化工进展 ›› 2019, Vol. 38 ›› Issue (06): 2707-2713.DOI: 10.16085/j.issn.1000-6613.2018-1968
5-羟甲基糠醛催化氢化制备2,5-呋喃二甲醇的研究进展
- 华南理工大学轻工科学与工程学院,制浆造纸工程国家重点实验室,广东 广州 510640
-
收稿日期:2018-09-30出版日期:2019-06-05发布日期:2019-06-05 -
通讯作者:刘颖 -
作者简介:张凯莉(1994—),女,硕士研究生,研究方向为生物质催化转化。E-mail:<email>1395702635@qq.com</email>。 -
基金资助:广东省自然科学基金(2016A030313489);广州市科学计划(201607020025);制浆造纸工程国家重点实验室项目(2017PY01,201829)
Advances in catalytic hydrogenation of 5-hydroxymethylfurfural to 2,5-bishydroxymethylfuran
Kaili ZHANG( ),Ying LIU(
),Ying LIU( ),Shubin WU
),Shubin WU
- State Key Laboratory of Pulp and Paper Engineering,School of Industry and Engineering,South China University of Technology,Guangzhou 510640,Guangdong,China
-
Received:2018-09-30Online:2019-06-05Published:2019-06-05 -
Contact:Ying LIU
摘要:
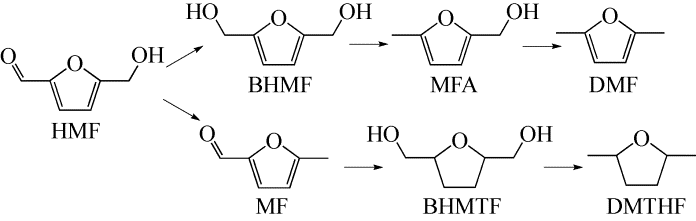
2,5-呋喃二甲醇(BHMF)在合成树脂、药物等方面具有重要应用。随着化石资源的日益缩减,由可再生的生物质基平台分子5-羟甲基糠醛(HMF)催化制备BHMF引起人们的广泛关注。本文在总结了HMF及BHMF物化性质的基础上,介绍了HMF在分子氢、醇类、甲酸3种不同的氢供体中催化加氢制备BHMF的研究近况,总结了贵金属、非贵金属、双金属或多金属协同催化体系在该加氢反应中的应用进展,同时分析了反应过程中温度、时间、催化剂载体、反应溶剂种类及酸值等因素对HMF转化率及BHMF得率的影响。最后对HMF催化转化制备BHMF的研究前景进行了总结和展望,提出了使用醇类代替氢气作为氢供体,开发非贵金属及金属协同催化体系将是该选择性氢化反应的重要研究方向之一。
中图分类号:
引用本文
张凯莉, 刘颖, 武书彬. 5-羟甲基糠醛催化氢化制备2,5-呋喃二甲醇的研究进展[J]. 化工进展, 2019, 38(06): 2707-2713.
Kaili ZHANG, Ying LIU, Shubin WU. Advances in catalytic hydrogenation of 5-hydroxymethylfurfural to 2,5-bishydroxymethylfuran[J]. Chemical Industry and Engineering Progress, 2019, 38(06): 2707-2713.
