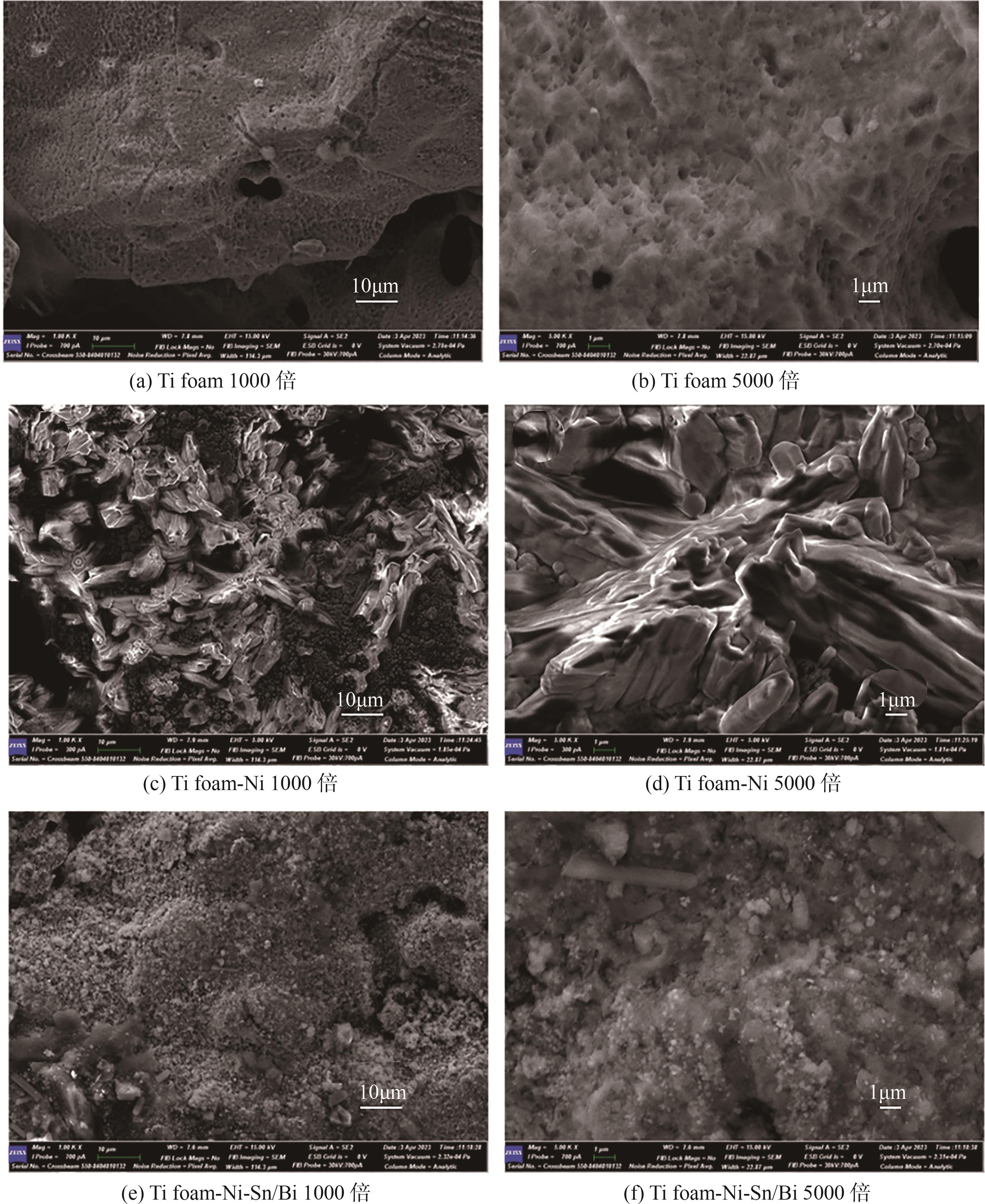
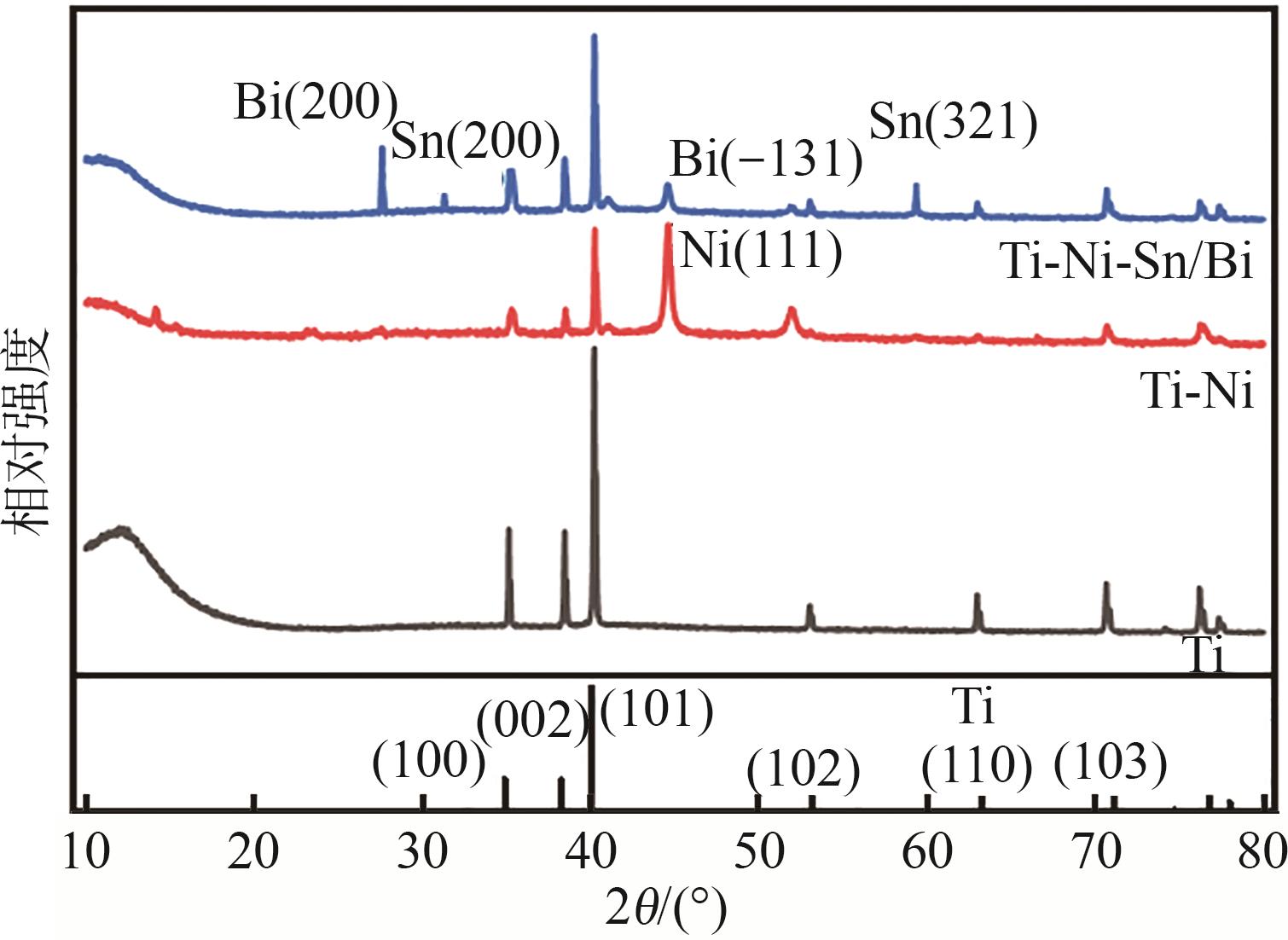
