化工进展 ›› 2019, Vol. 38 ›› Issue (06): 2746-2755.DOI: 10.16085/j.issn.1000-6613.2018-1749
Ir基催化剂用于CO选择性催化还原NO的研究进展
孟婧轩1( ),高凤雨1,2,唐晓龙1,2(
),高凤雨1,2,唐晓龙1,2( ),易红宏1,2,周远松1,2
),易红宏1,2,周远松1,2
- 1. 北京科技大学能源与环境工程学院,北京 100083
2. 工业典型污染物资源化处理北京市重点实验室,北京 100083
-
收稿日期:2018-08-31出版日期:2019-06-05发布日期:2019-06-05 -
通讯作者:唐晓龙 -
作者简介:孟婧轩(1992—),女,硕士研究生,研究方向为大气污染控制。E-mail:<email>jingxuan1127@163.com</email>。 -
基金资助:国家重点研发计划重点专项(2017YFC0210303);国家自然科学基金面上项目(21677010);国家自然科学基金(U1660109,21806009);中国博士后基金(2018M631344);中央高校基本科研业务费专项资金项目(FRF-TP-18-019A1)
Review of the Ir-based catalyst in selective catalytic reduction of NO with CO
Jingxuan MENG1( ),Fengyu GAO1,2,Xiaolong TANG1,2(
),Fengyu GAO1,2,Xiaolong TANG1,2( ),Honghong YI1,2,Yuansong ZHOU1,2
),Honghong YI1,2,Yuansong ZHOU1,2
