Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (11): 6334-6349.DOI: 10.16085/j.issn.1000-6613.2024-1750
• Industrial catalysis • Previous Articles
Research progress and prospect of hydrogen evolution catalysts for alkaline water electrolysis
CHEN Xinyue( ), CHEN Binjian(
), CHEN Binjian( ), MAO Yudong, YAN Min, XUE Lu
), MAO Yudong, YAN Min, XUE Lu
- School of Thermal Energy Engineering, Shandong Jianzhu University, Jinan 250100, Shandong, China
-
Received:2024-10-30Revised:2025-02-03Online:2025-12-08Published:2025-11-25 -
Contact:CHEN Binjian
碱性电解水析氢催化剂的研究进展及展望
- 山东建筑大学热能工程学院,山东 济南 250100
-
通讯作者:陈彬剑 -
作者简介:陈心悦(2000—),女,硕士研究生,研究方向为过渡金属析氢催化剂。E-mail:15550079736@163.com。
CLC Number:
Cite this article
CHEN Xinyue, CHEN Binjian, MAO Yudong, YAN Min, XUE Lu. Research progress and prospect of hydrogen evolution catalysts for alkaline water electrolysis[J]. Chemical Industry and Engineering Progress, 2025, 44(11): 6334-6349.
陈心悦, 陈彬剑, 毛煜东, 闫敏, 薛璐. 碱性电解水析氢催化剂的研究进展及展望[J]. 化工进展, 2025, 44(11): 6334-6349.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1750
| 项目 | 碱性电解水 | 质子交换膜电解水 | 固体氧化物电解水 |
|---|---|---|---|
| 电解质 | 30% KOH或25% NaOH | 纯水 | 水蒸气 |
| 工作温度/℃ | 60~80 | 50~80 | 500~1000 |
| 工作效率/% | 70~80 | 74~87 | 100 |
| 产氢纯度/% | 99.95 | >99.99 | >99.90 |
| 成本/CNY·kW-1 | 2600~4000 | 6500~9800 | 10000~13000 |
| 优点 | 成本低、使用寿命长、技术成熟 | 氢气纯度高、电流密度高 | 环境污染小、制氢效率高 |
| 缺点 | 电极腐蚀易失活、电流密度低 | 成本高、使用寿命短 | 高温稳定性差、成本高 |
| 项目 | 碱性电解水 | 质子交换膜电解水 | 固体氧化物电解水 |
|---|---|---|---|
| 电解质 | 30% KOH或25% NaOH | 纯水 | 水蒸气 |
| 工作温度/℃ | 60~80 | 50~80 | 500~1000 |
| 工作效率/% | 70~80 | 74~87 | 100 |
| 产氢纯度/% | 99.95 | >99.99 | >99.90 |
| 成本/CNY·kW-1 | 2600~4000 | 6500~9800 | 10000~13000 |
| 优点 | 成本低、使用寿命长、技术成熟 | 氢气纯度高、电流密度高 | 环境污染小、制氢效率高 |
| 缺点 | 电极腐蚀易失活、电流密度低 | 成本高、使用寿命短 | 高温稳定性差、成本高 |
| 催化剂 | 过电位(10mA·cm-2)/mV | Tafel斜率/mV·dec-1 | Rct/ | ECSA/cm2·mg-1 | Cdl/mF·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|
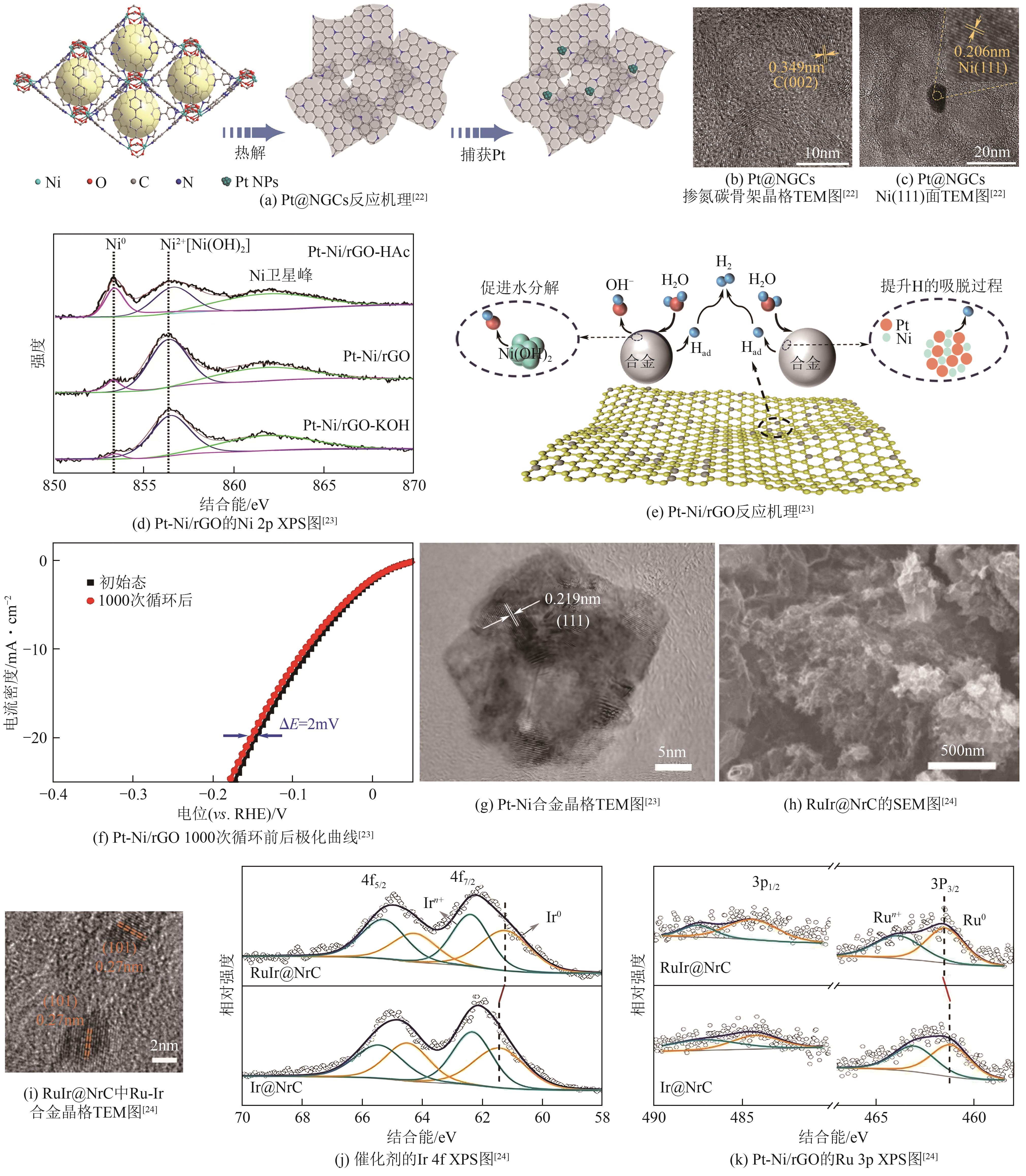
| Pt@NGCs | 27 | 30 | <16 | — | 8.78 | [ |
| Pt-Ni/rGO | 78 | 53 | — | 352 | — | [ |
| RuIr@NrC | 28 | 35 | — | 1602 | 64.7 | [ |
| 催化剂 | 过电位(10mA·cm-2)/mV | Tafel斜率/mV·dec-1 | Rct/ | ECSA/cm2·mg-1 | Cdl/mF·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|
| Pt@NGCs | 27 | 30 | <16 | — | 8.78 | [ |
| Pt-Ni/rGO | 78 | 53 | — | 352 | — | [ |
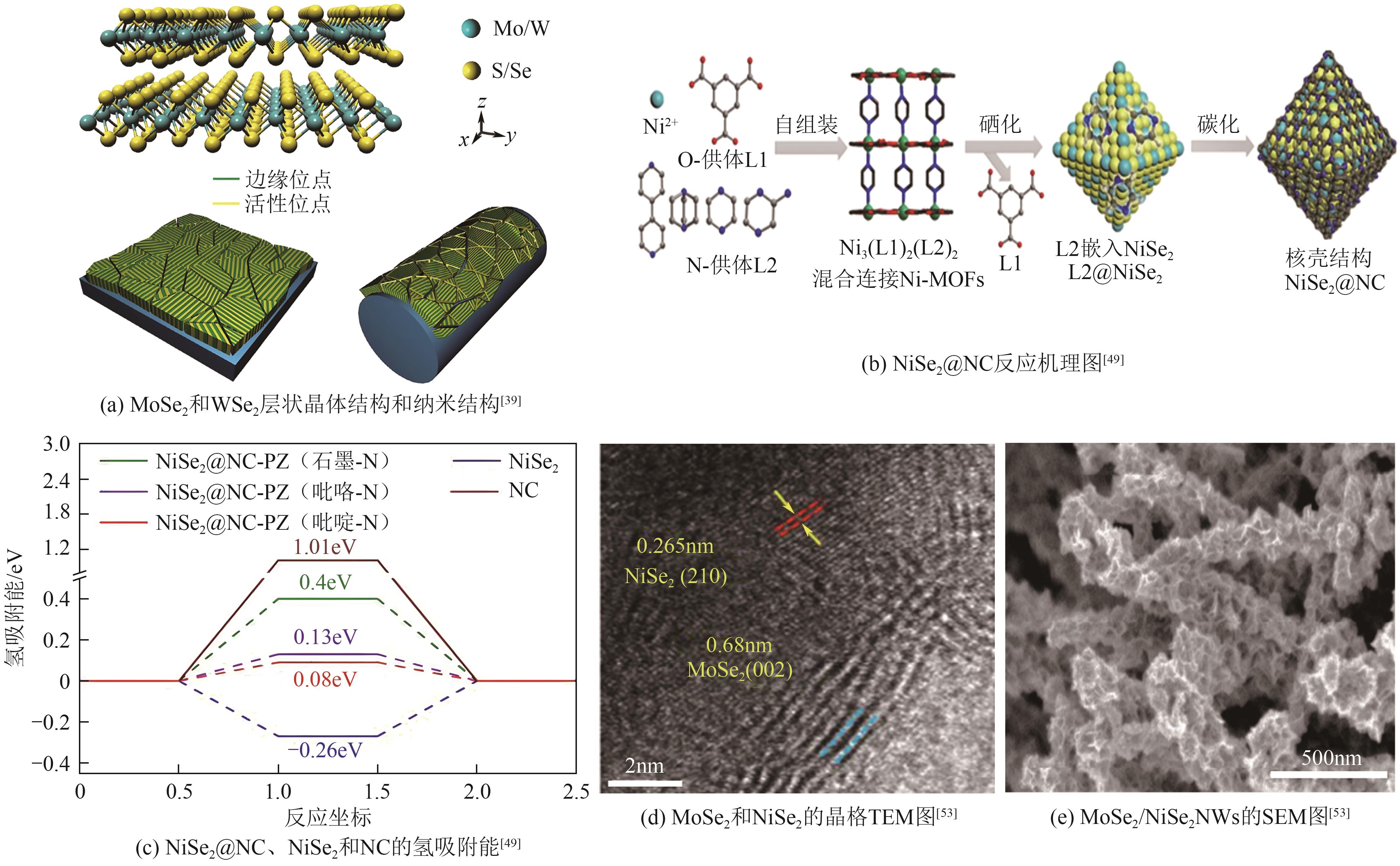
| RuIr@NrC | 28 | 35 | — | 1602 | 64.7 | [ |
| 过渡金属化合物 | 催化剂 | 过电位(10mA/cm2)/mV | Tafel斜率/mV·dec-1 | Rct/ | ECSA/cm2·mg-1 | Cdl/mF·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|---|
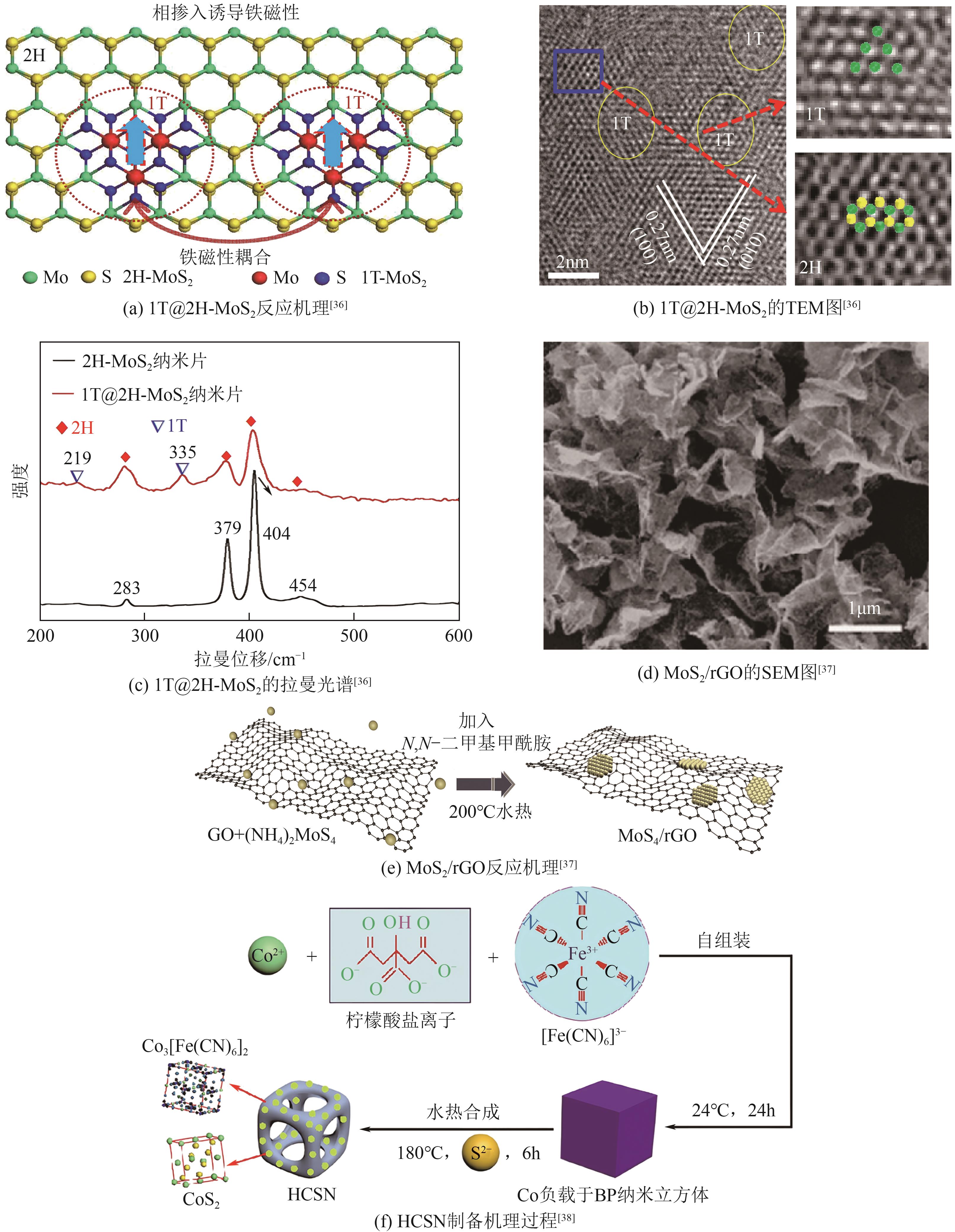
过渡金属硫化物 (TMSs) | 1T@2H-MoS2 | — | — | — | — | — | [ |
| MoS2/rGO | 100 | 41 | 250 | — | — | [ | |
| HCSN | — | — | 0.45 | 566 | — | [ | |
过渡金属硒化物 (TMSes) | MoSe2NWs | 259 | 59.8 | — | — | — | [ |
| WSe2NWs | 306 | 77.4 | — | — | — | [ | |
| NiSe2@NC-PZ | 162 | 88 | — | — | 5.88 | [ | |
| MoSe2 /NiSe2NWs | 249 (100mA/cm2) | 46.9 | 2.17 | — | 8.8 | [ | |
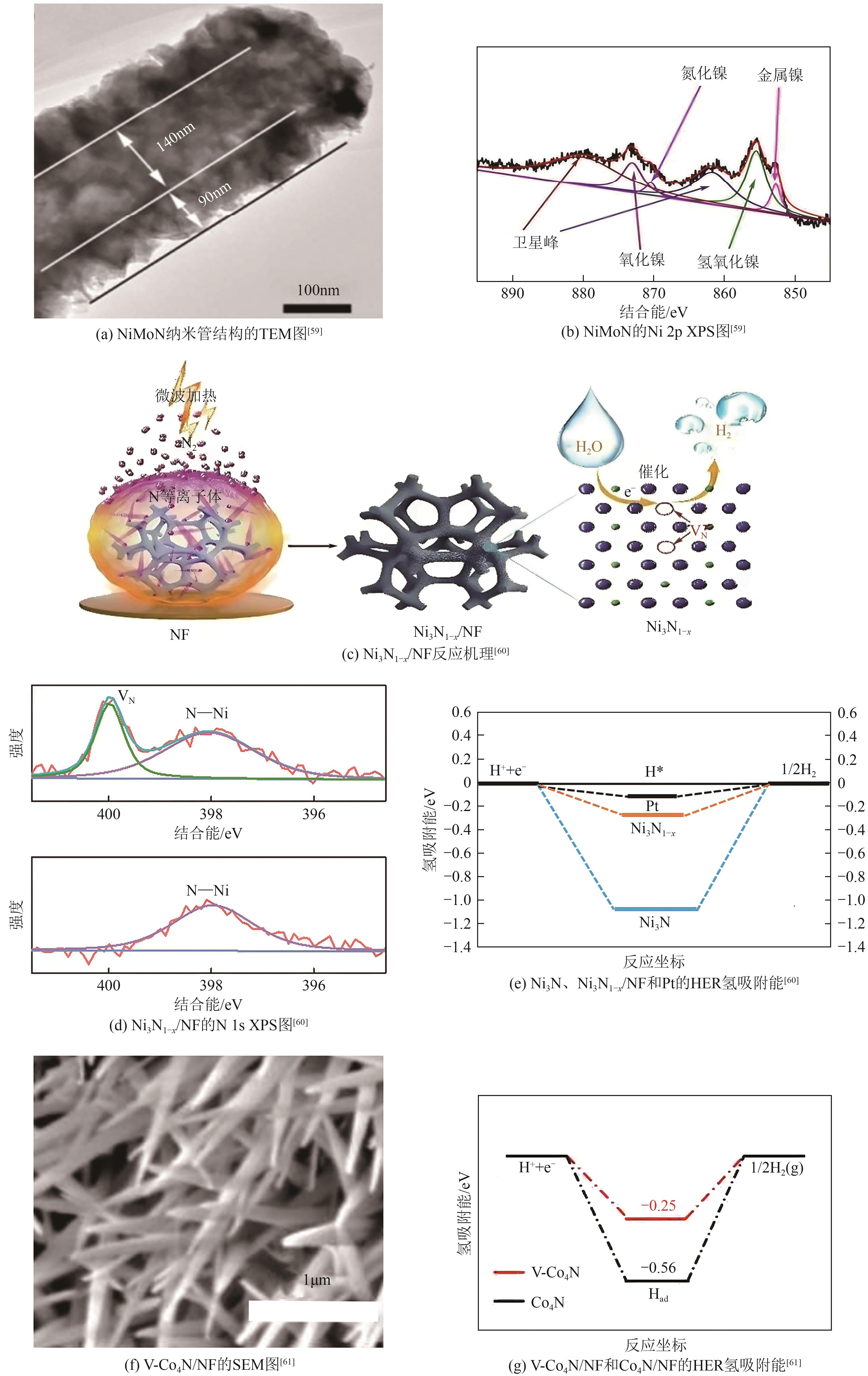
过渡金属氮化物 (TMNs) | NiMoN | 89 | 79 | 18.6 | — | — | [ |
| Ni3N1-x /NF | 55 | 54 | 18.1 | — | — | [ | |
| V-Co4N/NF | 37 | 44 | 6.2 | — | 152 | [ | |
过渡金属磷化物 (TMPs) | FeP@C | 115 | 56 | 29.2 | — | 65.3 | [ |
| Co2P@NPG | 61 (1mA/cm2) | 96 | 28 | — | 66.8 | [ | |
| Ni2P-NW | 139 | 92 | 6.8 | — | 65.1 | [ |
| 过渡金属化合物 | 催化剂 | 过电位(10mA/cm2)/mV | Tafel斜率/mV·dec-1 | Rct/ | ECSA/cm2·mg-1 | Cdl/mF·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|---|
过渡金属硫化物 (TMSs) | 1T@2H-MoS2 | — | — | — | — | — | [ |
| MoS2/rGO | 100 | 41 | 250 | — | — | [ | |
| HCSN | — | — | 0.45 | 566 | — | [ | |
过渡金属硒化物 (TMSes) | MoSe2NWs | 259 | 59.8 | — | — | — | [ |
| WSe2NWs | 306 | 77.4 | — | — | — | [ | |
| NiSe2@NC-PZ | 162 | 88 | — | — | 5.88 | [ | |
| MoSe2 /NiSe2NWs | 249 (100mA/cm2) | 46.9 | 2.17 | — | 8.8 | [ | |
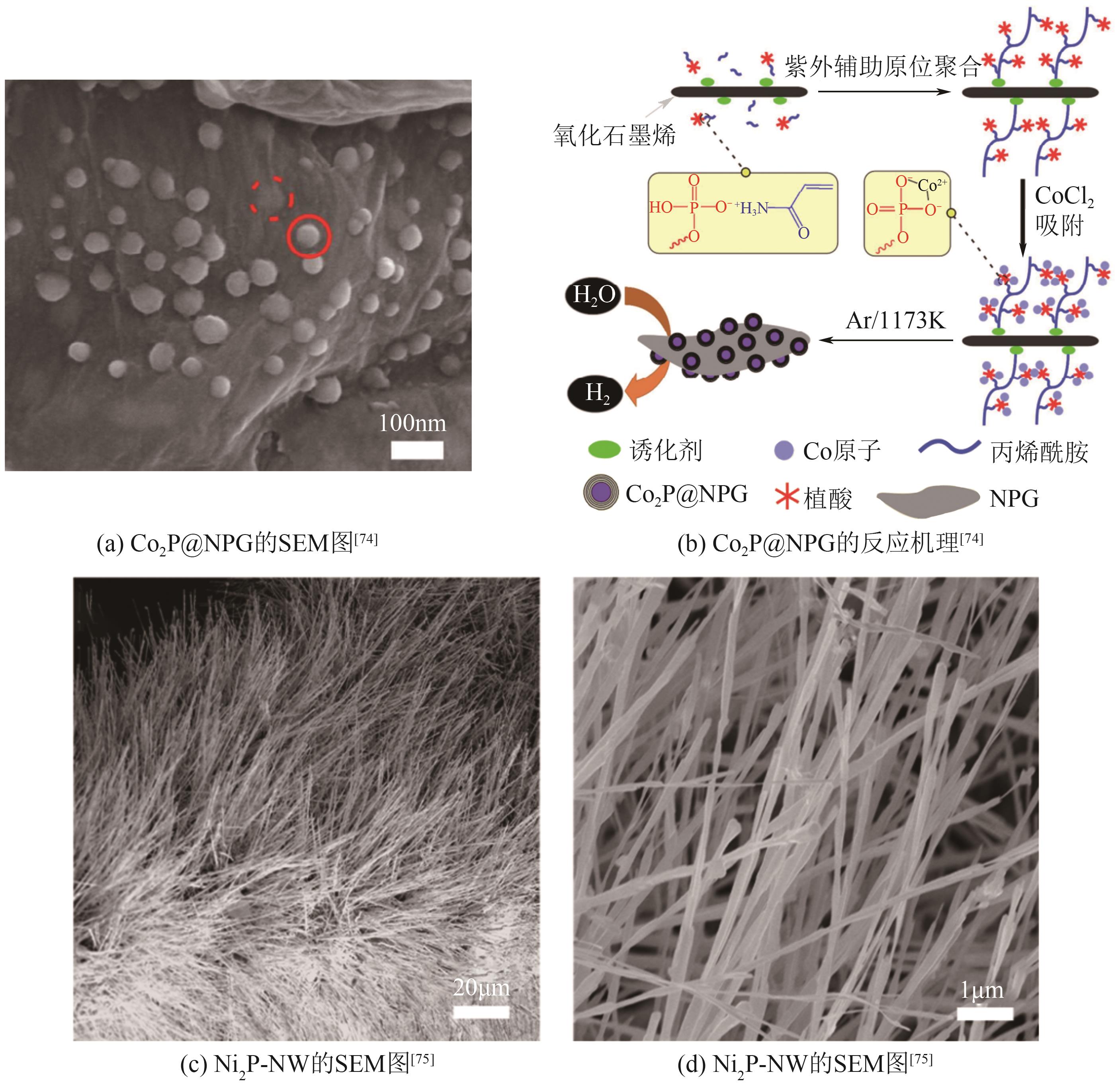
过渡金属氮化物 (TMNs) | NiMoN | 89 | 79 | 18.6 | — | — | [ |
