WANG L, SHEN B X, ZHAO J G, et al. Trimetallic Au-Cu-K/AC for acetylene hydrochlorination[J]. The Canadian Journal of Chemical Engineering, 2017, 95(6): 1069-1075. |
| 26 | ZHAO J, GU S C, XU X L, et al. Promotional effect of copper(Ⅱ) on an activated carbon supported low content bimetallic gold-cesium(Ⅰ) catalyst in acetylene hydrochlorination[J]. RSC Advances, 2015, 5(123): 101427-101436. |
| 27 | HU D, WANG L, WANG F, et al. Bimetallic Au-Li/SAC catalysts for acetylene hydrochlorination[J]. Catalysis Communications, 2018, 115: 45-48. |
| 28 | ZHANG H Y, LI W, LI X Q, et al. Non-mercury catalytic acetylene hydrochlorination over bimetallic Au-Ba(Ⅱ)/AC catalysts[J]. Catalysis Science & Technology, 2015, 5(3): 1870-1877. |
| 29 | PU Y F, ZHANG J L, WANG X, et al. Bimetallic Au-Ni/CSs catalysts for acetylene hydrochlorination[J]. Catalysis Science & Technology, 2014, 4(12): 4426-4432. |
| 30 | JOHNSTON P, CARTHEY N, HUTCHINGS G J. Discovery, development, and commercialization of gold catalysts for acetylene hydrochlorination[J]. Journal of the American Chemical Society, 2015, 137(46): 14548-14557. |
| 31 | XU H, ZHOU K, SI J K, et al. A ligand coordination approach for high reaction stability of an Au-Cu bimetallic carbon-based catalyst in the acetylene hydrochlorination process[J]. Catalysis Science & Technology, 2016, 6(5): 1357-1366. |
| 32 | ZHAO J, GU S C, XU X L, et al. Supported ionic-liquid-phase-stabilized Au(Ⅲ) catalyst for acetylene hydrochlorination[J]. Catalysis Science & Technology, 2016, 6(9): 3263-3270. |
| 33 | ZHAO J, YU Y, XU X L, et al. Stabilizing Au(Ⅲ) in supported-ionic-liquid-phase (SILP) catalyst using CuCl2via a redox mechanism[J]. Applied Catalysis B: Environmental, 2017, 206: 175-183. |
| 34 | YUE Y X, WANG B L, SHENG G F, et al. An ultra-high H2S-resistant gold-based imidazolium ionic liquid catalyst for acetylene hydrochlorination[J]. New Journal of Chemistry, 2019, 43(32): 12767-12775. |
| 35 | DI X X, ZHAO J, YU Y, et al. One-pot synthesis of nitrogen and sulfur co-doped activated carbon supported AuCl3 as efficient catalysts for acetylene hydrochlorination[J]. Chinese Chemical Letters, 2016, 27(9): 1567-1571. |
| 36 | ZHU M Y, KANG L H, SU Y, et al. MClx (M=Hg, Au, Ru; x=2,3) catalyzed hydrochlorination of acetylene—A density functional theory study[J]. Canadian Journal of Chemistry, 2013, 91(2): 120-125. |
| 37 | PU Y F, ZHANG J L, YU L, et al. Active ruthenium species in acetylene hydrochlorination[J]. Applied Catalysis A: General, 2014, 488: 28-36. |
| 38 | LI G B, LI W, ZHANG H Y, et al. Non-mercury catalytic acetylene hydrochlorination over Ru catalysts enhanced by carbon nanotubes[J]. RSC Advances, 2015, 5(12): 9002-9008. |
| 39 | HAN Y, SUN M, LI W, et al. Influence of chlorine coordination number on the catalytic mechanism of ruthenium chloride catalysts in the acetylene hydrochlorination reaction: a DFT study[J]. Physical Chemistry Chemical Physics, 2015, 17(12): 7720-7730. |
| 40 | MAN B C, ZHANG H Y, ZHANG C M, et al. Effect of Ru/Cl ratio on the reaction of acetylene hydrochlorination[J]. New Journal of Chemistry, 2017, 41(23): 14675-14682. |
| 41 | JIN Y H, LI G B, ZHANG J L, et al. Effects of potassium additive on the activity of Ru catalyst for acetylene hydrochlorination[J]. RSC Advances, 2015, 5(47): 37774-37779. |
| 42 | ZHANG H Y, LI W, JIN Y H, et al. Ru-Co(Ⅲ)-Cu(Ⅱ)/SAC catalyst for acetylene hydrochlorination[J]. Applied Catalysis B: Environmental, 2016, 189: 56-64. |
| 43 | GU J J, GAO Y M, ZHANG J L, et al. Hydrochlorination of acetylene catalyzed by an activated carbon-supported ammonium hexachlororuthenate complex[J]. Catalysts, 2017, 7(12): 17. |
| 44 | LI J, ZHANG H Y, CAI M, et al. Enhanced catalytic performance of activated carbon-supported Ru-based catalysts for acetylene hydrochlorination by azole ligands[J]. Applied Catalysis A: General, 2020, 592: 117431. |
| 45 | SHANG S S, ZHAO W, WANG Y, et al. Highly efficient Ru@IL/AC to substitute mercuric catalyst for acetylene hydrochlorination[J]. ACS Catalysis, 2017, 7(5): 3510-3520. |
| 46 | HU J Y, YANG Q W, YANG L F, et al. Confining noble metal (Pd, Au, Pt) nanoparticles in surfactant ionic liquid: active non-mercury catalysts for hydrochlorination of acetylene[J]. ACS Catalysis, 2015, 5(11): 6724-6731. |
| 47 | KRASNYAKOVA T V, ZHIKHAREV I V, MITCHENKO R S, et al. Acetylene catalytic hydrochlorination over mechanically pre-activated K2PdCl4 salt: a study of reaction mechanism[J]. Journal of Catalysis, 2012, 288: 33-43. |
| 48 | SONG Q L, WANG S J, SHEN B X, et al. Palladium-based catalysts for the hydrochlorination of acetylene: reason for deactivation and its regeneration[J]. Petroleum Science and Technology, 2010, 28:1825-1833. |
| 49 | WANG L, WANG F, WANG J. Catalytic properties of Pd/HY catalysts modified with NH4F for acetylene hydrochlorination[J]. Catalysis Communications, 2015, 65: 41-45. |
| 50 | WANG L, WANG F, WANG J. Enhanced stability of hydrochlorination of acetylene using polyaniline-modified Pd/HY catalysts[J]. Catalysis Communications, 2016, 74: 55-59. |
| 51 | LI P, DING M Z, HE L M, et al. The activity and stability of PdCl2/C-N catalyst for acetylene hydrochlorination[J]. Science China Chemistry, 2018, 61: 444-448. |
| 52 | HE H H, ZHAO J, WANG B L, et al. Design strategies for the development of a Pd-based acetylene hydrochlorination catalyst: improvement of catalyst stability by nitrogen-containing ligands[J]. RSC Advances, 2019, 9(37): 21557-21563. |
| 53 | CEN Y Q, YUE Y X, WANG S S, et al. Adsorption behavior and electron structure engineering of Pd-based catalysts for acetylene hydrochlorination[J]. Catalysts, 2019, 10(1): 24. |
| 54 | KAISER S K, FAKO E, MANZOCCHI G, et al. Nanostructuring unlocks high performance of platinum single-atom catalysts for stable vinyl chloride production[J]. Nature Catalysis, 2020, 3: 376-385. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [10] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [11] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [12] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [13] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [14] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [15] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||