| 化学品 | 5-羟甲基糠醛(HMF) | 2,5-呋喃二甲醇(BHMF) |
|---|---|---|
| 分子式 | C6H6O3 | C6H8O3 |
| 摩尔质量/g·mol-1 | 126.11 | 128.13 |
| 熔点/℃ | 28~34 | 74~77 |
| 沸点/℃ | 114~116 | 275 |
| 密度/g·cm-3 | 1.243 | 1.283 |
| 紫外吸收波长/nm | 284 | 219 |
表1 5-羟甲基糠醛及2,5-呋喃二甲醇的物化性质
| 化学品 | 5-羟甲基糠醛(HMF) | 2,5-呋喃二甲醇(BHMF) |
|---|---|---|
| 分子式 | C6H6O3 | C6H8O3 |
| 摩尔质量/g·mol-1 | 126.11 | 128.13 |
| 熔点/℃ | 28~34 | 74~77 |
| 沸点/℃ | 114~116 | 275 |
| 密度/g·cm-3 | 1.243 | 1.283 |
| 紫外吸收波长/nm | 284 | 219 |
| 序号 | 氢供体 | 溶剂 | 催化剂 | 温度/℃ | 时间/h | BHMF产率/% | 文献 |
|---|---|---|---|---|---|---|---|
| 1 | 氢气 | 甲苯 | Shvo’s | 90 | 1 | 99 | [24 ] |
| 2 | 氢气 | 水 | Ni/MCM-41 | 35 | 2 | 89 | [ 25] |
| 3 | 2-异丙醇 | 2-异丙醇 | Ru/C | 150 | 6 | 38 | [ 26] |
| 4 | 乙醇 | 乙醇 | Al(OH)3 | 150 | 2 | 58.8 | [ 27] |
| 5 | 乙醇 | 乙醇 | Ti(OH)4 | 150 | 2 | 18.7 | [ 27] |
| 6 | 乙醇 | 乙醇 | ZrO(OH)2 | 150 | 2 | 87.7 | [ 27] |
| 7 | 甲醇 | 甲醇 | MgO | 160 | 3 | 100 | [ 28] |
| 8 | 甲酸 | THF | Cp*Ir(TsDPEN) | 40 | 2 | 99 | [ |
| 9 | 甲酸 | THF | Pd/C | 120 | 4 | 94 | [ 30] |
| 10 | 氢气 | 水 | Au/Al2O3 | 120 | 2 | 96 | [ |
| 11 | 氢气 | 水 | Pd/Al2O3 | 140 | 4 | 20 | [ 31] |
| 12 | 氢气 | 水,正丁醇 | Ru/CeO x | 130 | 2 | 81 | [ 32] |
| 13 | 氢气 | 水,正丁醇 | Ru/CeO x | 130 | 6 | 32 | [32 ] |
| 14 | 1,4-丁二醇 | 1,4-丁二醇 | Cu/AlO x | 220 | 6 | 93 | [ 33] |
| 15 | 氢气 | 丁醇 | Ni-Fe/CNTs | 110 | 18 | 96.1 | [34 ] |
| 16 | 氢气 | 丁醇 | Ni/CNTs | 110 | 18 | 76.4 | [34 ] |
| 17 | 氢气 | 丁醇 | Fe/CNTs | 110 | 18 | — | [ 34] |
| 18 | 氢气 | 乙醇 | Cu/Zn | 120 | 3 | 95 | [35 ] |
| 19 | 氢气 | 水 | Ir-ReO x /SiO2 | 30 | 6 | 99 | [ 36] |
| 20 | 氢气 | 1,4-二氧己环 | Cu-ZnO | 100 | 2 | 99.1 | [ 37] |
| 21 | 氢气 | 水 | Ir/C | 50 | 3 | 95.4 | [ 38] |
| 22 | 氢气 | 水 | Ir/TiO2 | 50 | 3 | 69.7 | [38 ] |
表2 不同催化体系作用下HMF催化转化为BHMF
| 序号 | 氢供体 | 溶剂 | 催化剂 | 温度/℃ | 时间/h | BHMF产率/% | 文献 |
|---|---|---|---|---|---|---|---|
| 1 | 氢气 | 甲苯 | Shvo’s | 90 | 1 | 99 | [24 ] |
| 2 | 氢气 | 水 | Ni/MCM-41 | 35 | 2 | 89 | [ 25] |
| 3 | 2-异丙醇 | 2-异丙醇 | Ru/C | 150 | 6 | 38 | [ 26] |
| 4 | 乙醇 | 乙醇 | Al(OH)3 | 150 | 2 | 58.8 | [ 27] |
| 5 | 乙醇 | 乙醇 | Ti(OH)4 | 150 | 2 | 18.7 | [ 27] |
| 6 | 乙醇 | 乙醇 | ZrO(OH)2 | 150 | 2 | 87.7 | [ 27] |
| 7 | 甲醇 | 甲醇 | MgO | 160 | 3 | 100 | [ 28] |
| 8 | 甲酸 | THF | Cp*Ir(TsDPEN) | 40 | 2 | 99 | [ |
| 9 | 甲酸 | THF | Pd/C | 120 | 4 | 94 | [ 30] |
| 10 | 氢气 | 水 | Au/Al2O3 | 120 | 2 | 96 | [ |
| 11 | 氢气 | 水 | Pd/Al2O3 | 140 | 4 | 20 | [ 31] |
| 12 | 氢气 | 水,正丁醇 | Ru/CeO x | 130 | 2 | 81 | [ 32] |
| 13 | 氢气 | 水,正丁醇 | Ru/CeO x | 130 | 6 | 32 | [32 ] |
| 14 | 1,4-丁二醇 | 1,4-丁二醇 | Cu/AlO x | 220 | 6 | 93 | [ 33] |
| 15 | 氢气 | 丁醇 | Ni-Fe/CNTs | 110 | 18 | 96.1 | [34 ] |
| 16 | 氢气 | 丁醇 | Ni/CNTs | 110 | 18 | 76.4 | [34 ] |
| 17 | 氢气 | 丁醇 | Fe/CNTs | 110 | 18 | — | [ 34] |
| 18 | 氢气 | 乙醇 | Cu/Zn | 120 | 3 | 95 | [35 ] |
| 19 | 氢气 | 水 | Ir-ReO x /SiO2 | 30 | 6 | 99 | [ 36] |