- 1. School of Energy and Environmental Engineering,University of Science and Technology Beijing,Beijing 100083,China
2. Beijing Key Laboratory of Resource-oriented Treatment of Industrial Pollutants,Beijing 100083,China
-
Received:2018-08-31Online:2019-06-05Published:2019-06-05 -
Contact:Xiaolong TANG
摘要:
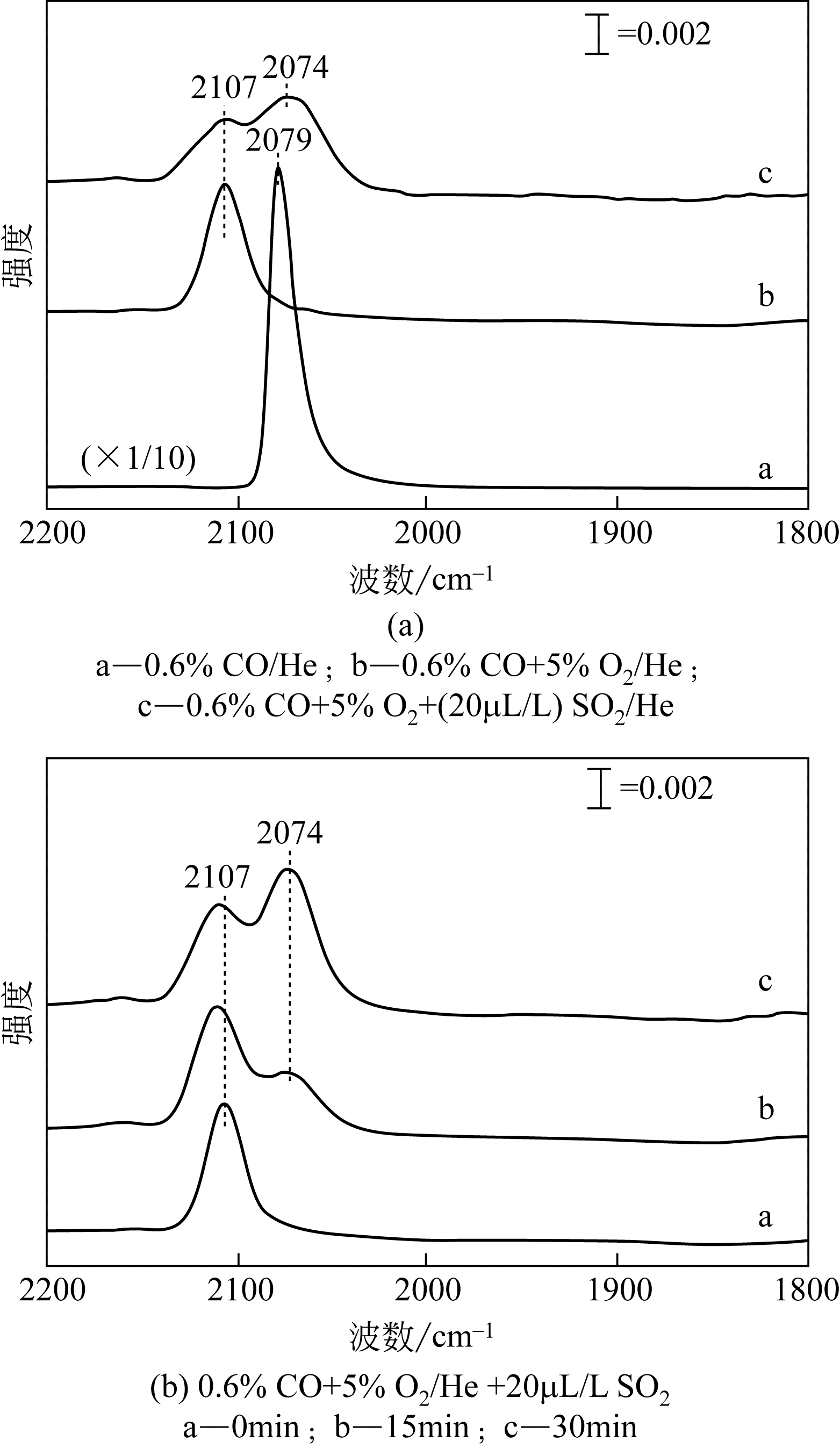
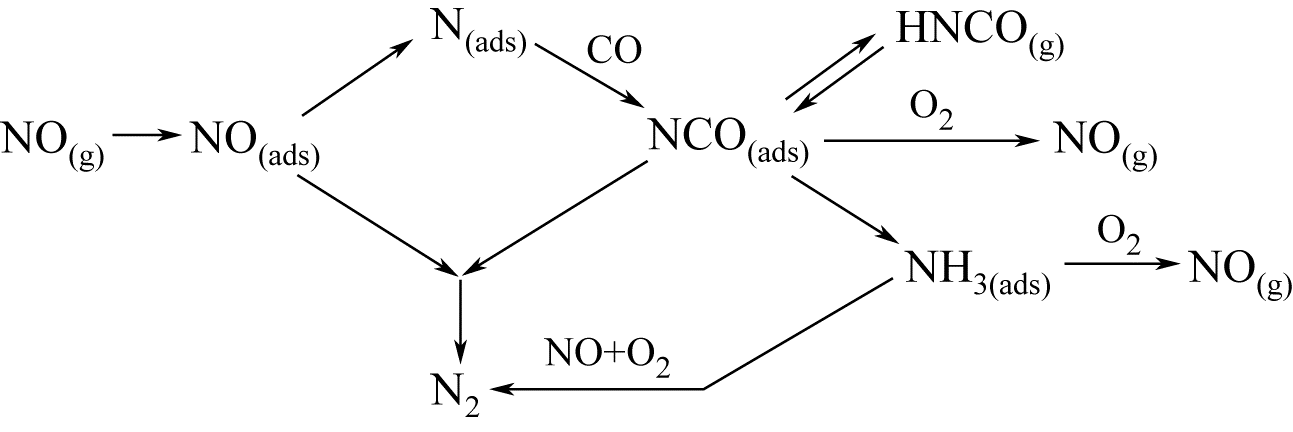
针对具有烟温低、氧含量高和CO浓度大等特征的固定源烟气(钢铁烧结/球团烟气和焦化烟气等)脱硝领域,CO选择性催化还原NO x (CO-SCR)技术具有良好的发展前景。Ir基贵金属催化剂因其在CO-SCR反应体系中表现出了良好的抗氧能力和较高的催化活性成为催化脱硝领域研究的热点之一。本文重点总结了单一载体、复合载体与复合活性组分三类Ir基催化剂在CO-SCR脱除NO x 中的催化性能,同时从制备条件和反应条件两大方面归纳了其对Ir基催化剂的CO-SCR脱硝性能的影响,简要阐述了Ir基催化剂表面反应机理,并对未来研究工作进行了展望,指出采用多种手段对催化剂进行改性,并通过降低Ir负载量、反应温度窗口以及提升其催化活性等方式来降低成本,为实现Ir基催化剂CO-SCR工业化应用提供借鉴。
中图分类号:
引用本文
孟婧轩, 高凤雨, 唐晓龙, 易红宏, 周远松. Ir基催化剂用于CO选择性催化还原NO的研究进展[J]. 化工进展, 2019, 38(06): 2746-2755.
