化工进展 ›› 2019, Vol. 38 ›› Issue (04): 1662-1670.DOI: 10.16085/j.issn.1000-6613.2018-0468
超临界二氧化碳与二苯醚相平衡研究
- 西安交通大学化学工程与技术学院,陕西 西安 710049
-
收稿日期:2018-03-07修回日期:2018-04-23出版日期:2019-04-05发布日期:2019-04-05 -
通讯作者:方涛 -
作者简介:杜博文(1993—),男,硕士研究生,研究方向为超临界流体。E-mail:<email>18182428965@163.com</email>。|方涛,教授,博士生导师,研究方向为生物质能、超临界流体、化学储氢、微纳米材料。E-mail:<email>taofang@mail.xjtu.edu.cn</email>。 -
基金资助:陕能源化工过程强化重点实验室项目(20160109-4)
Phase equilibrium for binary system of diphenyl ether-supercritical carbon dioxide
Bowen DU( ),Kang CHEN,Xin DING,Zhao JIANG,Tao FANG(
),Kang CHEN,Xin DING,Zhao JIANG,Tao FANG( )
)
- School of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2018-03-07Revised:2018-04-23Online:2019-04-05Published:2019-04-05 -
Contact:Tao FANG
摘要:
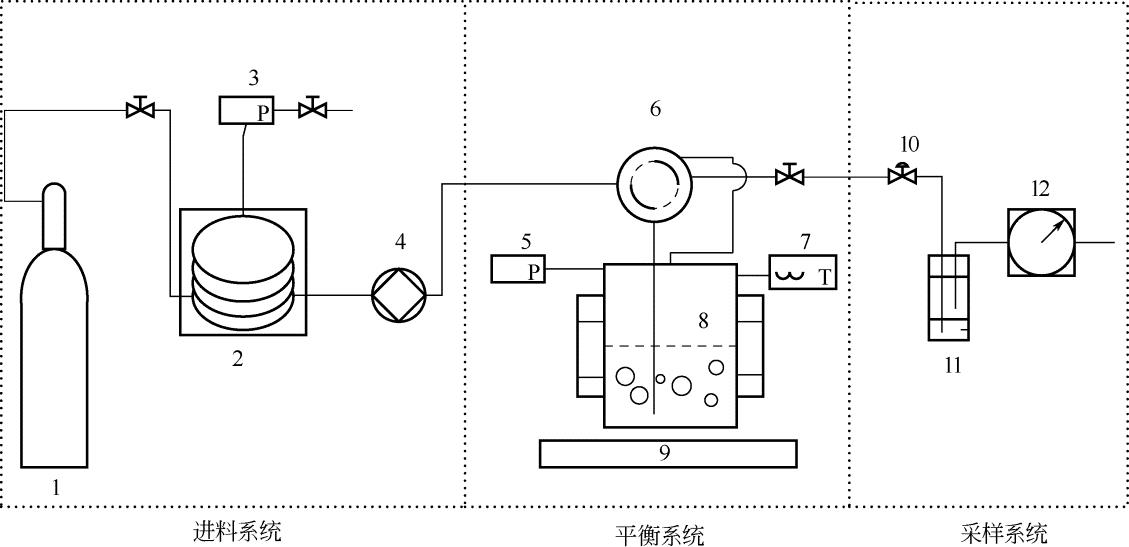
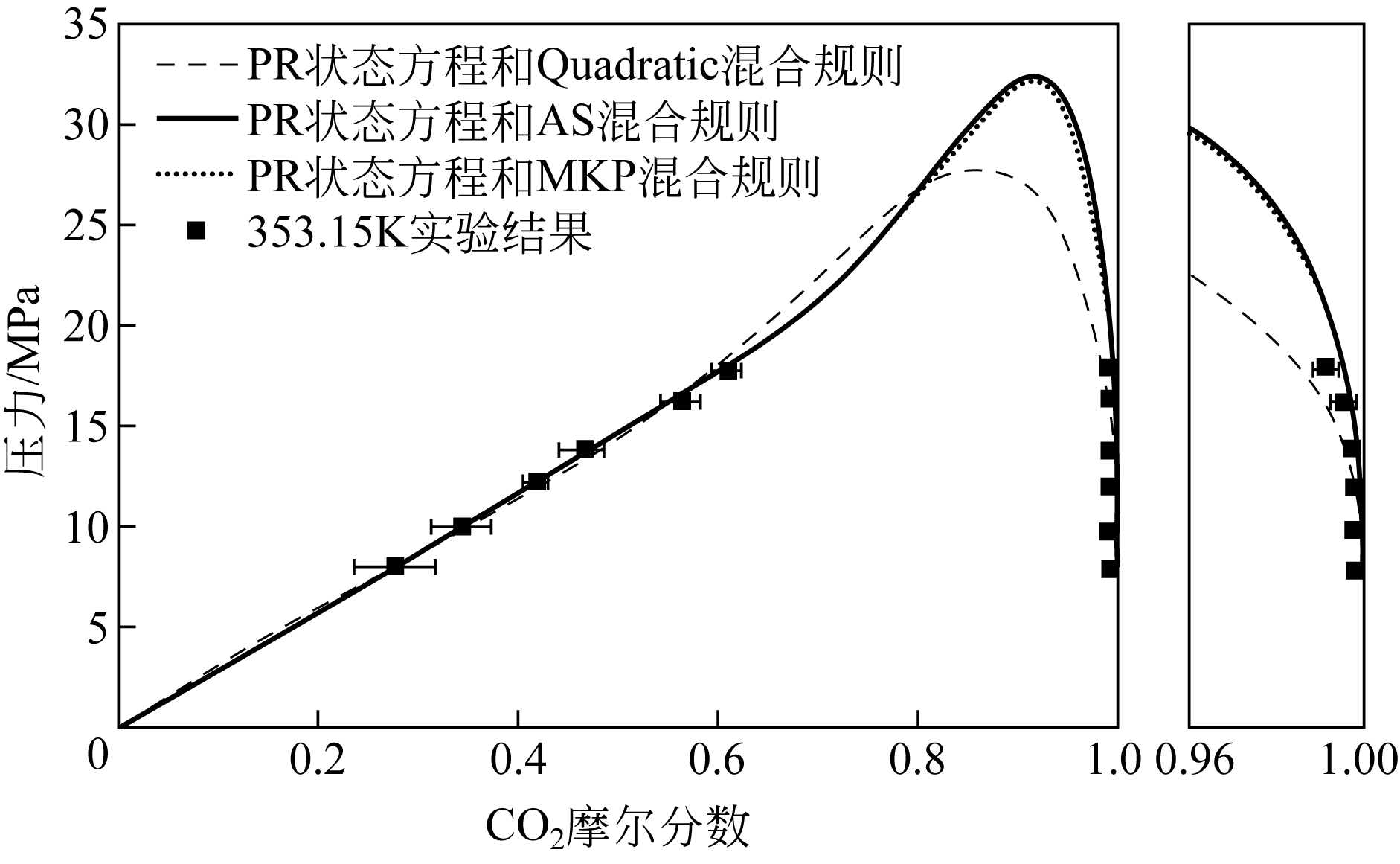
为了解决煤焦油及其轻质化产物中二苯醚的萃取精馏分离问题以及为后续的高效分离过程提供工程数据,本文自行设计并搭建了一套流动法可视化高温高压相平衡测定装置,对二苯醚-二氧化碳体系的相平衡数据进行了测定,测定温度为313.15K、333.15K和353.15K,测定压力为8~18MPa,并使用Peng-Robinson (PR)和Soave-Redlich-Kwong(SRK)状态方程结合Quadratic、Adachi-Sugie、Mathias-Klotz-Prausnitz混合规则对的超临界二氧化碳与二苯醚相平衡数据进行了关联计算,关联结果表明PR和SRK状态方程结合AS混合规则可以获得较为准确的关联结果,而传统二次型混合规则得到的计算结果误差相对较大。此外,关联计算还表明,选择合适的混合规则后,不同状态方程的关联结果相差不大。这表明,对于低挥发性的液体与二氧化碳这类非对称混合物体系,相平衡模拟计算的关键在于选取合适的混合规则。
中图分类号:
引用本文
杜博文, 陈康, 丁鑫, 姜召, 方涛. 超临界二氧化碳与二苯醚相平衡研究[J]. 化工进展, 2019, 38(04): 1662-1670.
Bowen DU, Kang CHEN, Xin DING, Zhao JIANG, Tao FANG. Phase equilibrium for binary system of diphenyl ether-supercritical carbon dioxide[J]. Chemical Industry and Engineering Progress, 2019, 38(04): 1662-1670.
| 序号 | 仪器名称和型号 | 生产厂家 |
|---|---|---|
| 1 | 普析G5气相色谱仪 | 北京普析通用仪器有限责任公司 |
| 2 | CP系列先行者TM电子天平 | 奥豪斯仪器(上海)有限公司 |
| 3 | 湿式气体流量计 | 天津市泰斯仪器有限公司 |
表1 主要检测仪器
| 序号 | 仪器名称和型号 | 生产厂家 |
|---|---|---|
| 1 | 普析G5气相色谱仪 | 北京普析通用仪器有限责任公司 |
| 2 | CP系列先行者TM电子天平 | 奥豪斯仪器(上海)有限公司 |
| 3 | 湿式气体流量计 | 天津市泰斯仪器有限公司 |
| 序号 | 试剂名称 | 规格 | 生产厂家 |
|---|---|---|---|
| 1 | 二氧化碳 | 分析纯 | 西安泰达低温设备有限公司气体厂 |
| 2 | 无水乙醇 | 分析纯 | 国药集团化学试剂有限公司 |
| 3 | 二苯醚 | ≥98% | 阿拉丁科技(中国)有限公司 |
表2 主要试剂
| 序号 | 试剂名称 | 规格 | 生产厂家 |
|---|---|---|---|
| 1 | 二氧化碳 | 分析纯 | 西安泰达低温设备有限公司气体厂 |
| 2 | 无水乙醇 | 分析纯 | 国药集团化学试剂有限公司 |
| 3 | 二苯醚 | ≥98% | 阿拉丁科技(中国)有限公司 |
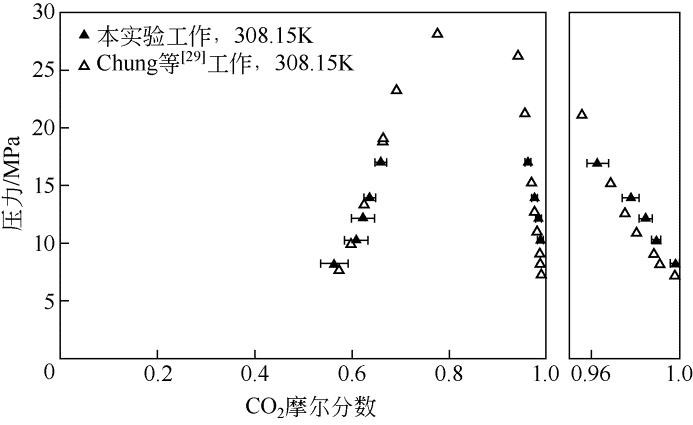
| 数据来源 | 压力 /MPa | x 1 | Sx /% | 压力 /MPa | y 1 | Sy /% |
|---|---|---|---|---|---|---|
| Chung等[ | 7.81 | 0.571 | — | 7.39 | 0.9982 | — |