| 20 | 氢气 | 1,4-二氧己环 | Cu-ZnO | 100 | 2 | 99.1 | [ 37] |
| 21 | 氢气 | 水 | Ir/C | 50 | 3 | 95.4 | [ 38] |
| 22 | 氢气 | 水 | Ir/TiO2 | 50 | 3 | 69.7 | [38 ] |
| 序号 | 醇 | 还原电势/kJ·mol-1 |
|---|---|---|
| 1 | 2-丁醇 | 69.3 |
| 2 | 异丙醇 | 70.0 |
| 3 | 1-丁醇 | 79.7 |
| 4 | 乙醇 | 85.4 |
| 5 | 甲醇 | 130.1 |
表3 不同醇的还原电势
| 序号 | 醇 | 还原电势/kJ·mol-1 |
|---|---|---|
| 1 | 2-丁醇 | 69.3 |
| 2 | 异丙醇 | 70.0 |
| 3 | 1-丁醇 | 79.7 |
| 4 | 乙醇 | 85.4 |
| 5 | 甲醇 | 130.1 |
| 1 | LUCIA L A , ARGROPOULOSD S , ADAMOPOULOS L ,et al . Chemicals and energy from biomass[J]. Canadian Journal of Chemistry,2006,84(7):960-970. |
| 2 | KLASS D L . Biomass for renewable energy,fuels,and chemicals[M]. Amsterdam: Elsevier , 1998:1028-1037. |
| 3 | KONUR O . The scientometric evaluation of the research on the production of bioenergy from biomass[J]. Biomass and Bioenergy, 2012,47(6):504-515. |
| 4 | STAMBOULI A B . Algerian renewable energy assessment:the challenge of sustainability[J]. Energy Policy,2011,39(8):4507-4519. |
| 5 | HABERL H , BERINGER T , BHATTACHARYA S C ,et al . The global technical potential of bio-energy in 2050 considering sustainability constraints[J]. Current Opinion in Environmental Sustainability,2010,2(5/6):394-403. |
| 6 | 杨真真 . 催化转化果糖制备5-羟甲基糠醛及其衍生物2,5-呋喃二甲醛[D]. 合肥:中国科学技术大学,2013. |
| YANG Z Z . Catalytic conversion of fructose to 5-hydroxymethylfurfural and its derivative 2, 5-diformylfuran[D]. Hefei: University of Science and Technology of China,2013. | |
| 7 | CLIMENT M J , CORMA A , IBORRA S . Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels[J]. Green Chemistry, 2014,16(2):516-547. |
| 8 | LESHKOV Y R T , CHHEDA J N , DUMESIC J A . Phase modifiers promote efficient production of hydroxymethylfurfural from fructose[J]. Science,2006,312(5782):1933-1937. |
| 9 | GOSWAMI S ,DEY S, JANA S . Design and synthesis of a unique ditopic macrocyclic fluorescent receptor containing furan ring as a spacer for the recognition of dicarboxylic acids[J]. Tetrahedron, 2008,64(27):6358–6363. |
| 10 | MAJDOUB M , LOUPY A , PETIT A ,et al . Coupling focused microwaves and solvent-free phase transfer catalysis: application to the synthesis of new furanic diethers[J]. Tetrahedron, 1996,52(2):617-628. |
| 11 | TILLET G , BOUTEVIN B , AMEDURI B . Chemical reactions of polymer crosslinking and post-crosslinking at room and medium temperature[J]. Progress in Polymer Science,2011,36(2):191-217. |
| 12 | TIMKO J M , CRAM D J . Furanyl unit in host compounds[J]. Journal of the American Chemical Society,1974,96(22):7159-7160. |
| 13 | COTTIER L , DESCOTES G , SORO Y . Heteromacrocycles from ring-closing metathesis of unsaturated furanic ethers[J]. Synth. Commun., 2003,33(24):4285–4295. |
| 14 | 马继平,于维强,王敏,等 . 催化选择转化多羟基化合物制备高附加值化学品研究进展[J]. 催化学报,2013,34(3):492-507. |
