化工进展 ›› 2021, Vol. 40 ›› Issue (2): 824-834.DOI: 10.16085/j.issn.1000-6613.2020-0710
非均相催化剂催化5-羟甲基糠醛氢解制备2,5-二甲基呋喃研究进展
王彤1( ), 安华良1,2, 李芳1,2, 薛伟1,2(
), 安华良1,2, 李芳1,2, 薛伟1,2( ), 王延吉1,2
), 王延吉1,2
- 1.河北工业大学化工学院,河北省绿色化工与高效节能重点实验室,天津 300130
2.天津市本质安全化工技术 重点实验室,天津 300130
-
收稿日期:2020-04-29修回日期:2020-09-26出版日期:2021-02-05发布日期:2021-02-09 -
通讯作者:薛伟 -
作者简介:王彤(1991—),女,博士研究生,研究方向为绿色化工。E-mail:15122865361@163.com 。 -
基金资助:国家自然科学基金(21776057);天津市自然科学基金(18JCYBJC42600)
Research progress of the heterogeneous catalysts for 2,5-dimethylfuran synthesis via hydrogenolysis of 5-hydroxymethylfufural
Tong WANG1( ), Hualiang AN1,2, Fang LI1,2, Wei XUE1,2(
), Hualiang AN1,2, Fang LI1,2, Wei XUE1,2( ), Yanji WANG1,2
), Yanji WANG1,2
- 1.Hebei Provincial Key Laboratory of Green Chemical Technology and High Efficient Energy Saving, School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300130, China
2.Tianjin Key Laboratory of Chemical Process Safety, Tianjin 300130, China
-
Received:2020-04-29Revised:2020-09-26Online:2021-02-05Published:2021-02-09 -
Contact:Wei XUE
摘要:
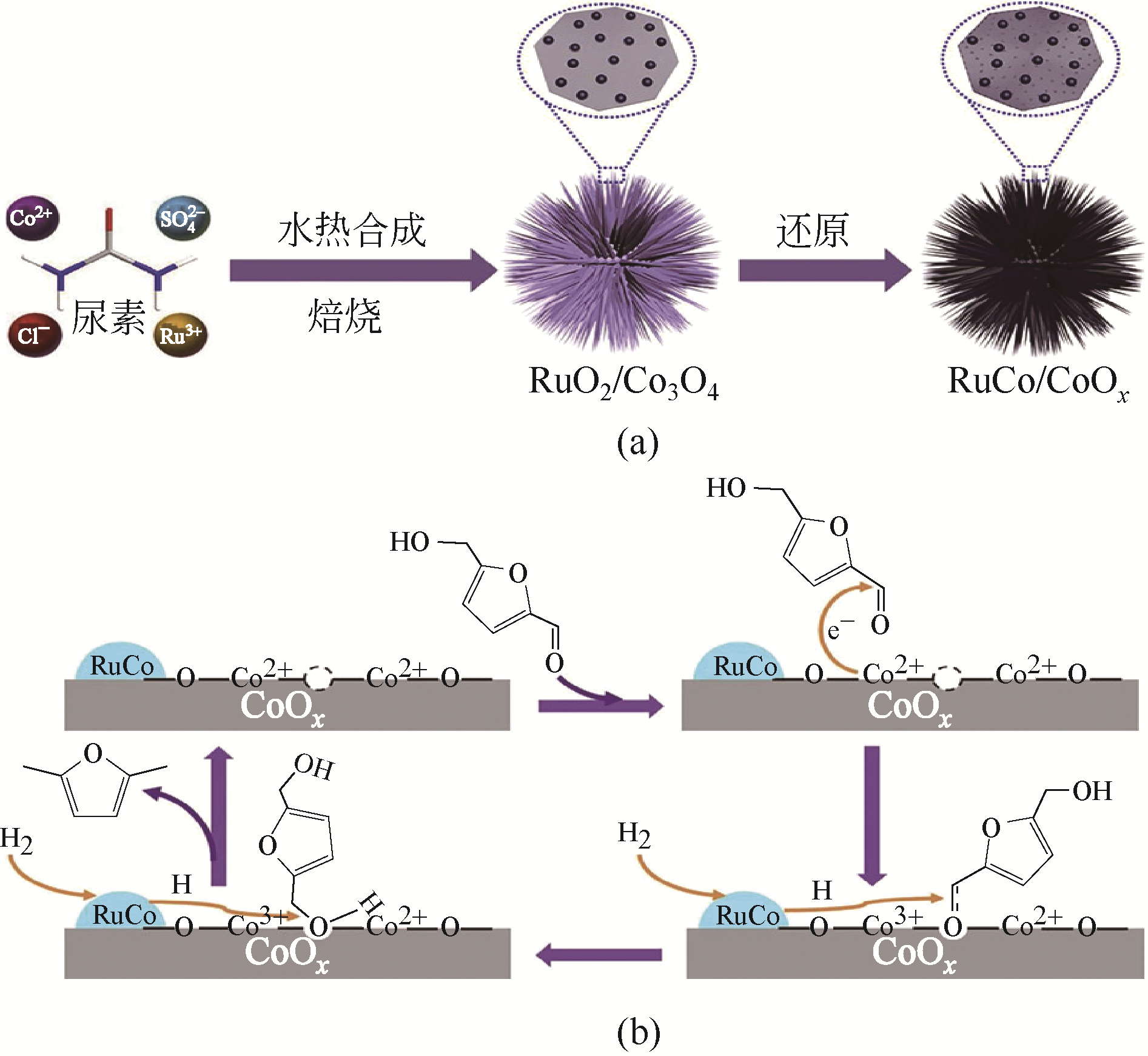
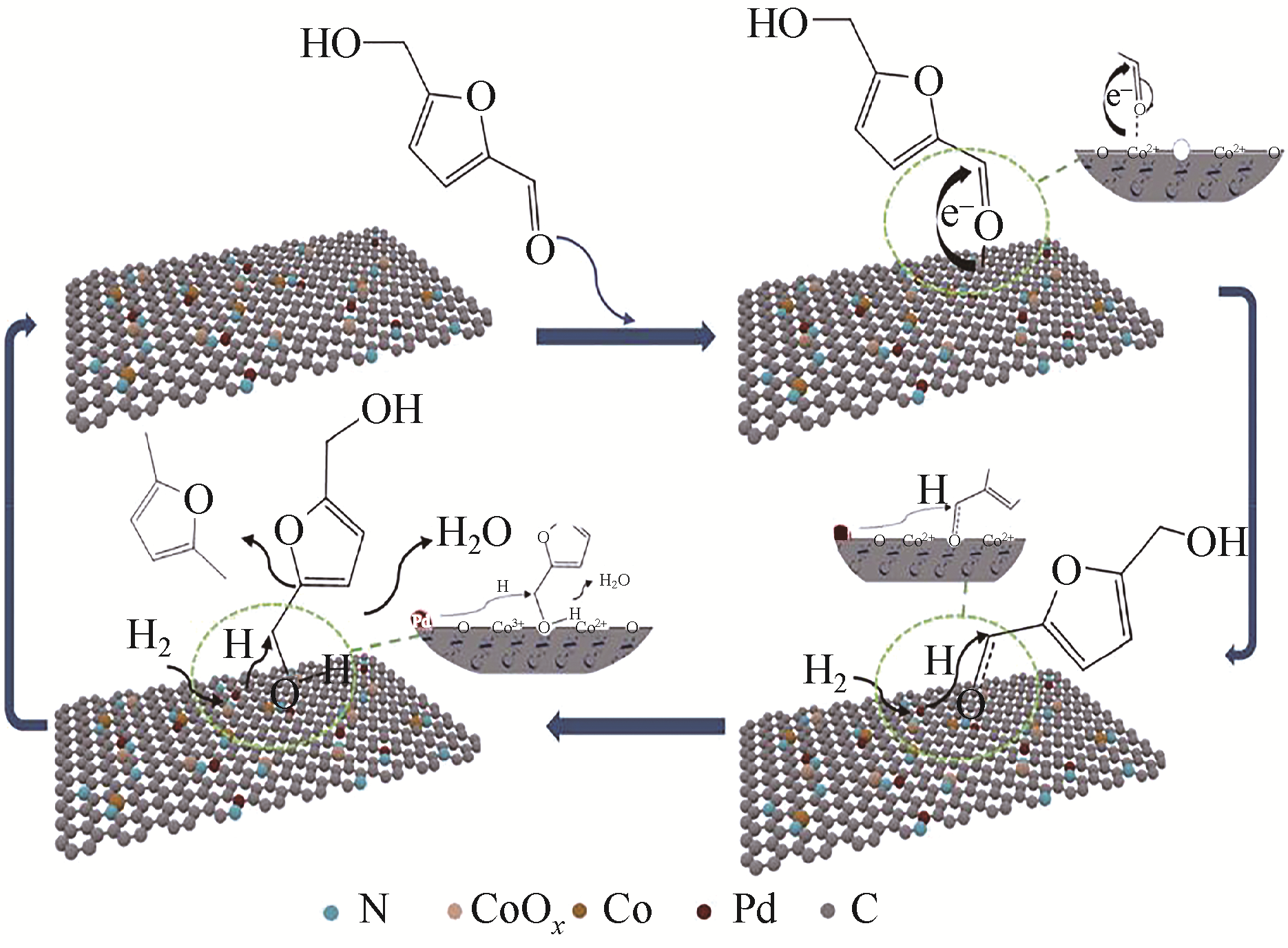
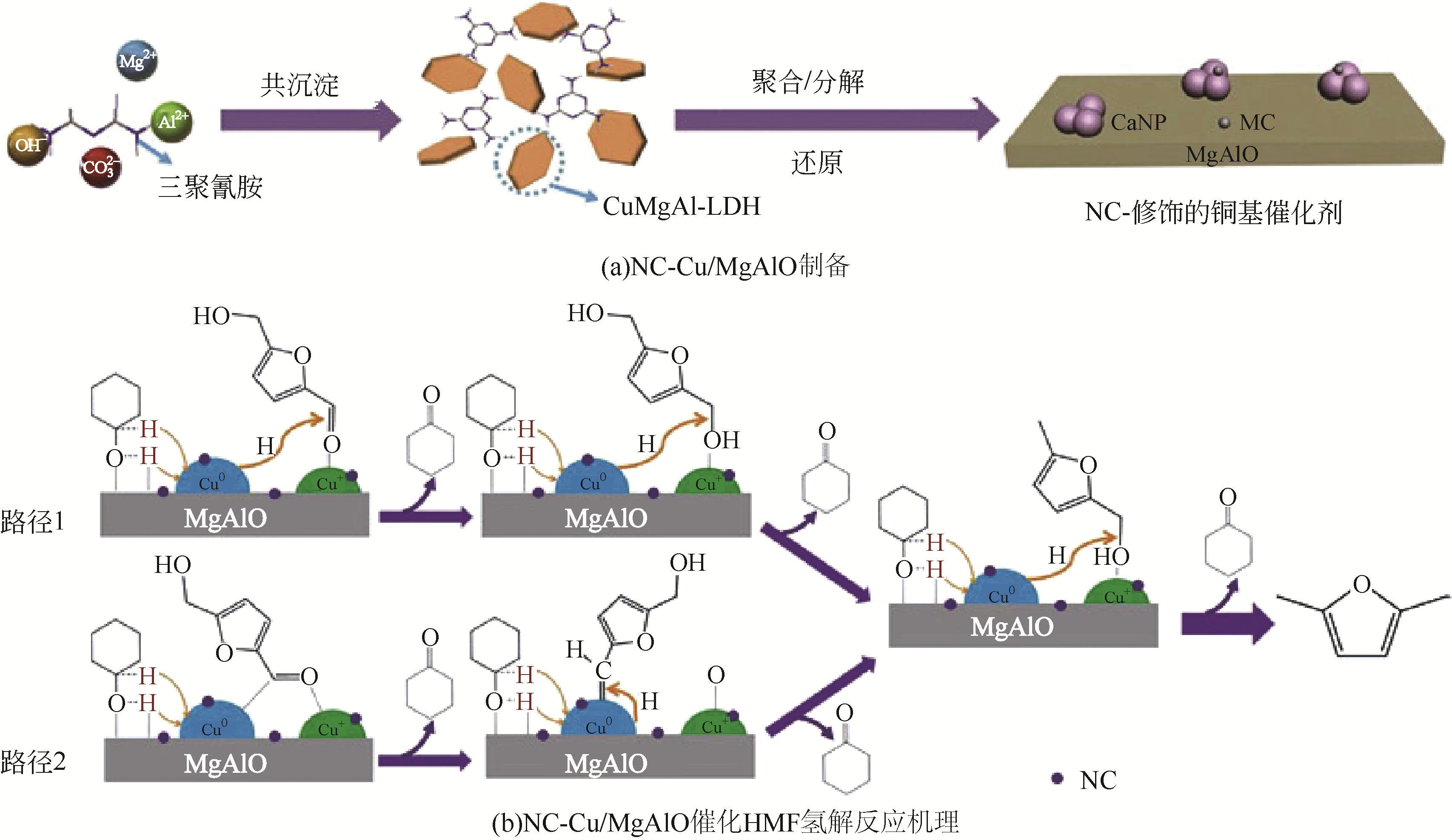
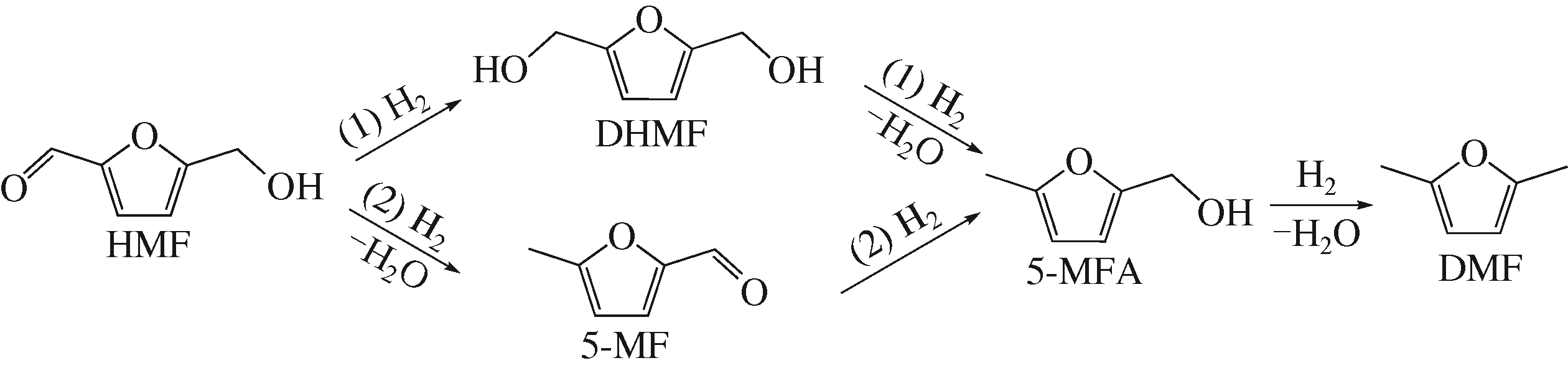
