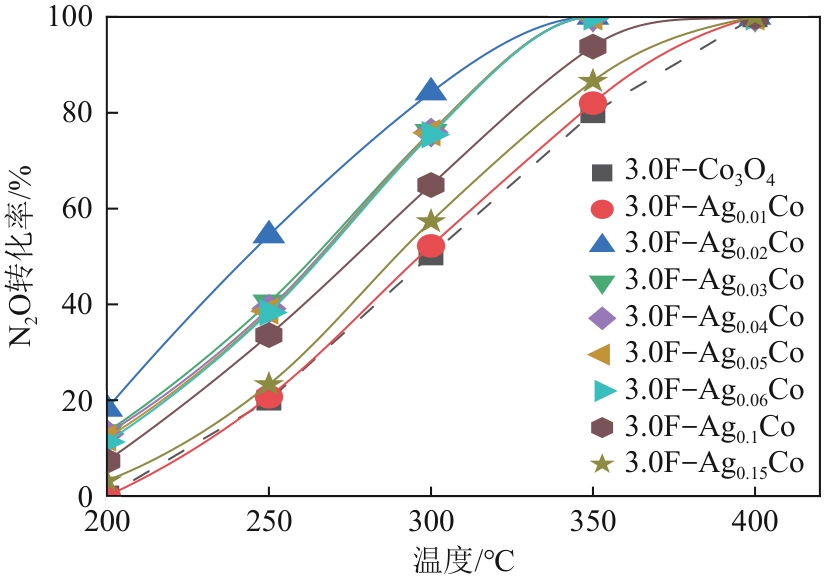
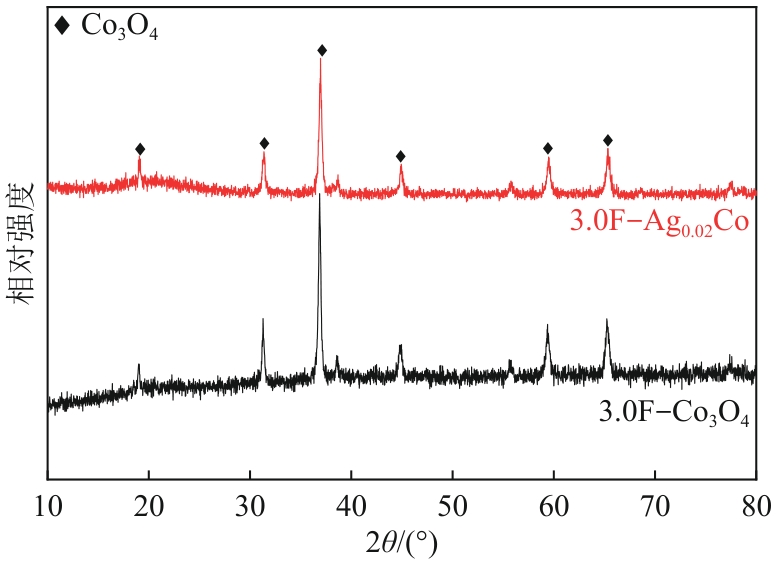
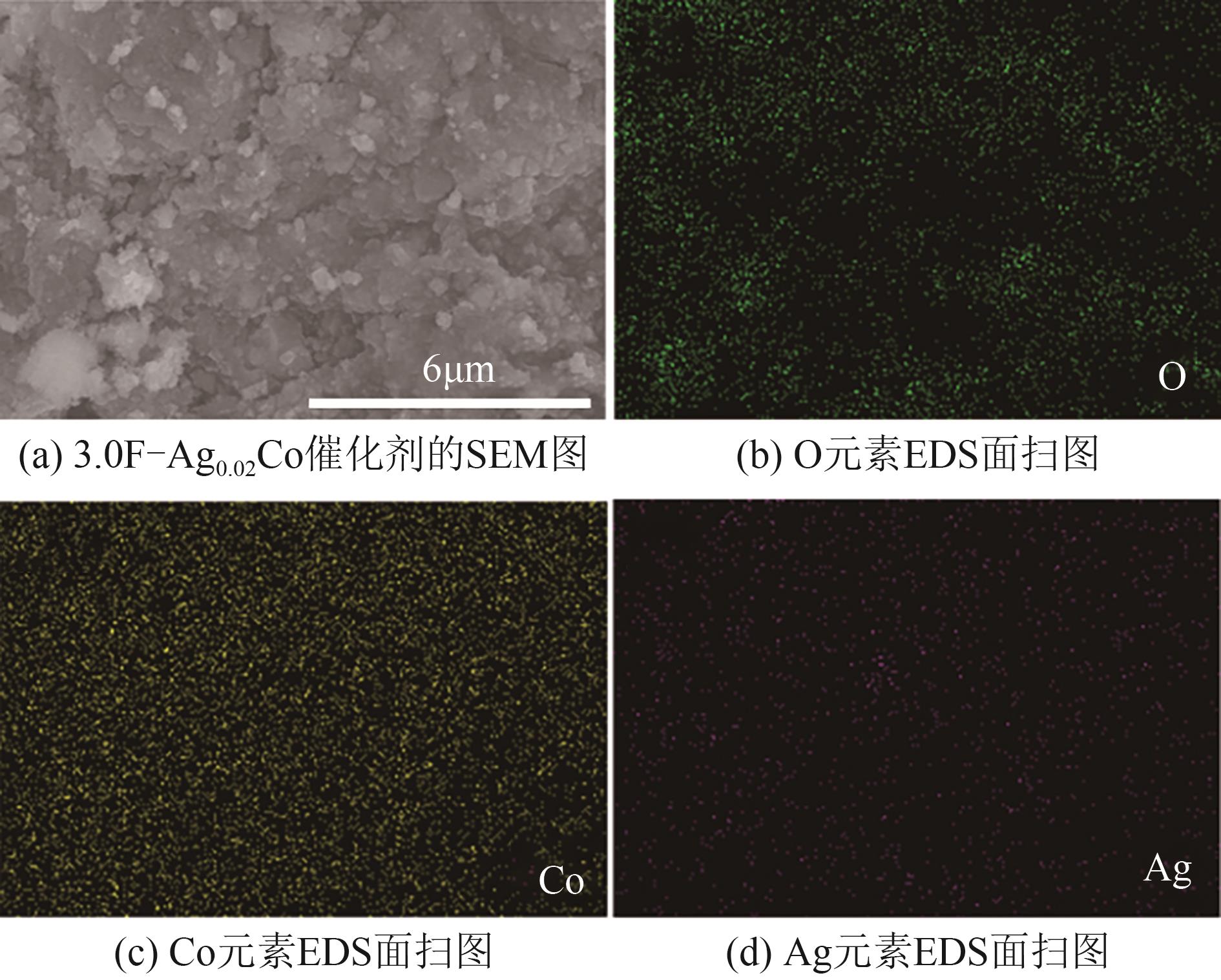
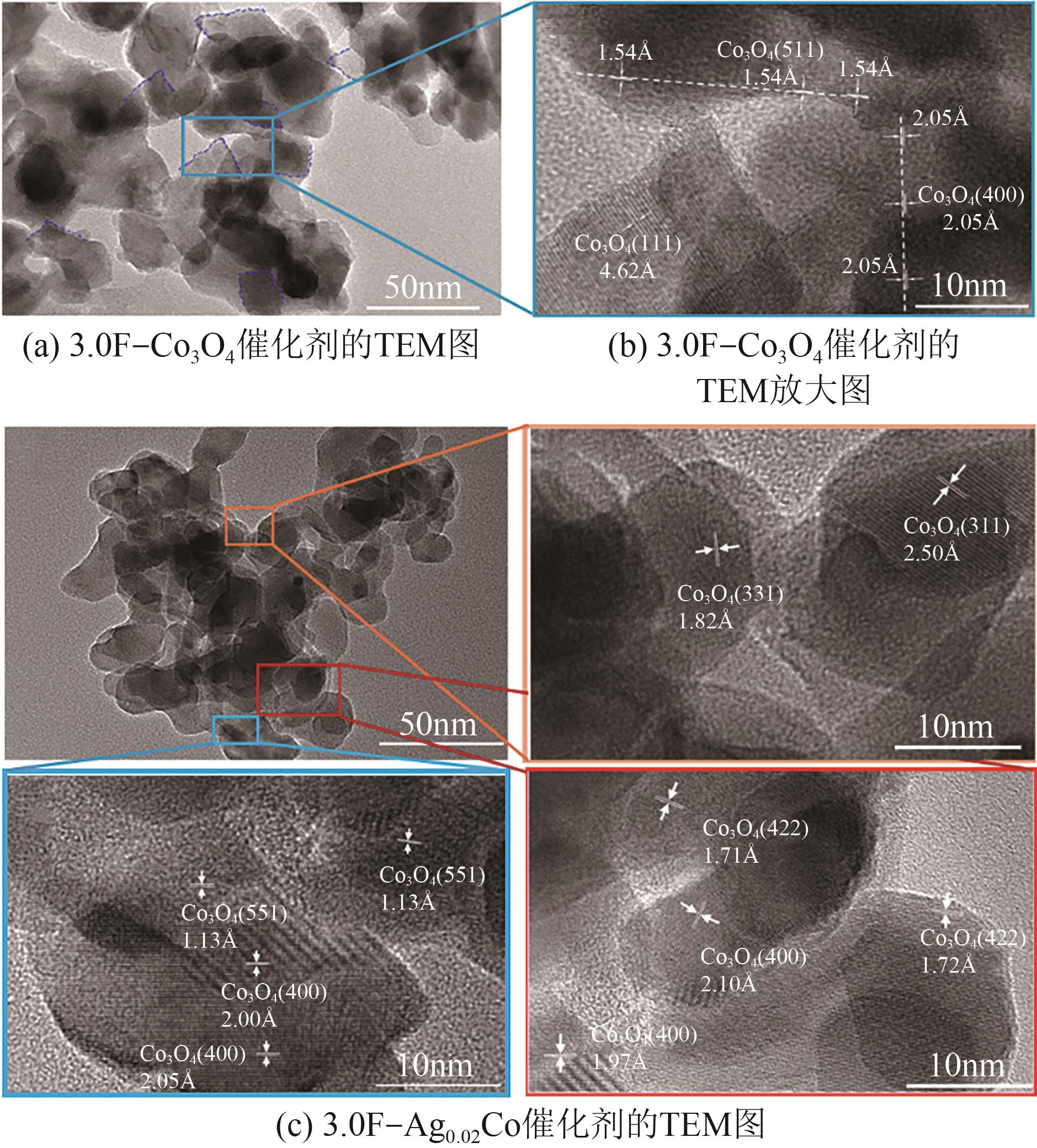
| [1] |
KAPTEIJN Freek, José RODRIGUEZ-MIRASOL, MOULIJN Jacob A. Heterogeneous catalytic decomposition of nitrous oxide[J]. Applied Catalysis B: Environmental, 1996, 9(1/2/3/4): 25-64.
|
| [2] |
PÉREZ-RAMı́REZ J, KAPTEIJN F, SCHÖFFEL K, et al. Formation and control of N2O in nitric acid production: Where do we stand today?[J]. Applied Catalysis B: Environmental, 2003, 44(2): 117-151.
|
| [3] |