| Ni3N1-x /NF | 55 | 54 | 18.1 | — | — | [ | |
| V-Co4N/NF | 37 | 44 | 6.2 | — | 152 | [ | |
过渡金属磷化物 (TMPs) | FeP@C | 115 | 56 | 29.2 | — | 65.3 | [ |
| Co2P@NPG | 61 (1mA/cm2) | 96 | 28 | — | 66.8 | [ | |
| Ni2P-NW | 139 | 92 | 6.8 | — | 65.1 | [ |
| 催化剂 | 过电位(10mA/cm2)/mV | Tafel斜率/mV·dec-1 | Rct/ | ECSA/cm2·mg-1 | Cdl/mF·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|
| Er-CoP NMs | 66 | 61 | — | 1234 | — | [ |
| — | 143.1 | |||||
| Co4N-CeO2/GP | 24 | 61 | 27 | — | — | [ |
| 催化剂 | 过电位(10mA/cm2)/mV | Tafel斜率/mV·dec-1 | Rct/ | ECSA/cm2·mg-1 | Cdl/mF·cm-2 | 参考文献 |
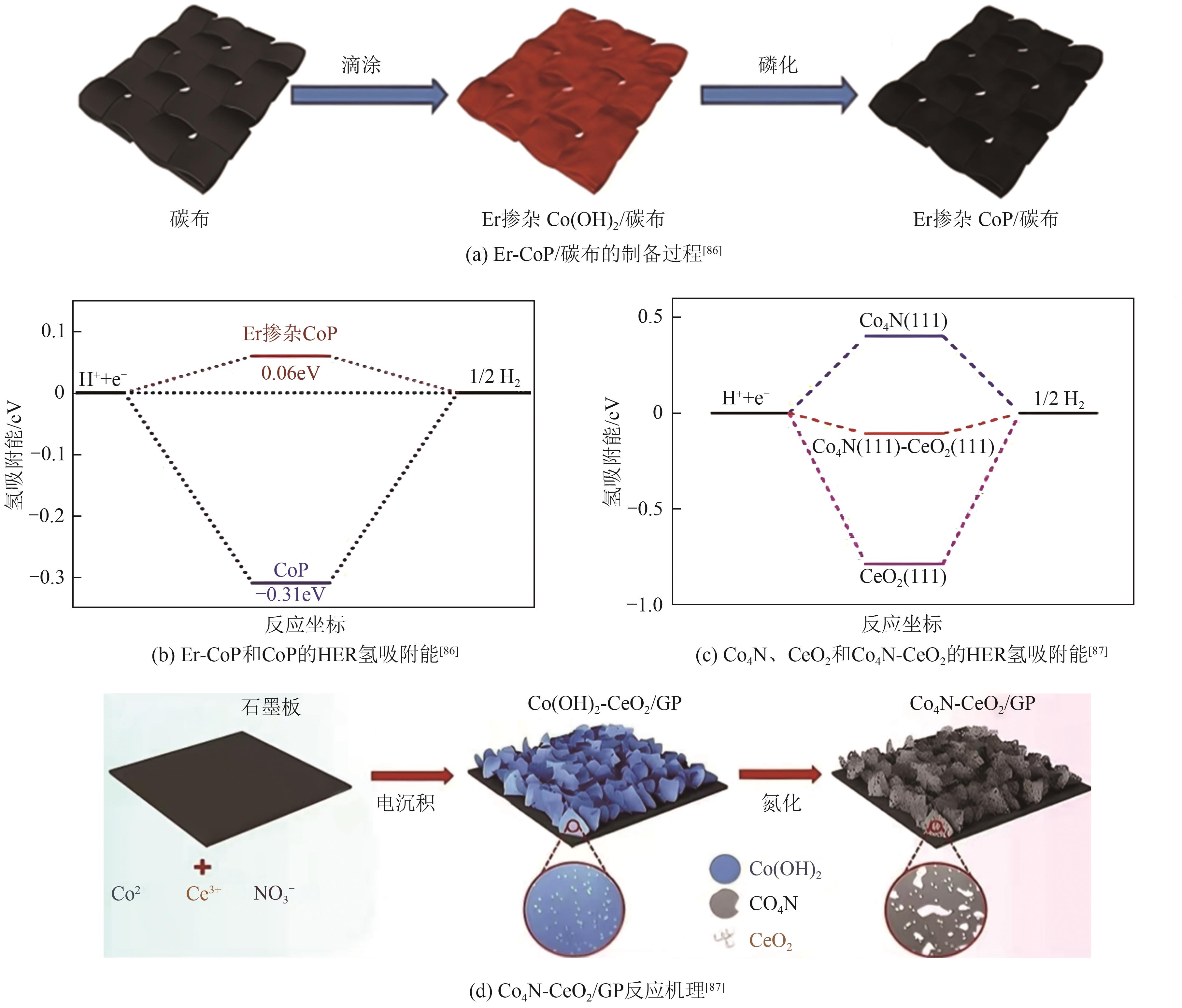
|---|---|---|---|---|---|---|
| Er-CoP NMs | 66 | 61 | — | 1234 | — | [ |
| — | 143.1 | |||||
| Co4N-CeO2/GP | 24 | 61 | 27 | — | — | [ |
| 催化剂 | 过电位(10mA/cm2)/mV | Tafel斜率/mV·dec-1 | Rct/ | ECSA/cm2·mg-1 | Cdl/mF·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|
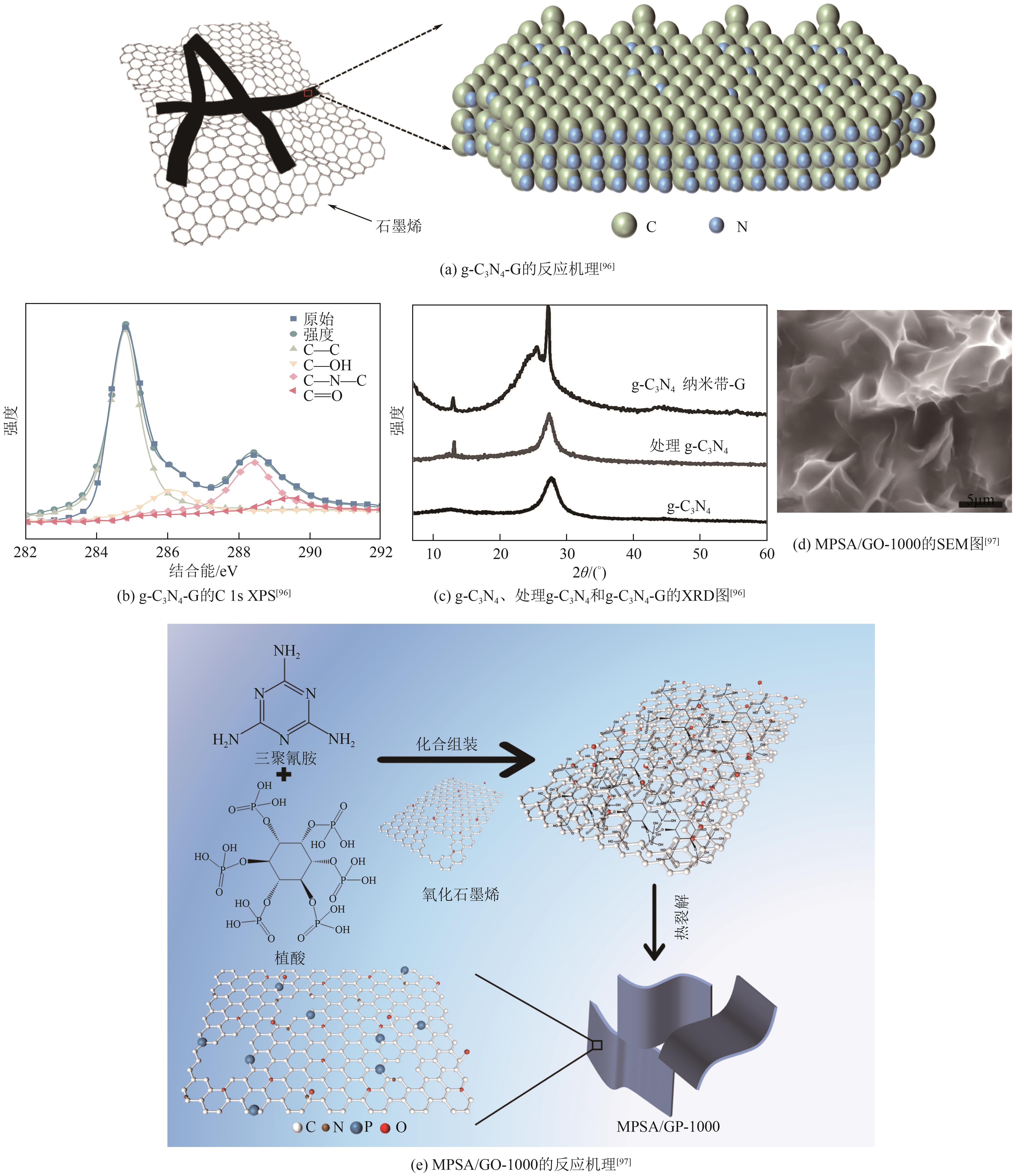
| g-C3N4-G | 207 | 54 | — | — | 13 | [ |
| MPSA/GO-1000 | 210(30mA/cm2) | 89 | — | — | — | [ |
| 催化剂 | 过电位(10mA/cm2)/mV | Tafel斜率/mV·dec-1 | Rct/ | ECSA/cm2·mg-1 | Cdl/mF·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|
| g-C3N4-G | 207 | 54 | — | — | 13 | [ |
| MPSA/GO-1000 | 210(30mA/cm2) | 89 | — | — | — | [ |
| 优化方式 | 反应机理 | 制备策略 |
|---|---|---|
| 改变形貌 | 改变催化剂本身结构,增大反应面积;改变催化剂晶格相提升性能;缺陷工程增加成核位点 | 并非使用单一优化方法,采用多种优化方式结合以制备高效稳定的析氢催化剂 |
| 掺杂元素 | 元素协同作用优化催化剂电子结构;优化表面电子-质子耦合机制;杂原子提升催化剂稳定性和活性;掺杂具有独特电子结构的稀土元素,调节催化剂电子态密度 | |
| 使用载体 | 均匀分散催化剂;载体与催化剂耦合界面提升传质和电子转移;提供成核位点;非金属材料互相耦合掺杂调整碳晶格,提升稳定性 |
| 优化方式 | 反应机理 | 制备策略 |
|---|---|---|
| 改变形貌 | 改变催化剂本身结构,增大反应面积;改变催化剂晶格相提升性能;缺陷工程增加成核位点 | 并非使用单一优化方法,采用多种优化方式结合以制备高效稳定的析氢催化剂 |
| 掺杂元素 | 元素协同作用优化催化剂电子结构;优化表面电子-质子耦合机制;杂原子提升催化剂稳定性和活性;掺杂具有独特电子结构的稀土元素,调节催化剂电子态密度 | |
| 使用载体 | 均匀分散催化剂;载体与催化剂耦合界面提升传质和电子转移;提供成核位点;非金属材料互相耦合掺杂调整碳晶格,提升稳定性 |
| [1] | WANG Gang, YU Minghao, FENG Xinliang. Carbon materials for ion-intercalation involved rechargeable battery technologies[J]. Chemical Society Reviews, 2021, 50(4): 2388-2443. |
| [2] | SUN Jianpeng, ZHAO Zhan, LI Jiao, et al. Recent advances in transition metal selenides-based electrocatalysts: Rational design and applications in water splitting[J]. Journal of Alloys and Compounds, 2022, 918: 165719. |
| [3] | GE Zhenhua, FU Bin, ZHAO Jinping, et al. A review of the electrocatalysts on hydrogen evolution reaction with an emphasis on Fe, Co and Ni-based phosphides[J]. Journal of Materials Science, 2020, 55(29): 14081-14104. |
| [4] | 宋东新, 王世乐, 苗剑, 等. 可再生能源制氢技术经济性探讨及成本分析[J]. 中外能源, 2023, 28(11): 24-29. |
| SONG Dongxin, WANG Shile, MIAO Jian, et al. Economics and cost analysis of technologies for hydrogen production from renewable energy[J]. Sino-Global Energy, 2023, 28(11): 24-29. | |
| [5] | LIANG Jia, YANG Yingchao, ZHANG Jing, et al. Metal diselenide nanoparticles as highly active and stable electrocatalysts for the hydrogen evolution reaction[J]. Nanoscale, 2015, 7(36): 14813-14816. |
| [6] | 李锐. Zif-67衍生的硒化钴基复合材料的制备及其电解水析氢性能研究[D]. 扬州: 扬州大学, 2023. |
| LI Rui. Preparation of cobalt selenide-based composite derived from ZIF-67 and its hydrogen evolution performance by electrolytic water[D]. Yangzhou: Yangzhou University, 2023. | |
| [7] | 纪志愿, 周琴. 氢气在能源领域发展中的作用[C]//2013年年会暨工业气体供应技术论坛论文集(上海). 上海. 中国动力工程学会工业气体专业委员会, 2014: 67-76. |
| JI Zhiyuan, ZHOU Qin. The role of hydrogen in the development of energy[C]//Proceedings of the 2013 Annual Meeting and Industrial Gas Supply Technology Forum (Shanghai). Shanghai: China Power Engineering Society industrial gas professional committee, 2014: 67-76. | |
| [8] | 廖龙飞, 李明雨, 尹永利, 等. 碱性水电解制氢催化剂研究进展[J]. 工业催化, 2023, 31(2): 7-17. |
| LIAO Longfei, LI Mingyu, YIN Yongli, et al. Research progress on catalysts of alkaline water electrolysis for hydrogen production[J]. Industrial Catalysis, 2023, 31(2): 7-17. | |