Jingxuan MENG, Fengyu GAO, Xiaolong TANG, Honghong YI, Yuansong ZHOU. Review of the Ir-based catalyst in selective catalytic reduction of NO with CO[J]. Chemical Industry and Engineering Progress, 2019, 38(06): 2746-2755.
| 催化剂 | 反应条件 | 温度/℃ | NO转化率/% | CO转化率/% | 参考 文献 |
|---|---|---|---|---|---|
| Ce0.67Sn0.33O2 | [NO]=5%,[CO]=10%,He 平衡气, GHSV=12000h-1 | 325 | 70 | — | [ |
| Cu/Ce/Al(20∶1,Ce和Al体积比) | [NO]=5%,[CO]=10%, He平衡气, GHSV=12000h-1 | 350 | 100 | — | [ |
| CuO-V2O5/γ-Al2O3 | [NO]=5%,[CO]=10%, Ar平衡气, GHSV=24000h-1 | 350 | 100 | — | [ |
| CuO/Ni x O y /γ-Al2O3 | [NO]=5%,[CO]=10%, He平衡气, GHSV=24000h-1 | 350 | 100 | — | [ |
| Cu/CeMn-10∶1 | [NO]=5%,[CO]=5%, He平衡气, GHSV=24000h-1 | 225 | 98 | 48 | [ |
| Co2.9Cu0.1O4 | [NO]=5%,[CO]=5%,[O2]=2.5%,[H2O]=2.5%, Ar平衡气, GHSV=5000h-1 | 200 | 70 | 100 | [ |
表1 部分典型非贵金属催化剂的CO-SCR脱硝活性
| 催化剂 | 反应条件 | 温度/℃ | NO转化率/% | CO转化率/% | 参考 文献 |
|---|---|---|---|---|---|
| Ce0.67Sn0.33O2 | [NO]=5%,[CO]=10%,He 平衡气, GHSV=12000h-1 | 325 | 70 | — | [ |
| Cu/Ce/Al(20∶1,Ce和Al体积比) | [NO]=5%,[CO]=10%, He平衡气, GHSV=12000h-1 | 350 | 100 | — | [ |
| CuO-V2O5/γ-Al2O3 | [NO]=5%,[CO]=10%, Ar平衡气, GHSV=24000h-1 | 350 | 100 | — | [ |
| CuO/Ni x O y /γ-Al2O3 | [NO]=5%,[CO]=10%, He平衡气, GHSV=24000h-1 | 350 | 100 | — | [ |
| Cu/CeMn-10∶1 | [NO]=5%,[CO]=5%, He平衡气, GHSV=24000h-1 | 225 | 98 | 48 | [ |
| Co2.9Cu0.1O4 | [NO]=5%,[CO]=5%,[O2]=2.5%,[H2O]=2.5%, Ar平衡气, GHSV=5000h-1 | 200 | 70 | 100 | [ |
| 催化剂 | 反应条件 | 温度 /℃ | NO转化率/% | CO转化率/% | 参考文献 |
|---|---|---|---|---|---|
| 3% Pt/TiO2-SG | [NO]=0.5%,[CO]=1.5%,[O2]=1.5%, He平衡气 | 300 | 73 | — | [ |
| Pt/WO3/ZrO2 | [NO]=0.5%,[CO]=1.5%,[O2]=2%, N2平衡气 | 380 | 16 | — | [ |
| Pd/Ce0.6Zr0.4O2 | [NO]= 0.023%,[CO]= 0.0685%,[O2]=10.5%, N2平衡气 | 350 | 96 | 100 | [ |
| Rh/Na-Beta zeolite | [NO]=0.05%,[CO]=1.5%,[O2]=9%,[H2O]=6%, N2平衡气 | 350 | 45 | 79 | [ |
| LaCu-ZSM-5/cordierite | [NO]=0.05%,[CO]=0.1%,[O2]=6.8%,[SO2]=100μL/L, [H2O]=10.5%, He平衡气 | 250 | 50 | 100 | [ |
| Ba/Ir/WO3-SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[SO2]=1μL/L, [H2O]=6%,He平衡气, GHSV=60000h-1 | 260 | 100 | — | [ |
表2 部分典型贵金属催化剂的CO-SCR脱硝活性
| 催化剂 | 反应条件 | 温度 /℃ | NO转化率/% | CO转化率/% | 参考文献 |
|---|---|---|---|---|---|
| 3% Pt/TiO2-SG | [NO]=0.5%,[CO]=1.5%,[O2]=1.5%, He平衡气 | 300 | 73 | — | [ |
| Pt/WO3/ZrO2 | [NO]=0.5%,[CO]=1.5%,[O2]=2%, N2平衡气 | 380 | 16 | — | [ |
| Pd/Ce0.6Zr0.4O2 | [NO]= 0.023%,[CO]= 0.0685%,[O2]=10.5%, N2平衡气 | 350 | 96 | 100 | [ |
| Rh/Na-Beta zeolite | [NO]=0.05%,[CO]=1.5%,[O2]=9%,[H2O]=6%, N2平衡气 | 350 | 45 | 79 | [ |
| LaCu-ZSM-5/cordierite | [NO]=0.05%,[CO]=0.1%,[O2]=6.8%,[SO2]=100μL/L, [H2O]=10.5%, He平衡气 | 250 | 50 | 100 | [ |
| Ba/Ir/WO3-SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[SO2]=1μL/L, [H2O]=6%,He平衡气, GHSV=60000h-1 | 260 | 100 | — | [ |
| WO3 质量分数/% | 反应温度/℃ | NO x 转化率/% | N2 选择性/% |
|---|---|---|---|
| 0 | 250 | 11 | 81 |
| 1 | 260 | 85 | 89 |
| 10 | 260 | 86 | 89 |
| 30 | 265 | 82 | 87 |
| 40 | 265 | 69 | 81 |
| 50 | 255 | 49 | 79 |
| 80 | 275 | 42 | 72 |
| 100 | 270 | 39 | 79 |
表3 不同WO3 含量Ir/WO3-SiO2催化剂 的CO-SCR