| 9.89 | 0.599 | — | 8.32 | 0.9911 | — | |
| 13.52 | 0.625 | — | 9.13 | 0.9885 | — | |
| 19.04 | 0.665 | — | 11.09 | 0.9807 | — | |
| 19.26 | 0.665 | — | 12.75 | 0.9751 | — | |
| 23.40 | 0.692 | — | 15.40 | 0.9687 | — | |
| 28.25 | 0.776 | — | 17.69 | 0.9838 | — | |
| 21.33 | 0.9560 | — | ||||
| 26.39 | 0.9419 | — | ||||
| 本文测定 | 8.3 | 0.5646 | 2.82 | 8.3 | 0.9994 | 0.03 |
| 10.3 | 0.60875 | 2.36 | 10.3 | 0.9897 | 0.02 | |
| 12.2 | 0.6228 | 2.49 | 12.2 | 0.9849 | 0.03 | |
| 14.0 | 0.6361 | 1.13 | 14.0 | 0.9779 | 0.04 | |
| 17.0 | 0.6598 | 1.03 | 17.0 | 0.9628 | 0.05 |
表3 二氧化碳+二苯甲烷二元体系相平衡实验数据与文献
| 数据来源 | 压力 /MPa | x 1 | Sx /% | 压力 /MPa | y 1 | Sy /% |
|---|---|---|---|---|---|---|
| Chung等[ | 7.81 | 0.571 | — | 7.39 | 0.9982 | — |
| 9.89 | 0.599 | — | 8.32 | 0.9911 | — | |
| 13.52 | 0.625 | — | 9.13 | 0.9885 | — | |
| 19.04 | 0.665 | — | 11.09 | 0.9807 | — | |
| 19.26 | 0.665 | — | 12.75 | 0.9751 | — | |
| 23.40 | 0.692 | — | 15.40 | 0.9687 | — | |
| 28.25 | 0.776 | — | 17.69 | 0.9838 | — | |
| 21.33 | 0.9560 | — | ||||
| 26.39 | 0.9419 | — | ||||
| 本文测定 | 8.3 | 0.5646 | 2.82 | 8.3 | 0.9994 | 0.03 |
| 10.3 | 0.60875 | 2.36 | 10.3 | 0.9897 | 0.02 | |
| 12.2 | 0.6228 | 2.49 | 12.2 | 0.9849 | 0.03 | |
| 14.0 | 0.6361 | 1.13 | 14.0 | 0.9779 | 0.04 | |
| 17.0 | 0.6598 | 1.03 | 17.0 | 0.9628 | 0.05 |
| 温度/K | 压力/MPa | x 1 | Sx /% | y 1/% | Sy /% |
|---|---|---|---|---|---|
| 353.15 | 8.0 | 0.2765 | 8.36 | 0.9994 | 0.04 |
| 10.0 | 0.3433 | 6.67 | 0.9991 | 0.02 | |
| 12.2 | 0.4171 | 1.55 | 0.9983 | 0.01 | |
| 13.8 | 0.4636 | 2.56 | 0.9976 | 0.02 | |
| 16.2 | 0.5628 | 2.42 | 0.9954 | 0.03 | |
| 17.8 | 0.6088 | 2.06 | 0.9913 | 0.03 | |
| 333.15 | 7.8 | 0.3671 | 7.75 | 0.9996 | 0.04 |
| 9.8 | 0.4655 | 4.03 | 0.9985 | 0.02 | |
| 12.0 | 0.5556 | 2.49 | 0.9935 | 0.02 | |
| 13.8 | 0.6296 | 3.53 | 0.9873 | 0.02 | |
| 16.4 | 0.6785 | 2.78 | 0.9861 | 0.01 | |
| 18.0 | 0.7039 | 3.37 | 0.9818 | 0.04 | |
| 313.15 | 7.8 | 0.6298 | 6.63 | 0.9982 | 0.02 |
| 10.0 | 0.7064 | 3.14 | 0.9949 | 0.03 | |
| 11.6 | 0.7239 | 3.55 | 0.9928 | 0.02 | |
| 13.8 | 0.7463 | 2.96 | 0.9820 | 0.04 | |
| 16.0 | 0.7757 | 4.63 | 0.9769 | 0.01 | |
| 18.0 | 0.8002 | 3.03 | 0.9669 | 0.02 |
表4 二氧化碳(1)+二苯醚(2)体系相平衡测定结果
| 温度/K | 压力/MPa | x 1 | Sx /% | y 1/% | Sy /% |
|---|---|---|---|---|---|
| 353.15 | 8.0 | 0.2765 | 8.36 | 0.9994 | 0.04 |