| [9] | ANANTHARAJ Sengeni, Sivasankara Rao EDE, SAKTHIKUMAR Kuppan, et al. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of Fe, Co, and Ni: A review[J]. ACS Catalysis, 2016, 6(12): 8069-8097. |
| [10] | PENG Yuanting, LIAO Yucong, YE Donghao, et al. Recent advances regarding precious metal-based electrocatalysts for acidic water splitting[J]. Nanomaterials, 2022, 12(15): 2618. |
| [11] | 张舒涵. 质子交换膜电解水制氢中阴阳极催化剂及膜电极的制备研究[D]. 杭州: 浙江大学, 2023. |
| ZHANG Shuhan. Study on preparation of anode and cathode catalysts and membrane electrodes in proton exchange membrane water electrolysis for H2 production[D]. Hangzhou: Zhejiang University, 2023. | |
| [12] | PARSONS Roger. The rate of electrolytic hydrogen evolution and the heat of adsorption of hydrogen[J]. Transactions of the Faraday Society, 1958, 54: 1053-1063. |
| [13] | LI Haiyao, GUO Jun, LI Zhishan, et al. Research progress of hydrogen production technology and related catalysts by electrolysis of water[J]. Molecules, 2023, 28(13): 5010. |
| [14] | CONWAY B E, JERKIEWICZ G. Relation of energies and coverages of underpotential and overpotential deposited H at Pt and other metals to the ‘volcano curve’ for cathodic H2 evolution kinetics[J]. Electrochimica Acta, 2000, 45(25/26): 4075-4083. |
| [15] | KHAN Noureen Amir, RAHMAN Gul, NGUYEN Tung M, et al. Recent development of nanostructured nickel metal-based electrocatalysts for hydrogen evolution reaction: A review[J]. Topics in Catalysis, 2023, 66(1): 149-181. |
| [16] | LI Huan, WANG Yanan, YANG Xiaofan, et al. Structure and superconducting properties of multiple phases of (NH3) y M x FeSe (M: Ca, Sr and Ba) at ambient and high pressures[J]. Summary Collection of Lectures of the Japanese Physical Society, 2020, 803(2189): 1723. |
| [17] | 张泽霞, 吕瑞涛, 黄正宏, 等. 碳基材料在电催化析氢反应中的应用[J]. 新型炭材料, 2019, 34(2): 115-131. |
| ZHANG Zexia, Ruitao LYU, HUANG Zhenghong, et al. Carbon materials for use in the electrocatalytic hydrogen evolution reaction[J]. New Carbon Materials, 2019, 34(2): 115-131. | |
| [18] | GHOSH Srabanti, BASU Rajendra N. Multifunctional nanostructured electrocatalyts for energy conversion and storage: Current status and perspectives [J]. Nanoscale, 2018, 10(24): 11241-11280. |
| [19] | GUO Desheng, WEN Lingling, WANG Tiantian, et al. Electrodeposition synthesis of cobalt-molybdenum bimetallic phosphide on nickel foam for efficient water splitting[J]. Journal of Colloid and Interface Science, 2024, 659: 707-717. |
| [20] | MALTA O L. Mechanisms of non-radiative energy transfer involving lanthanide ions revisited[J]. Journal of Non-Crystalline Solids, 2008, 354(42/43/44): 4770-4776. |
| [21] | CHENG Niancai, STAMBULA Samantha, WANG Da, et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction[J]. Nature Communications, 2016, 7: 13638. |
| [22] | FAN Lili, DU Xinxin, ZHOU Sainan, et al. Efficient platinum harvesting of MOF-derived N-doped carbon through cathodic cyclic voltammetry for hydrogen evolution[J]. Electrochimica Acta, 2019, 317: 173-181. |
| [23] | DU Zuokai, WANG Yilong, LI Junsheng, et al. Facile fabrication of Pt-Ni alloy nanoparticles supported on reduced graphene oxide as excellent electrocatalysts for hydrogen evolution reaction in alkaline environment[J]. Journal of Nanoparticle Research, 2019, 21(1): 13. |
| [24] | YU Jie, DAI Yawen, WU Xinhao, et al. Ultrafine ruthenium-iridium alloy nanoparticles well-dispersed on N-rich carbon frameworks as efficient hydrogen-generation electrocatalysts[J]. Chemical Engineering Journal, 2021, 417: 128105. |
| [25] | 李宪荣. Ni-MOFs衍生过渡金属硫属化物复合材料的制备及储钠性能研究[D]. 哈尔滨: 哈尔滨工业大学, 2022. |
| LI Xianrong. Synthesis of nickel-MOFs derived transition metal-sulfide composites and their sodium-storage properties[D]. Harbin: Harbin Institute of Technology, 2022. | |
| [26] | Azad MALIK M, REVAPRASADU Neerish, Paul O’BRIEN. Air-stable single-source precursors for the synthesis of chalcogenide semiconductor nanoparticles[J]. Chemistry of Materials, 2001, 13(3): 913-920. |
