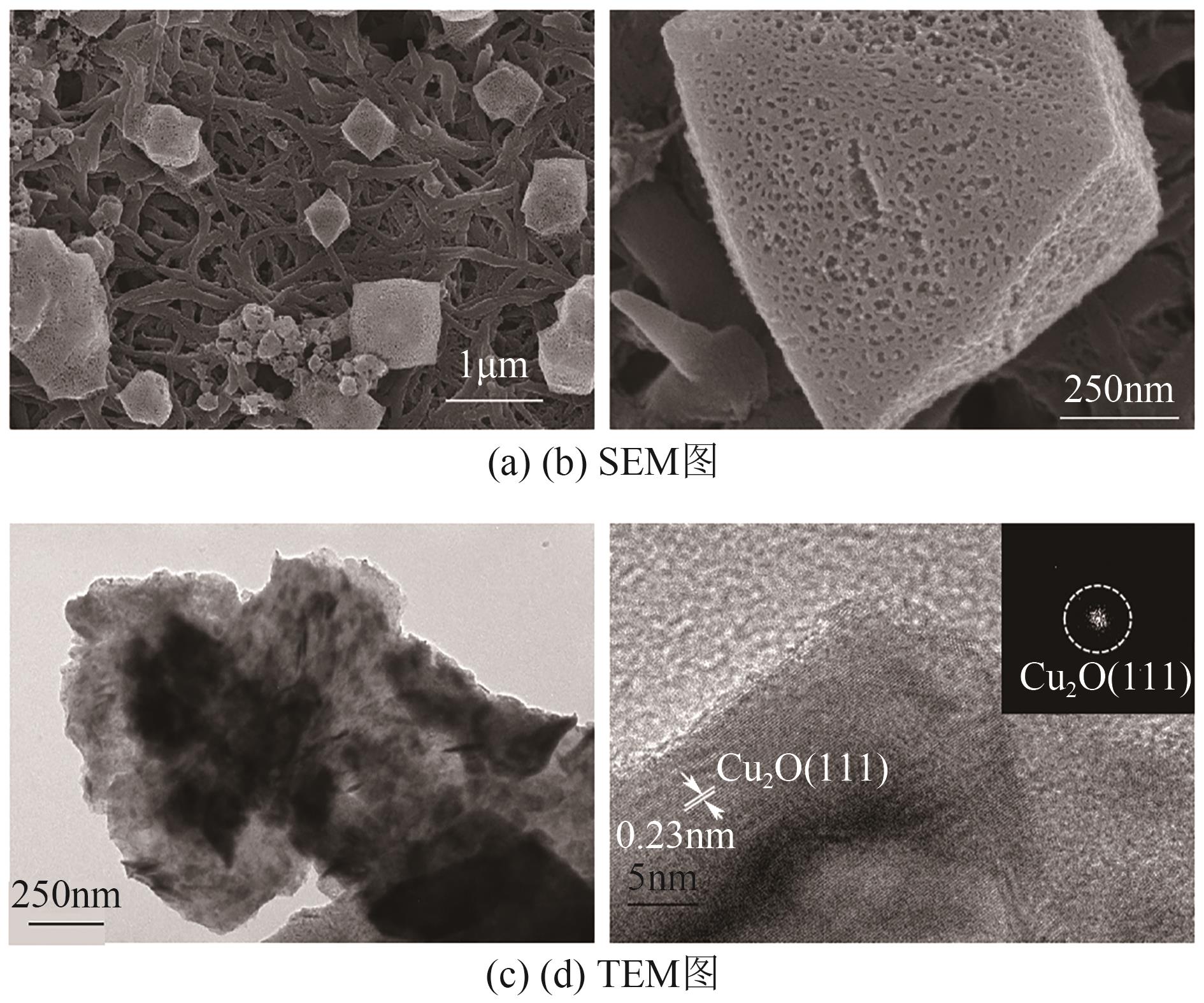
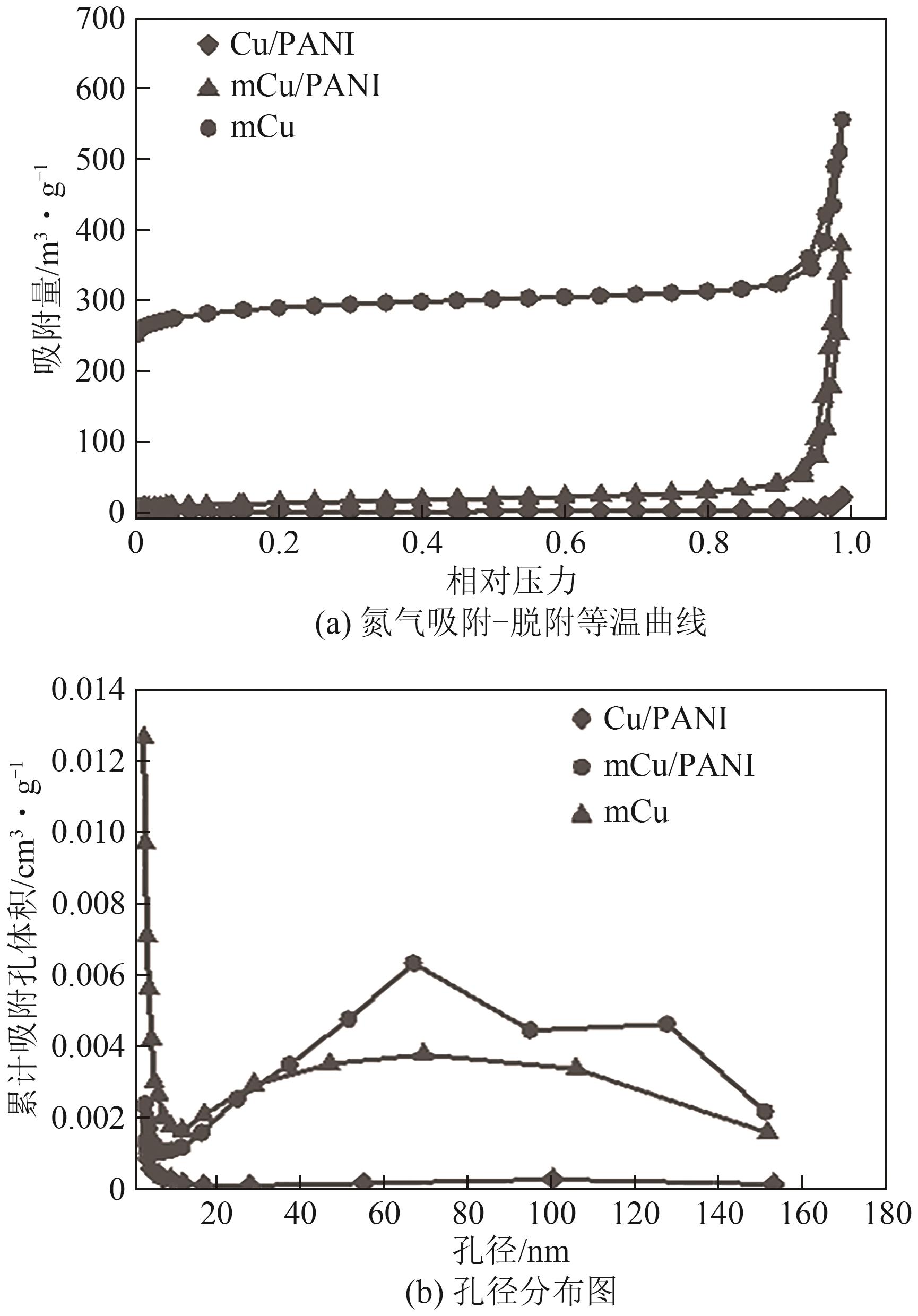
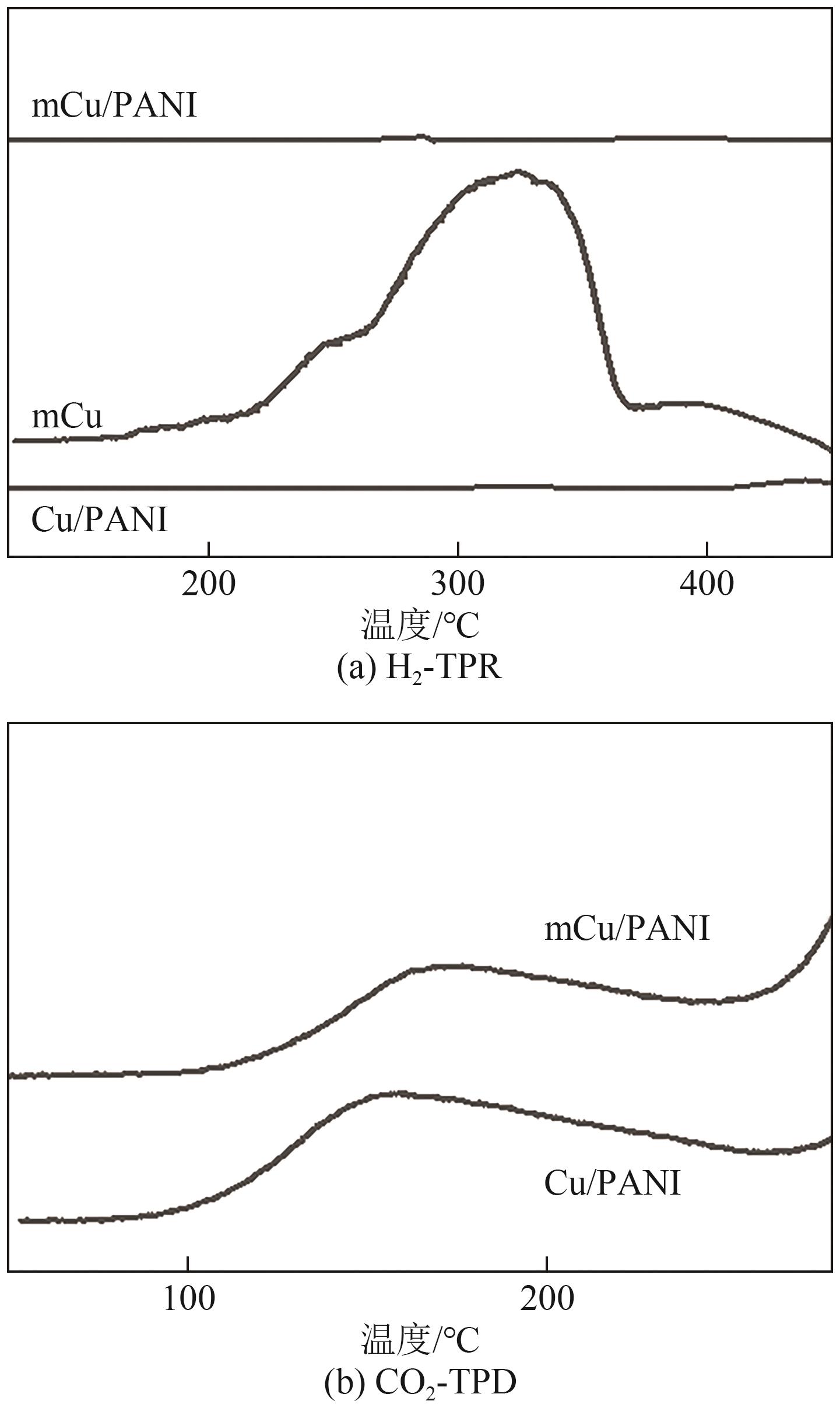
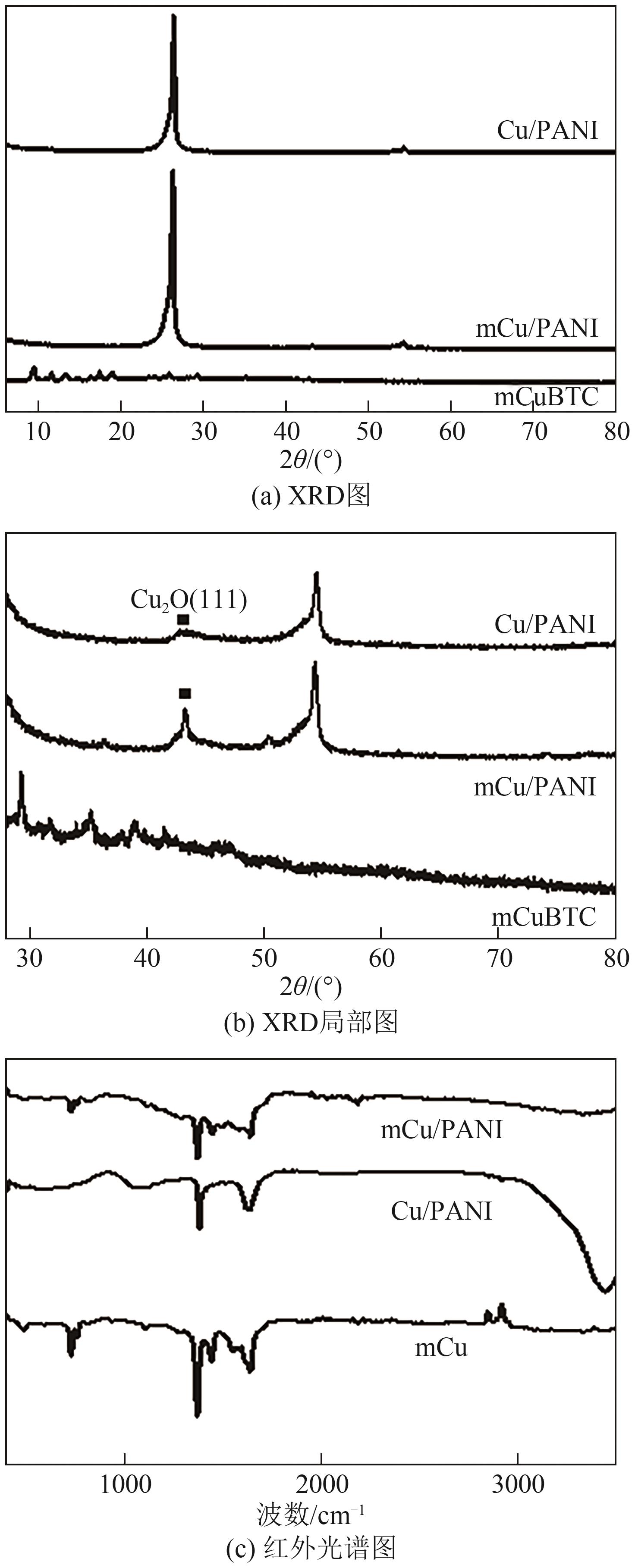
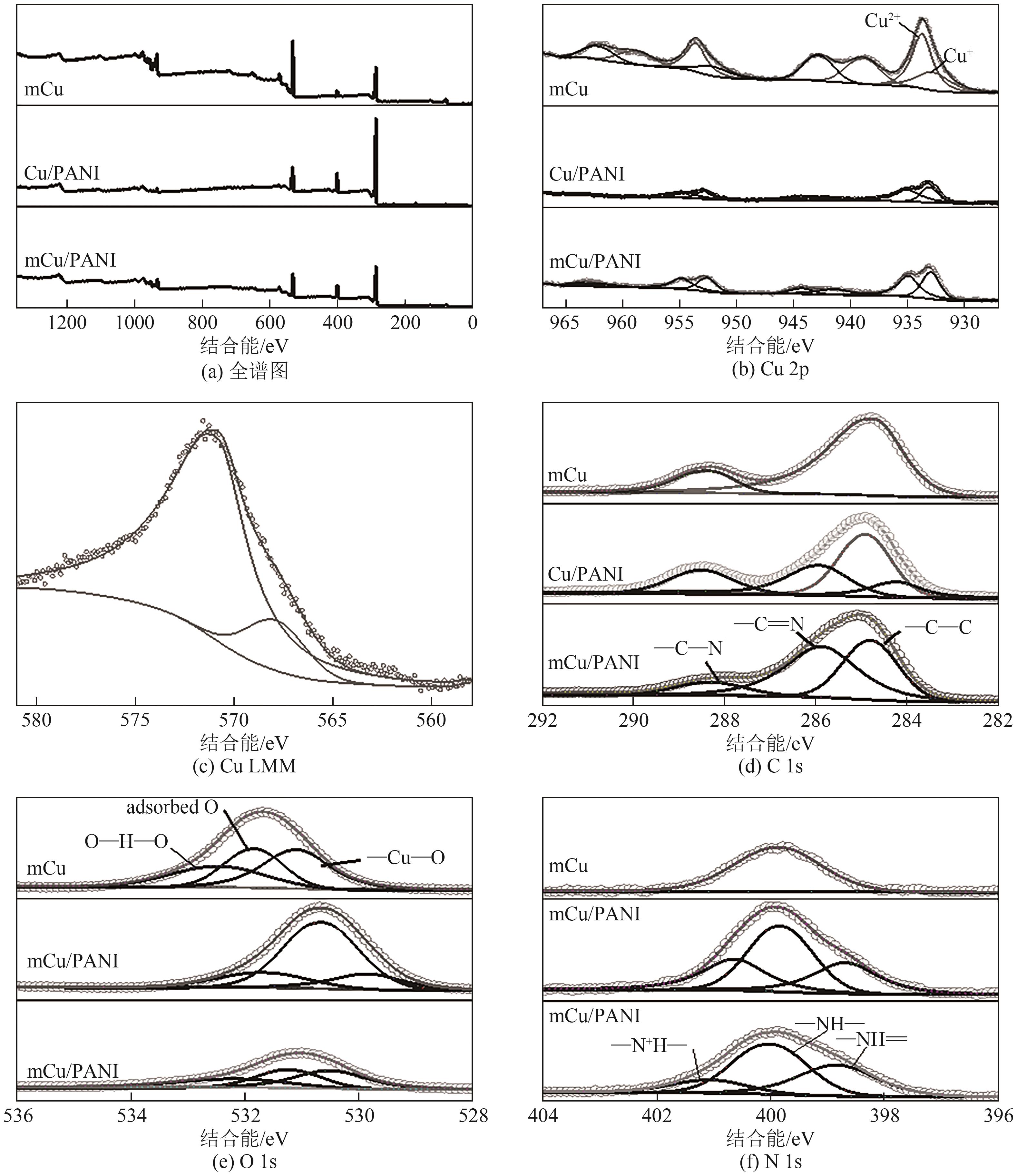
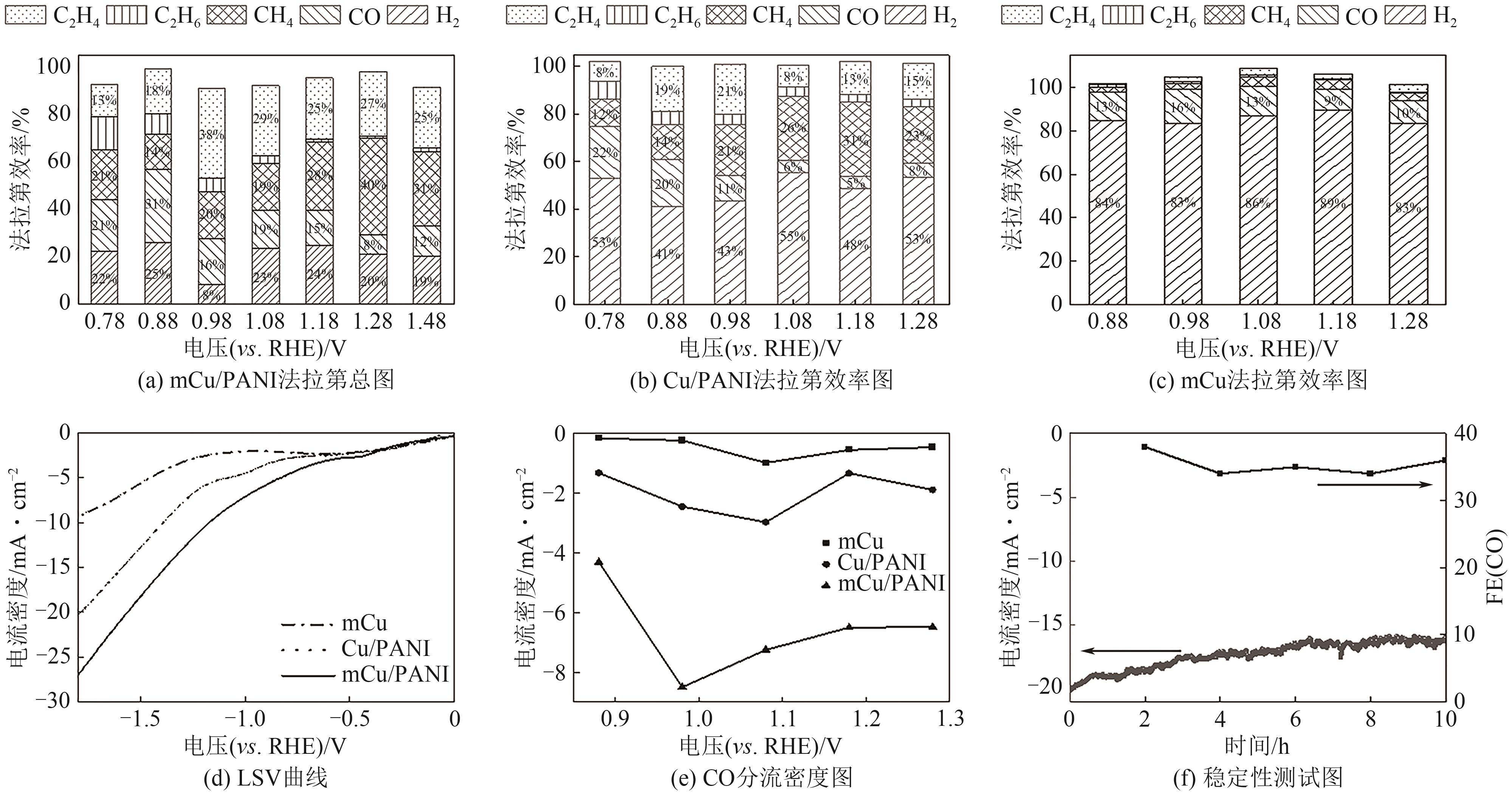
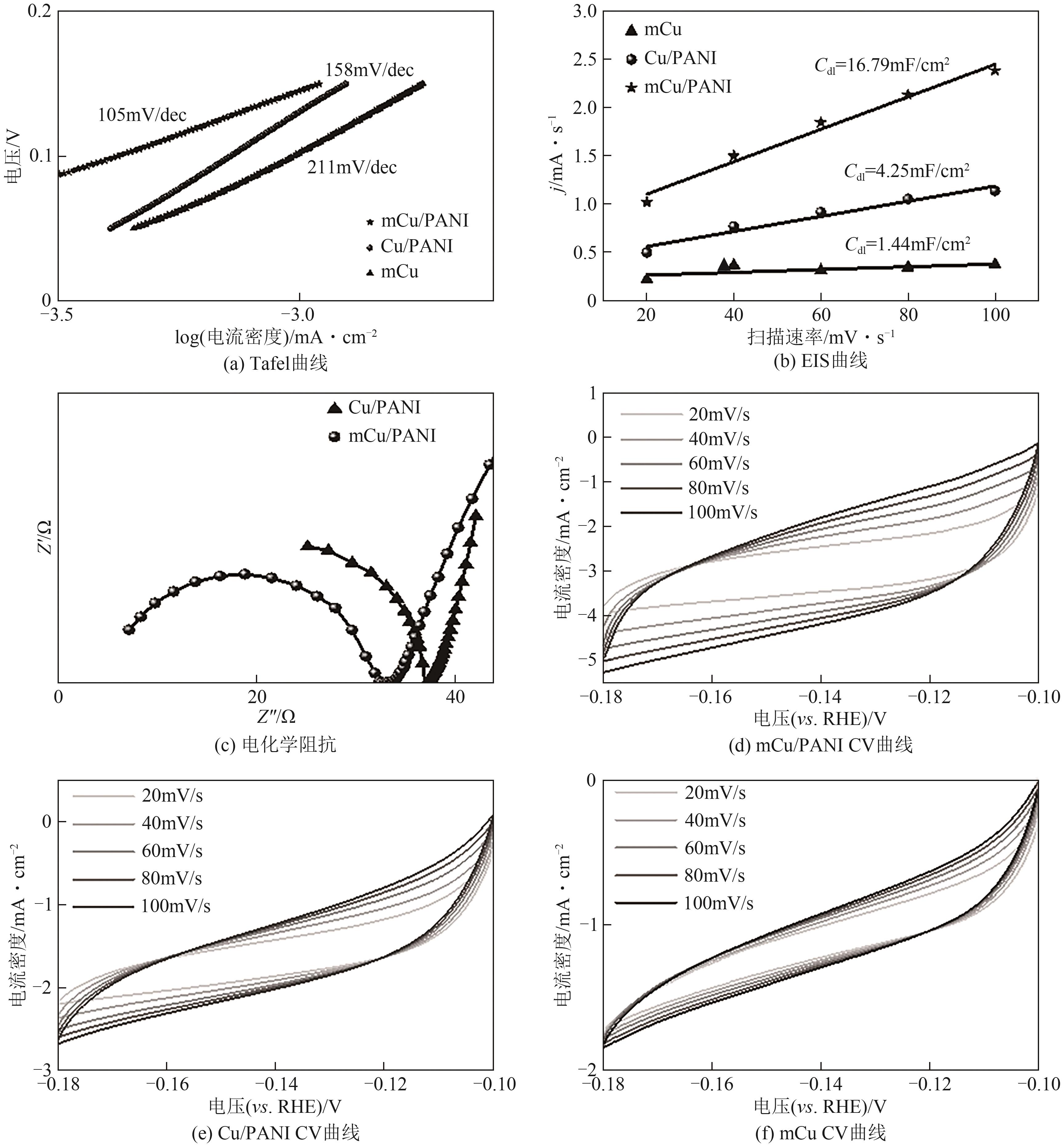
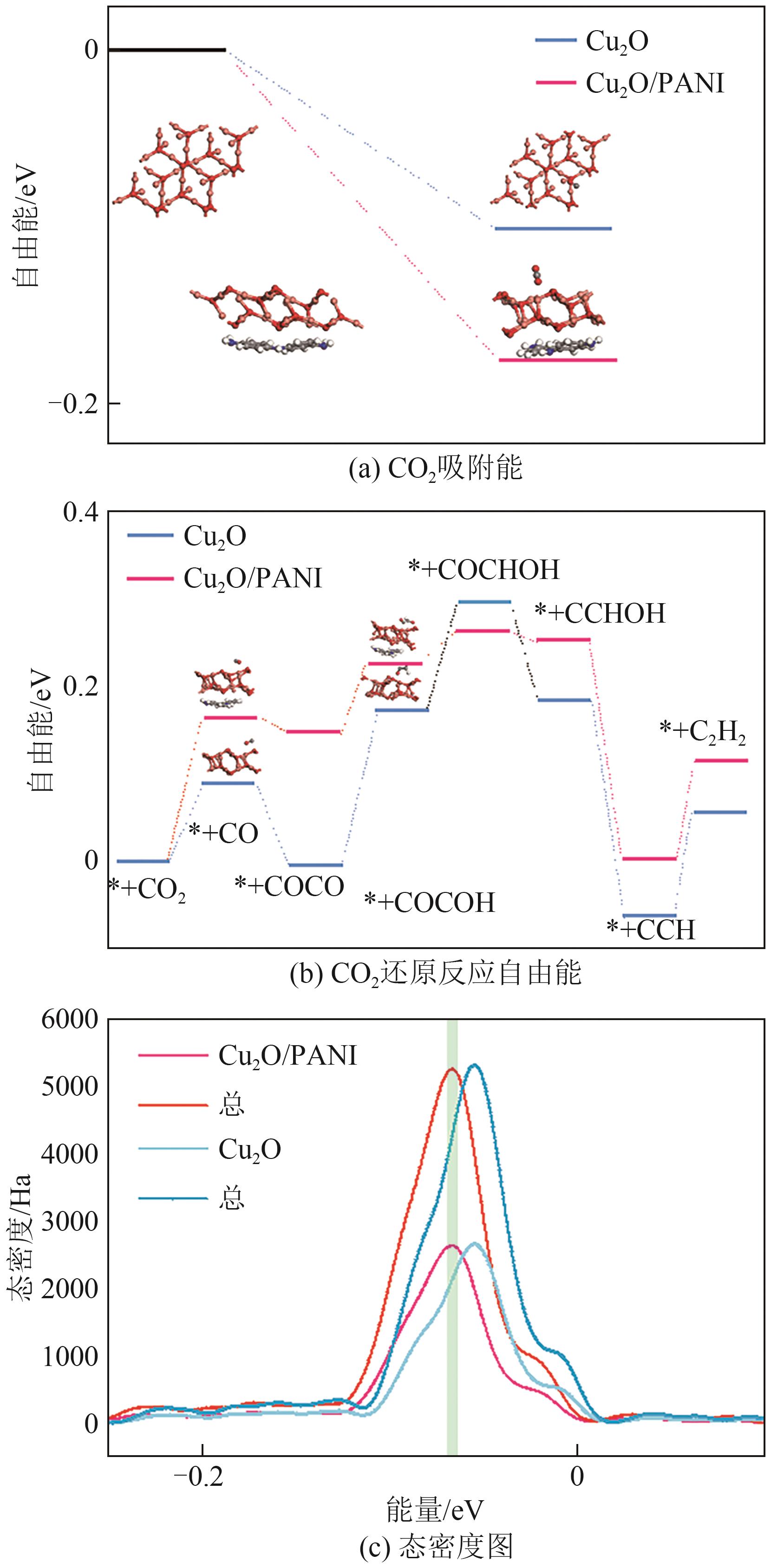
| [15] |