随着化石能源的日益短缺,清洁可再生生物质资源的利用,尤其是制备高品质生物燃料逐渐成为研究热点。2,5-二甲基呋喃(DMF)具有优良的物理化学性质,被认为是最有前途的液体生物燃料之一,可通过生物质平台分子5-羟甲基糠醛(HMF)选择性氢解制备。HMF化学性质非常活泼,可以转化成多种下游产品,因此设计制备高选择性催化剂对于靶向合成DMF至关重要。本文依据贵金属和非贵金属对催化剂进行分类,详细综述了非均相催化剂在HMF氢解制备DMF反应中的研究新进展;针对目前研究中存在的局限性和问题,提出了催化剂和反应体系的研究方向。此外,指出以生物质为原料直接制备DMF及建立有效的分离技术是实现DMF工业化生产的重要途径。
中图分类号:
引用本文
王彤, 安华良, 李芳, 薛伟, 王延吉. 非均相催化剂催化5-羟甲基糠醛氢解制备2,5-二甲基呋喃研究进展[J]. 化工进展, 2021, 40(2): 824-834.
Tong WANG, Hualiang AN, Fang LI, Wei XUE, Yanji WANG. Research progress of the heterogeneous catalysts for 2,5-dimethylfuran synthesis via hydrogenolysis of 5-hydroxymethylfufural[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 824-834.