| WO3 质量分数/% | 反应温度/℃ | NO x 转化率/% | N2 选择性/% |
|---|---|---|---|
| 0 | 250 | 11 | 81 |
| 1 | 260 | 85 | 89 |
| 10 | 260 | 86 | 89 |
| 30 | 265 | 82 | 87 |
| 40 | 265 | 69 | 81 |
| 50 | 255 | 49 | 79 |
| 80 | 275 | 42 | 72 |
| 100 | 270 | 39 | 79 |
| 催化剂 | 反应条件 | 温度/℃ | NO 转化率/% | CO转化率/% | 参考 文献 |
|---|---|---|---|---|---|
| Ir/SiO2 | [NO]=0.1%,[CO]=0.75%,[O2]=1%,He平衡气,GHSV=40000h-1 | 365 | 80 | — | [ |
| Ir/Al2O3 | [NO]=0.1%,[CO]=0.75%,[O2]=1%,He平衡气,GHSV=40000h-1 | 400 | 50 | — | [ |
| Ir/SiO2 | [NO]=0.1%,[CO]=0.6%,[O2]=5%,[SO2]=20μL/L,[H2O]=6%,He平衡气, GHSV=75000h-1 | 350 | 62 | 90 | [ |
| Ir/WO3 | [NO]=0.1%,[CO]=1%,[O2]=2%,He平衡气 | 250 | 79.5 | — | [ |
| Ir/WO3-SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[SO2]=1μL/L,[H2O]=6%,He平衡气, GHSV=75000h-1 | 300 | 70 | 55 | [ |
| Ir/WO3-SiO2 | [NO]=0.05%,[CO]=0.5%,[O2]=10%,[H2O]=1%,He平衡气,GHSV=34000h-1 | 260 | 86 | — | [ |
| Nb2O5/Ir/SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[H2O]=6%,He平衡气,GHSV=75000h-1 | 280 | 80 | — | [ |
| Ba/Ir/SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[SO2]=1μL/L,[H2O]=6%,He平衡气, GHSV=75000h-1 | 280 | 65 | 60 | [ |
| Ba-Ir/WO3-SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[SO2]=1μL/L,[H2O]=6%,He平衡气, GHSV=75000h-1 | 280 | 94 | 90 | [ |
| Ba/Ir/WO3-SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[SO2]=1μL/L,[H2O]=6%,He平衡气, GHSV=60000h-1 | 260 | 100 | — | [ |
表4 部分典型Ir基贵金属催化剂的CO-SCR脱硝活性
| 催化剂 | 反应条件 | 温度/℃ | NO 转化率/% | CO转化率/% | 参考 文献 |
|---|---|---|---|---|---|
| Ir/SiO2 | [NO]=0.1%,[CO]=0.75%,[O2]=1%,He平衡气,GHSV=40000h-1 | 365 | 80 | — | [ |
| Ir/Al2O3 | [NO]=0.1%,[CO]=0.75%,[O2]=1%,He平衡气,GHSV=40000h-1 | 400 | 50 | — | [ |
| Ir/SiO2 | [NO]=0.1%,[CO]=0.6%,[O2]=5%,[SO2]=20μL/L,[H2O]=6%,He平衡气, GHSV=75000h-1 | 350 | 62 | 90 | [ |
| Ir/WO3 | [NO]=0.1%,[CO]=1%,[O2]=2%,He平衡气 | 250 | 79.5 | — | [ |
| Ir/WO3-SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[SO2]=1μL/L,[H2O]=6%,He平衡气, GHSV=75000h-1 | 300 | 70 | 55 | [ |
| Ir/WO3-SiO2 | [NO]=0.05%,[CO]=0.5%,[O2]=10%,[H2O]=1%,He平衡气,GHSV=34000h-1 | 260 | 86 | — | [ |
| Nb2O5/Ir/SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[H2O]=6%,He平衡气,GHSV=75000h-1 | 280 | 80 | — | [ |
| Ba/Ir/SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[SO2]=1μL/L,[H2O]=6%,He平衡气, GHSV=75000h-1 | 280 | 65 | 60 | [ |
| Ba-Ir/WO3-SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[SO2]=1μL/L,[H2O]=6%,He平衡气, GHSV=75000h-1 | 280 | 94 | 90 | [ |
| Ba/Ir/WO3-SiO2 | [NO]=0.05%,[CO]=0.3%,[O2]=5%,[SO2]=1μL/L,[H2O]=6%,He平衡气, GHSV=60000h-1 | 260 | 100 | — | [ |
| 前体 | NO x 转化率/% | N2 选择性/% |
|---|---|---|
| Ir(NO3)4 | 44 | 84 |
| H2IrCl6 | 43 | 88 |
| Ir(NH3)6(NO3)3 | 64 | 86 |
| Ir(NH3)6(OH)3 | 72 | 89 |
表5 前体对 CO-SCR 催化活性的影响[40]
| 前体 | NO x 转化率/% | N2 选择性/% |
|---|---|---|
| Ir(NO3)4 | 44 | 84 |
| H2IrCl6 | 43 | 88 |
| Ir(NH3)6(NO3)3 | 64 | 86 |
| Ir(NH3)6(OH)3 | 72 | 89 |
| 1 | SKALSKA K , MILLER J S , LEDAKOWICZ S . Trends in NO x abatement: a review[J]. The Science of the Total Environment, 2010, 408(19): 3976-3989. |