ESMAEILIZADSHALI Hossein, ALIREZA Nikfarjam, AMIR Ghasemi Kordlar. The effect of Cu ions concentration on electrochromic properties of Cu embedded PANI thin films[J]. Physica Scripta, 2023, 98(9): 095915.
|
| [16] |
NING Shangbo, SUN Yanhui, OUYANG Shuxin, et al. Solar light-induced injection of hot electrons and photocarriers for synergistically enhanced photothermocatalysis over Cu-Co/SrTiO3 catalyst towards boosting CO hydrogenation into C2-C4 hydrocarbons[J]. Applied Catalysis B: Environmental, 2022, 310, 121063.
|
| [17] |
NING Hui; WANG Xiaoshang, WANG Wenhang, et al. Cubic Cu2O on nitrogen-doped carbon shells for electrocatalytic CO2 reduction to C2H4 [J]. Carbon, 2019, 146: 218-223.
|
| [18] |
Dae-Hyun NAM, Bushuyev OLEKSANDR S., LI Jun, et al. Metal-organic frameworks mediate Cu coordination for selective CO electroreduction[J]. Journal of the American Chemical Society, 2018, 140(36): 11378-11386.
|
| [19] |
ZHOU Jianjun, PAN Fan, YAO Qiaofeng, et al. Achieving efficient and stable electrochemical