化工进展 ›› 2019, Vol. 38 ›› Issue (04): 1815-1822.DOI: 10.16085/j.issn.1000-6613.2018-1313
以生物质为原料合成丙烯腈的研究进展
- 中国石化上海石油化工研究院,上海 201208
-
收稿日期:2018-06-26修回日期:2018-09-04出版日期:2019-04-05发布日期:2019-04-05 -
作者简介:周晓峰(1979 —),男,博士,副研究员,研究方向为丙烯腈催化剂。E-mail:<email>zhouxf.sshy@sinopec.com</email>。
Research progress of acrylonitrile production from renewable biomass
Xiaofeng ZHOU( ),Lianghua WU,Jiale JIANG
),Lianghua WU,Jiale JIANG
- SINOPEC Shanghai Research Institute of Petrochemical Technology,Shanghai 201208,China
-
Received:2018-06-26Revised:2018-09-04Online:2019-04-05Published:2019-04-05
摘要:
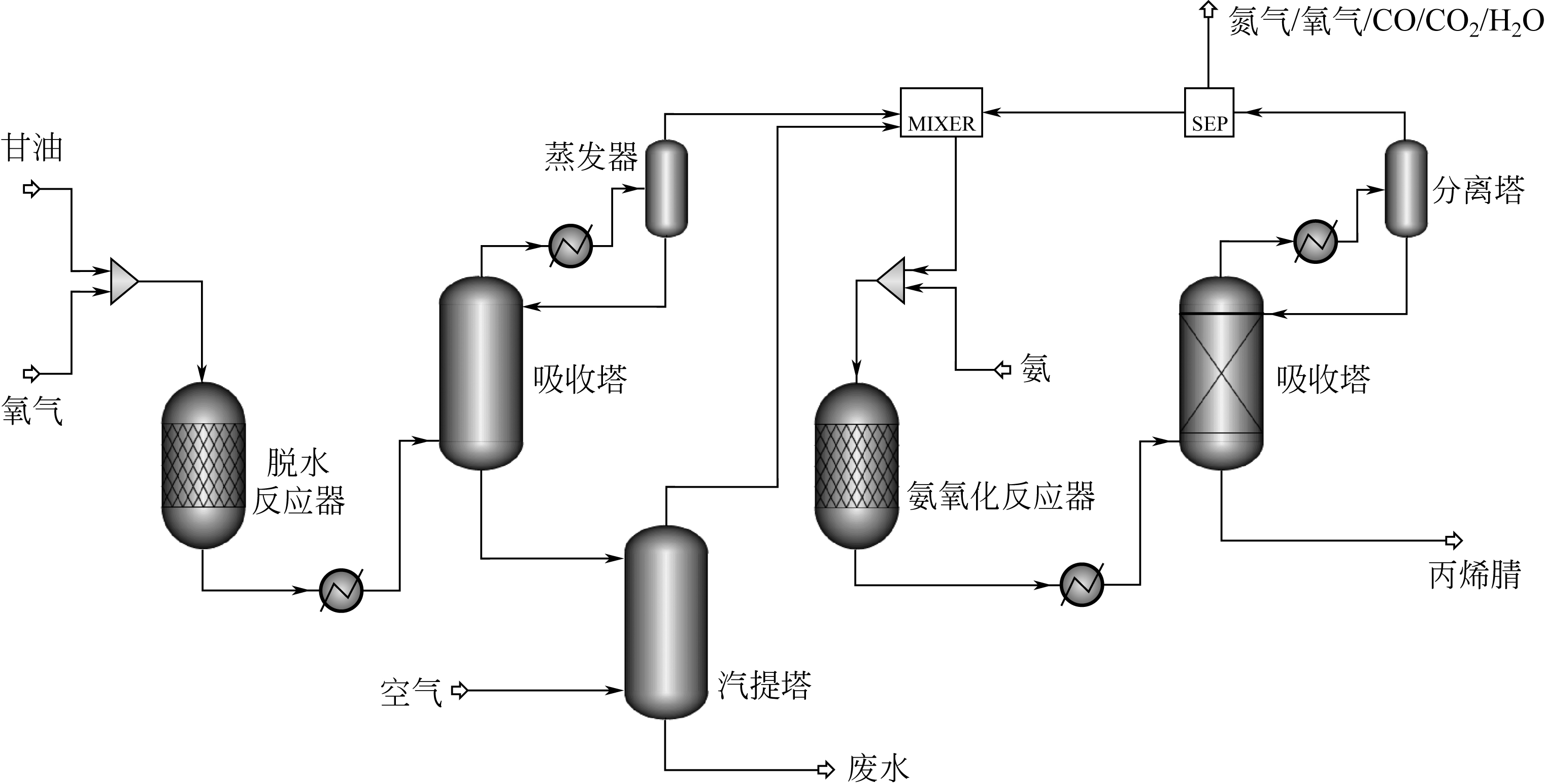
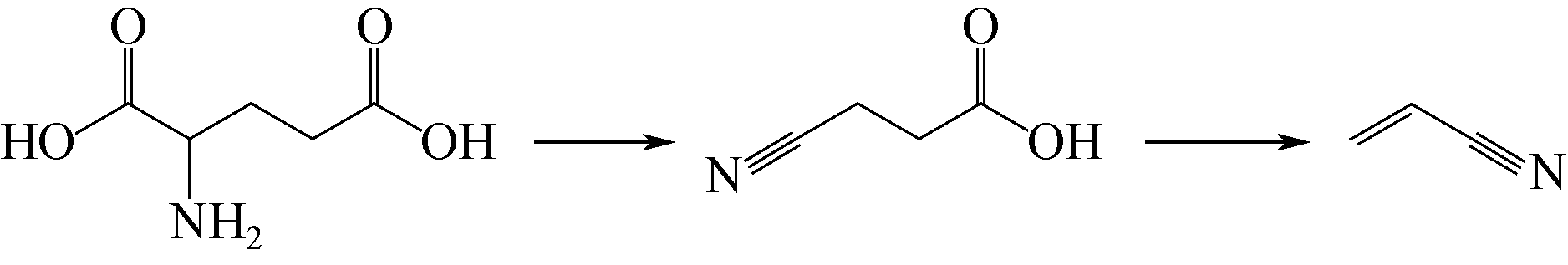
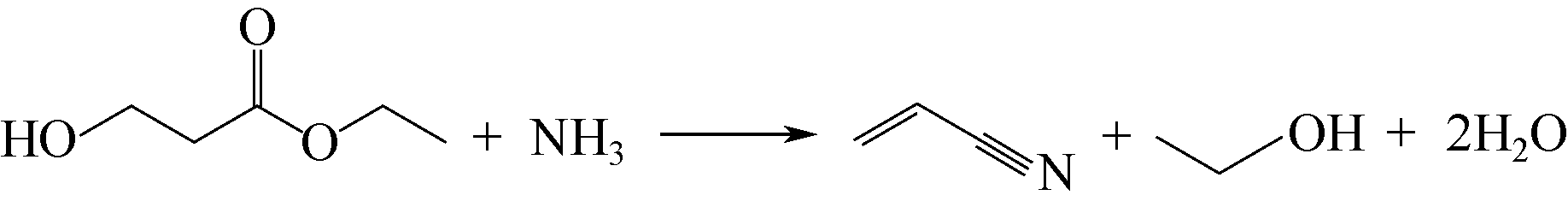
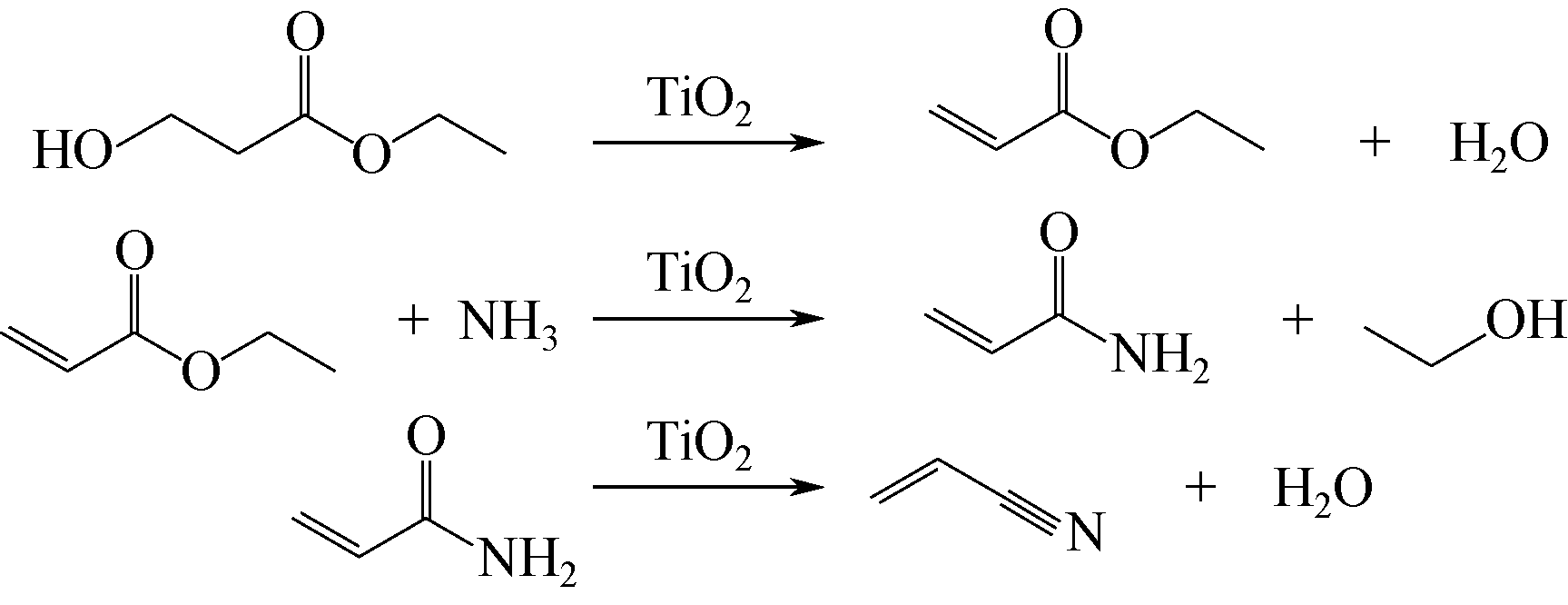