| 15 | MA J P, YU W Q , WANG M ,et al . Research progress in the preparation of high value-added chemicals by catalytic selective conversion of polyhydroxyl compounds[J]. Journal of Catalysis,2013,34(3):492-507. |
| 16 | TAKAGAKI A , NISHIMURA S , EBITANI K . Syntheses of 5-hydroxymethylfurfural and levoglucosan by selective dehydration of glucose using solid acid and base catalysts[J]. Applied Catalysis A: General,2010,383: 149-155. |
| 17 | LIU Y , LI H , HE J ,et al . Catalytic conversion of carbohydrates to levulinic acid with mesoporous niobium-containing oxides[J]. Catalysis Communications,2017,93: 20-24. |
| 18 | ZHANG J , CHEN J . Selective transfer hydrogenation of biomass-based furfural and 5-hydroxymethylfurfural over hydrotalcite-derived copper catalysts using methanol as a hydrogen donor[J]. ACS Sustainable Chemistry & Engineering,2017, 5(7): 5982-5993. |
| 19 | GYNGAZOVA M S , NEGAHDAR L , BLUMENTHAL L C ,et al . Experimental and kinetic analysis of the liquid phase hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over carbon-supported nickel catalysts[J]. Chemical Engineering Science,2017,173: 455-464. |
| 20 | BUNTARA T , NOEL S , PHUA P H ,et al . From 5-hydroxymethylfurfural(HMF) to polymer precursors: catalyst screening studies on the conversion of 1,2,6-hexanetriol to 1,6-hexanediol[J]. Topics in Catalysis,2012,55(7/8/9):612-619. |
| 21 | LI D , LIU Q Y , ZHU C H ,et al . Selective hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Co3O4,catalyst by controlled reduction[J]. Journal of Energy Chemistry,2019,30:34-41. |
| 22 | GAO Z , LI C Y , FAN G L ,et al . Nitrogen-doped carbon-decorated copper catalyst for highly efficient transfer hydrogenolysis of 5-hydroxymethylfurfural to convertibly produce 2,5-dimethylfuran or 2,5-dimethyltetrahydrofuran[J]. Applied Catalysis B: Environmental,2018,226:523-533. |
| 23 | YAO S , WANG X , JIANG Y ,et al . One-step conversion of biomass-derived 5-hydroxymethylfurfural to 1,2,6-hexanetriol over Ni-Co-Al mixed oxide catalysts under mild conditions[J]. ACS Sustainable Chemistry & Engineering,2014,2(2):173-180. |
| 24 | TANG X , WEI J , DING N ,et al . Chemoselective hydrogenation of biomass derived 5-hydroxymethylfurfural to diols: key intermediates for sustainable chemicals,materials and fuels[J]. Renewable & Sustainable Energy Reviews,2017,77:287-296. |
| 25 | MAZZONI R , PASINI T , SOLINAS G ,et al . Substrate and product role in the Shvo’s catalyzed selective hydrogenation of the platform bio-based chemical 5-hydroxymethylfurfural[J]. Dalton Trans, 2014,43:10224-10234. |
| 26 | CHATTERJEE M , ISHIZAKA T , KAWANAMI H . Selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis-(hydroxymethyl)furan using Pt/MCM-41 in an aqueous medium:a simple approach[J]. Green Chemistry,2014,16(11):4734-4739. |