nitrate removal by in-situ reconstruction of Cu2O/Cu electroactive nanocatalysts on Cu foam[J]. Applied Catalysis B: Environmental, 2022, 317: 121811.
|
| [20] |
Pawar ATUL A, Bandal HARSGAD A, ANAND Rajkamal, et al. Understanding the impact of reaction parameters on electrochemical reduction of CO2 to methanol: Activity relationship of cuprite@polyaniline electrodes[J]. Journal of Electroanalytical Chemistry, 2023, 946: 117721.
|
| [21] |
WEI Xing, YIN Zhenglei, Kangjie LYU, et al. Highly selective reduction of CO2 to C2+ hydrocarbons at copper polyaniline interfaces[J]. ACS Catalysis, 2020, 10(7): 4103-4111.
|
| [22] |
张瑞, 齐仲, 史军军, 等. Au/Cu2O@Au可见光催化CO2还原性能[J]. 石油学报(石油加工), 2023, 39(5): 1092-1103.
|
|
ZHANG Rui, QI Zhong, SHI Junjun, et al. Photocatalytic CO2 reduction of Au/Cu2O@Au photocatalyst under visible-light irradiation[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2023, 39(5): 1092-1103.
|
| [23] |
吴宇晗, 张栋栋, 尹宏宇, 等. “双碳”目标下Janus In2S2X光催化还原CO2的密度泛函理论研究[J]. 化学学报, 2023, 81(9): 1148-1156.