KONSOLAKIS Michalis. Recent advances on nitrous oxide (N2O) decomposition over non-noble-metal oxide catalysts: Catalytic performance, mechanistic considerations, and surface chemistry aspects[J]. ACS Catalysis, 2015, 5(11): 6397-6421.
|
| [4] |
ZHUANG Zhongqi, GUAN Bin, CHEN Junyan, et al. Review of nitrous oxide direct catalytic decomposition and selective catalytic reduction catalysts[J]. Chemical Engineering Journal, 2024, 486: 150374.
|
| [5] |
LIU Hao, YANG Shan, WANG Guimin, et al. Strong electronic orbit coupling between cobalt and single-atom praseodymium for boosted nitrous oxide decomposition on Co3O4 catalyst[J]. Environmental Science & Technology, 2022, 56(22): 16325-16335.
|
| [6] |
YU Haibiao, WANG Xinping, LI Ye. Strong impact of cobalt distribution on the activity for Co3O4/CaCO3 catalyzing N2O decomposition[J]. Catalysis Today, 2020, 339: 274-280.
|
| [7] |
XIONG Ying, ZHAO Yumei, SHAN Weijun, et al. Potassium promoted Gd0.06Co catalysts for highly efficient catalytic N2O decomposition in presence of impurity gases at low temperature[J]. Chemosphere, 2022, 303: 135257.
|
| [8] |
XIONG Ying, ZHAO Yumei, QI Xingkun, et al. Strong structural modification of Gd to Co3O4 for catalyzing N2O decomposition under simulated real tail gases[J]. Environmental Science & Technology, 2021, 55(19): 13335-13344.