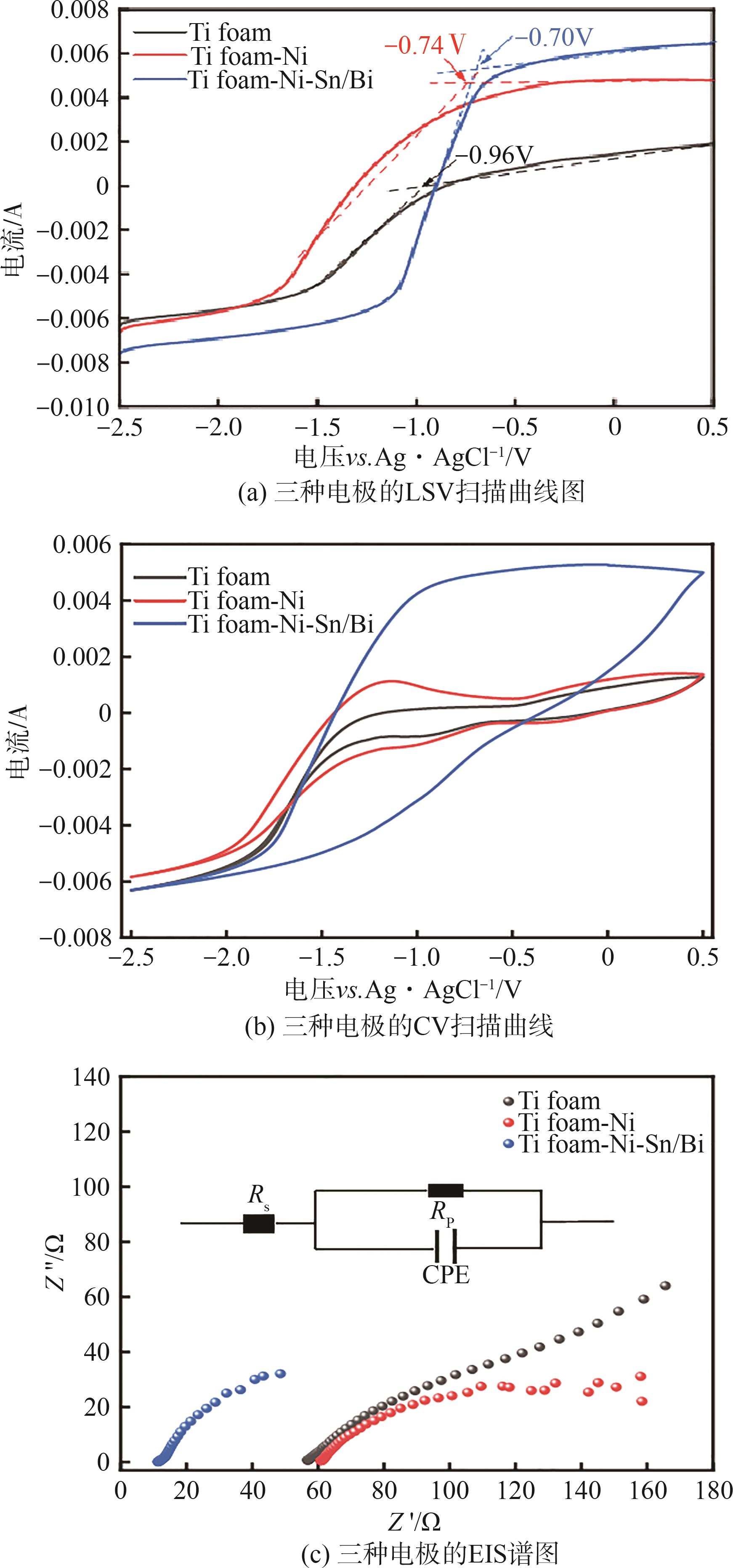
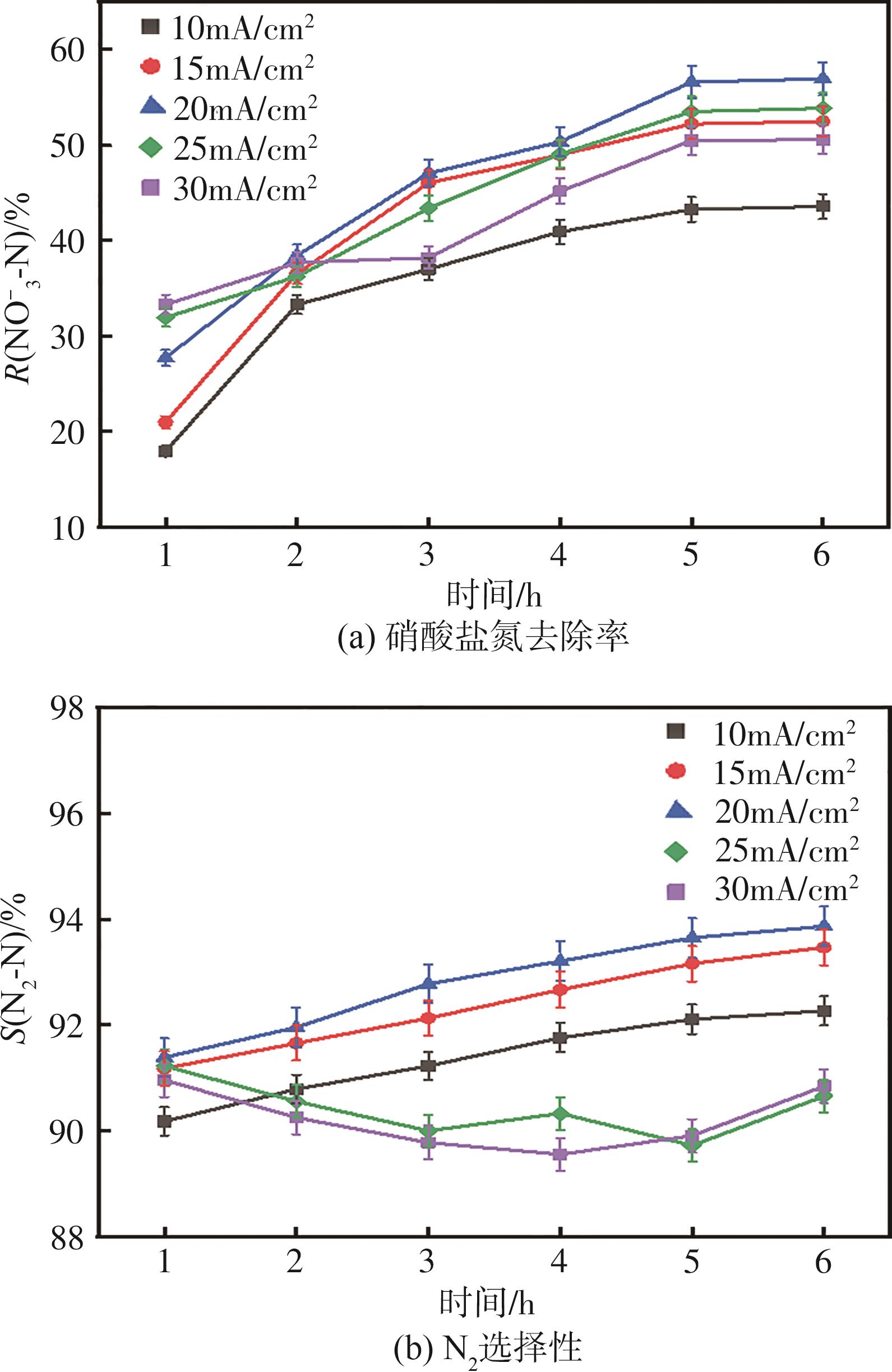