| 27 | JAE J, ZHENG W , LOBO R F ,et al . Production of dimethylfuran from hydroxymethylfurfural through catalytic transfer hydrogenation with ruthenium supported on carbon[J],ChemSusChem, 2013,6(7):1158-1162. |
| 28 | HAO W , LI W , TANG X ,et al . Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethyl furfural to the building block 2,5-bishydroxymethylfuran[J]. Green Chemistry, 2016,18(4):1080-1088. |
| 29 | PASINI T , LOLLI A , ALBONETTI S ,et al . Methanol as a clean and efficient H-transfer reactant for carbonyl reduction:scope,limitations,and reaction mechanism[J]. Journal of Catalysis,2014,317:206-219. |
| 30 | THANANATTHANACHON T , RAUCHFUSS T B . Efficient production of the liquid fuel 2,5-dimethylfuran from fructose using formic acid as a reagent[J]. Angew. Chem.: Int. Ed., 2010,49(37):6616-6618. |
| 31 | OHYAMA J , ESAKI A , YAMAMOTO Y ,et al .Selective hydrogenation of 2-hydroxymethyl-5-furfural to 2,5-bis(hydroxymethyl)furan over gold sub-nano clusters[J]. RSC Adv., 2013,3(4):1033-1036. |
| 32 | ALAMILLO R , TUCKER M , MEI C ,et al . The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts[J]. Green Chemistry,2012,14(5):1413-1419. |
| 33 | AELLIG C , JENNY F , SCHOLZ D , et al . Combined 1,4-butanediol lactonization and transfer hydrogenation/hydrogenolysis of furfural-derivatives under continuous flow conditions[J]. Catalysis Science & Technology,2014,4(8):2326-2331. |
| 34 | CUI Y , WU B , MAO L ,et al . Facilely constructing robust nanohybrids comprising high dispersion of platinum-ruthenium nanoparticles on carbon nanotubes and their enhanced electrocatalytic performance[J]. Physica Status Solidi,2012,209(12):2532-2538. |
| 35 | BOTTARI G , KUMALAPUTRI A J , KRAWCZYK K K ,et al . Copper-zinc alloy nanopowder:a robust precious-metal-free catalyst for the conversion of 5-hydroxymethylfurfural[J]. ChemSusChem,2015,8(8):1323. |
| 36 | MACK D J , GUO B , NJARDARSON J T . ChemInform abstract: synthesis of allylic and homoallylic alcohols from unsaturated cyclic ethers using a mild and selective C—O reduction approach[J]. Chemical Communications,2012,48(63):7844-7846. |
| 37 | ZHU Y , KONG X , ZHENG H ,et al . Efficient synthesis of 2,5- dihydroxymethylfuran and 2,5-dimethylfuran from 5-hydroxymethylfurfural using mineral-derived Cu catalysts as versatile catalysts[J]. Catalysis Science & Technology 2015,5(8):4208-4217. |
| 38 | CAO Q , LIANG W , GUAN J ,et al . Catalytic synthesis of 2,5-bis-methoxymethylfuran: a promising cetane number improver for diesel[J]. Appl. Catal. A , 2014,481:49–53. |
| 39 | LIU Y , MELLMER M A , ALONSO D M ,et al . Effects of water on the copper-catalyzed conversion of hydroxymethylfurfural in tetrahydrofuran[J]. ChemSusChem,2016,8(23):3983-3986. |