|
| [9] |
HU Xiaobo, WANG Yongzhao, WU Ruifang, et al. N-doped Co3O4 catalyst with a high efficiency for the catalytic decomposition of N2O[J]. Molecular Catalysis, 2021, 509: 111656.
|
| [10] |
WANG Qian, YANG Weiwei, DANG Hui, et al. Enhancement of N2O decomposition performance by co-doping of Ni and Y to Co3O4 catalyst[J]. Journal of Environmental Chemical Engineering, 2024, 12(2): 112463.
|
| [11] |
ABU-ZIED Bahaa M, BAWAKED Salem M, KOSA Samia A, et al. Effect of Pr, Sm, and Tb doping on the morphology, crystallite size, and N2O decomposition activity of Co3O4 nanorods[J]. Journal of Nanomaterials, 2015, 2015(1): 580582.
|
| [12] |
SUN Jinru, WANG Lu, ZHANG Lu, et al. Taming the redox property of A0.5Co2.5O4 (A=Mg, Ca, Sr, Ba) toward high catalytic activity for N2O decomposition[J]. ACS Applied Energy Materials, 2021, 4(8): 8496-8505.
|
| [13] |
LI Ye, TANG Fan, WANG Dongqi, et al. A key step for preparing highly active Mg-Co composite oxide catalysts for N2O decomposition[J]. Catalysis Science & Technology, 2021, 11(11): 3737-3745.
|
| [14] |
孙巾茹, 宋傲磊, 赵明新, 等. 沉淀剂对Co3O4催化分解N2O的性能影响[J]. 化工进展, 2024, 43(4): 1823-1831.
|
|
SUN Jinru, SONG Aolei, ZHAO Mingxin, et al. Effect of precipitating agent on the performance of Co3O4-catalyzed decomposition of N2O[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1823-1831.
|
| [15] |
LIU Hao, CHEN Jianjun, WANG Ya, et al. Boosting nitrous oxide direct decomposition performance based on samarium doping effects[J]. Chemical Engineering Journal, 2021, 414: 128643.
|
| [16] |
SONG Wenqiao, REN Zheng, CHEN Shengyu, et al. Ni- and Mn-promoted mesoporous Co3O4: A stable bifunctional catalyst with surface-structure-dependent activity for oxygen reduction reaction and oxygen evolution reaction[J]. ACS Applied Materials & Interfaces, 2016, 8(32): 20802-20813.
|
| [17] |
YU Haibiao, QI Xingkun, DU Xinwei, et al. The preparation of 3.0F-Co3O4 catalyst with “Yardang Landform” structure and its performance for catalyzing N2O decomposition[J]. Molecular Catalysis, 2023, 537: 112960.
|
| [18] |
于海彪. Co基复合氧化物催化N2O分解反应的研究[D]. 大连: 大连理工大学, 2019.
|
|
YU Haibiao. Study on decomposition of N2O catalyzed by co-based composite oxides[D]. Dalian: Dalian University of Technology, 2019.
|
| [19] |
贾瑞, 董文阳, 张磊, 等. Co3O4/KIT-6催化剂催化分解N2O反应研究[J]. 分子科学学报, 2023, 39(2): 154-164.
|
|
JIA Rui, DONG Wenyang, ZHANG Lei, et al. Catalytic decomposition of N2O over Co3O4/KIT-6 catalyst[J]. Journal of Molecular Science, 2023, 39(2): 154-164.
|
| [20] |
马木提江·吐尔逊. Co基复合氧化物催化剂催化分解N2O的研究[D]. 大连: 大连理工大学, 2015.
|
|
MAMUTJAN Tursun. Study on catalytic decomposition of N2O with co-based composite oxide catalyst[D]. Dalian: Dalian University of Technology, 2015.
|
| [21] |
齐兴堃. Co基氧化物催化剂微纳结构构筑及其催化净化N2O研究[D]. 沈阳: 辽宁大学, 2023.
|
|
QI Xingkun. Study on micro-nano structure construction of co-based oxide catalyst and its catalytic purification of N2O[D]. Shenyang: Liaoning University, 2023.
|
| [22] |
YU Haibiao, WANG Xinping, WU Xingxing, et al. Promotion of Ag for Co3O4 catalyzing N2O decomposition under simulated real reaction conditions[J]. Chemical Engineering Journal, 2018, 334: 800-806.
|
 ), QIN Shanshan2, WANG Yuxuan2, YU Haibiao2(
), QIN Shanshan2, WANG Yuxuan2, YU Haibiao2( )
)