| [27] | SAGADE Abhay A, SHARMA Ramphal. Copper sulphide (Cu x S) as an ammonia gas sensor working at room temperature[J]. Sensors and Actuators B: Chemical, 2008, 133(1): 135-143. |
| [28] | NIU H J, HAMPSHIRE D P. Critical parameters of disordered nanocrystalline superconducting Chevrel-phase PbMo6S8 [J]. Physical Review B, 2004, 69(17): 174503. |
| [29] | YU Xiaolong, WANG Yu, CHAN Huiwen, et al. Novel gas sensoring materials based on CuS hollow spheres[J]. Microporous and Mesoporous Materials, 2009, 118(1/2/3): 423-426. |
| [30] | MURRAY C B, NORRIS D J, BAWENDI M G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites[J]. Journal of the American Chemical Society, 1993, 115(19): 8706-8715. |
| [31] | COLEMAN Jonathan N, LOTYA Mustafa, Arlene O'NEILL, et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials[J]. Science, 2011, 331(6017): 568-571. |
| [32] | CHEN Xuanwa, YU Yanhui, LI Jing, et al. Recent advances in cobalt disulfide for electrochemical hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2023, 48(25): 9231-9243. |
| [33] | ZHANG Jing, WU Jingjie, GUO Hua, et al. Unveiling active sites for the hydrogen evolution reaction on monolayer MoS2 [J]. Advanced Materials, 2017, 29(42): 1701955. |
| [34] | 方健, 许志志, 李雨贝, 等. 2H-MoS2定向调控生成1T-MoS2及应用[J]. 精细化工, 2022, 39(7): 1339-1351. |
| FANG Jian, XU Zhizhi, LI Yubei, et al. Directional regulation of 2H-MoS2 to produce 1T-MoS2 and its application[J]. Fine Chemicals, 2022, 39(7): 1339-1351. | |
| [35] | GENG Xiumei, JIAO Yucong, HAN Yang, et al. Freestanding metallic 1T MoS2 with dual ion diffusion paths as high rate anode for sodium‐ion batteries[J]. Advanced Functional Materials, 2017, 27(40): 1702998. |
| [36] | CAI Liang, HE Jingfu, LIU Qinghua, et al. Vacancy-induced ferromagnetism of MoS2 nanosheets[J]. Journal of the American Chemical Society, 2015, 137(7): 2622-2627. |
| [37] | LI Yanguang, WANG Hailiang, XIE Liming, et al. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction[J]. Journal of the American Chemical Society, 2011, 133(19): 7296-7299. |
| [38] | ZENG Xiaojun, YANG Bai, LI Xiaopan, et al. Three-dimensional hollow CoS2 nanoframes fabricated by anion replacement and their enhanced pseudocapacitive performances[J]. Electrochimica Acta, 2017, 240: 341-349. |
| [39] | WANG Haotian, KONG Desheng, JOHANES Petr, et al. MoSe2 and WSe2 nanofilms with vertically aligned molecular layers on curved and rough surfaces[J]. Nano Letters, 2013, 13(7): 3426-3433. |
| [40] | TAN Gangjian, ZHAO Lidong, KANATZIDIS Mercouri G. Rationally designing high-performance bulk thermoelectric materials[J]. Chemical Reviews, 2016, 116(19): 12123-12149. |
| [41] | CHEN Fan, TANG Qing, MA Tian, et al. Structures, properties, and challenges of emerging 2D materials in bioelectronics and biosensors[J]. InfoMat, 2022, 4(5): e12299. |
| [42] | VIVANCO Hector K, RODRIGUEZ Efrain E. The intercalation chemistry of layered iron chalcogenide superconductors[J]. Journal of Solid State Chemistry, 2016, 242: 3-21. |
| [43] | 孟祥宇, 邓德会. 二维催化材料在电解水中的研究进展[J]. 科学通报, 2017, 62(27): 3154-3174. |
| MENG Xiangyu, DENG Dehui. Two-dimensional materials for electrocatalytic water splitting[J]. Chinese Science Bulletin, 2017, 62(27): 3154-3174. | |
| [44] | DENG Yuting, XIAO Sutong, ZHENG Yijuan, et al. Emerging electrocatalytic activities in transition metal selenides: Synthesis, electronic modulation, and structure-performance correlations[J]. Chemical Engineering Journal, 2023, 451: 138514. |
| [45] | 严若薇. 纳米碳材料负载过渡金属硫属化合物的制备及其在超级电容器上的应用研究[D]. 福州: 福州大学, 2020. |
| YAN Ruowei. Synthesis of carbon materials loaded metal chalcogenide and its application on high-performance supercapacitor[D]. Fuzhou: Fuzhou University, 2020. | |
| [46] | 吴晓琳. 镍钴基氧族催化电极的制备及其电解水性能研究[D]. 杭州: 浙江大学, 2018. |