| 10.0 | 0.3433 | 6.67 | 0.9991 | 0.02 | |
| 12.2 | 0.4171 | 1.55 | 0.9983 | 0.01 | |
| 13.8 | 0.4636 | 2.56 | 0.9976 | 0.02 | |
| 16.2 | 0.5628 | 2.42 | 0.9954 | 0.03 | |
| 17.8 | 0.6088 | 2.06 | 0.9913 | 0.03 | |
| 333.15 | 7.8 | 0.3671 | 7.75 | 0.9996 | 0.04 |
| 9.8 | 0.4655 | 4.03 | 0.9985 | 0.02 | |
| 12.0 | 0.5556 | 2.49 | 0.9935 | 0.02 | |
| 13.8 | 0.6296 | 3.53 | 0.9873 | 0.02 | |
| 16.4 | 0.6785 | 2.78 | 0.9861 | 0.01 | |
| 18.0 | 0.7039 | 3.37 | 0.9818 | 0.04 | |
| 313.15 | 7.8 | 0.6298 | 6.63 | 0.9982 | 0.02 |
| 10.0 | 0.7064 | 3.14 | 0.9949 | 0.03 | |
| 11.6 | 0.7239 | 3.55 | 0.9928 | 0.02 | |
| 13.8 | 0.7463 | 2.96 | 0.9820 | 0.04 | |
| 16.0 | 0.7757 | 4.63 | 0.9769 | 0.01 | |
| 18.0 | 0.8002 | 3.03 | 0.9669 | 0.02 |
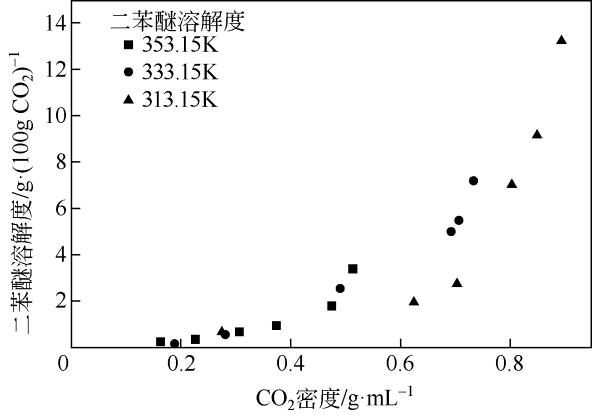
| 温度/K | 压力 /MPa | CO2密度 /g·mL-1 | 二苯醚溶解度 /g·(100g CO2)-1 |
|---|---|---|---|
| 353.15 | 8.0 | 0.1640 | 0.2322 |
| 10.0 | 0.2266 | 0.3484 | |
| 12.2 | 0.3078 | 0.6586 | |
| 13.8 | 0.3731 | 0.9304 | |
| 16.2 | 0.4764 | 1.7873 | |
| 17.8 | 0.5138 | 3.3940 | |
| 333.15 | 7.8 | 0.1884 | 0.1547 |
| 9.8 | 0.2820 | 0.5810 | |
| 12.0 | 0.4918 | 2.5304 | |
| 13.8 | 0.6926 | 4.9752 | |
| 16.4 | 0.7080 | 5.4519 | |
| 18.0 | 0.7350 | 7.1698 | |
| 313.15 | 7.8 | 0.2753 | 0.6974 |
| 10.0 | 0.6259 | 1.9826 | |
| 11.6 | 0.7051 | 2.8049 | |
| 13.8 | 0.8052 | 7.0895 | |
| 16.0 | 0.8512 | 9.1457 | |
| 18.0 | 0.8949 | 13.2405 |
表5 二苯醚在超临界CO2中的溶解度
| 温度/K | 压力 /MPa | CO2密度 /g·mL-1 | 二苯醚溶解度 /g·(100g CO2)-1 |
|---|---|---|---|
| 353.15 | 8.0 | 0.1640 | 0.2322 |
| 10.0 | 0.2266 | 0.3484 | |
| 12.2 | 0.3078 | 0.6586 | |
| 13.8 | 0.3731 | 0.9304 | |
| 16.2 | 0.4764 | 1.7873 | |
| 17.8 | 0.5138 | 3.3940 | |
| 333.15 | 7.8 | 0.1884 | 0.1547 |
| 9.8 | 0.2820 | 0.5810 | |
| 12.0 | 0.4918 | 2.5304 | |
| 13.8 | 0.6926 | 4.9752 | |
| 16.4 | 0.7080 | 5.4519 | |
| 18.0 | 0.7350 | 7.1698 | |
| 313.15 | 7.8 | 0.2753 | 0.6974 |
| 10.0 | 0.6259 | 1.9826 | |
| 11.6 | 0.7051 | 2.8049 | |
| 13.8 | 0.8052 | 7.0895 | |
| 16.0 | 0.8512 | 9.1457 | |
| 18.0 | 0.8949 | 13.2405 |
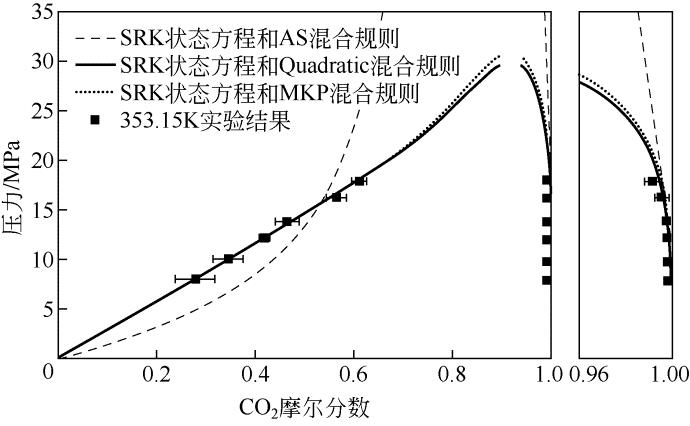
| 状态方程 | 混合规则 | 交互参数个数 | 优化后的参数 | Δx 1 /% | Δy 1 /% | ||
|---|---|---|---|---|---|---|---|
| k 1,2 | l 1,2 | λ 1,2 | |||||
| PR | Quadratic | 2 | 0.0670 | ?0.056 | — | 6.05 | 1.28 |
| Adachi-Sugie | 3 | 1.4667 | 0.7892 | ?1.0467 | 4.86 | 0.89 | |