| 催化剂 | 氢供体 | 温度/℃ | 反应时间/h | HMF转化率/% | DMF产率/% | 参考文献 |
|---|---|---|---|---|---|---|
| Ru/C+RuO2 | 异丙醇 | 190 | 6 | 100 | 72 | [ |
| r-Ru2@MSC-30 | H2(0.5MPa) | 125 | 1 | 100 | 69.52 | [ |
| Ru-ZrO2-MCM-41 | H2(1.5MPa) | 160 | 1 | 100 | 90 | [ |
| Ru@Mg(Al)O | 异丙醇 | 220 | 5 | — | 14 | [ |
| Ru-MoOx/C | H2(1.5MPa) | 180 | 1 | 100 | 79.8 | [ |
| RuCo/CoOx | H2(0.5MPa) | 200 | 2 | 100 | 96.5 | [ |
| Pt-Co/MWCNTs | H2(1MPa) | 160 | 8 | 100 | 92.3 | [ |
| Pt3Co2 | H2(3.3MPa) | 160 | — | 100 | 98 | [ |
| Pt3Ni/C | H2(3.3MPa) | 200 | — | 100 | 98 | [ |
| PtCu/C | H2(3.3MPa) | 200 | — | 100 | 96 | [ |
| Pt2Zn/C | H2(3.3MPa) | 200 | — | 100 | 97 | [ |
| PtCo/HCS | H2(1MPa) | 180 | 2 | 100 | 98 | [ |
| Pd/Fe2O3 | 异丙醇 | 180 | 24 | 100 | 72 | [ |
| Pd/C | H2(1MPa) | 80 | 2 | 100 | 100 | [ |
| Pd/C | H2(2MPa) | 120 | 15 | 95 | 85 | [ |
| Pd-Co9S8/S-CNT | H2(0.3MPa) | 120 | 13 | 96 | 83.7 | [ |
| Pd50Au50/C | H2(0.1MPa) | 60 | 6 | >99 | >99 | [ |
| PdAu4/GC800 | H2(1MPa) | 150 | 4 | 86.8 | 81.9 | [ |
| Pd-Cs2.5H0.5PW12O40/K-10 | H2(1MPa) | 90 | 2 | 98 | 79 | [ |
| Pd/C/Zn | H2(0.8MPa) | 150 | 8 | >99 | 85 | [ |
| Cu-Pd@C | H2(1.5MPa) | 120 | 7 | 100 | 96 | [ |
| Pd/Co-CoOx@NC | H2(1.5MPa) | 180 | 2 | 100 | 97.8 | [ |
| Raney Ni | H2(1.5MPa) | 180 | 15 | 100 | 88.5 | [ |
| Ni-Al2O3 | H2(1.2MPa) | 180 | 4 | 100 | 91.5 | [ |
| NiSi-PS | H2(1.5MPa) | 130 | 3 | 100 | 72.9 | [ |
| 7Ni-30W2C/AC | H2(4MPa) | 180 | 3 | 100 | 96 | [ |
| 2%Ni-20%Co/C | 甲酸 | 210 | 24 | 99 | 90 | [ |
| Ni-OMD3 | H2(3MPa) | 200 | 6 | >99.9 | 98.7 | [ |
| Ni/ZrP | H2(3MPa) | 240 | 20 | 100 | 68.1 | [ |
| Ni/ZSM-5 | H2(0.25MPa) | 180 | 7 | 91.2 | 96.2 | [ |
| Ni/TiO2 | H2(3MPa) | 220 | 2 | 100 | 85 | [ |
| Ni/C | H2(4.5MPa) | 180 | 2 | 100 | 75 | [ |
| Ni@SAZn-PC | H2(0.6MPa) | 150 | 1 | 56 | 50 | [ |
| NiCu3/C | H2(3.3MPa) | 180 | — | 100 | 98.7 | [ |
| Ni2-Fe1/CNTs | H2(3MPa) | 200 | 3 | 100 | 91.3 | [ |
| Cu-PMO | 甲醇 | 260 | 3 | 100 | 48 | [ |
| Cu20-Ru2-PMO | H2(5MPa) | 220 | 1 | 100 | 62.6 | [ |
| Cu/Al2O3 | 甲醇 | 240 | 6 | >99.9 | 73.9 | [ |
| NC-Cu/MgAlO | 环己醇 | 220 | 0.5 | 100 | 96.1 | [ |
| Co@Cu/3CoAlOx | H2(1.5MPa) | 180 | 5 | >99 | 98.5 | [ |
| CuZn | H2(2MPa) | 200 | 6 | 100 | 89 | [ |
| CnZn-2 | H2(1.5MPa) | 220 | 5 | 100 | 91.8 | [ |
| CuZnO(P) | H2(3MPa) | 220 | 100 | 79 | [ | |
| CuCo?/NGr/α-Al2O3 | H2(2MPa) | 180 | 6 | 100 | >99 | [ |
| Cu-Co@C(Cu∶Co=1∶3) | H2(5MPa) | 180 | 8 | 100 | 99.4 | [ |
| CuZnCox | 乙醇 | 210 | 5 | 100 | 99 | [ |
| FeNx/C | H2(4MPa) | 240 | 5 | 100 | 86.2 | [ |
| Fe-N-C | H2(4MPa) | 240 | 3 | 100 | 85.7 | [ |
| Fe-Co-Ni/h-BN | H2(2MPa) | 180 | 4.5 | 100 | 94 | [ |
| Co-CoOx | H2(1MPa) | 170 | 12 | 100 | 83.3 | [ |
| Co@NGs | H2(2MPa) | 200 | 6 | 99.9 | 94.7 | [ |
| 20Co/β-DAC723R | H2(1.5MPa) | 150 | 3 | 100 | 83.1 | [ |
| Co-(ZnO-ZnAl2O4) | H2(0.7MPa) | 130 | 6 | 100 | 74.2 | [ |
表1 用于HMF氢解制备DMF的非均相催化剂
| 催化剂 | 氢供体 | 温度/℃ | 反应时间/h | HMF转化率/% | DMF产率/% | 参考文献 |
|---|---|---|---|---|---|---|
| Ru/C+RuO2 | 异丙醇 | 190 | 6 | 100 | 72 | [ |
| r-Ru2@MSC-30 | H2(0.5MPa) | 125 | 1 | 100 | 69.52 | [ |
| Ru-ZrO2-MCM-41 | H2(1.5MPa) | 160 | 1 | 100 | 90 | [ |
| Ru@Mg(Al)O | 异丙醇 | 220 | 5 | — | 14 | [ |
| Ru-MoOx/C | H2(1.5MPa) | 180 | 1 | 100 | 79.8 | [ |
| RuCo/CoOx | H2(0.5MPa) | 200 | 2 | 100 | 96.5 | [ |
| Pt-Co/MWCNTs | H2(1MPa) | 160 | 8 | 100 | 92.3 | [ |
| Pt3Co2 | H2(3.3MPa) | 160 | — | 100 | 98 | [ |
| Pt3Ni/C | H2(3.3MPa) | 200 | — | 100 | 98 | [ |
| PtCu/C | H2(3.3MPa) | 200 | — | 100 | 96 | [ |
| Pt2Zn/C | H2(3.3MPa) | 200 | — | 100 | 97 | [ |
| PtCo/HCS | H2(1MPa) | 180 | 2 | 100 | 98 | [ |
| Pd/Fe2O3 | 异丙醇 | 180 | 24 | 100 | 72 | [ |
| Pd/C | H2(1MPa) | 80 | 2 | 100 | 100 | [ |
| Pd/C | H2(2MPa) | 120 | 15 | 95 | 85 | [ |
| Pd-Co9S8/S-CNT | H2(0.3MPa) | 120 | 13 | 96 | 83.7 | [ |
| Pd50Au50/C | H2(0.1MPa) | 60 | 6 | >99 | >99 | [ |
| PdAu4/GC800 | H2(1MPa) | 150 | 4 | 86.8 | 81.9 | [ |
| Pd-Cs2.5H0.5PW12O40/K-10 | H2(1MPa) | 90 | 2 | 98 | 79 | [ |
| Pd/C/Zn | H2(0.8MPa) | 150 | 8 | >99 | 85 | [ |
| Cu-Pd@C | H2(1.5MPa) | 120 | 7 | 100 | 96 | [ |
| Pd/Co-CoOx@NC | H2(1.5MPa) | 180 | 2 | 100 | 97.8 | [ |
| Raney Ni | H2(1.5MPa) | 180 | 15 | 100 | 88.5 | [ |
| Ni-Al2O3 | H2(1.2MPa) | 180 | 4 | 100 | 91.5 | [ |