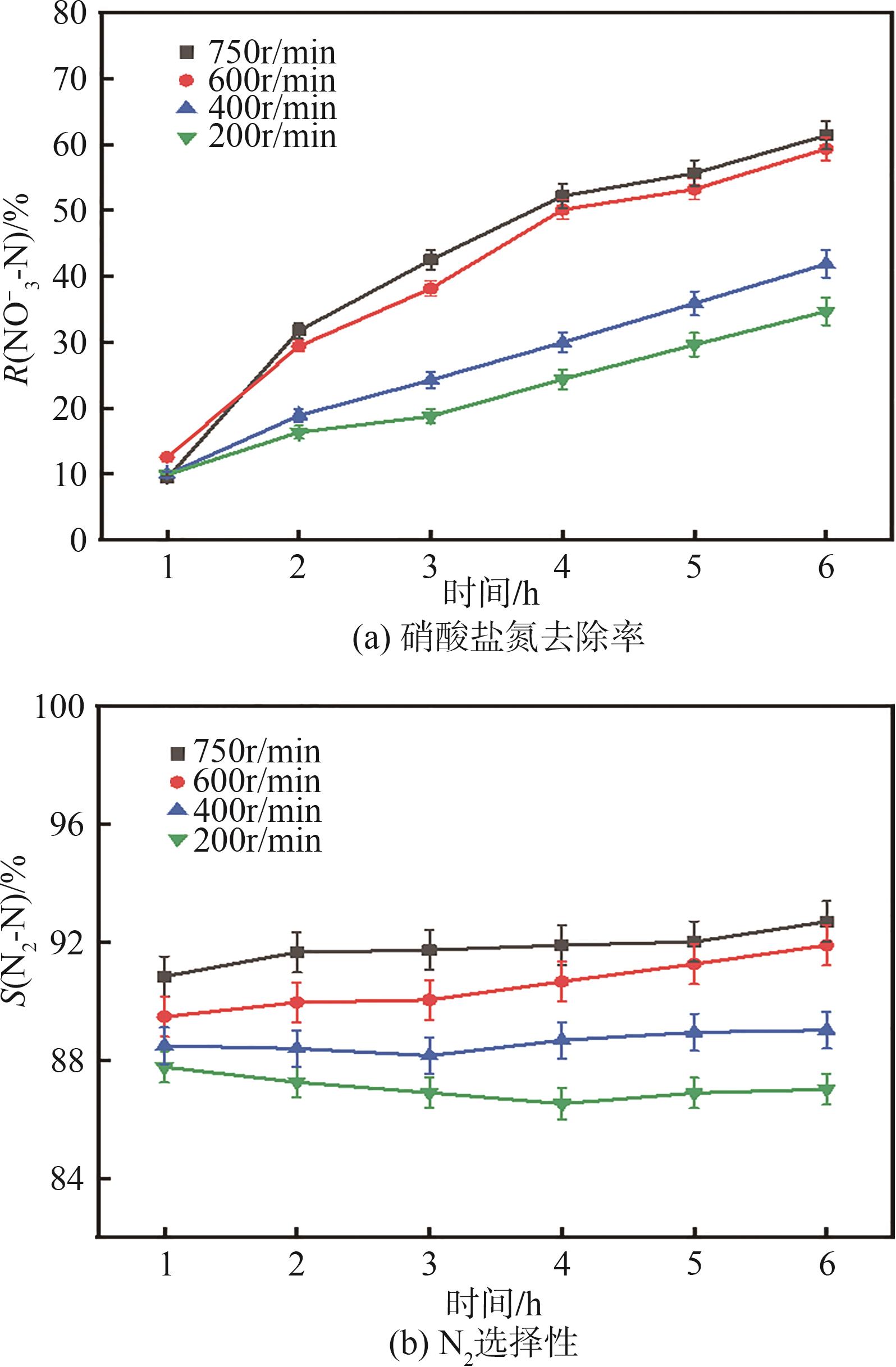
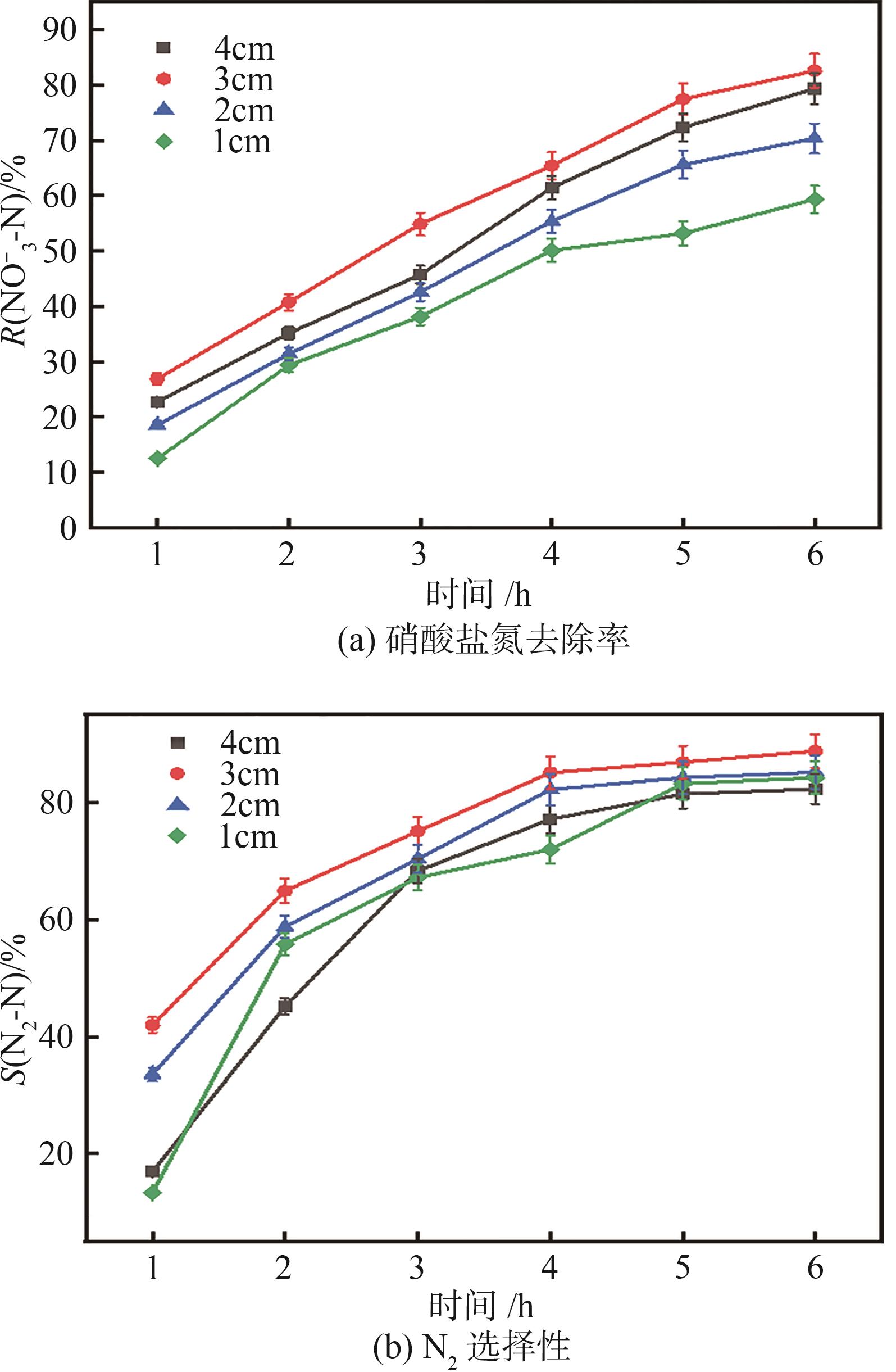