| Mathias-Klotz-Prausnitz | 3 | 1.4709 | 0.7922 | 2.0988 | 4.89 | 0.97 | |
| SRK | Quadratic | 2 | 0.1502 | 0.0471 | — | 51.61 | 1.03 |
| Adachi-Sugie | 3 | 1.4449 | 0.7498 | ?1.0597 | 4.90 | 0.87 | |
| Mathias-Klotz-Prausnitz | 3 | 1.5036 | 0.7832 | 2.2060 | 4.96 | 0.96 | |
表6 353.15K下SRK、PR状态方程使用不同混合规则关联结果
| 状态方程 | 混合规则 | 交互参数个数 | 优化后的参数 | Δx 1 /% | Δy 1 /% | ||
|---|---|---|---|---|---|---|---|
| k 1,2 | l 1,2 | λ 1,2 | |||||
| PR | Quadratic | 2 | 0.0670 | ?0.056 | — | 6.05 | 1.28 |
| Adachi-Sugie | 3 | 1.4667 | 0.7892 | ?1.0467 | 4.86 | 0.89 | |
| Mathias-Klotz-Prausnitz | 3 | 1.4709 | 0.7922 | 2.0988 | 4.89 | 0.97 | |
| SRK | Quadratic | 2 | 0.1502 | 0.0471 | — | 51.61 | 1.03 |
| Adachi-Sugie | 3 | 1.4449 | 0.7498 | ?1.0597 | 4.90 | 0.87 | |
| Mathias-Klotz-Prausnitz | 3 | 1.5036 | 0.7832 | 2.2060 | 4.96 | 0.96 | |
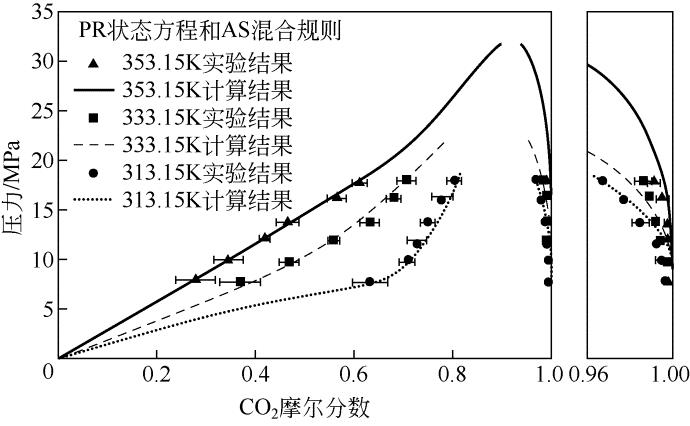
| 温度/K | k | l | λ | Δy 1/% | Δx 1/% |
|---|---|---|---|---|---|
| 313.15 | 1.6097 | 0.8897 | ?1.127817 | 4.76 | 1.02 |
| 333.15 | ?0.0410 | ?0.1187 | 0.0815 | 4.77 | 1.09 |
| 353.15 | 1.4667 | 0.7892 | ?1.0467 | 4.86 | 0.95 |
表7 不同温度下PR状态结合AS混合规则关联结果
| 温度/K | k | l | λ | Δy 1/% | Δx 1/% |
|---|---|---|---|---|---|
| 313.15 | 1.6097 | 0.8897 | ?1.127817 | 4.76 | 1.02 |
| 333.15 | ?0.0410 | ?0.1187 | 0.0815 | 4.77 | 1.09 |
| 353.15 | 1.4667 | 0.7892 | ?1.0467 | 4.86 | 0.95 |
| a | —— | 状态方程的能量参数 |
| a 0 | —— | 能量项的参数 |
| B | —— | 状态方程的体积参数 |
| c 1 | —— | 能量项的参数 |
| kij | —— | 二元交互因子 |
| lij | —— | 二元交互因子 |
| p | —— | 压力,MPa |
| p c | —— | 临界压力,MPa |
| R | —— | 气体常数,J/(mol·K) |
| T | —— | 温度,K |
| T c | —— | 临界温度,K |
| T r | —— | 对比温度,K |
| v | —— | 摩尔体积,m3/mol |
| x | —— | 液相摩尔分数 |
| Δx | —— | 液相标准误差 |
| y | —— | 气相摩尔分数 |
| Δy | —— | 气相标准误差 |
| γ | —— | 逸度系数 |
| λij | —— | 二元交互因子 |
| ρ | —— | 密度 |
| ω | —— | 偏心因子 |
| 下角标 | ||
| c | —— | 临界值 |
| i | —— | 组分指数 |
| j | —— | 组分指数 |
| r | —— | 对比 |
| 缩写 | ||
| AS | —— | Adachi-Sugie |
| EOS | —— | Equation of State状态方程 |
| MKP | —— | Mathias-Klotz-Prausnitz |
| PR | —— | Peng-Robinson |
| Q | —— | Quadratic 二次型 |
| SRK | —— | Soave-Redlich-Kwong |
符号说明
| a | —— | 状态方程的能量参数 |
| a 0 | —— | 能量项的参数 |
| B | —— | 状态方程的体积参数 |
| c 1 | —— | 能量项的参数 |
| kij | —— | 二元交互因子 |
| lij | —— | 二元交互因子 |
| p | —— | 压力,MPa |
| p c | —— | 临界压力,MPa |
| R | —— | 气体常数,J/(mol·K) |
| T | —— | 温度,K |
| T c | —— | 临界温度,K |