| NiSi-PS | H2(1.5MPa) | 130 | 3 | 100 | 72.9 | [ |
| 7Ni-30W2C/AC | H2(4MPa) | 180 | 3 | 100 | 96 | [ |
| 2%Ni-20%Co/C | 甲酸 | 210 | 24 | 99 | 90 | [ |
| Ni-OMD3 | H2(3MPa) | 200 | 6 | >99.9 | 98.7 | [ |
| Ni/ZrP | H2(3MPa) | 240 | 20 | 100 | 68.1 | [ |
| Ni/ZSM-5 | H2(0.25MPa) | 180 | 7 | 91.2 | 96.2 | [ |
| Ni/TiO2 | H2(3MPa) | 220 | 2 | 100 | 85 | [ |
| Ni/C | H2(4.5MPa) | 180 | 2 | 100 | 75 | [ |
| Ni@SAZn-PC | H2(0.6MPa) | 150 | 1 | 56 | 50 | [ |
| NiCu3/C | H2(3.3MPa) | 180 | — | 100 | 98.7 | [ |
| Ni2-Fe1/CNTs | H2(3MPa) | 200 | 3 | 100 | 91.3 | [ |
| Cu-PMO | 甲醇 | 260 | 3 | 100 | 48 | [ |
| Cu20-Ru2-PMO | H2(5MPa) | 220 | 1 | 100 | 62.6 | [ |
| Cu/Al2O3 | 甲醇 | 240 | 6 | >99.9 | 73.9 | [ |
| NC-Cu/MgAlO | 环己醇 | 220 | 0.5 | 100 | 96.1 | [ |
| Co@Cu/3CoAlOx | H2(1.5MPa) | 180 | 5 | >99 | 98.5 | [ |
| CuZn | H2(2MPa) | 200 | 6 | 100 | 89 | [ |
| CnZn-2 | H2(1.5MPa) | 220 | 5 | 100 | 91.8 | [ |
| CuZnO(P) | H2(3MPa) | 220 | 100 | 79 | [ | |
| CuCo?/NGr/α-Al2O3 | H2(2MPa) | 180 | 6 | 100 | >99 | [ |
| Cu-Co@C(Cu∶Co=1∶3) | H2(5MPa) | 180 | 8 | 100 | 99.4 | [ |
| CuZnCox | 乙醇 | 210 | 5 | 100 | 99 | [ |
| FeNx/C | H2(4MPa) | 240 | 5 | 100 | 86.2 | [ |
| Fe-N-C | H2(4MPa) | 240 | 3 | 100 | 85.7 | [ |
| Fe-Co-Ni/h-BN | H2(2MPa) | 180 | 4.5 | 100 | 94 | [ |
| Co-CoOx | H2(1MPa) | 170 | 12 | 100 | 83.3 | [ |
| Co@NGs | H2(2MPa) | 200 | 6 | 99.9 | 94.7 | [ |
| 20Co/β-DAC723R | H2(1.5MPa) | 150 | 3 | 100 | 83.1 | [ |
| Co-(ZnO-ZnAl2O4) | H2(0.7MPa) | 130 | 6 | 100 | 74.2 | [ |
| 1 | BICKER M, HIRTH J, VOGEL H. Dehydration of fructose to 5-hydroxymethylfurfural in sub-and supercritical acetone[J]. Green Chemistry, 2003, 5(2): 280-284. |
| 2 | RINALDI R, SCHÜTH F. Acid hydrolysis of cellulose as the entry point into biorefinery schemes[J]. ChemSusChem, 2009, 2: 1096-1107. |
| 3 | 方向晨. 生物质在能源资源替代中的途径及前景展望[J]. 化工进展, 2011, 30(11): 2333-2339. |
| FANG X C. Routes and prospects in the energy resources replacing by biomasses[J]. Chemical Industry and Engineering Progress, 2011, 30(11): 2333-2339. | |
| 4 | 王泽, 林伟刚, 宋文立, 等. 生物质热化学转化制备生物燃料及化学品[J]. 化学进展, 2007, 19(7): 1190-1197. |
| WANG Z, LIN W G, SONG W L, et al. Bio-fuel and chemicals by thermochemical treating of biomass[J]. Progress in Chemistry, 2007, 19(7): 1190-1197. | |
| 5 | 林鹿, 何北海, 孙润仓, 等. 木质生物质转化高附加值化学品[J]. 化学进展, 2007, 19(7): 1206-1216. |
| LIN L, HE B H, SUN R C, et al. High-value chemicals from lignocellulosic biomass[J]. Progress in Chemistry, 2007, 19(7): 1206-1216. | |
| 6 | SERRANO-RUIZ J C, DUMESIC J A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels[J]. Energy & Environmental Science, 2011, 4(1): 83-99. |
| 7 | ZHANG X H, ZHANG Q, WANG T J, et al. Hydrodeoxygenation of lignin-derived phenolic compounds to hydrocarbons over Ni/SiO2-ZrO2 catalysts[J]. Bioresource Technology, 2013, 134: 73-80. |
| 8 | NORONHA F B, SCHMAL M, MORAWECK B, et al. Characterization of niobia-supported palladium-cobalt catalysts[J]. The Journal of Physical Chemistry B, 2000, 104(23): 5478-5485. |
| 9 | SAHA B, ABU-OMAR M M. Current technologies, economics, and perspectives for 2,5-dimethylfuran production from biomass-derived intermediates[J]. ChemSusChem, 2015, 8(7): 1133-1142. |
| 10 | GALKIN K I, ANANIKOV V P. When will 5-hydroxymethylfurfural, the “sleeping giant” of sustainable chemistry, awaken?[J]. ChemSusChem, 2019, 12: 2976-2982. |
| 11 | WANG X F, LIANG X H, LI J M, et al. Catalytic hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to biofuel 2,5-dimethylfuran[J]. Applied Catalysis A: General, 2019, 576: 85-95. |
| 12 | TAN J, LIU Q, CHEN L, et al. Efficient production of ethyl levulinate from cassava over Al2(SO4)3 catalyst in ethanol-water system[J]. Journal of Energy Chemistry, 2017, 26(1):115-120. |
| 13 | ANTONYRAJ C A, JEONG J, KIM B, et al. Selective oxidation of HMF to DFF using Ru/γ-alumina catalyst in moderate boiling solvents toward industrial production[J]. Industrial and Engineering Chemistry Research, 2013, 19(3): 1056-1059. |
| 14 | SHUAI X, ZHOU P, ZHANG Z, et al. Selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid using O2 and a photocatalyst of Co-thioporphyrazine bonded to g-C3N4[J]. Journal of the American Chemical Society, 2017, 139(41): 14775-14782. |