| WU Xiaolin. Synthesis and studies of nickel/cobalt-based oxides and chalcogenides for electrocatalytic water splitting[D]. Hangzhou: Zhejiang University, 2018. | |
| [47] | 张丽丽. 过渡金属硫属化物和磷化物异质结电催化析氢性能研究[D]. 长春: 长春工业大学, 2023. |
| ZHANG Lili. Study on the electrocatalytic hydrogen evolution performance of transition metal dichalcogenides and phosphides heterojunctions[D]. Changchun: Changchun University of Technology, 2023. | |
| [48] | SWESI A T, MASUD J, NATH M. Nickel selenide as a high-efficiency catalyst for oxygen evolution reaction[J]. Energy & Environmental Science, 2016, 9(5): 1771-1782. |
| [49] | HUANG Zhaodi, YUAN Shuai, ZHANG Tiantian, et al. Selective selenization of mixed-linker Ni-MOFs: NiSe2@NC core-shell nano-octahedrons with tunable interfacial electronic structure for hydrogen evolution reaction[J]. Applied Catalysis B: Environmental, 2020, 272: 118976. |
| [50] | JIAO Chuanlai, BO Xiangjie, ZHOU Ming. Electrocatalytic water splitting at nitrogen-doped carbon layers-encapsulated nickel cobalt selenide[J]. Journal of Energy Chemistry, 2019, 34: 161-170. |
| [51] | WANG Guoxu, CHEN Wei, CHEN Guangliang, et al. Trimetallic Mo-Ni-Co selenides nanorod electrocatalysts for highly-efficient and ultra-stable hydrogen evolution[J]. Nano Energy, 2020, 71: 104637. |
| [52] | HUANG Jingbin, JIANG Yan, MENG Tao, et al. Regulating electronic structure and adsorptivity in molybdenum selenide for boosting electrocatalytic water splitting[J]. Electrochimica Acta, 2021, 390: 138888. |
| [53] | ZHANG Long, WANG Tao, SUN Lan, et al. Hydrothermal synthesis of 3D hierarchical MoSe2/NiSe2 composite nanowires on carbon fiber paper and their enhanced electrocatalytic activity for the hydrogen evolution reaction[J]. Journal of Materials Chemistry A, 2017, 5(37): 19752-19759. |
| [54] | HOU Jungang, SUN Yiqing, LI Zhuwei, et al. Electrical behavior and electron transfer modulation of nickel-copper nanoalloys confined in nickel-copper nitrides nanowires array encapsulated in nitrogen‐doped carbon framework as robust bifunctional electrocatalyst for overall water splitting[J]. Advanced Functional Materials, 2018, 28(37): 1803278. |
| [55] | MA Zhiyuan, LI Zhicheng, LI Shuhua, et al. Nanostructured Ni2N thin films magnetron-sputtered on nickel foam as efficient electrocatalyst for hydrogen evolution reaction[J]. Materials Letters, 2018, 229: 148-151. |
| [56] | SCHWARZ Karlheinz. Band structure and chemical bonding in transition metal carbides and nitrides[J]. Critical Reviews in Solid State and Materials Sciences, 1987, 13(3): 211-257. |
| [57] | 杜其星. 过渡金属氮化物(M x N, M=Ni, Mo, Co)基催化剂的制备及其电解水性能研究[D]. 上海: 上海大学, 2022. |
| DU Qixing. Preparation and water electrolysis performance of transition metal nitride(M x N, M=Ni, Mo, Co)-based catalysts[D]. Shanghai: Shanghai University, 2022. | |
| [58] | 梁书芹. 镍基氮化物复合催化剂的制备以及高效电解水性能的研究[D]. 北京: 中国石油大学(北京), 2022. |
| LIANG Shuqin. Preparation of nickel-based nitride composite catalyst and its high efficiency in water electrolysis[D]. Beijing: China University of Petroleum (Beijing), 2022. | |
| [59] | YIN Zhuoxun, SUN Yue, ZHU Chunling, et al. Bimetallic Ni-Mo nitride nanotubes as highly active and stable bifunctional electrocatalysts for full water splitting[J]. Journal of Materials Chemistry A, 2017, 5(26): 13648-13658. |
| [60] | LIU Bin, HE Bin, PENG Huiqing, et al. Unconventional nickel nitride enriched with nitrogen vacancies as a high-efficiency electrocatalyst for hydrogen evolution[J]. Advanced Science, 2018, 5(8): 1800406. |
| [61] | CHEN Zhiyan, SONG Yao, CAI Jinyan, et al. Tailoring the d-band centers enables Co4N nanosheets to be highly active for hydrogen evolution catalysis[J]. Angewandte Chemie International Edition, 2018, 57(18): 5076-5080. |
| [62] | 高岩峰, 贾少培, 刘奇鹏, 等. 金属磷化物在电解水制氢中的应用研究进展[J]. 工业催化, 2024, 32(1): 1-13. |