| T r | —— | 对比温度,K |
| v | —— | 摩尔体积,m3/mol |
| x | —— | 液相摩尔分数 |
| Δx | —— | 液相标准误差 |
| y | —— | 气相摩尔分数 |
| Δy | —— | 气相标准误差 |
| γ | —— | 逸度系数 |
| λij | —— | 二元交互因子 |
| ρ | —— | 密度 |
| ω | —— | 偏心因子 |
| 下角标 | ||
| c | —— | 临界值 |
| i | —— | 组分指数 |
| j | —— | 组分指数 |
| r | —— | 对比 |
| 缩写 | ||
| AS | —— | Adachi-Sugie |
| EOS | —— | Equation of State状态方程 |
| MKP | —— | Mathias-Klotz-Prausnitz |
| PR | —— | Peng-Robinson |
| Q | —— | Quadratic 二次型 |
| SRK | —— | Soave-Redlich-Kwong |
| 1 | BRUNNER G . Supercritical process technology related to energy and future directions — An introduction[J]. Journal of Supercritical Fluids, 2015, 96: 11-20. |
| 2 | SAVAGE P E . Organic chemical reactions in supercritical water[J]. Chemical Reviews, 1999, 99(2): 603-621. |
| 3 | GOTO M , NADA T , KAWAJIRI S , et al . Decomposition of municipal sludge by supercritical water oxidation[J]. Journal of Chemical Engineering of Japan, 1997, 30(5): 813-818. |
| 4 | GOTO M , NADA T , OGATA A , et al . Supercritical water oxidation for the destruction of municipal excess sludge and alcohol distillery wastewater of molasses[J]. Journal of Supercritical Fluids, 1998, 13(1/2/3): 277-282. |
| 5 | WAHYUDIONO, SASAKI M , GOTO M . Kinetic study for liquefaction of tar in sub- and supercritical water[J]. Polymer Degradation and Stability, 2008, 93(6): 1194-1204. |
| 6 | WAHYUDIONO, FUJINAGA S , SASAKI M , et al . Recovery of phenol through the decomposition of tar under hydrothermal alkaline conditions[J]. Chemical Engineering & Technology, 2006, 29(7): 882-889. |
| 7 | NICOLAOU K C , BODDY C N C , BRASE S , et al . Chemistry, biology, and medicine of the glycopeptide antibiotics[J]. Angewandte Chemie: International Edition, 1999, 38(15): 2096-2152. |
| 8 | AZPIROZ M D G , BLANCO C G , BANCIELLA C . The use of solvents for purifying industrial naphthalene from coal tar distilled oils[J]. Fuel Processing Technology, 2008, 89(2): 111-117. |
| 9 | YAMAMOTO Y , SATO Y , EBINA T , et al . Separation of high-purity indole from coal-tar by high-pressure crystallization[J]. Fuel, 1991, 70(4): 565-566. |
| 10 | 王汝成, 王宁波, 王明峰, 等 . 中低温煤焦油中酚类化合物的柱层析分离[J]. 煤化工, 2013, 41(6): 53-56. |
| WANG R C , WANG N B , WANG M F , et al . Column chromatography isolation of phenolic compounds in the low temperature coal tar[J]. Coal Chemical Industry, 2013, 41(6): 53-56. | |