| 2 | CHENG X , BI X T . A review of recent advances in selective catalytic NO x reduction reactor technologies[J]. Particuology, 2014, 16: 1-18. |
| 3 | 李晨露, 唐晓龙, 易红宏,等 . Mn基低温SCR催化剂的抗H2O、抗SO2研究进展[J]. 化工进展, 2017, 36(3): 934-943. |
| LI C L , TANG X L , YI H H ,et al . Review on manganese based catalysts resistant to H2O and SO2 for SCR reduction at low temperature[J]. Chemical Industry and Engineering Progress, 2017, 36(3): 934-943. | |
| 4 | ZHANG X , MA C, CHENG X , et al . Performance of Fe-Ba/ZSM-5 catalysts in NO + O2 adsorption and NO + CO reduction[J]. International Journal of Hydrogen Energy, 2017, 42(10): 7077-7088. |
| 5 | WANG L , CHENG X , WANG Z , et al . Investigation on Fe-Co binary metal oxides supported on activated semi-coke for NO reduction by CO[J]. Applied Catalysis B: Environmental, 2017, 201: 636-51. |
| 6 | SALKER A V , DESAI M S F . CO-NO/O2 redox reactions over Cu substituted cobalt oxide spinels[J]. Catalysis Communications, 2016, 87: 116-119. |
| 7 | GU X , LI H , LIU L , et al . Promotional effect of CO pretreatment on CuO/CeO2 catalyst for catalytic reduction of NO by CO[J]. Journal of Rare Earths, 2014, 32(2): 139-145. |
| 8 | LIU T , QIAN J , YAO Y , et al . Research on SCR of NO with CO over the Cu0.1La0.1Ce0.8O mixed-oxide catalysts: effect of the grinding[J]. Molecular Catalysis, 2017, 430: 43-53. |
| 9 | LV Y , ZHANG H , YAO X , et al . Investigation of the physicochemical properties of CuO/Sm2O3/γ-Al2O3 catalysts and their activity for NO removal by CO[J]. Journal of Molecular Catalysis A: Chemical, 2016, 420: 34-44. |
| 10 | XIONG Y , YAO X , TANG C , et al . Effect of CO-pretreatment on the CuO-V2O5/γ-Al2O3 catalyst for NO reduction by CO[J]. Catal. Sci. Technol., 2014, 4(12): 4416-4425. |
| 11 | DENG C , LI B , DONG L , et al . NO reduction by CO over CuO supported on CeO2-doped TiO2: the effect of the amount of a few CeO2 [J]. Physical Chemistry Chemical Physics : PCCP, 2015, 17(24): 16092-16109. |
| 12 | YAO X , XIONG Y , ZOU W , et al . Correlation between the physicochemical properties and catalytic performances of Ce x Sn1– x O2 mixed oxides for NO reduction by CO[J]. Applied Catalysis B: Environmental, 2014, 144: 152-165. |
| 13 | SALKER A V , DESAI M S F . Catalytic activity and mechanistic approach of NO reduction by CO over M0.05 Co2.95O4 (M = Rh, Pd & Ru) spinel system[J]. Applied Surface Science, 2016, 389: 344-353. |
| 14 | SALKER A V , DESAI M S FAL . Low-temperature nitric oxide reduction over silver-substituted cobalt oxide spinels[J]. Catalysis Science & Technology, 2016, 6(2): 430-433. |
| 15 | ZHU H-O , J-R KIM , S-K IHM . Selective catalytic reduction of NO with CO on Pt/W–Ce–Zr catalysts[J]. Reaction Kinetics and Catalysis Letters, 2009, 97(2): 207-215. |
| 16 | ZHU H-O , J-R KIM , S-K IHM . Characteristics of Pt/WO3/CeO2/ZrO2 catalysts for catalytic reduction of NO by CO[J]. Applied Catalysis B: Environmental, 2009, 86(1/2): 87-92. |