| 40 | KUZNETSOV V A , PESTOV A V , PERVOVA M G ,et al . ChemInform abstract:new synthesis of dialkyl carbonates from alkylene carbonates and titanium alkoxides[J]. Russian Journal of Organic Chemistry,2013,49(7):1078-1079. |
| 41 | LIU F , ZHANG X , LU C ,et al . Non-specific lipid transfer proteins in plants: presenting new advances and an integrated functional analysis[J]. Journal of Experimental Botany,2015,66(19):5663. |
| 42 | WAAL J C VAN DER , KUNKELER P J , TAN K ,et al . Zeolite titanium beta:a selective catalyst for the gas-phase meerwein-ponndorf-verley,and oppenauer reactions[J]. Journal of Catalysis,1998,173(1):74-83. |
| 43 | CHU G , YU Z , GAO F ,et al . Cheminform abstract:One-pot formylation and dimerization of p-alkyl phenols using DCMT‐activated DMSO[J]. Synthetic Communications,2013,43(1):44-51. |
| 44 | LAURENCZY G , GRASEMANN M . Formic acid as hydrogen source- recent developments and future trends[J]. Energy & Environ. Science,2012,5:8171-8181. |
| 45 | SHEN J , WYMAN C E . Hydrochloric acid-catalyzed levulinic acid formation from cellulose: data and kinetic model to maximize yields[J]. AIChE Journal,2011,58(1):236–246. |
| 46 | GIRISUTA B , JANSSEN L P B M , HEERES H J . Green chemicals:a kinetic study on the conversion of glucose to levulinic acid[J]. Chemical Engineering Research & Design,2006,84(5):339-349. |
| 47 | CHANG C ,MA X J, CEN P L . Kinetics of levulinic acid formation from glucose decomposition at high temperature[J]. Chinese Journal of Chemical Engineering,2006,14(5):708-712. |
| 48 | LIU F , ZHANG X , LU C ,et al . Non-specific lipid transfer proteins in plants: presenting new advances and an integrated functional analysis[J]. Journal of Experimental Botany,2015,66(19):5663. |
| 49 | THANANATTHANACHON T , RAUCHFUSS T B . Efficient route to hydroxymethylfurans from sugars via transfer hydrogenation[J]. ChemSusChem, 2010,3(10):1139. |
| 50 | KUMALAPUTRI A J , BOTTARI G , ERNE P M ,et al . Tunable and selectiveconversion of 5-HMF to 2,5-furandimethanol and 2,5-dimethylfuran over copperdopedporous metal oxides[J]. ChemSusChem, 2014,7(8):2266-2275. |
| 51 | CAI H , LI C , WANG A ,et al . Biomass into chemicals: one-pot production of furan-based diols from carbohydrates via tandem reactions[J]. Catal. Today, 2014,234(1):59-65. |
| 52 | LIU F , AUDEMAR M , OLIVEIRA VIGIER K DE ,et al . Combination of Pd/C and amberlys-15 in a single reactor for the acid/hydrogenating catalytic conversion of carbohydrates to 5-hydroxy-2,5-hexanedione[J]. Green Chemistry,2014,16(9):4110-4114. |
| 53 | ALAMILLO R , TUCKER M , CHIA M ,et al . The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts[J]. Green Chemistry,2012,14(5):1413-1419. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||