| GAO Yanfeng, JIA Shaopei, LIU Qipeng, et al. A review on metal phosphides in hydrogen production by electrolysis of water[J]. Industrial Catalysis, 2024, 32(1): 1-13. | |
| [63] | 雷琬莹, 吴攀, 司渭滨, 等. 过渡金属磷化物基材料在电催化析氢中的改性策略:现状及展望[J]. 复合材料学报, 2024, 41(4): 1737-1749. |
| LEI Wanying, WU Pan, SI Weibin, et al. Modification strategies of transition metal phosphide-based materials in electrocatalytic hydrogen evolution: Current status and prospect[J]. Acta Materiae Compositae Sinica, 2024, 41(4): 1737-1749. | |
| [64] | 董美君, 张超, 郝敏彤, 等. 过渡金属磷化物的制备及电催化性能研究进展[J]. 现代化工, 2023, 43(12): 69-73, 82. |
| DONG Meijun, ZHANG Chao, HAO Mintong, et al. Progress in preparation and electrocatalysis properties of transition metal phosphates[J]. Modern Chemical Industry, 2023, 43(12): 69-73, 82. | |
| [65] | 贾珺然. 过渡金属氮化物的制备及其在电解水领域的应用[D]. 南京: 南京大学, 2019. |
| JIA Junran. Preparation of transition metal nitrides and their application in water splitting[D]. Nanjing: Nanjing University, 2019. | |
| [66] | 罗义宽. 过渡金属(Ni,Co)基磷化物的制备及其电催化性能研究[D]. 桂林: 桂林电子科技大学, 2023. |
| LUO Yikuan. Study of electrocatalytic properties and preparation of transition metal (Ni,Co) based phosphides[D]. Guilin: Guilin University of Electronic Technology, 2023. | |
| [67] | WENG Chenchen, REN Jintao, YUAN Zhongyong. Transition metal phosphide-based materials for efficient electrochemical hydrogen evolution: A critical review[J]. ChemSusChem, 2020, 13(13): 3357-3375. |
| [68] | BAI Yuanjuan, FANG Ling, XU Haitao, et al. Strengthened synergistic effect of metallic M x P y (M = Co, Ni, and Cu) and carbon layer via peapod-like architecture for both hydrogen and oxygen evolution reactions[J]. Small, 2017, 13(16): 1603718. |
| [69] | ZHOU Lei, SHAO Mingfei, LI Jianbo, et al. Two-dimensional ultrathin arrays of CoP: Electronic modulation toward high performance overall water splitting[J]. Nano Energy, 2017, 41: 583-590. |
| [70] | WU Rui, XIAO Bing, GAO Qiang, et al. A janus nickel cobalt phosphide catalyst for high-efficiency neutral-pH water splitting[J]. Angewandte Chemie International Edition, 2018, 57(47): 15445-15449. |
| [71] | WANG Xiujuan, CHEN Kai, WANG Gang, et al. Rational design of three-dimensional graphene encapsulated with hollow FeP@Carbon nanocomposite as outstanding anode material for lithium ion and sodium ion batteries[J]. ACS Nano, 2017, 11(11): 11602-11616. |
| [72] | ZHANG Shen, ZHANG Xing, RUI Yuan, et al. Recent advances in non-precious metal electrocatalysts for pH-universal hydrogen evolution reaction[J]. Green Energy & Environment, 2021, 6(4): 458-478. |
| [73] | ZHU Xiaohua, LIU Mengjia, LIU Yang, et al. Carbon-coated hollow mesoporous FeP microcubes: An efficient and stable electrocatalyst for hydrogen evolution[J]. Journal of Materials Chemistry A, 2016, 4(23): 8974-8977. |
| [74] | ZHUANG Minghao, Xuewu OU, DOU Yubing, et al. Polymer-embedded fabrication of Co2P nanoparticles encapsulated in N,P-doped graphene for hydrogen generation[J]. Nano Letters, 2016, 16(7): 4691-4698. |
| [75] | QU Guoxing, ZHAO Yawen, ZHAO Guoliang, et al. Ultrahigh length-to-diameter ratio nickel phosphide nanowires as pH-wide electrocatalyst for efficient hydrogen evolution[J]. Electrochimica Acta, 2019, 298: 943-949. |
| [76] | KIM Jun-Hyuk, SHIN Kihyun, KAWASHIMA Kenta, et al. Enhanced activity promoted by CeO x on a CoO x electrocatalyst for the oxygen evolution reaction[J]. ACS Catalysis, 2018, 8(5): 4257-4265. |
| [77] | GAO Wei, WEN Dan, Johnny C HO, et al. Incorporation of rare earth elements with transition metal-based materials for electrocatalysis: A review for recent progress[J]. Materials Today Chemistry, 2019, 12: 266-281. |
| [78] | MILES Melvin H. Evaluation of electrocatalysts for water electrolysis in alkaline solutions[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1975, 60(1): 89-96. |
| [79] | SUN Zhaomei, ZHANG Jiayu, XIE Junfeng, et al. High-performance alkaline hydrogen evolution electrocatalyzed by a Ni3 N-CeO2 nanohybrid[J]. Inorganic Chemistry Frontiers, 2018, 5(12): 3042-3045. |