| 17 | WANG J A , CUAN A , SALMONES J , et al . Studies of sol-gel TiO2 and Pt/TiO2 catalysts for NO reduction by CO in an oxygen-rich condition[J]. Applied Surface Science, 2004, 230(1-4): 94-105. |
| 18 | LI M S , SESHAN K , LEFFERTS L . Influence of NO on the reduction of NO2 with CO over Pt/SiO2 in the presence of O2 [J]. Chinese Journal of Chemistry, 2007, 25(4): 435-438. |
| 19 | HUANG K , LIN L , YANG K , et al . Promotion effect of ultraviolet light on NO + CO reaction over Pt/TiO2 and Pt/CeO2-TiO2 catalysts[J]. Applied Catalysis B: Environmental, 2015, 179: 395-406. |
| 20 | CHEN L F , GONZ L G , WANG J A , et al . Surfactant-controlled synthesis of Pd/Ce0.6Zr0.4O2 catalyst for NO reduction by CO with excess oxygen[J]. Applied Surface Science, 2005, 243(1-4): 319-328. |
| 21 | NAKATSUJI T , YAMAGUCHI T , SATO N , et al . A selective NO x reduction on Rh-based catalysts in lean conditions using CO as a main reductant[J]. Applied Catalysis B: Environmental, 2008, 85(1/2): 61-70. |
| 22 | DOI Y, HANEDA M . Catalytic performance of supported Ir catalysts for NO reduction with C3H6 and CO in slight lean conditions[J]. Catalysis Today, 2018, 303: 8-12. |
| 23 | HANEDA M , HAMADA H . Promotional role of H2O in the selective catalytic reduction of NO with CO over Ir/WO3/SiO2 catalyst[J]. Journal of Catalysis, 2010, 273(1): 39-49. |
| 24 | INOMATA H , SHIMOKAWABE M , KUWANA A , et al . Selective reduction of NO with CO in the presence of O2 with Ir/WO3 catalysts: influence of preparation variables on the catalytic performance[J]. Applied Catalysis B: Environmental, 2008, 84(3/4): 783-789. |
| 25 | NANBA T , K-I WADA , MASUKAWA S , et al . Enhancement of activity of Ir catalysts for selective catalytic reduction of NO with CO by physical mixing with SiO2 [J]. Applied Catalysis A: General, 2010, 380(1/2): 66-71. |
| 26 | 付玉秀,仲雪梅,常化振,等 . 铈钴复合氧化物催化剂CO-SCR反应机理研究[J]. 中国环境科学, 2018,38(8):2934-2940. |
| FU Y X , ZHONG X M , CHANG Z H ,et al . Study on reaction mechanism of CO-SCR of samarium cobalt composite oxide catalyst[J]. Chinese Environmental Science, 2018, 38(8):2934-2940. | |
| 27 | TAUSTER S J , MURRELL L . The NO CO reaction in the presence of excess O2 as catalyzed by iridium[J]. Journal of Catalysis, 1976, 41(1): 192-195. |
| 28 | HANEDA M , PUSPARATU, KINTAICHI Y , et al . Promotional effect of SO2 on the activity of Ir/SiO2 for NO reduction with CO under oxygen-rich conditions[J]. Journal of Catalysis, 2005, 229(1): 197-205. |
| 29 | YOSHINARI T , SATO K , HAMADA M . Remarkable promoting effcet of coexisting SO2 on the catalytic of Ir/SiO2 for NO reduction in the presence of oxygen[J]. Catalysis Communications, 2001,2:155-158. |
| 30 | YOSHINARI T , SATO K , HANEDA M , et al . Positive effect of coexisting SO2 on the activity of supported iridium catalysts for NO reduction in the presence of oxygen[J]. Applied Catalysis B: Environmental, 2003, 41(1/2): 157-169. |
| 31 | YAO X , TANG C , JI Z , et al . Investigation of the physicochemical properties and catalytic activities of Ce0.67M0.33O2(M = Zr4+, Ti4+, Sn4+) solid solutions for NO removal by CO[J]. Catal. Sci. Technol., 2013, 3(3): 688-698. |