生物质原料由于具有来源广泛、绿色环保和可再生循环利用等特点,近年来逐渐应用于丙烯腈的合成研究。本文简述了以生物质(甘油、谷氨酸和3-羟基丙酸)为原料合成丙烯腈3种新工艺的研究进展,主要介绍和讨论了甘油两段法、谷氨酸脱羧基法和3-羟基丙酸腈化法的合成工艺及其催化剂的性能。目前甘油两段法丙烯腈总收率在60%左右,远低于丙烯法工业装置高于80%的水平,并且催化剂较易积炭而失活,今后仍需努力提高丙烯腈收率和催化剂的稳定性;谷氨酸脱羧基法的优点是反应过程中不需要引入氨,但是工艺复杂且对环境影响较大,丙烯腈单收只有20%左右,合成工艺仍待进一步优化;而3-羟基丙酸腈化法不需要氧气参与反应,副产物较少,碳氧化物的排放显著降低且不产生氢氰酸,丙烯腈单收大于90%远高于丙烯法,通过该工艺生产的丙烯腈具有一定的成本优势,比较适合应用于国内低成本聚丙烯腈基碳纤维的生产。
中图分类号:
引用本文
周晓峰, 吴粮华, 姜家乐. 以生物质为原料合成丙烯腈的研究进展[J]. 化工进展, 2019, 38(04): 1815-1822.