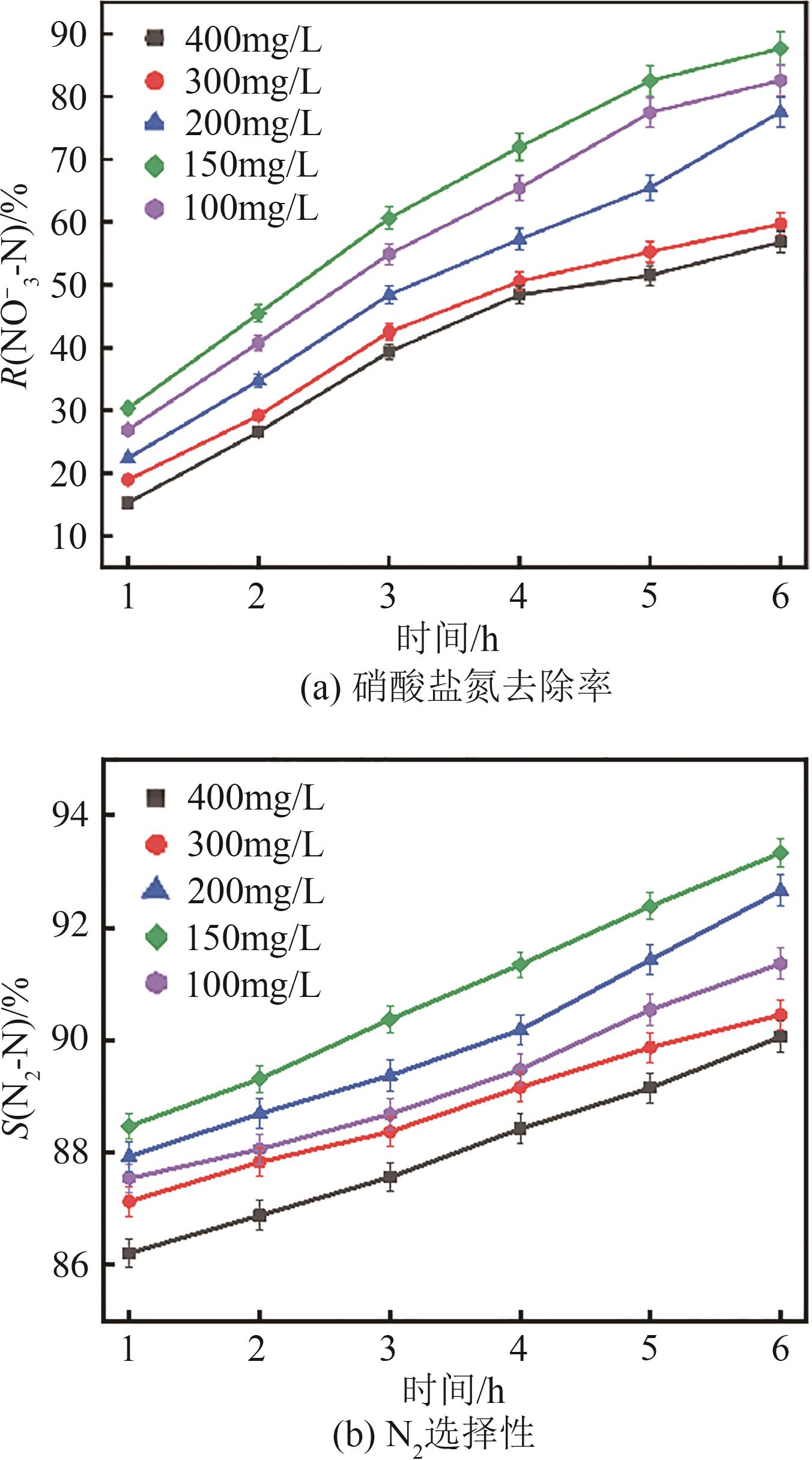
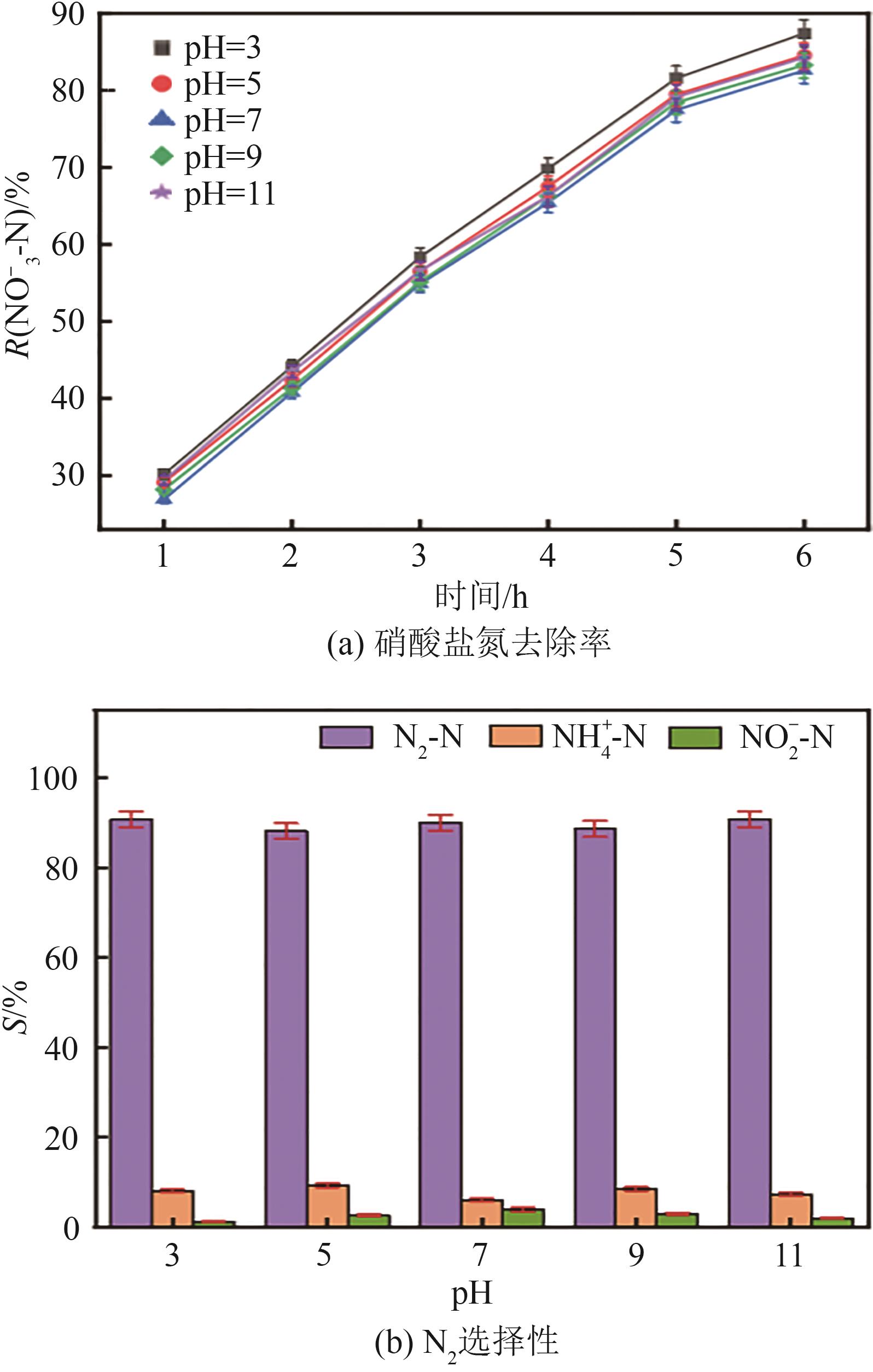
| 1 |
KAUSHAL Sujay S, GROFFMAN Peter M, BAND Lawrence E, et al. Tracking nonpoint source nitrogen pollution in human-impacted watersheds[J]. Environmental Science & Technology, 2011, 45(19): 8225-8232.