| [80] | WANG Xixi, YANG Yu, DIAO Lechen, et al. CeO x -decorated NiFe-layered double hydroxide for efficient alkaline hydrogen evolution by oxygen vacancy engineering[J]. ACS Applied Materials & Interfaces, 2018, 10(41): 35145-35153. |
| [81] | ZHANG Shuai, SAJI Sandra Elizabeth, YIN Zongyou, et al. Rare‐earth incorporated alloy catalysts: Synthesis, properties, and applications[J]. Advanced Materials, 2021, 33(16): 2005988. |
| [82] | HUANG Haiping, ZHU Junjie. The electrochemical applications of rare earth-based nanomaterials[J]. Analyst, 2019, 144(23): 6789-6811. |
| [83] | ZHENG Bingzhu, FAN Jingyue, CHEN Bing, et al. Rare-earth doping in nanostructured inorganic materials[J]. Chemical Reviews, 2022, 122(6): 5519-5603. |
| [84] | WANG Xuan, TANG Yawen, LEE Jong-Min, et al. Recent advances in rare-earth-based materials for electrocatalysis[J]. Chem Catalysis, 2022, 2(5): 967-1008. |
| [85] | 张宇超. 稀土修饰钴基纳米材料的控制合成、表面重构及电催化性能研究[D]. 呼和浩特: 内蒙古大学, 2023. |
| ZHANG Yuchao. Controlled synthesis of rare earth modified cobalt-based nanomaterials and their surface reconstruction and electrocatalytic performancs[D]. Hohhot: Inner Mongolia University, 2023. | |
| [86] | ZHANG Gengwei, WANG Bin, BI Jinglei, et al. Constructing ultrathin CoP nanomeshes by Er-doping for highly efficient bifunctional electrocatalysts for overall water splitting[J]. Journal of Materials Chemistry A, 2019, 7(10): 5769-5778. |
| [87] | SUN Hongming, TIAN Caiying, FAN Guilan, et al. Boosting activity on Co4N porous nanosheet by coupling CeO2 for efficient electrochemical overall water splitting at high current densities[J]. Advanced Functional Materials, 2020, 30(32): 1910596. |
| [88] | WANG Jiong, ZHANG Hua, WANG Xin. Recent methods for the synthesis of noble-metal-free hydrogen-evolution electrocatalysts: From nanoscale to sub-nanoscale[J]. Small Methods, 2017, 1(6): 1700118. |
| [89] | 贺艳静. 离子液体-氧化石墨烯复合体系的模型优化及模拟计算[D]. 锦州: 渤海大学, 2021. |
| HE Yanjing. Model optimization and simulation calculation of ionic liquid-graphene oxide composite system[D]. Jinzhou: Bohai University, 2021. | |
| [90] | CHATTOPADHYAY Jayeeta, PATHAK Tara Sankar, Daewon PAK. Heteroatom-doped metal-free carbon nanomaterials as potential electrocatalysts[J]. Molecules, 2022, 27(3): 670. |
| [91] | ANTHONYSAMY Shahreen Binti Izwan, AFANDI Syahidah Binti, KHAVARIAN Mehrnoush, et al. A review of carbon-based and non-carbon-based catalyst supports for the selective catalytic reduction of nitric oxide[J]. Beilstein Journal of Nanotechnology, 2018, 9: 740-761. |
| [92] | 沈燕, 袁维, 刘可, 等. 镍基催化剂电催化析氢应用研究进展[J]. 山西化工, 2023, 43(12): 34-35, 43. |
| SHEN Yan, YUAN Wei, LIU Ke, et al. Research progress of electrocatalytic hydrogen evolution with nickel-based catalysts[J]. Shanxi Chemical Industry, 2023, 43(12): 34-35, 43. | |
| [93] | 周旋, 李梦锐, 陈一尘, 等. 镍基磷化物复合材料在催化电解水析氢性能提升方面的研究进展[J]. 无机盐工业, 2024, 56(4): 8-15, 33. |
| ZHOU Xuan, LI Mengrui, CHEN Yichen, et al. Research progress of nickel-based phosphide composites in improving of catalytic water electrolysis for hydrogen evolution performance[J]. Inorganic Chemicals Industry, 2024, 56(4): 8-15, 33. | |
| [94] | 瞿国兴. 非金属元素掺杂型电催化剂的制备及其催化电解水性能研究[D]. 成都: 电子科技大学, 2020. |
| QU Guoxing. The study of nonmetal-doped electrocatalyst synthesis and their performances in catalyzing water electrolysis[D]. Chengdu: University of Electronic Science and Technology of China, 2020. | |
| [95] | WANG Xuesi, VASILEFF Anthony, JIAO Yan, et al. Electronic and structural engineering of carbon-based metal-free electrocatalysts for water splitting[J]. Advanced Materials, 2019, 31(13): 1803625. |
| [96] | ZHAO Yang, ZHAO Fei, WANG Xiaopeng, et al. Graphitic carbon nitride nanoribbons: Graphene-assisted formation and synergic function for highly efficient hydrogen evolution[J]. Angewandte Chemie International Edition, 2014, 53(50): 13934-13939. |