| 11 | 姜广策, 张生娟, 王永刚, 等 . 低温煤焦油中特定芳烃组分的选择性分离[J]. 化工学报, 2015, 66(6): 2131-2138. |
| JIANG G C , ZHANG S J , WANG Y G , et al . Selective separation of aromatic hydrocarbons from low temperature coal tar[J]. CIESC Journal, 2015, 66(6): 2131-2138. | |
| 12 | 张生娟, 高亚男, 李晓宏, 等 . 煤焦油组分分离与分析技术研究进展[J]. 煤化工, 2017, 45(1): 45-49. |
| ZHANG S J , GAO Y N , LI X H , et al . Research progress of the separation and analysis technology of coal tar composition[J]. Coal Chemical Industry, 2017, 45(1): 45-49. | |
| 13 | ASIABI H , YAMINI Y , LATIFEH F , et al . Solubilities of four macrolide antibiotics in supercritical carbon dioxide and their correlations using semi-empirical models[J]. Journal of Supercritical Fluids, 2015, 104: 62-69. |
| 14 | CELIK H T , GURU M . Extraction of oil and silybin compounds from milk thistle seeds using supercritical carbon dioxide[J]. Journal of Supercritical Fluids, 2015, 100: 105-109. |
| 15 | MARKOVI Z , MARKOVI S , ENGELBRECHT J P , et al . Extraction of coal-tar pitch by supercritical carbon dioxide. Dependence of chemical composition of the extracts on temperature, pressure and extraction time[J]. South African Journal of Chemistry-Suid-Afrikaanse Tydskrif Vir Chemie, 2000, 53: 1-12. |
| 16 | KWIATKOWSKI J , LISICKI Z , MAJEWSKI W . An experimental-method for measuring solubilities of solids in supercritical fluids[J]. Berichte Der Bunsen-Gesellschaft-Physical Chemistry Chemical Physics, 1984, 88(9): 865-869. |
| 17 | GOODARZNIA I , ESMAEILZADEH F . Solubility of an anthracene, phenanthrene, and carbazole mixture in supercritical carbon dioxide[J]. Journal of Chemical & Engineering Data, 2002, 47(2): 333-338. |
| 18 | BAGHERI H , ABDUL MANAP M Y B , SOLATI Z . Response surface methodology applied to supercritical carbon dioxide extraction of Piper nigrum L. essential oil[J]. LWT—Food Science and Technology, 2014, 57(1): 149-155. |
| 19 | 陈华, 骆赞椿, 章寿华, 等 . 混合规则对超临界流体相平衡计算的影响——低挥发性液体体系[J]. 华东化工学院学报, 1993(3): 280-284. |
| CHEN H , LUO Z C , ZHANG S H , et al . Effects of mixing rules on phase equilibrium calculations for supercritical fluid—Low volatile liquid systems[J]. Journal of East China Institute of Chemical Technology, 1993(3): 280-284. | |