Xiaofeng ZHOU, Lianghua WU, Jiale JIANG. Research progress of acrylonitrile production from renewable biomass[J]. Chemical Industry and Engineering Progress, 2019, 38(04): 1815-1822.
| 1 | 姜其舟 . 丙烯腈行业生产现状分析[J]. 山东化工, 2018, 47(7): 56-57, 59. |
| JIANG Q Z . An overview of the production capacity of acrylonitrile industry[J]. Shandong Chemical Industry, 2018, 47(7): 56-57, 59. | |
| 2 | GRASSELLI R K , LUGMAIR C G , VOLPE J A F . Towards an understanding of the reaction pathways in propane ammoxidation based on the distribution of elements at the active centers of the M1 phase of the MoV(Nb,Ta)TeO system[J]. Topics in Catalysis, 2011, 54(10): 595-604. |
| 3 | 钱伯章 . 旭化成在泰国的丙烯腈、甲基丙烯酸甲酯和纺黏设施投产[J]. 合成纤维, 2013, 42(4): 15. |
| QIAN B Z . The PTT Asahi new acrylonitrile and methyl methacrylate plants in Thailand were commissioned[J]. Synthetic Fiber in China, 2013, 42(4): 15. | |
| 4 | Gardner Business Media, Inc . Southern Research Inst. receives DOE award to advance low cost carbon fiber from biomass [EB/OL]. [2014-09-01].. |
| 5 | 孙启梅, 王崇辉, 王领民, 等 .生物柴油副产物粗甘油的综合利用[J].化工进展, 2017, 36(s1): 161-166. |
| SUN Q M , WANG C H , WANG L M , et al . Integrated utilization of crude glycerol as a by-product of biodiesel production[J]. Chemical Industry and Engineering Progress, 2017, 36(s1): 161-166. | |
| 6 | 李春义 . 甘油催化脱水制备丙烯醛的研究[D].青岛: 中国石油大学(华东), 2013. |
| LI C Y . Study on the catalytic dehydration of glycerol to acrolein [D].Qingdao: China University of Petroleum(East China), 2013. | |
| 7 | GUERRERO-PÉREZ M O , BAÑARES M A . New reaction: conversion of glycerol into acrylonitrile[J]. ChemSusChem, 2008, 1: 511-513. |
| 8 | CARSTEN L , SÉBASTIEN P , BENJAMIN K , et al . Glycerol conversion to acrylonitrile by consecutive dehydration over WO3/TiO2 and ammoxidation over Sb-(Fe,V)-O[J]. Applied Catalysis B: Environmental, 2013, 132/133: 170-182. |
| 9 | CARSTEN L , SÉBASTIEN P , BENJAMIN K , et al . Reply to the letter to the editor concerning the comments of M.A. Banares and M.O. Guerrero-Pérez to the article “Glycerol conversion to acrylonitrile by consecutive dehydration over WO3/TiO2 and ammoxidation over Sb-(Fe,V)-O”[J].Applied Catalysis B: Environmental, 2014, 148/149: 604-605. |
| 10 | BAÑARES M A , GUERRERO-PÉREZ M O . Comments on “Glycerol conversion to acrylonitrile by consecutive dehydration over WO3/TiO2 and ammoxidation over Sb-(Fe,V)-O”[J]. Applied Catalysis B: Environmental, 2014, 148/149: 601-603. |
| 11 | CALVINO-CASILDA V , GUERRERO-PÉREZ M O , BAÑARES M A . Microwave-activated direct synthesis of acrylonitrile from glycerol under mild conditions: effect of niobium as dopant of the V-Sb oxide catalytic system[J]. Applied Catalysis B: Environmental, 2010, 95: 192-196. |
| 12 | France Arkema . Method for the synthesis of acrylonitrile from glycerol: US 8829223[P].2014-09-09. |
| 13 | GRASSELLI R K , TRIFIRO F . Acrylonitrile from biomass: still far from being a sustainable process[J]. Topics in Catalysis, 2016, 59: 1651-1658. |
| 14 | 阿肯马法国公司 . 从甘油合成丙烯醛的方法:CN101896451[P]. 2010-11-24. |
| France Arkema . Method for the synthesis of acrolein from glycerol: CN 101896451[P]. 2010-11-24. | |
| 15 | 阿肯马法国公司 . 丙烯醛的制备方法:CN101379017[P]. 2013-01-02. |