| 15 | KUBOTA S R, CHOI K S. Electrochemical oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid (FDCA) in acidic media enabling spontaneous FDCA separation[J]. ChemSusChem, 2018, 11(13): 2138-2145. |
| 16 | ZHANG X Y, ZONG M H, LI N. Whole-cell biocatalytic selective oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid[J]. Green Chemistry, 2017, 19(19): 4544-4551. |
| 17 | JAE J, ZHENG W, KARIM A M, et al. The role of Ru and RuO2 in the catalytic transfer hydrogenation of 5-hydroxymethylfurfural for the production of 2,5-dimethylfuran[J]. ChemCatChem, 2014, 6(3): 848-856. |
| 18 | TZENG T W, LIN C Y, PAO C W, et al. Understanding catalytic hydrogenolysis of 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) using carbon supported Ru catalysts[J]. Fuel Processing Technology, 2020, 199: 106225. |
| 19 | RAUT A B, NANDA B, PARIDA K M, et al. Hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to produce 2,5-dimethylfuran over Ru-ZrO2-MCM-41 catalyst[J]. Chemistry Select, 2019, 4(20): 6080-6089. |
| 20 | NAGPURE A S, VENUGOPAL A K, LUCAS N, et al. Renewable fuels from biomass-derived compounds: Ru-containing hydrotalcites as catalysts for conversion of HMF to 2,5-dimethylfuran[J]. Catalysis Science & Technology, 2015, 5(3): 1463-1472. |
| 21 | YANG Y, LIU Q Y, LI D, et al. Selective hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran on Ru-MoOx/C catalysts[J]. RSC Advances, 2017, 7(27): 16311-16318. |
| 22 | GAO Z, FAN G, LIU M, et al. Dandelion-like cobalt oxide microsphere-supported RuCo bimetallic catalyst for highly efficient hydrogenolysis of 5-hydroxymethylfurfural[J]. Applied Catalysis B: Environmental, 2018, 237(5): 649-659. |
| 23 | WANG X, LIU Y, LIANG X. Hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran over supported Pt-Co bimetallic catalysts under mild conditions[J]. Green Chemistry, 2018, 20(12): 2894-2902. |
| 24 | LUO J, YUN H, MIRONENKO A V, et al. Mechanisms for high selectivity in the hydrodeoxygenation of 5-hydroxymethylfurfural over PtCo nanocrystals[J]. ACS Catalysis, 2016, 6(7): 4095-4104. |
| 25 | LUO J, LEE J D, YUN H, et al. Base metal-Pt alloys: a general route to high selectivity and stability in the production of biofuels from HMF[J]. Applied Catalysis B: Environmental, 2016, 199(15): 439-446. |
| 26 | WANG G H, HILGERT J, RICHTER F H, et al. Platinum-cobalt bimetallic nanoparticles in hollow carbon nanospheres for hydrogenolysis of 5-hydroxymethylfurfural[J]. Nature Materials, 2014, 13(3): 293-300. |
| 27 | SCHOLZ D, AELLIG C, HERMANS I. Catalytic transfer hydrogenation/hydrogenolysis for reductive upgrading of furfural and 5-(hydroxymethyl)furfural[J]. ChemSusChem, 2014, 7(1): 268-275. |
| 28 | CHATTERJEE M, ISHIZAKA T, KAWANAMI H. Hydrogenation of 5-hydroxymethylfurfural in supercritical carbon dioxide-water: a tunable approach to dimethylfuran selectivity[J]. Green Chemistry, 2014, 16(3): 1543-1551. |
| 29 | MITRA J, ZHOU X, RAUCHFUSS T. Pd/C-catalyzed reactions of HMF: decarbonylation, hydrogenation, and hydrogenolysis[J]. Green Chemistry, 2015, 17(1): 307-313. |
| 30 | LIAO W P, ZHU Z G, CHEN N M, et al. Highly active bifunctional Pd-Co9S8/S-CNT catalysts for selective hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran[J]. Molecular Catalysis, 2020, 482: 110756. |
| 31 | NISHIMURA S, IKEDA N, EBITANI K. Selective hydrogenation of biomass-derived 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) under atmospheric hydrogen pressure over carbon supported PdAu bimetallic catalyst[J]. Catalysis Today, 2014, 232(1): 89-98. |
| 32 | ZHANG F, LIU Y, YUAN F, et al. Efficient production of the liquid fuel 2,5-dimethylfuran from 5-hydroxymethylfurfural in the absence of acid additive over bimetallic PdAu supported on graphitized carbon[J]. Energy and Fuels, 2017, 31(6): 6364-6373. |
| 33 | GAWADE A B, TIWARI M S, YADAV G D. Biobased green process: selective hydrogenation of 5-hydroxymethylfurfural to 2,5-dimethyl furan under mild conditions using Pd-Cs2.5H0.5PW12O40/K-10 clay[J]. ACS Sustainable Chemistry and Engineering, 2016, 4(8): 4113-4123. |
| 34 | SAHA B, BOHN C M, ABU-OMAR M M. Zinc-assisted hydrodeoxygenation of biomass-derived 5-hydroxymethylfurfural to 2, 5-dimethylfuran[J]. ChemSusChem, 2014, 7(11): 3095-3101. |