|
| 2 |
ESSIEN Eno E, ABASSE Kassim Said, André CÔTÉ, et al. Drinking-water nitrate and cancer risk: A systematic review and meta-analysis[J]. Archives of Environmental & Occupational Health, 2022, 77(1): 51-67.
|
| 3 |
COTRUVO Joseph A. 2017 WHO guidelines for drinking water quality: First addendum to the fourth edition[J]. Journal AWWA, 2017, 109(7): 44-51.
|
| 4 |
中华人民共和国国家卫生健康委员会. 生活饮用水卫生标准 [S]. 国家市场监督管理总局、中国国家标准化管理委员会, 2022-03-15.
|
|
National Health Commission, PRC. Hygienic standards for drinking water [S]. State Administration for Market Regulation, National Standardization Administration, 2022-03-15.
|
| 5 |
DUCA Matteo, KOPER Marc T M. Powering denitrification: The perspectives of electrocatalytic nitrate reduction[J]. Energy & Environmental Science, 2012, 5(12):9726-9742.
|
| 6 |
SHEN Zhanhui, LIU Daoru, PENG Gege, et al. Electrocatalytic reduction of nitrate in water using Cu/Pd modified Ni foam cathode: High nitrate removal efficiency and N2-selectivity[J]. Separation and Purification Technology, 2020, 241: 116743.
|
| 7 |
Sergi GARCIA-SEGURA, Mariana LANZARINI-LOPES, HRISTOVSKI Kiril, et al. Electrocatalytic reduction of nitrate: Fundamentals to full-scale water treatment applications[J]. Applied Catalysis B: Environmental, 2018, 236: 546-568.
|
| 8 |
HAMME Roberta C, EMERSON Steven R. The solubility of neon, nitrogen and argon in distilled water and seawater[J]. Deep Sea Research Part Ⅰ: Oceanographic Research Papers, 2004, 51(11): 1517-1528.
|
| 9 |
REYTER David, CHAMOULAUD Gwenaël, Daniel BÉLANGER, et al. Electrocatalytic reduction of nitrate on copper electrodes prepared by high-energy ball milling[J]. Journal of Electroanalytical Chemistry, 2006, 596(1): 13-24.
|
| 10 |
WANG Lele, LI Miao, LIU Xiang, et al. Electrochemical behavior of Ti-based nano-electrode for highly efficient denitrification in synthetic groundwater[J]. Journal of the Electrochemical Society, 2017, 164(12): E326-E331.
|
| 11 |
任柯丞, 许彦桐, 曹晏. 铁系无机材料用于新能源电催化硝酸盐还原制氨[J]. 新能源进展, 2022, 10(3): 209-217.
|
|
REN Kecheng, XU Yantong, CAO Yan. Iron-based inorganic materials for new energy-based electrocatalytic nitrate-to-ammonia reduction reaction[J]. Advances in New and Renewable Energy, 2022, 10(3): 209-217.
|
| 12 |
RAO Xufeng, SHAO Xiaolin, XU Jie, et al. Efficient nitrate removal from water using selected cathodes and Ti/PbO2 anode: Experimental study and mechanism verification[J]. Separation and Purification Technology, 2019, 216: 158-165.
|
| 13 |
YAO Fubing, YANG Qi, ZHONG Yu, et al. Indirect electrochemical reduction of nitrate in water using zero-valent titanium anode: Factors, kinetics, and mechanism[J]. Water Research, 2019, 157: 191-200.
|
| 14 |
SHIH YuJen, WU Zhilun, HUANG Yaohui, et al. Electrochemical nitrate reduction as affected by the crystal morphology and facet of copper nanoparticles supported on nickel foam electrodes (Cu/Ni)[J]. Chemical Engineering Journal, 2020, 383: 123157.
|
| 15 |
GAO Weichun, GAO Lulu, LI Dan, et al. Removal of nitrate from water by the electrocatalytic denitrification on the Cu-Bi electrode[J]. Journal of Electroanalytical Chemistry, 2018, 817: 202-209.
|
| 16 |
KATSOUNAROS I, IPSAKIS D, POLATIDES C, et al. Efficient electrochemical reduction of nitrate to nitrogen on tin cathode at very high cathodic potentials[J]. Electrochimica Acta, 2006, 52(3): 1329-1338.
|
| 17 |
LI Fukuan, ZHANG Weizhe, ZHANG Peng, et al. Strategies of selective electroreduction of aqueous nitrate to N2 in chloride-free system: A critical review[J]. Green Energy & Environment, 2024, 9(2): 198-216.