|
|
WU Yuhan, ZHANG Dongdong, YIN Hongyu, et al. Density functional theory study of janus In2S2X photocatalytic reduction of CO2 under “double carbon” target[j]. Acta Chimica Sinica, 2023, 81(9): 1148-1156.
|
| [24] |
LIN Fan, ANDANA Tahrizi, WU Yiqing, et al. Catalytic site requirements for N2O decomposition on Cu-, Co-, and Fe-SSZ-13 zeolites[J]. Journal of Catalysis, 2021, 401: 70-80.
|
| [1] |
KHEZRI Bahareh, FISHER Adrian C. PUMERA Martin. CO2 reduction: The quest for electrocatalytic materials[J]. Journal of Materials Chemistry A, 2017, 5(18): 8230-8246.
|
| [2] |
GOODPASTER Jason D, BELL Alexis T, Martin HEAD-GORDON. Identification of possible pathways for C—C bond formation during electrochemical reduction of CO2: New theoretical insights from an improved electrochemical model[J]. The Journal of Physical Chemistry Letters, 2016, 7(8): 1471-1477.
|
| [3] |
Elena PEREZ-GALLENT, FIGUEIREDO Marta C. CALLE-VALLEJO Federico,et al. Spectroscopic observation of a hydrogenated CO dimer intermediate during CO reduction on Cu(100) electrodes[J]. Angewandte Chemie, 2017, 129(13): 3675-3678.
|
| [4] |
HAN Xu, Sara THOI V. Non-innocent role of porous carbon toward enhancing C2-3 products in the electroreduction of carbon dioxide[J]. ACS Applied Materials & Interfaces, 2020, 12(41): 45929-45935.
|
| [5] |
张强, 姚章权, 周蓉, 等. Ag/Au/Pt复合催化剂的制备及其对甲酸的电催化氧化[J]. 化学学报, 2012(20): 2149-2154.
|
|
ZHANG Qiang, YAO Zhangquan, ZHOU Rong, et al. Fabrication of Ag/Au/Pt Composite Catalysts and Their Electrocatalytic Oxidation for Formic Acid[J]. Acta Chimica Sinica, 2012(20): 2149-2154.
|
| [6] |
FENG Liang, WANG Kunyu, DOWELL Joshua, et al. Controllable synthesis of metal-organic frameworks and their hierarchical assemblies[J]. Matter, 2019, 1(4): 801-824.
|
| [7] |
CHEN Yajie, DING Yi, HAN Wei, et al. Cu-BTC-confined synthesis of Cu-Cu2O-CuS nanojunctions embedded in a porous carbon matrix for remarkable photothermal CO2 conversion[J]. Journal of Materials Chemistry A, 2023, 11(15): 8342-8351.
|
| [8] |
ZHANG Chengming, WANG Meng, GAO Kaiyue, et al. Constructing N-Cu-S interface chemical bonds over SnS2 for efficient solar-driven photoelectrochemical water splitting[J]. Small, 2023, 19(3): 2205706.
|
| [9] |
LIU Zhifang, ZHU Aixin, Chikeung LAM, et al. Oriented growth of a single crystalline Cu(111) flake synthesized by pyrolysis of coordination polymer [(CuBr)2(bpy)] n (bpy=2,2′-bipyridine)[J]. CrystEngComm, 2009, 11(7): 1303-1305.
|
| [10] |
LEGARE Marc-André, Guillaume BELANGER-CHABOT, DEWHURST Rian D, et al. Nitrogen fixation and reduction at boron[J]. Science, 2018, 359(6378): 896-900.
|
| [11] |
GUAN Hongyu, LEBLANC R J, XIE Shuya, et al. Recent progress in the syntheses of mesoporous metal-organic framework materials[J]. Coordination Chemistry Reviews, 2018, 369: 76-90.
|
| [12] |
ZHI Wenya, LIU Yuting, SHAN Sili, et al. Efficient electroreduction of CO2 to C2-C3 products on Cu/Cu2O@N-doped graphene[J]. Journal of CO2 Utilization, 2021, 50: 101594.
|
| [13] |
刘毅, 房强, 钟达忠, 等. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142.
|
|
LIU Yi, FANG Qiang, ZHONG Dazhong, et al. Fabrication of Ag/Au/Pt composite catalysts and their electrocatalytic oxidation for formic acid[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142.
|
| [14] |
CAI Guorui, YAN Peng, ZHANG Liangliang, et al. Metal-organic framework-based hierarchically porous materials: Synthesis and applications[J]. Chemical Reviews, 2021, 121(20): 12278-12326.
|
 ), LIU Yuhan1, SHENG Yu1, LIU Fang1(
), LIU Yuhan1, SHENG Yu1, LIU Fang1( ), CHANG Huazhen2
), CHANG Huazhen2