| France Arkema . Acrolein preparation method: CN101379017[P]. 2013-01-02. | |
| 16 | 阿肯马法国公司 . 由甘油制造丙烯醛和/或丙烯酸的方法:CN 104220409[P]. 2017-02-8. |
| France Arkema . Method for producing acrolein and/or acrylic acid from glycerol: CN104220409[P]. 2017-02-08. | |
| 17 | 阿肯马法国公司 . 使甘油脱水为丙烯醛的方法: CN101119955[P]. 2011-06-22. |
| France Arkema . Process for dehydrating glycerol to acrolein: CN 101119955[P]. 2011-06-22. | |
| 18 | 阿肯马法国公司 . 由甘油制造丙烯醛的方法:CN105348054[P]. 2016-02-24. |
| France Arkema . Process for manufacturing acrolein from glycerol: CN 105348054 [P]. 2016-02-24. | |
| 19 | 阿肯马法国公司 . 由甘油制造丙烯醛的方法:CN101801902[P]. 2015-01-28. |
| France Arkema . Process for manufacturing acrolein from glycerol: CN101801902[P]. 2015-01-28. | |
| 20 | 阿肯马法国公司 . 由甘油制造丙烯醛的方法:CN102197015[P]. 2011-09-21. |
| France Arkema . Process for manufacturing acrolein from glycerol: CN 102197015[P]. 2011-09-21. | |
| 21 | 阿肯马法国公司 . 制备丙烯醛/丙烯酸的改进方法:CN103328428 [P]. 2015-12-23. |
| France Arkema . Improved process for manufacturing acrolein/acrylic acid: CN 103328428[P]. 2015-12-23. | |
| 22 | 阿肯马法国公司 . 由甘油制备丙烯醛或丙烯酸的方法:CN 102066301[P]. 2011-05-18. |
| France Arkema . Process for manufacturing acrolein or acrylic acid from glycerin: CN102066301[P]. 2011-05-18. | |
| 23 | 阿肯马法国公司 . 使甘油脱水为丙烯醛的方法:CN101119956 [P]. 2012-02-15. |
| France Arkema . Process for dehydrating glycerol to acrolein: CN101119956[P]. 2012-02-15. | |
| 24 | 阿肯马法国公司 . 通过丙三醇脱水制造丙烯醛的方法:CN 102046574 [P]. 2011-05-04. |
| France Arkema . Method for producing acrolein by means of dehydration of glycerol: CN102046574 [P]. 2011-05-04. | |
| 25 | Group Arkema . Successful Arkema & hte research project in glycerol to acrolein and acrylic acid conversion [EB/OL]. [2019-03-12]. . |
| 26 | HIROKAZU K , SHOGO I , KENJI H , et al . Conversion of glycerol to acrolein by mesoporous sulfated zirconia-silica catalyst[J]. Chinese Journal of Catalysis, 2017, 38: 420-425. |
| 27 | RODRIGUES M V , VIGNATTI C , GARETTO T , et al . Glycerol dehydration catalyzed by MWW zeolites and the changes in the catalyst deactivation caused by porosity modification[J]. Applied Catalysis A: General, 2015, 495: 84-91. |
| 28 | CHRISTIAN H , ANDREAS L , JAN G M B . Pore condensation in glycerol dehydration: modification of a mixed oxide catalyst[J]. Topics in Catalysis, 2017, 60: 1462-1472. |
| 29 | 清华大学 . 丙烯醛制备方法:CN104387249[P]. 2017-03-01. |
| University Tsinghua . Preparation method of acraldehyde: CN104387249[P]. 2017-03-01. | |
| 30 | 复旦大学 . 甘油脱水制备丙烯醛用的多级孔ZSM-5催化剂及其制备方法:CN105621452[P]. 2016-06-01. |
| University Fudan . Multistage pore ZSM-5 catalyst for preparing acrolein by glycerol dehydration and preparation method of catalyst: CN105621452[P]. 2016-06-01. | |
| 31 | 复旦大学 . 用于甘油脱水制丙烯醛的多级孔ZSM-5沸石催化剂及其制备方法和应用: CN103638965[P]. 2015-12-30. |
| University Fudan . Hierarchical porous ZSM-5 zeolite catalyst for preparing acrolein through glycerin dehydration as well as preparation method and application of hierarchical porous ZSM-5 zeolite catalyst: CN103638965[P]. 2015-12-30. | |
| 32 | 复旦大学 . 一种用于甘油脱水制丙烯醛的纳米ZSM-5/γ-Al2O3复合催化剂及其制备方法和应用:CN104475147[P]. 2017-01-25. |