| 20 | LUCAS M A , BORGES G R , ROCHA I C C DA , et al . Use of real crude oil fractions to describe the high pressure phase behavior of crude oil in carbon dioxide[J]. Journal of Supercritical Fluids, 2016, 118: 140-147. |
| 21 | AUGELLETTI R , FRATTARI S , GIRONI F , et al . Phase equilibria and thermodynamic modeling of systems CO2 - bergamot oil and CO2 - linalyl acetate[J]. Journal of Supercritical Fluids, 2016, 116: 1-9. |
| 22 | PENG D , ROBINSON D B . A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals, 1976, 15(1): 59-64. |
| 23 | VALDERRAMA J O . The state of the cubic equations of state[J]. Industrial & Engineering Chemistry Research, 2003, 42(8): 1603-1618. |
| 24 | COSTA G M N , CARDOSO S G , SOARES R O , et al . Modeling high pressure vapor-liquid equilibrium of ternary systems containing supercritical CO2 and mixed organic solvents using Peng-Robinson equation of state[J]. Journal of Supercritical Fluids, 2014, 93: 82-90. |
| 25 | HIGASHI H , FURUYA T , ISHIDAO T , et al . An exponent-type mixing rule for energy parameters[J]. Journal of Chemical Engineering of Japan, 1994, 27(5): 677-679. |
| 26 | ADACHI Y , SUGIE H . A new mixing rule modified conventional mixing rule[J]. Fluid Phase Equilibria, 1986, 28(2): 103-118. |
| 27 | FANG T , GOTO M , SASAKI M , et al . Phase equilibria for the ternary system methyl oleate + tocopherol + supercritical CO2 [J]. Journal of Chemical & Engineering Data, 2005, 50(2): 390-397. |
| 28 | MEIER U , GROSS F , TREPP C . High pressure phase equilibrium studies for the carbon dioxide/α-tocopherol (vitamin E) system[J]. Fluid Phase Equilibria, 1994, 92: 289-302. |
| 29 | CHUNG S T , SHING K S . Multiphase behavior of binary and ternary systems of heavy aromatic hydrocarbons with supercritical carbon dioxide: Part I. Experimental results[J]. Fluid Phase Equilibria, 1992, 81: 321-341. |
| 30 | 陈华, 骆赞椿, 章寿华, 等 . 超临界二氧化碳萃取低挥发性液体混合物的相平衡[J]. 高校化学工程学报, 1994(1): 46-54. |
| CHEN H , LUO Z C , ZHANG S H , et al . Extraction of low volatility liquid mixtures with supercritical carbon dioxide[J]. Journal of Chemical Engineering of Chinese Universities, 1994(1): 46-54. | |
| 31 | LATSKY C , KOUAKOU A C , SCHWARZ C E . Phase equilibria of CO2 with components in the light naphtha cut of tyre derived oil[J]. Journal of Supercritical Fluids, 2018, 131: 58-65. |