| 35 | SARKAR C, KOLEY P, SHOWN I, et al. Integration of interfacial and alloy effects to modulate catalytic performance of metal-organic-framework-derived Cu-Pd nanocrystals toward hydrogenolysis of 5-hydroxymethylfurfural[J]. ACS Sustainable Chemistry and Engineering, 2019, 7: 10349-10362. |
| 36 | SHANG Y N, LIU C W, ZHANG Z N, et al. Insights into the synergistic effect in Pd immobilized to MOF-derived Co-CoOx@ N-doped carbon for efficient selective hydrogenolysis of 5-hydroxylmethylfurfural[J]. Industrial and Engineering Chemistry Research, 2020, 59: 6532-6542. |
| 37 | KONG X, ZHU Y, ZHENG H, et al. Switchable synthesis of 2,5-dimethylfuran and 2,5-dihydroxymethyltetrahydrofuran from 5-hydroxymethylfurfural over Raney Ni catalyst[J]. RSC Advances, 2014, 4(105): 60467-60472. |
| 38 | KONG X, ZHENG R, ZHU Y, et al. Rational design of Ni-based catalysts derived from hydrotalcite for selective hydrogenation of 5-hydroxymethylfurfural[J]. Green Chemistry, 2015, 17(4): 2504-2514. |
| 39 | KONG X, ZHU Y, ZHENG H, et al. Ni nanoparticles inlaid nickel phyllosilicate as a metal-acid bifunctional catalyst for low-temperature hydrogenolysis reactions[J]. ACS Catalysis, 2015, 5(10): 5914-5920. |
| 40 | HUANG Y B, CHEN M Y, YAN L, et al. Nickel-tungsten carbide catalysts for the production of 2,5-dimethylfuran from biomass-derived molecules[J]. ChemSusChem, 2014, 7(4): 1068-1072. |
| 41 | YANG P, XIA Q, LIU X, et al. High-yield production of 2,5-dimethylfuran from 5-hydroxymethylfurfural over carbon supported Ni-Co bimetallic catalyst[J]. Journal of Energy Chemistry, 2016, 25(6): 1015-1020. |
| 42 | GOYAL R, SARKAR B, BAG A, et al. Studies of synergy between metal-support interfaces and selective hydrogenation of HMF to DMF in water[J]. Journal of Catalysis, 2016, 340: 248-260. |
| 43 | ZHU C, LIU Q, LI D, et al. Selective hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Ni supported on zirconium phosphate catalysts[J]. ACS Omega, 2018, 3(7): 7407-7417. |
| 44 | SUN Y, XIONG C X, LIU Q C, et al. Catalytic transfer hydrogenolysis/hydrogenation of biomass-derived 5-formyloxymethylfurfural to 2,5-dimethylfuran over Ni-Cu bimetallic catalyst with formic acid as a hydrogen donor[J]. Industrial & Engineering Chemistry Research, 2019, 58(14): 5414-5422. |
| 45 | GUO D W, LIU X X, CHENG F, et al. Selective hydrogenolysis of 5-hydroxymethylfurfural to produce biofuel 2,5-dimethylfuran over Ni/ZSM-5 catalysts[J]. Fuel, 2020, 274: 117853. |
| 46 | PRZYDACZ M, JEDRZEJCZYK M, BRZEZINSKA M, et al. Solvothermal hydrodeoxygenation of hydroxymethylfurfural derived from biomass towards added value chemicals on Ni/TiO2 catalysts[J]. The Journal of Supercritical Fluids, 2020, 163: 104827. |
| 47 | GYNGAZOVA M S, NEGAHDAR L, BLUMENTHAL L C, et al. Experimental and kinetic analysis of the liquid phase hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over carbon-supported nickel catalysts[J]. Chemical Engineering Science, 2017, 173: 455-464. |
| 48 | MANI C M, BRAUN M, MOLINARI V, et al. A high-throughput composite catalyst based on nickel carbon cubes for the hydrogenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran[J]. ChemCatChem, 2017, 9(17): 3388-3394. |
| 49 | LUO J, MONAI M, WANG C, et al. Unraveling the surface state and composition of highly selective nanocrystalline Ni-Cu alloy catalysts for hydrodeoxygenation of HMF[J]. Catalysis Science and Technology, 2017, 7(8): 1735-1743. |
| 50 | YU L, HE L, CHEN J, et al. Robust and recyclable nonprecious bimetallic nanoparticles on carbon nanotubes for the hydrogenation and hydrogenolysis of 5-hydroxymethylfurfural[J]. ChemCatChem, 2015, 7(11): 1701-1707. |
| 51 | ROMÁN-LESHKOV Y, BARRETT C J, LIU Z Y, et al. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates[J]. Nature, 2007, 447(7147): 982-985. |
| 52 | HANSEN T S, BARTA K, ANASTAS P T, et al. One-pot reduction of 5-hydroxymethylfurfural via hydrogen transfer from supercritical methanol[J]. Green Chemistry, 2012, 14(9): 2457-2461. |
| 53 | KUMALAPUTRI A J, BOTTARI G, ERNE P M, et al. Tunable and selective conversion of 5-HMF to 2,5-furandimethanol and 2,5-dimethylfuran over copper-doped porous metal oxides[J]. ChemSusChem, 2014, 7(8): 2266-2275. |