| [97] | ZHANG Jintao, QU Liangti, SHI Gaoquan, et al. N,P‐codoped carbon networks as efficient metal-free bifunctional catalysts for oxygen reduction and hydrogen evolution reactions[J]. Angewandte Chemie International Edition, 2016, 55(6): 2230-2234. |
| [1] | LIU Zhe, ZHOU Shunli, LI Yongxiang, ZHANG Chengxi, LIU Yipeng. Research progress on alkyl naphthalene synthesis catalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 144-158. |
| [2] | LIN Yijie, QIAO Peng, LI Xinrui, ZHANG Hongbin, WANG Xueqin. Construction and application of heterostructures of photocatalyst TiO2 nanomaterials [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 159-177. |
| [3] | WANG Tao, ZHANG Xuebing, ZHANG Qi, CHEN Qiang, ZHANG Kui, MEN Zhuowu. Effects of reduction-carburization temperature and inlet CO concentration on industrial precipitated iron-based catalyst for Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 178-184. |
| [4] | BAO Xinde, LIU Biye, HUANG Renwei, HONG Yuhao, GUAN Xin, LIN Jinguo. Preparation of biomass-based@CuNiOS composite catalysts for the reduction of organic dye [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 185-196. |
| [5] | ZHAO Siyang, LI Chenran, LIU Yang. Process optimization for regulating diene selectivity of MTO regenerated catalyst through pre-carbon deposition using C4 by-product [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 205-212. |
| [6] | ZHAO Yulong, CAI Kai, YU Shanqing. Influence of pore structure of alumina on the adsorption, diffusion and reactivity of hydrocarbon molecules in catalytic cracking [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 213-221. |
| [7] | LI Junliang, LI Yue, SUN Daolai. Hydrodeoxygenation of 1,2-butanediol to 1-butanol over Cu/SiO2-Al2O3 catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 222-231. |
| [8] | LIU Chao, DING Chengao, WU Baoshun, LEI Xinyu, WANG Guangying, YU Zhengwei. Effect of TiO2 support particle size on the denitrification and water/sulfur poisoning resistance of RuO x -V2O5-WO3/TiO2 catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 232-242. |
| [9] | ZHANG Hanlin, YUE Xuehai, LIU Junxi, YIN Fengjun. Fabrication of high stability electrocatalyst for oxygen evolution reaction by Ru-Sr-Ir electrodeposition [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 243-251. |
| [10] | WANG Wenjun, LIU Ruixin, WANG Jun, ZHANG Qinglei, HOU Li’an. Research progress of visible light degradation of indoor VOCs by titanium dioxide materials [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5351-5362. |
| [11] | ZENG Jin, GAO Yan, WANG Zhaopeng, XIE Yuyun, LIU Jun, LIANG Qi, WANG Chunying. Degradation mechanism of 2,4-dichlorophenoxyacetic acid by NaYF4:Yb,Tm composite TiO2/Bi2WO6 photocatalyst and evaluation of products toxicity [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5416-5431. |
| [12] | WANG Zhen, ZHANG Yaoyuan, WU Qin, SHI Daxin, CHEN Kangcheng, LI Hansheng. Development of Ni/Al2O3-based catalysts for the dry reforming of methane [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4979-4998. |
| [13] | ZHANG Haipeng, QIN Shanshan, WANG Yuxuan, YU Haibiao. Preparation of 3.0F-Ag x Co catalysts for N2O decomposition [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4999-5005. |
| [14] | SUN Mengyuan, LU Shijian, LIU Ling, XUE Yanyang, ZHANG Yunrong, DONG Qi, KANG Guojun. Research progress of MOF and their derivatives in carbon capture [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5339-5350. |
| [15] | CHEN Zizhao, HE Fangshu, HU Qiang, YANG Yang, CHEN Hanping, YANG Haiping. Research progress on anti-carbon deposition Ni-based catalysts for dry reforming of methane [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4968-4978. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||