|
| 18 |
SHIH YuJen, WU Zhilun. Electroplating of surfactant-modified tin catalyst over a nickel foam electrode (Sn/Ni) for selective N2 yield from nitrate reduction as affected by Sn(200) and Sn(101) crystal facets[J]. Applied Catalysis B: Environmental, 2021, 285: 119784.
|
| 19 |
DORTSIOU M, KYRIACOU G. Electrochemical reduction of nitrate on bismuth cathodes[J]. Journal of Electroanalytical Chemistry, 2009, 630(1/2): 69-74.
|
| 20 |
FAJARDO Ana S, WESTERHOFF Paul, SANCHEZ-SANCHEZ Carlos M, et al. Earth-abundant elements a sustainable solution for electrocatalytic reduction of nitrate[J]. Applied Catalysis B: Environmental, 2021, 281: 119465.
|
| 21 |
LI Duo, TANG Jingyan, ZHOU Xiezhen, et al. Electrochemical degradation of pyridine by Ti/SnO2-Sb tubular porous electrode[J]. Chemosphere, 2016, 149: 49-56.
|
| 22 |
ZHAO Lu, ZHANG Yun, ZHAO Zhonglong, et al. Steering elementary steps towards efficient alkaline hydrogen evolution via size-dependent Ni/NiO nanoscale heterosurfaces[J]. National Science Review, 2020, 7(1): 27-36.
|
| 23 |
TAGUCHI Satoshi, FELIU Juan M. Kinetic study of nitrate reduction on Pt(110) electrode in perchloric acid solution[J]. Electrochimica Acta, 2008, 53(10): 3626-3634.
|
| 24 |
GAO Yihong, WANG Kunpeng, XU Chao, et al. Enhanced electrocatalytic nitrate reduction through phosphorus-vacancy-mediated kinetics in heterogeneous bimetallic phosphide hollow nanotube array[J]. Applied Catalysis B: Environmental, 2023, 330: 122627.
|
| 25 |
AUFA Muhamad H, WATZELE Sebastian A, HOU Shujin, et al. Fast and accurate determination of the electroactive surface area of MnO x [J]. Electrochimica Acta, 2021, 389: 138692.
|
| 26 |
SHAO Dan, YAN Wei, LI Xiaoliang, et al. A highly stable Ti/TiH x /Sb-SnO2 anode: Preparation, characterization and application[J]. Industrial & Engineering Chemistry Research, 2014, 53(10): 3898-3907.
|
| 27 |
ZHANG Lihao, WU Yuqing, ZHU Zongqiang, et al. Synergistically enhancing nitrate reduction into N2 in water by N-doped Pd-Cu biochar bimetallic single-atom electrocatalysis[J]. Biochar, 2024, 6(1): 8.
|
| 28 |
MENG Shuo, LING Yan, YANG Mingyu, et al. Recent research progress of electrocatalytic reduction technology for nitrate wastewater: A review[J]. Journal of Environmental Chemical Engineering, 2023, 11(2): 109418.
|
| 29 |
LAN Huachun, LIU Xiaobin, LIU Huijuan, et al. Efficient nitrate reduction in a fluidized electrochemical reactor promoted by Pd-Sn/AC particles[J]. Catalysis Letters, 2016, 146(1): 91-99.
|
| 30 |
CLEMATIS Davide, CERISOLA Giacomo, PANIZZA Marco. Electrochemical oxidation of a synthetic dye using a BDD anode with a solid polymer electrolyte[J]. Electrochemistry Communications, 2017, 75: 21-24.
|
| 31 |
ZHUO Qiongfang, WANG Jinbao, NIU Junfeng, et al. Electrochemical oxidation of perfluorooctane sulfonate (PFOS) substitute by modified boron doped diamond (BDD) anodes[J]. Chemical Engineering Journal, 2020, 379: 122280.
|
| 32 |
MINAKSHI Manickam, IONESCU Mihail. Anodic behavior of zinc in Zn-MnO2 battery using ERDA technique[J]. International Journal of Hydrogen Energy, 2010, 35(14): 7618-7622.
|
| 33 |
DE GROOT M T, KOPER M T M. The influence of nitrate concentration and acidity on the electrocatalytic reduction of nitrate on platinum[J]. Journal of Electroanalytical Chemistry, 2004, 562(1): 81-94.
|
| 34 |
DIMA G E, DE VOOYS A C A, KOPER M T M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions[J]. Journal of Electroanalytical Chemistry, 2003, 554: 15-23.
|
| 35 |
WANG Zixuan, RICHARDS Danielle, SINGH Nirala. Recent discoveries in the reaction mechanism of heterogeneous electrocatalytic nitrate reduction[J]. Catalysis Science & Technology, 2021, 11(3): 705-725.
|
| 36 |
SUN Jie, GAO Weiqi, FEI Honghan, et al. Efficient and selective electrochemical reduction of nitrate to N2 by relay catalytic effects of Fe-Ni bimetallic sites on MOF-derived structure[J]. Applied Catalysis B: Environmental, 2022, 301: 120829.
|
 ), LIU Yun’e2, HUANG Zhihao1, SHEN Rong1
), LIU Yun’e2, HUANG Zhihao1, SHEN Rong1