| 54 | ZHANG Z H, WANG C X, GOU X, et al. Catalytic in-situ hydrogenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Cu-based catalysts with methanol as a hydrogen donor[J]. Applied Catalysis A, General, 2019, 570: 245-250. |
| 55 | GAO Z, LI C, FAN G, et al. Nitrogen-doped carbon-decorated copper catalyst for highly efficient transfer hydrogenolysis of 5-hydroxymethylfurfural to convertibly produce 2,5-dimethylfuran or 2,5-dimethyltetrahydrofuran[J]. Applied Catalysis B: Environmental, 2018, 226: 523-533. |
| 56 | WANG Q, FENG J T, ZHENG L R, et al. Interfacial structure-determined reaction pathway and selectivity for 5-(hydroxymethyl)furfural hydrogenation over Cu-based catalysts[J]. ACS Catalysis, 2019, 10(2): 1353-1365. |
| 57 | BOTTARI G, KUMALAPUTRI A J, KRAWCZYK K K, et al. Copper-zinc alloy nanopowder: a robust precious-metal-free catalyst for the conversion of 5-hydroxymethylfurfural[J]. ChemSusChem, 2015, 8(8): 1323-1327. |
| 58 | ZHU Y, KONG X, ZHENG H, et al. Efficient synthesis of 2,5-dihydroxymethylfuran and 2,5-dimethylfuran from 5-hydroxymethylfurfural using mineral-derived Cu catalysts as versatile catalysts[J]. Catalysis Science and Technology, 2015, 5(8): 4208-4217. |
| 59 | BRZEZIŃSKA M, KELLER N, RUPPERT A M. Self-tuned properties of CuZnO catalysts for hydroxymethylfurfural hydrodeoxygenation towards dimethylfuran production[J]. Catalysis Science & Technology, 2020, 10(3): 658-670. |
| 60 | GUO W W, LIU H Y, ZHANG S Q, et al. Efficient hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran over a cobalt and copper bimetallic catalyst on N-graphene-modified Al2O3[J]. Green Chemistry, 2016, 18(23): 6222-6228. |
| 61 | CHEN B F, LI F B, HUANG Z J, et al. Carbon-coated Cu-Co bimetallic nanoparticles as selective and recyclable catalysts for production of biofuel 2,5-dimethylfuran[J]. Applied Catalysis B: Environmental, 2017, 200: 192-199. |
| 62 | ZHANG Z H, YAO S Y, WANG C X, et al. CuZnCoOx multifunctional catalyst for in situ hydrogenation of 5-hydroxymethylfurfural with ethanol as hydrogen carrier[J]. Journal of Catalysis, 2019, 373: 314-321. |
| 63 | LI J, LIU J L, LIU H Y, et al. Selective hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over heterogeneous iron catalysts[J]. ChemSusChem, 2017, 10(7): 1436-1447. |
| 64 | LI J, ZHANG J J, LIU H Y, et al. Graphitic carbon nitride (g-C3N4)-derived Fe-N-C catalysts for selective hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran[J]. ChemistrySelect, 2017, 2(34): 11062-11070. |
| 65 | CHEN N M, ZHU Z G, SU T, et al. Catalytic hydrogenolysis of hydroxymethylfurfural to highly selective 2,5-dimethylfuran over FeCoNi/h-BN catalyst[J]. Chemical Engineering Journal, 2020, 381: 122755. |
| 66 | LI D, LIU Q Y, ZHU C H, et al. Selective hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Co3O4 catalyst by controlled reduction[J]. Journal of Energy Chemistry, 2019, 30: 34-41. |
| 67 | WANG J S, WEI Q H, MA Q X, et al. Constructing Co@N-doped graphene shell catalyst via Mott-Schottky effect for selective hydrogenation of 5-hydroxylmethylfurfural[J]. Applied Catalysis B: Environmental, 2020, 263: 118339-118349. |
| 68 | CHEN N M, ZHU Z G, MA H K, et al. Catalytic upgrading of biomass-derived 5-hydroxymethylfurfural to biofuel 2,5-dimethylfuran over Beta zeolite supported non-noble Co catalyst[J]. Molecular Catalysis, 2020, 486: 110882. |
| 69 | AN Z, WANG W L, DONG S H, et al. Well-distributed cobalt-based catalysts derived from layered double hydroxides for efficient selective hydrogenation of 5-hydroxymethyfurfural to 2,5-methylfuran[J]. Catalysis Today, 2019, 319: 128-138. |
| 70 | 刘迎新,曾茂,楼炯涛,等. 5-羟甲基糠醛选择性加氢制备2,5-二甲基呋喃研究进展[J]. 高校化学工程学报, 2018, 32(2): 255-265. |
| LIU Y X, ZENG M, LOU J T, et al. Review on 2,5-dimethylfuran synthesis via selectivity hydrogenation of 5-hydroxymethylfufural[J]. Journal of Chemical Engineering of Chinese Universities, 2018, 32(2): 255-265. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [11] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [15] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||