| 32 | GE C , LIU L , LIU Z , et al . Improving the dispersion of CeO2 on γ-Al2O3 to enhance the catalytic performances of CuO/CeO2/γ-Al2O3 catalysts for NO removal by CO[J]. Catalysis Communications, 2014, 51: 95-99. |
| 33 | GE C , LIU L , YAO X , et al . Treatment induced remarkable enhancement of low-temperature activity and selectivity of copper-based catalysts for NO reduction[J]. Catalysis Science & Technology, 2013, 3(6): 1547. |
| 34 | DENG C , HUANG Q , ZHU X , et al . The influence of Mn-doped CeO2 on the activity of CuO/CeO2 in CO oxidation and NO + CO model reaction[J]. Applied Surface Science, 2016, 389: 1033-1049. |
| 35 | WANG T , LI L , GUAN N . Combination catalyst for the purification of automobile exhaust from lean-burn engine[J]. Fuel Processing Technology, 2013, 108: 41-46. |
| 36 | SASAKI M , SULTANA A , HANEDA M , et al . Practical evaluation of the catalytic performance of Ir/SiO2-based catalysts for selective reduction of NO with CO[J]. Topics in Catalysis, 2009, 52(13-20): 1803-1807. |
| 37 | OGURA M , KAWAMURA A , MATSUKATA M . Catalytic activity of Ir for NO-CO reaction in the presence of SO2 and excess oxygen[J]. Chemistry Letters, 2000, 29(2): 146-147. |
| 38 | SHIMOKAWABE M , UMEDA N . Selective catalytic reduction of NO by CO over supported iridium and rhodium catalysts[J]. Chemistry Letters, 2004, 33(5): 534-535. |
| 39 | TAKAHASHI A , NAKAMURA I , HANEDA M , et al . Role of tungsten in promoting selective reduction of NO with CO over Ir/WO3-SiO2 catalysts[J]. Catalysis Letters, 2006, 112(3/4): 133-138. |
| 40 | NANBA T , SHINOHARA S , UCHISAWA J , et al . Enhancement of activity of Ir catalysts for the selective catalytic reduction of NO by CO[J]. Chemistry Letters, 2006, 35(4): 450-451. |
| 41 | NANBA T , SHINOHARA S , MASUKAWA S , et al . Formation of active sites on Ir/WO3-SiO2 for selective catalytic reduction of NO by CO[J]. Applied Catalysis B: Environmental, 2008, 84(3/4): 420-425. |
| 42 | TAKAHASHI A , FUJITANI T , NAKAMURA I , et al . Excellent promoting effect of Ba addition on the catalytic activity of Ir/WO3-SiO2 for the selective reduction of NO with CO[J]. Chemistry Letters, 2006, 35(4): 420-421. |
| 43 | HANEDA M , HAMADA H . Promoting effect of coexisting H2O on the activity of Ir/WO3/SiO2 catalyst for the selective reduction of NO with CO[J]. Chemistry Letters, 2008, 37(8): 830-831. |
| 44 | TAMAI T , HANEDA M , FUJITANI T , et al . Promotive effect of Nb2O5 on the catalytic activity of Ir/SiO2 for NO reduction with CO under oxygen-rich conditions[J]. Catalysis Communications, 2007, 8(6): 885-888. |
| 45 | HANEDA M , KUDO H , NAGAO Y , et al . Enhanced activity of Ba-doped Ir/SiO2 catalyst for NO reduction with CO in the presence of O2 and SO2 [J]. Catalysis Communications, 2006, 7(7): 423-426. |
| 46 | HANEDA M , AOKI N , ARIMITSU K , et al . Activity enhancement of WO3-promoted Ir/SiO2 catalysts by high-temperature calcination for the selective reduction of NO with CO[J]. Bulletin of the Chemical Society of Japan, 2009, 82(8): 1023-1029. |