| University Fudan . Nano ZSM-5/gamma-Al2O3 composite catalyst for preparing acraldehyde by glycerol dehydration, and preparation method and application thereof: CN104475147[P]. 2017-01-25. | |
| 33 | 阿肯马法国公司 . 从甘油合成丙烯腈的方法:CN101636381[P]. 2014-10-15. |
| France Arkema . Method for the synthesis of acrylonitrile from glycerol: CN101636381[P]. 2014-10-15. | |
| 34 | KOLTUNOV K Y , SOBOLEY V I , BONDAREVA V M . Oxidation, oxidative esterification and ammoxidation of acrolein overmetal oxides: do these reactions include nucleophilic acylsubstitution?[J]. Catalysis Today, 2017, 279: 90-94. |
| 35 | THANH-BINH N , DUBOIS J , KALIAGUINEA S . Ammoxidation of acrolein to acrylonitrile over bismuth molybdatecatalysts[J]. Applied Catalysis A: General, 2016, 520: 7-12. |
| 36 | 肖雁秋 . 玉米秸秆原料生物炼制生产谷氨酸的研究[D]. 上海: 华东理工大学, 2014. |
| XIAO Y Q . Study on glutamic acid fermentation using corn stover as raw material through biorefinery technology[D]. Shanghai: East University of Science and Technology, 2014. | |
| 37 | JÉRÔME L N , ELINOR L S , MAURICE C R , et al . Biobased synthesis of acrylonitrile from glutamic acid[J]. Green Chemistry, 2011, 13: 807-809. |
| 38 | ANDRADA B , JÉRÔME L N , ELINOR L S , et al . Selective oxidative decarboxylation of amino acids to produce industrially relevant nitriles by vanadium chloroperoxidase[J]. ChemSusChem, 2012, 5: 1199-1202. |
| 39 | MIRANDA M O , PIETRANGELO A , HILLMYER M A , et al . Catalytic decarbonylation of biomass-derived carboxylic acids as efficient route to commodity monomers[J]. Green Chemistry, 2012, 14: 490-494. |
| 40 | LAMMENS T M , POTTING J , SANDERS J P M , et al . Environmental comparison of bio-based chemicals from glutamic acid with their petrochemical equivalents[J]. Environmental Science & Technology, 2011, 45: 8521-8528. |
| 41 | LAMMENS T M , GANGARAPU S , FRANSSEN M C R , et al . Techno-economic assessment of the production of bio-based chemicals from glutamic acid[J]. Biofuels Bioproducts Biorefining, 2012, 6 (2): 177-187. |
| 42 | ISIKGORA F H , BECER C R . Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers[J]. Polymer Chemistry, 2015, 6: 4497-4559. |
| 43 | KARP E M , EATON T R , NOGUÉ V S , et al . Renewable acrylonitrile production[J]. Science, 2017, 358: 1307-1310. |
| 44 | GUERRERO-PÉREZ M O , BAÇARES M A . Metrics of acrylonitrile: from biomass vs. petrochemical route[J]. Catalysis Today, 2015, 239: 25-30. |
| 45 | ROMAND S , GOH J . Acrylonitrile process evaluation/research planing[R]. San Francisco: Nexant,Inc, 2015: 8. |
| 46 | 芦长椿 . 碳纤维供求状况与生产成本[J].合成纤维, 2013, 42(2): 1-5. |
| LU C C . The demand and supply status of carbon fibers and its production cost[J]. Synthetic Fiber in China, 2013, 42(2): 1-5. | |
| 47 | 赵稼祥 . 碳纤维低成本制备技术[J]. 高科技纤维与应用, 2003, 28(6): 12-14. |
| ZHAO J X . Low cost manufacturing technology for carbon fibers[J]. Hi-Tech Fiber & Application, 2003, 28(6): 12-14. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [13] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [14] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [15] | 王兰江, 梁瑜, 汤琼, 唐明兴, 李学宽, 刘雷, 董晋湘. 快速热解铂前体合成高分散的Pt/HY催化剂及其萘深度加氢性能[J]. 化工进展, 2023, 42(8): 4159-4166. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||