| 32 | KORDIKOWSKI A , SCHNEIDER G M . Fluid phase equilibria of binary and ternary mixtures of supercritical carbon dioxide with low-volatility organic substances up to 100 MPa and 393 K[J]. Fluid Phase Equilibria, 1993, 90(1): 149-162. |
| 33 | PENG D Y , ROBINSON D B . A new two-constant equation of state[J]. Industrial and Engineering Chemistry, Fundamentals, 1976, 15(1): 59-64. |
| 34 | ADACHI Y , SUGIE H . A new mixing rule — Modified conventional mixing rule[J]. Fluid Phase Equilibria, 1986, 28(2): 103-118. |
| 35 | MATHIAS P M , KLOTZ H C , PRAUSNITZ J M . Equation-of-State mixing rules for multicomponent mixtures: the problem of invariance[J]. Fluid Phase Equilibria, 1991, 67: 31-44. |
| 36 | WEBER W , PETKOV S , BRUNNER G . Vapour-liquid-equilibria and calculations using the Redlich-Kwong-Aspen-equation of state for tristearin, tripalmitin, and triolein in CO2 and propane[J]. Fluid Phase Equilibria, 1999, 158/159/160: 695-706. |
| [1] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [2] | 罗成, 范晓勇, 朱永红, 田丰, 崔楼伟, 杜崇鹏, 王飞利, 李冬, 郑化安. 中低温煤焦油加氢反应器不同分配器中液体分布的CFD模拟[J]. 化工进展, 2023, 42(9): 4538-4549. |
| [3] | 张乐乐, 钱运东, 朱华曈, 冯思龙, 杨秀娜, 于颖, 杨强, 卢浩. 加氢原料煤焦油脱水除盐预处理工艺优化限值[J]. 化工进展, 2023, 42(5): 2298-2305. |
| [4] | 徐贤, 崔楼伟, 刘杰, 施俊合, 朱永红, 刘姣姣, 刘涛, 郑化安, 李冬. 原料组成对半焦中间相结构发展的影响[J]. 化工进展, 2023, 42(5): 2343-2352. |
| [5] | 杨鑫, 许红, 胡卫勋, 刘泓佐, 龙泉芝, 朱立业. 基于超临界CO2萃取再生废润滑油[J]. 化工进展, 2023, 42(10): 5399-5405. |
| [6] | 岳子瀚, 龙臻, 周雪冰, 臧小亚, 梁德青. sⅡ型水合物储氢研究进展[J]. 化工进展, 2023, 42(10): 5121-5134. |
| [7] | 贾文龙, 宋硕硕, 李长俊, 吴瑕, 杨帆, 张员瑞. 超临界CO2萃取含油污泥研究现状与进展[J]. 化工进展, 2022, 41(12): 6573-6585. |
| [8] | 杨永斌, 董寅瑞, 钟强, 李骞, 王林, 姜涛. 高温煤焦油沥青黏结剂碳化固结作用在炭质型材中的应用与研究进展[J]. 化工进展, 2022, 41(12): 6419-6429. |
| [9] | 王逸伟, 刘智琪, 孙强, 刘爱贤, 杨兰英, 宫敬, 郭绪强. 聚苯乙烯磺酸钠作用下Ⅰ型水合物的生成热力学与动力学[J]. 化工进展, 2021, 40(S1): 168-181. |
| [10] | 王军良, 杨丽丽, 林春绵, 潘志彦. 超临界二氧化碳化学反应研究进展[J]. 化工进展, 2021, 40(8): 4127-4134. |
| [11] | 胡倩, 周诗岽, 郭宇, 张雪艳, 王娇娇, 王国栋, 姬浩洋. 蜡晶析出对CO2水合物相平衡及诱导特性的影响[J]. 化工进展, 2021, 40(5): 2452-2460. |
| [12] | 陈国华, 杨棚, 赵一新, 李小峰, 赵远飞. 温度载荷与爆炸碎片冲击载荷耦合作用下储罐易损性分析[J]. 化工进展, 2021, 40(2): 1130-1136. |
| [13] | 韩文亮, 刘豫. 城市水源水库及入库河流沉积物中总有机碳和黑碳的时空分异及其对多溴二苯醚的影响[J]. 化工进展, 2021, 40(2): 1085-1096. |
| [14] | 杨董, 陈林. 跨/超临界多相射流过程瞬态密度场可视化实验[J]. 化工进展, 2021, 40(12): 6432-6440. |
| [15] | 孙宪航, 朱忠泉, 黄维秋, 浮历沛, 王雨雨, 吕爱华. 超临界CO2法再生油气回收用活性炭机理研究进展[J]. 化工进展, 2020, 39(S2): 346-351. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||