| 47 | HANEDA M , FUJITANI T , HAMADA H . Effect of iridium dispersion on the catalytic activity of Ir/SiO2 for the selective reduction of NO with CO in the presence of O2 and SO2 [J]. Journal of Molecular Catalysis A: Chemical, 2006, 256(1/2): 143-148. |
| 48 | SHIMOKAWABE M , NIITSU M , INOMATA h , et al . A highly active Ir/WO3 catalyst for the selective reduction of NO by CO in the presence of O2 or O2+SO2 [J]. Chemistry Letters, 2005, 34(10): 1426-1427. |
| 49 | INOMATA H , SHIMOKAWABE M , ARAI M . An Ir/WO3 catalyst for selective reduction of NO with CO in the presence of O2 and/or SO2 [J]. Applied Catalysis A: General, 2007, 332(1): 146-152. |
| 50 | HANEDA M , CHIBA K , TAKAHASHI A , et al . Enhancing effect of H2 on the selective reduction of NO with CO over Ba-doped Ir/WO3/SiO2 catalyst[J]. Catalysis Letters, 2007, 118(3/4): 159-164. |
| 51 | FUJITANI T , NAKAMURA I , KOBAYASHI Y , et al . Adsorption and reactivity of SO2 on Ir(111) and Rh(111) [J]. Surface Science, 2007, 601(6): 1615-1622. |
| 52 | CHEN W , SHEN Q , BARTYNSKI R A , et al . Reduction of nitric oxide by acetylene on Ir surfaces with different morphologies: comparison with reduction of NO by CO[J]. Langmuir : the ACS Journal of Surfaces and Colloids, 2013, 29(4): 1113-1121. |
| 53 | NAKAMURA I , FUJITANI T . Adsorption behavior and reaction properties of NO and CO on Ir(111) and Rh(111) [J]. Catalysis Surveys from Asia, 2009, 13(1): 22-29. |
| 54 | LYONS K J , XIE J , MITCHELL W J . Adsorption of carbon monoxide on Ir (110) investigated by infrared reflection-absorption spectroscopy[J]. Surface Science, 1995, 325(1/2): 85-92. |
| 55 | BURGHAUS U , DING J , WEINBERG W H . Effect of preadsorbed oxygen on the adsorption of CO on Ir (110) [J]. Surface Science, 1997, 384(1-3): L869-L874. |
| 56 | TANG C , SEVERSON M W . Coverage-dependent infrared spectroscopy of carbon monoxide on Iridium (111) in aqueous solution: a benchmark comparison between chemisorption in ordered electrochemical and ultrahigh-vacuum environments[J]. The Journal of Physical Chemistry B, 1998, 102(44): 8796-8806. |
| 57 | ZOU S , WEAVER M J . Spatial structure of ordered electrochemical adlayers from in situ scanning tunneling microscopy and infrared spectroscopy: single-site carbon monoxide binding on iridium (111) and comparisons with related systems[J]. Surface Science, 2000, 446(1/2): L95-L100. |
| 58 | FUJITANI T , NAKAMURA I , KOBAYASHI Y . Adsorption and reactions of NO on clean and CO-precovered Ir (111) [J]. The Journal of Physical Chemistry B, 2005, 109(37): 17603-17607. |
| 59 | FUJITANI T , NAKAMURA I , TAKAHASHI A , et al . Kinetics and mechanism of NO reduction with CO on Ir surfaces[J]. Journal of Catalysis, 2008, 253(1): 139-147. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 李东泽, 张祥, 田键, 胡攀, 姚杰, 朱林, 卜昌盛, 王昕晔. 基于水泥窑脱硝的碳基还原NO x 研究进展[J]. 化工进展, 2023, 42(9): 4882-4893. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||